Abstract
Purpose of Review
Cardiovascular disease (CVD) increasingly afflicts people living with HIV (PLWH) in the contemporary era of antiretroviral therapy (ART). Coronary artery disease (CAD) is the most widely studied cardiovascular problem in PLWH; however, less is known about other clinically relevant subtypes of CVD such as heart failure (HF), cerebrovascular disease, sudden cardiac death, pericardial diseases, and pulmonary hypertension. This paper reviews evidence of other subtypes of CVD as emerging issues in the post-ART era.
Recent Findings
Recent studies have shown that PLWH have higher risk of HF as well as subclinical impairment of left ventricular (LV) mechanics (systolic and diastolic dysfunction) and myocardial abnormalities (fibrosis and steatosis). The underlying mechanisms, however, are not well-understood. A few studies have also shown higher rates of atrial fibrillation and sudden cardiac death in PLWH. Ischemic stroke is the most common stroke type in the post-ART era, with underlying mechanisms like those identified in CAD: chronic inflammation and associated vasculopathy. Studies of great vessels (carotid artery and aorta) and peripheral arterial disease show heterogeneous results. Small subclinical pericardial effusions are common in PLWH in post-ART era. Pulmonary hypertension continues to be an underdiagnosed and potentially fatal complication of HIV infection.
Summary
PLWH remain at higher risk for all types of CVD including heart failure, stroke, and arrhythmias in the post-ART era. Chronic inflammation may play an important role in this increased risk. More studies are needed to further elucidate the extent of non-coronary CVD in PLWH and the underlying mechanisms for them.
Keywords: HIV infection, Cardiovascular disease, Stroke, Heart failure, Atrial fibrillation, Sudden cardiac death
Introduction
The effects of HIV infection on the cardiovascular system have changed in post-antiretroviral therapy (ART) era. As discussed in our companion article published in this issue [1], people living with HIV infection (PLWH) now live longer and thus will encounter more chronic diseases that are traditionally associated with aging, such as cardiovascular diseases (CVD). Coronary artery disease (CAD) is one of the first cardiovascular diseases that were reported in the post-ART era and is the most widely studied. However, recent studies have shown that the risk of CVD goes beyond coronary artery disease. In fact, the increased risk of conditions such as myocardial diseases appears to be even more dramatic than that of coronary artery disease. This article expands the discussion of CVD in HIV patients beyond coronary artery disease.
Heart Failure and Myocardial Diseases in HIV-Infected Patients
The clinical narrative of HIV-associated myocardial disease has changed with the widespread availability of ART. While HIV-associated cardiomyopathy was reported in 10–40% of HIV+ patients prior to ART [2•], there is a significant reduction in this rate after introduction of ART [3]. This reduction in cardiomyopathy and overt myocarditis (due to HIV or other co-infections) resulted in the initial underestimation of the risk of myocardial involvement and heart failure (HF) in HIV+ patients on ART. However, recent studies have shown that even on ART, HIV-infected patients remain at higher risk of HF [4–6]. More importantly, recent studies using cardiac imaging have shown the involvement of myocardium and impairment of global and regional systolic function as well as diastolic function even in asymptomatic HIV+ individuals on ART.
Heart Failure
Heart failure remains a significant problem in HIV-infected patients [4, 5]. A study on more than 8,000 veterans enrolled in two cohorts reported an age- and race/ethnicity-adjusted incidence of 7.1 (95% CI 6.9–7.3) and 4.8 (95% CI 4.7–4.9) per 1000 person-years for HIV-infected and uninfected veterans, respectively [6]. After adjustment for traditional risk factors, HIV-infected patients had a hazard ratio of 1.8 (95% CI 1.4–2.4) for developing HF and this increased risk persisted even among those without CAD. In this study, veterans on ART whose viral load was less than 500 copies/mL did not have an increased risk of symptomatic HF. However, a recent study of 36,400 patients with HIV and more than 12 million controls using medical records showed that the prevalence of HF was only marginally lower in HIV+ patients on ART compared to untreated patients (prevalence rates of 6.4 and 7.7%, respectively) [4]. The overall prevalence of HF in this study was 7.2% in HIV+ patients and 4.4% in controls. HF admission is also more common in HIV+ patients and per our previous study using Health Care Utilization Project (HCUP) data, the age of first hospitalization for HF in PLWH is on average 20 years younger than those without HIV infection (53 vs 73 years) [5].
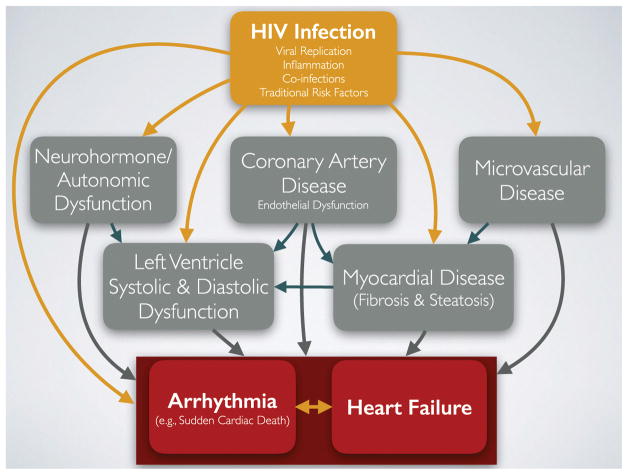
The sustained increased HF risk despite significant reduction in HIV-associated cardiomyopathy in HIV+ patients on ART can be attributed to other mechanisms involved in pathogenesis of HF [7]. HIV+ patients continue to have higher rates of traditional risk factors for CHF, drug abuse, and CAD. Additionally, HIV+ patients are shown to have higher levels of autonomic dysfunction [8, 9] and inflammation [10–12], which are considered novel risk factors for HF in the general population [13]. Finally, even though HIV+ patients on ART do not express overt cardiomyopathy, studies have shown increased subclinical cardiac mechanical dysfunction (both systolic and diastolic) and presence of myocardial abnormalities (fibrosis and steatosis). The natural history of these changes has not been studied yet, but if these processes are progressive, they can lead to even higher risk of HF as the HIV-infected population become older. Figure 1 provides a general paradigm for mechanisms associated with increased risk of HF based on the current state of our knowledge. Importantly, we believe many of the same pathways are involved in causing cardiac arrhythmias, as explained below.
Fig. 1.
HIV infection, through several known mechanisms such as viremia, inflammation, co-infections, and traditional risk factors such as dyslipidemia, smoking, and substance abuse, leads to higher risk of coronary artery disease, microvascular disease, and autonomic/neurohormone dysfunction. Subsequently, these abnormalities along with other unknown mechanisms result in myocardial disease and left ventricular dynamic dysfunction, which in turn give rise to higher risk of heart failure and arrhythmia
Left Ventricular Systolic and Diastolic Dysfunction
Several studies have evaluated the systolic and diastolic left ventricular (LV) dysfunction in HIV+ patients [2•, 14, 15•, 16]. Cerrato et al. [2•] conducted a meta-analysis of 11 studies that have assessed systolic and diastolic LV dysfunction using echocardiography (9 studies) or single photon emission computed tomography (SPECT; 2 studies). They reported that both systolic and diastolic dysfunctions are common problems in paucisymptomatic HIV+ patients (mostly on ART). In this study, the incidence of systolic dysfunction was 8.3% (95% CI 2.2–14.3%) and the incidence rates of grades 1, 2, and 3 diastolic dysfunctions (DD) were 31.9 (95% CI 24.9–43.7%), 8.5 (95% CI 2.1–14.9%), and 3.0% (95% CI 1.8–4.3%), respectively. High levels of high sensitivity C-reactive protein (hsCRP), active tobacco smoking, and history of myocardial infarction were significantly associated with systolic dysfunction, while older age and hypertension were related to higher risk of DD. Other studies have suggested that longer duration of HIV infection [17], higher body mass index [17], and exposure to zidovudine [18] were also associated with higher rates of DD. Hsue et al. [15•] reported an OR of 2.4 for DD in HIV+ patients compared to uninfected controls (p = 0.02) after adjustment for age and traditional risk factors. Studies have also shown that PLWH have higher LV mass, which has been associated with lower nadir CD4 counts [15•, 19]. Using tagged MRI and strain analyses, Lai et al. [20] also showed that in addition to global LV dysfunction, there is a higher rate of regional LV dysfunction in HIV+ patients.
Myocardial Fibrosis and Regional Myocardial Dysfunction
A recent study on 90 HIV+ patients undergoing combination ART and 39 uninfected controls with no history of cardiovascular disease using cardiac MRI studies reported that HIV-infected patients had a 6-fold higher rate of patchy myocardial fibrosis than uninfected individuals (76 versus 13%, respectively, p < 0.001) and 15% of HIV+ patients also had scars in the basal septum [21••]. The volumetric analyses also showed that the burden of patchy fibrosis in HIV patients was about twice of that of controls (p < 0.001). They also showed increased myocardial steatosis (higher lipid content) detected by MR spectroscopy. In a follow-up study from the same group [16], after adding 13 ART-naive HIV+ patients and 53 controls to the previous study population, patchy fibrosis was found in 83% of HIV+ patients (including ART-naive patients) and 16% in controls (p < 0.001). Another study also reported increased myocardial fibrosis and steatosis in HIV+ patients [22]. The underlying mechanisms involved in pathogenesis of these changes in the myocardium, their associated risk factors, and the clinical consequences of these findings have yet to be elucidated. Several ongoing studies are trying to answer these questions and investigate whether changes in LV function and the increased risk of arrhythmia (including sudden cardiac death) in these patients are related to these changes in the myocardium.
Disparities in HIV-Associated Heart Failure
HF in PLWH has attracted the attention of researchers interested in health disparities, including racial/ethnic disparities and disparities in access to health care. Important differences in the incidence and mechanisms of HF [23] and even therapeutic choices [24] by race/ethnicity have also been shown to exist in non-HIV-infected patients. Considering these racial/ethnic differences in the general population and the disproportionately higher incidence of HIV infection in African Americans and Hispanics in the USA [25], race/ethnicity should be taken into account in studies on HF in PLWH and particularly their comparison with general population.
Disparities in access to health care include the differences between low-to-middle income and higher income countries as well as disparities in the care provided to HIV+ patients compared to HIV-uninfected individuals. In low-to-middle income countries, systolic HF remains a more important contributor to the CVD burden in PLWH, even as access to ART expands [26]. On the other hand, studies have suggested that HIV+ patients with HF are less likely to be optimally treated with antiplatelet drugs, statins, diuretics, and angiotensin-converting enzyme inhibitors/aldosterone receptor blockers (ARBs; p < 0.0001 for all comparisons) compared to uninfected patients with heart failure [4, 5]. HIV+ patients who are admitted with HF undergo less coronary angiograms, percutaneous interventions, and coronary artery bypass grafts [5]. These disparities may be related to their younger age and different risk profile or clinical presentation—a notion that is supported by lower in-hospital mortality in HIV+ patients admitted for HF, despite receiving less procedures [5]. However, further studies are needed in this field to determine the sources of these disparities and the possibility of reducing or eliminating them.
Biomarkers in HIV-Associated Heart Failure
In a companion article, we have discussed the role of inflammation in CVD, including HF. In addition, another important system that plays a significant role in pathogenesis of LV dysfunction and HF is the autonomic system and associated neurohormones, such as B-type natriuretic peptide (BNP), which provides more opportunities to study biomarkers. Both C-reactive protein (CRP) and BNP serum levels were significantly higher in HIV-infected patients, but no association was found between these markers and diastolic dysfunction [15•]. N-terminal pro-BNP (NT-proBNP), which is secreted by ventricular myocytes under increased ventricular stretch and wall tension, has been associated with CVD in the general population [27, 28]. A case-control study nested in SMART found that NT-proBNP was associated with increased risk of having a CVD event among PLWH, after adjusting for traditional CVD risk factors, as well as levels of serum interleukin-6 (IL-6), highly sensitive CRP (hsCRP), and D-dimer [29]. A recent study aimed to examine biomarkers primarily expressed or secreted by cardiovascular tissue in response to pathological stress, including soluble ST2, growth differentiation factor (GDF)-15, NT-proBNP, and high-sensitivity troponin I. All of these biomarkers, except ST2, were elevated in HIV+ patients compared to HIV− individuals. Only ST2 was significantly associated with DD. GDF-15, NT-proBNP, and cystatin C were significantly associated with pulmonary hypertension. ST2, GDF-15, hsCRP, and D-dimer were all independently associated with all-cause mortality in adjusted analyses, and these associations remained significant when restricting analyses to virally suppressed HIV-infected patients undergoing ART [30].
Treatment of Heart Failure in HIV-Infected Patients
There are no published clinical trials on treatment of HF in HIV+ patients. Among drugs used in HF, digoxin is found to be highly active against HIV-1 in vitro [31] and if this is validated in human studies, this may be an attractive choice in HIV+ patients with HF. While the specific benefits of angiotensin-converting enzyme inhibitors and ARBs on heart failure are not reported, a pilot study has suggested that ARB (telmisartan) may increase endothelial progenitor cells (EPC) and improve vascular repair in older HIV+ patients (median age, 60) [32]. Also, an older retrospective study on 49 HIV+ children showed that intravenous immunoglobulin (IVIG) could improve LV wall thickness [33]. HIV+ patients should also be considered for LV-assisted device (LVAD) placement and heart transplant. Unfortunately, more than half of transplant centers in the USA consider HIV+ serostatus as a contraindication [34], while the guidelines from the 2001 United Network for Organ Sharing (UNOS) states that asymptomatic HIV+ patients should not necessarily be excluded from transplant lists. The limited available data do not show any difference in the outcomes of heart transplant and LVAD in HIV+ patients on ART compared to the general population [34, 35].
Cardiac Arrhythmia
Atrial Fibrillation
Atrial fibrillation (AF) is the most common form of arrhythmia in the general population and a major contributor to several comorbidities such as stroke and heart failure [36]. However, little is currently known about the relationship of HIV infection and AF. In a large study of veterans, the age-adjusted rates of AF found in HIV+ veterans were noticeably higher than the rates reported in several other cohort studies in the general population [37•]. As expected, this difference was even more striking in younger age groups. For example, the crude incidence rates of AF in age groups 35–44, 45–54, and 55–64 in HIV+ veterans were 1.8, 3.7, and 8.7 per 1000 person-years, while these rates in HIV− participants of the Manitoba study were 0.1–0.25, 0.5–0.8, and 2.3–3.7 per 1000 person-years [38]. In multivariable analyses, after adjusting for traditional risk factors, markers of HIV disease severity such as a lower CD4(+) cell count and higher viral load were associated with higher risk of AF. Other important risk factors for increased risk of AF included older age, Caucasian ethnicity, CAD, HF, alcoholism, proteinuria, renal dysfunction, and hypothyroidism [37•].
Another important consideration in management of HIV+ patients with AF is anticoagulation. Recent analyses using Veterans Affairs HIV Clinical Case Registry [39] suggest that CHADS2VASc score was not a strong predictor of thromboembolic events in this subgroup. Furthermore, they found that warfarin therapy was actually associated with more thromboembolic events, even after adjusting for CHADS2VASc score (HR 2.2 [95% CI 1.1–4.7, p = 0.04]. These findings have important clinical implications, suggesting that more complex pathophysiologic mechanisms may be at play in HIV-associated AF than is known about AF in non-infected patients. Further studies are needed to elucidate the underlying mechanisms and risk factors for AF, particularly in view of our increasing knowledge of other structural changes in the heart of HIV+ patients.
Sudden Cardiac Death
Sudden cardiac death (SCD) is another subtype of CVD that is suggested to be more common in HIV+ population. As summarized above and in the companion article published with this paper [1], there is growing evidence indicating that HIV+ patients are at higher risk for most common causes of SCD, including CAD [15•, 20, 21••, 40–45], cardiomyopathy and myocardial fibrosis [21••, 22, 46], heart failure [4, 5, 47], pulmonary hypertension [48], and some arrhythmias such as prolonged QT interval [49]. However, only a few studies have evaluated the risk of SCD in this population. In a single-center retrospective cohort study of 2,860 consecutive HIV+ patients in a public HIV clinic from 2000 to 2009, SCD accounted for 13% (30 out of 230) of deaths and the SCD rate was 2.6/1000 person-years, demonstrating a 4.5-fold higher rate than the general population [50]. Another study in the same population suggested that, as expected, LV systolic dysfunction and diastolic dysfunction were strongly associated with higher risk of SCD [51]. However, given that HIV infection is associated with higher risk for most of common causes of SCD that are listed above, further studies are needed to determine the relative contribution of each of these etiologies as well as other HIV-specific factors such as ART medications (or their interruptions) and chronic inflammation.
Cerebrovascular Disease and Stroke
Data is steadily emerging to help us further understand the burden, causes, pathogenesis, and management of cerebrovascular disease in PLWH. It is estimated that 1–5% of PLWH experience clinical stroke, although ischemic lesions are seen in 4–34% of brain autopsies in this population [52]. Studies have reported increased risks of all-cause stroke (adjusted HR of 1.6–1.8) [53, 54], ischemic stroke (adjusted HR of 1.2–1.4) [53, 55, 56], and hemorrhagic stroke (adjusted HR of 2–3.3) in PLWH compared to uninfected individuals [53, 57]. Risk factors associated with these increased risks vary in different studies, but a lower CD4 count before initiation of ART [54], exposure to abacavir [54], higher HIV RNA levels [55], having AIDS-defining conditions [57], African American ethnicity [58], hepatitis C infection [57], illicit drug or alcohol abuse [57], heavy alcohol consumption [59], intracranial lesions [57], and coagulopathy [57] were all strongly associated with a higher risk of stroke. As reported for other CVD, PLWH appear to experience stroke at younger age, with a reported median age in 40s in the USA [60] and 30s in low-income countries [61, 62]. Given the low incidence of stroke in the general population at this age range, the HR of stroke associated with HIV infection appears to be higher in younger age groups. For example, in a study by Chow et al. [63], the HRs for hemorrhagic stroke at age 30, 40, and 50 were 2.7, 1.9, and 1.3, respectively, when comparing HIV+ to uninfected individuals. This is also consistent with the higher rates of atrial fibrillation (see above) and atherosclerotic involvement of the carotid arteries (see below) in HIV infection at younger ages.
With the introduction of ART, ischemic stroke has become the more common subtype of stroke identified in PLWH [52, 53, 64]. Some mechanisms that are thought to contribute to the higher prevalence of ischemic stroke in PLWH include: HIV-associated vasculopathy (20–32% prevalence) [60, 61], coagulopathy (19–49%) [60, 61, 65], opportunistic infection (13–28%) [61, 65], and cardioembolism (5–15%) [61, 65]. HIV-associated vasculopathy, the mechanism most associated with HIV-associated ischemic stroke, is characterized by specific cerebrovascular changes including stenosis and aneurysm formation, vasculitis, and accelerated atherosclerosis. Further studies are needed to determine the pathogenesis of this vasculopathy, but mechanisms similar to those explained for CAD, such as inflammation and endothelial damage, appear to play an important role [66•]. Even HIV+ patients on ART have higher levels of inflammatory markers [10] and baseline levels of markers such as IL-6, soluble tissue necrosis factors α I and II, soluble CD14, and D-dimer are significantly associated with increased risk of stroke [12]. In addition, HIV-associated coagulopathy (e.g., antiphospholipid syndrome) has emerged as another important mechanism of ischemic stroke in PLWH. Cardioembolic events also appear to play an important role, given the increased rates of atrial fibrillation and the challenges in anticoagulation in these patients, as detailed above.
Researchers are currently moving in the direction of developing systemic evidence-based approaches to help appropriately diagnose and classify the subtypes of HIV-related etiologies in ischemic stroke. For example, Benjamin et al. (2016) developed a diagnostic algorithm for stroke that was designed to give greater weight to etiologies that are well-established and/or have treatment implications [64]. In addition to the work up for routine causes of stroke, this algorithm includes diagnostic steps to evaluate specific causes of stroke-like symptoms in HIV-infected individuals, such as co-infections and HIV-associated vasculopathy. Approaches like this will have to be validated in prospective cohort studies to evaluate their clinical utility.
There are other non-stroke processes that can present with acute focal neurologic deficits and have been referred to as stroke-like syndromes or stroke mimics. In the absence of large prospective studies, the high prevalence of these stroke-like syndromes, which are estimated to present in up to 15% of patients in HIV-endemic populations [67], it is a challenge to estimate the accurate burden of true stroke. These syndromes are frequently associated with opportunistic infections that cause focal central nervous system (CNS) lesions such as tuberculosis (tuberculoma), fungal infections (cryptococcoma), progressive multifocal leukoencephalopathy (associated with John Cunningham (JC) virus), and/or HIV-associated lymphoma (commonly associated with Epstein-Barr virus (EBV) infection). Other opportunistic infections, such as meningovascular syphilis, have been shown to cause ischemic stroke in several documented case studies [68]. With the reemergence of syphilis, particularly among PLWH, syphilis testing seems to be an important component of stroke work-up in PLWH.
As in other CVD, the role of some ART drugs (such as abacavir) in increased risk of stroke remains controversial. However, as mentioned above, studies have consistently shown that poor control of HIV infection is associated with higher risk of stroke [54, 55, 57]. Stroke management and prevention should include identification and treatment of the specific cause of stroke and stroke risk factors, and judicious adjustment of an appropriate ART regimen.
Vascular Diseases
The mechanisms that are involved in the pathogenesis of CAD in PLWH, as detailed elsewhere [1], can also affect the extra-cardiac vascular system to a varying degree. However, the results of the limited available studies on the involvement of the vascular system have been less consistent than studies on CAD. This may be due to several reasons, including smaller sample size of these studies, the inability to control for confounders, and varying approaches used to assess the vascular system.
Carotid Artery
Among the great vessels, atherosclerosis of the carotid artery is studied most widely, partially because of the ease of measuring carotid intima-media thickness (CIMT) with ultrasound and its recognition as a measure of subclinical atherosclerosis and its association with CVD outcomes. In fact, a significant part of our knowledge about atherosclerosis in PLWH has originated from studies on carotid arteries, rather than on coronary arteries. Detailed review of all these studies is beyond this article. Briefly, some studies have shown increased CIMT in PLWH [40, 69–71], while others have shown that this involvement is limited to only some subgroups [72]. A pooled analysis of 5 large cohorts of the National Heart, Lung, and Blood Institute (NHLBI) HIV-CVD Collaborative concluded that HIV infection was associated with higher CIMT (both in common carotid artery and in carotid bifurcation) in younger ages (6–29 years), but not in middle-aged (30–49 years) and older (50–75 years) adults [73]. These findings are consistent with what was described for differences in risk of ischemic stroke in PLWH across lifespan (see above). In a systematic review, although CRP, IL-6, and D-dimer were positively related to CVD in three out of four studies, these biomarkers were not related to CIMT, and there was a significant heterogeneity in studies of inflammatory markers and CIMT [74]. While these findings are inconclusive and still need to be investigated further, they highlight the need for better measures of subclinical atherosclerosis in HIV+ patients.
Aorta
Evaluation of the aorta requires more advanced imaging modalities than ultrasound. Therefore, there are fewer studies investigating the association of HIV seropositivity with inflammation in the aorta. A recent study comparing the uptake of 18F-fluorodeoxyglucose (FDG) by PET in the ascending, descending, and abdominal aorta in 26 HIV+ patients on stable ART and 25 controls [75] found no difference in arterial FDG uptake in any of the aortic regions between the two groups. There were also no notable correlations between FDG uptake, intima-media thickness (IMT), and soluble biomarkers of inflammation in either group. This contradicts a previous FDG PET study that found significantly higher aortic uptake in 27 HIV+ patients on stable ART compared to controls who were individually matched on age, sex, and Framingham risk score. Additionally, the investigators found a significant association between aortic FDG uptake and sCD163, but not with CRP or D-dimer [76]. A third study, using MRI to examine the thoracic aorta in 84 participants, found significantly greater mean vessel wall area and wall thickness in HIV+ patients with detectable viremia and significantly greater mean vessel wall area but not thickness in HIV+ patients with undetectable viremia, when both were compared to uninfected controls [77].
Peripheral Arterial Disease
Most of the studies evaluating peripheral arterial disease (PAD) in PLWH have used ankle-brachial index (ABI), the ratio of systolic arterial pressure at the ankle over that in the brachial artery, as a non-invasive method of detecting subclinical PAD. In the general population, the overall prevalence of PAD has been estimated to be in the range of 3 to 10% [78]. However, studies have been inconsistent on whether the prevalence of PAD in HIV+ populations is higher or lower than in the general population [79–81]. Recent studies have shown low prevalence of PAD as measured by ABI in HIV+ populations that is similar to the prevalence in the general population [82, 83]. More importantly, ABI in HIV+ patients was not correlated with CIMT or traditional risk factors for CVD [83]. Overall, while PAD is considered as a CAD risk equivalent in the general population, it does not show as strong of a correlation with CAD in PLWH. More studies are needed to elaborate the causes for this inconsistency.
Pericardial Diseases
Pericardial effusion was a common cardiovascular sequela in pre-ART HIV patients, especially among those with advanced AIDS, and it was independently associated with increased mortality [84]. With the advent of ART, mortality due to pericardial effusion among HIV patients has dramatically decreased, and in a study of 802 HIV patients, 85% of whom were on ART, only 2 patients had echocardiographic evidence of pericardial effusions [85]. A more recent study using cardiac MRI, which is a more sensitive method that can detect small pericardial effusions, in 103 HIV+ patients (mostly on ART) and 92 non-HIV-infected controls found that small pericardial effusions (<1 cm maximal diameter) were found in 57% of HIV patients compared to 21% of controls (p < 0.001) [16]. Although these small pericardial effusions were not hemodynamically significant, they support the presence of low-grade inflammation even in patients on ART.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a serious progressive disease characterized by elevated pulmonary arterial pressures and pulmonary vascular resistance, resulting in right ventricular failure and premature death [86]. PAH has been a known complication of HIV infection, with HIV-associated PAH having similar clinical presentation and histology to idiopathic PAH [87]. The prevalence of HIV-PAH was estimated to be about 0.5% among PLWH before widespread use of ART, and this prevalence seems to not have changed over time since then [88]. HIV+ individuals are 2500 times more likely to develop PAH than the general population [87]. HIV-PAH is well-studied and the literature is abundant, so we will attempt to discuss it briefly and broadly here. The most recent systematic review of HIV-PAH in 2010 [89] found that the baseline CD4 count at PAH diagnosis was 352 ± 304 cells/μL, and 53% of individuals diagnosed with PAH were classified as having AIDS. The most common symptom was dyspnea, present in 93% of patients. Common findings from ancillary tests were cardiomegaly (80%), pulmonary arterial enlargement (75%), right ventricular hypertrophy (81%), dilated right atrium (59%) and ventricle (97%), and tricuspid regurgitation (70%). Mean pulmonary arterial pressure via right heart catheterization (RHC) was 55 ± 13 mmHg, right ventricular pressure (RVP) via echocardiography was 75 ± 19 mmHg, pulmonary capillary wedge pressure (PCWP) was 12 ± 6 mmHg, and the cardiac index (CI) was 2.6 ± 0.3 L/min/m2. Treatments were variable, and the most common were ART (32%), prostaglandins (28%), and diuretics (22%). Bosentan, ART, and prostaglandins were all reported to be beneficial in improving hemodynamic and functional status [89]. A 2001 systematic review [90] aimed to determine associations between HIV-PAH and the following: HIV risk factor, degree of immunosuppression, AIDS, and liver cirrhosis. No correlation was found between pulmonary systolic arterial pressure and CD4+ cell counts, but a statistically significant difference in the degree of PAH was found between those with AIDS and those without. The authors concluded that HIV-PAH seems to be related to cytokine-related stimulation and endothelial proliferation. High cytokine levels, caused by a HLA-subtype-mediated host response, can favor PAH [90]. A recent retrospective review [91] of National Hospital Discharge Survey data and multiple-cause mortality data from the National Vital Statistics Systems in the USA found that the prevalence of HIV-PAH among PLWH at hospital discharge and death was significantly lower than prevalence estimates in the literature. The authors concluded that HIV-PAH is underdiagnosed at discharge and can remain undetected until death, and recommended that screening guidelines for HIV-PAH be implemented [91].
Conclusions
The increased risk of CVD in PLWH is not limited to CAD. HF appears to be another important subtype of CVD in this population and subclinical impairment of LV dynamics (systolic and diastolic function) and myocardial changes (fibrosis and steatosis) are common in PLWH. The underlying mechanisms of these findings as well as their clinical importance in the aging HIV population may become clear in future studies. There is also some evidence for increased risk of atrial fibrillation and sudden cardiac death in PLWH. These patients are also at higher risk of stroke, particularly ischemic stroke. More studies are needed to elucidate the risk of vascular diseases in this population. Small pericardial effusions with no significant hemodynamic compromise continue to be a common finding in post-ART era. Finally, PLWH remain at significantly higher risk of pulmonary hypertension, and this risk has not changed since introduction of ART. The risk of most types of CVD, including HF, stroke, atrial fibrillation, and atherosclerosis of carotid arteries is higher at younger ages. As HIV+ patients live longer to encounter more of these cardiovascular diseases, further studies are needed to determine the epidemiology and clinical importance of these diseases and develop diagnostic and therapeutic guidelines specific to this population. More importantly, the research on risk of CVD in PLWH could provide insights into our understanding of the role of inflammation in pathogenesis of CVD in general population.
Acknowledgments
The authors would like to thank Dr. Farin Kamangar, Dr. Sonia Singh, and Dr. Gillian Buckley for their valuable contributions to this paper. This work is supported by National Institutes of Health/National Heart, Lung, and Blood Institute grant 5K23HL128164A. The senior author (HB) has also received NIH LRP grant and JCAL is supported by NIH/NIGMS T32 Training Grant GM067587-09.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Revery P. Barnes, John Charles A. Lacson, and Hossein Bahrami declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Coronary Heart Disease
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lacson JC, Barnes RP, Bahrami H. Coronary artery disease in HIV infected patients: downside of living. Curr Atheroscler Rep. 2017;19(4) doi: 10.1007/s11883-017-0651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2•.Cerrato E, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34(19):1432–6. doi: 10.1093/eurheartj/ehs471. The paper is a systematic review of recent studies on cardiac dysfunction in HIV-infected patients. [DOI] [PubMed] [Google Scholar]
- 3.Barbaro G. Reviewing the cardiovascular complications of HIV infection after the introduction of highly active antiretroviral therapy. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5(4):337–43. doi: 10.2174/1568006054553444. [DOI] [PubMed] [Google Scholar]
- 4.Al-Kindi SG, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology and management disparities. Int J Cardiol. 2016;218:43–6. doi: 10.1016/j.ijcard.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrami H, Fonarow G, Heidenreich P. Heart failure admission in HIV-infected patients. J Am Coll Cardiol. 2014;63(12_S) [Google Scholar]
- 6.Butt AA, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannillo M, et al. Heart failure in patients with human immunodeficiency virus: a review of the literature. J Cardiovasc Med (Hagerstown) 2015;16(5):383–9. doi: 10.2459/JCM.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 8.McIntosh RC. A meta-analysis of HIV and heart rate variability in the era of antiretroviral therapy. Clin Auton Res. 2016;26(4):287–94. doi: 10.1007/s10286-016-0366-6. [DOI] [PubMed] [Google Scholar]
- 9.Lebech AM, et al. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. Clin Physiol Funct Imaging. 2007;27(6):363–7. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 10.Bahrami H, et al. Inflammatory markers associated with subclinical coronary artery disease: the multicenter AIDS cohort study. J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuhaus J, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenorio AR, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. doi: 10.1093/infdis/jiu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami H, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Nayak G, et al. Cardiac diastolic dysfunction is prevalent in HIV-infected patients. AIDS Patient Care STDS. 2009;23(4):231–8. doi: 10.1089/apc.2008.0142. [DOI] [PubMed] [Google Scholar]
- 15•.Hsue PY, et al. Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail. 2010;3(1):132–9. doi: 10.1161/CIRCHEARTFAILURE.109.854943. This article showed the increased rates of diastolic dysfunction in PLWH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ntusi N, et al. HIV-1-related cardiovascular disease is associated with chronic inflammation, frequent pericardial effusions, and probable myocardial edema. Circ Cardiovasc Imaging. 2016;9(3):e004430. doi: 10.1161/CIRCIMAGING.115.004430. [DOI] [PubMed] [Google Scholar]
- 17.Blaylock JM, et al. Longitudinal assessment of cardiac diastolic function in HIV-infected patients. Int J STD AIDS. 2012;23(2):105–10. doi: 10.1258/ijsa.2011.011099. [DOI] [PubMed] [Google Scholar]
- 18.Luo L, et al. Assessment of cardiac diastolic dysfunction in HIV-infected people without cardiovascular symptoms in China. Int J STD AIDS. 2010;21(12):814–8. doi: 10.1258/ijsa.2010.010168. [DOI] [PubMed] [Google Scholar]
- 19.Reinsch N, et al. Echocardiographic findings and abnormalities in HIV-infected patients: results from a large, prospective, multicenter HIV-heart study. Am J Cardiovasc Dis. 2011;1(2):176–84. [PMC free article] [PubMed] [Google Scholar]
- 20.Lai H, et al. HIV infection and abnormal regional ventricular function. Int J Cardiovasc Imaging. 2009;25(8):809–17. doi: 10.1007/s10554-009-9493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Holloway CJ, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–22. doi: 10.1161/CIRCULATIONAHA.113.001719. This is an important study that used advanced cardiac MRI to show higher rates of myocardial fibrosis and steatosis in HIV-infected patients, even on ART. [DOI] [PubMed] [Google Scholar]
- 22.Thiara DK, et al. Abnormal myocardial function is related to myocardial steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212(10):1544–51. doi: 10.1093/infdis/jiv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahrami H, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AL, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351(20):2049–57. doi: 10.1056/NEJMoa042934. [DOI] [PubMed] [Google Scholar]
- 25.CDC, Centers for Disease Control and Prevention. HIV Surveillance Report. 2015. [Google Scholar]
- 26.Bloomfield GS, et al. Human immunodeficiency virus and heart failure in low- and middle-income countries. JACC Heart Fail. 2015;3(8):579–90. doi: 10.1016/j.jchf.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniels LB, et al. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2015;170(6):1170–83. doi: 10.1016/j.ahj.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi EY, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5(6):727–34. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duprez DA, et al. N-terminal-proB-type natriuretic peptide predicts cardiovascular disease events in HIV-infected patients. AIDS. 2011;25(5):651–7. doi: 10.1097/QAD.0b013e32834404a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Secemsky EA, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC Heart Fail. 2015;3(8):591–9. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong RW, et al. Digoxin suppresses HIV-1 replication by altering viral RNA processing. PLoS Pathog. 2013;9(3):e1003241. doi: 10.1371/journal.ppat.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lake JE, et al. Telmisartan increases vascular reparative capacity in older HIV-infected adults: a pilot study. HIV Clin Trials. 2016;17(6):225–32. doi: 10.1080/15284336.2016.1234222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipshultz SE, et al. Immunoglobulins and left ventricular structure and function in pediatric HIV infection. Circulation. 1995;92(8):2220–5. doi: 10.1161/01.cir.92.8.2220. [DOI] [PubMed] [Google Scholar]
- 34.Uriel N, et al. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant. 2014;33(9):924–30. doi: 10.1016/j.healun.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Aguero F, et al. An update on heart transplantation in human immunodeficiency virus-infected patients. Am J Transplant. 2016;16(1):21–8. doi: 10.1111/ajt.13496. [DOI] [PubMed] [Google Scholar]
- 36.Dries DL, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol. 1998;32(3):695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 37•.Hsu JC, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61(22):2288–95. doi: 10.1016/j.jacc.2013.03.022. The study showed higher risk of atrial fibrillation in PLWH. [DOI] [PubMed] [Google Scholar]
- 38.Krahn AD, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98(5):476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 39.Chau KH, et al. Abstract 13842: association of CHADS2VASc score and warfarin use with thromboembolic risk among HIV-infected persons with atrial fibrillation. Circulation. 2016;134(Suppl 1):A13842. [Google Scholar]
- 40.Grunfeld C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsue PY, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 42.Sims A, et al. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J Am Soc Echocardiogr. 2012;25(7):741–8. doi: 10.1016/j.echo.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker JV, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28(6):831–40. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esser S, et al. Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol. 2013;102(3):203–13. doi: 10.1007/s00392-012-0519-0. [DOI] [PubMed] [Google Scholar]
- 45.Lo J, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsue PY, Tawakol A. Inflammation and fibrosis in HIV: getting to the heart of the matter. Circ Cardiovasc Imaging. 2016;9(3):e004427. doi: 10.1161/CIRCIMAGING.116.004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng B, et al. Heart failure in HIV infection: focus on the role of atherosclerosis. Curr Opin Cardiol. 2014;29(2):174–9. doi: 10.1097/HCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 48.Barnett CF, Hsue PY. Human immunodeficiency virus-associated pulmonary arterial hypertension. Clin Chest Med. 2013;34(2):283–92. doi: 10.1016/j.ccm.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogunmola OJ, Oladosu YO, Olamoyegun MA. QTc interval prolongation in HIV-negative versus HIV-positive subjects with or without antiretroviral drugs. Ann Afr Med. 2015;14(4):169–76. doi: 10.4103/1596-3519.152072. [DOI] [PubMed] [Google Scholar]
- 50.Tseng ZH, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–6. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moyers BS, et al. Effect of left ventricular dysfunction and viral load on risk of sudden cardiac death in patients with human immunodeficiency virus. Am J Cardiol. 2014;113(7):1260–5. doi: 10.1016/j.amjcard.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benjamin LA, et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–90. doi: 10.1016/S1474-4422(12)70205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yen YF, et al. Association of HIV and opportunistic infections with incident stroke: a nationwide population-based cohort study in Taiwan. J Acquir Immune Defic Syndr. 2016 doi: 10.1097/QAI.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmussen LD, et al. Risk of cerebrovascular events in persons with and without HIV: a Danish nationwide population-based cohort study. AIDS. 2011;25(13):1637–46. doi: 10.1097/QAD.0b013e3283493fb0. [DOI] [PubMed] [Google Scholar]
- 55.Chow FC, et al. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sico JJ, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–40. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durand M, et al. Risk of spontaneous intracranial hemorrhage in HIV-infected individuals: a population-based cohort study. J Stroke Cerebrovasc Dis. 2013;22(7):e34–41. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Thakur KT, et al. Stroke in HIV-infected African Americans: a retrospective cohort study. J Neurovirol. 2016;22(1):50–5. doi: 10.1007/s13365-015-0363-x. [DOI] [PubMed] [Google Scholar]
- 59.Kelso NE, Sheps DS, Cook RL. The association between alcohol use and cardiovascular disease among people living with HIV: a systematic review. Am J Drug Alcohol Abuse. 2015;41(6):479–88. doi: 10.3109/00952990.2015.1058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ortiz G, et al. Mechanisms of ischemic stroke in HIV-infected patients. Neurology. 2007;68(16):1257–61. doi: 10.1212/01.wnl.0000259515.45579.1e. [DOI] [PubMed] [Google Scholar]
- 61.Tipping B, et al. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry. 2007;78(12):1320–4. doi: 10.1136/jnnp.2007.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heikinheimo T, et al. Stroke outcomes in Malawi, a country with high prevalence of HIV: a prospective follow-up study. PLoS One. 2012;7(3):e33765. doi: 10.1371/journal.pone.0033765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chow FC, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83(19):1705–11. doi: 10.1212/WNL.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benjamin LA, et al. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e254. doi: 10.1212/NXI.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke. 2003;34(1):10–5. doi: 10.1161/01.str.0000043821.35051.fa. [DOI] [PubMed] [Google Scholar]
- 66•.Benjamin LA, et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–90. doi: 10.1016/S1474-4422(12)70205-3. The paper summarizes some of the most important studies on stroke in PLWH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamin LA, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86(4):324–33. doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lachaud S, Suissa L, Mahagne MH. Stroke, HIV and meningovascular syphilis: study of three cases. Rev Neurol (Paris) 2010;166(1):76–82. doi: 10.1016/j.neurol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Hsue PY, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1(2) doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sainz T, et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: the Caro VIH Study. J Acquir Immune Defic Syndr. 2014;65(1):42–9. doi: 10.1097/QAI.0b013e3182a9466a. [DOI] [PubMed] [Google Scholar]
- 71.Hsue PY, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. Am J Cardiol. 2012;109(5):742–7. doi: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnsen S, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91(12):4916–24. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hanna DB, et al. HIV infection and carotid artery intima-media thickness: pooled analyses across 5 cohorts of the NHLBI HIV-CVD collaborative. Clin Infect Dis. 2016;63(2):249–56. doi: 10.1093/cid/ciw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vos AG, et al. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One. 2016;11(1):e0147484. doi: 10.1371/journal.pone.0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knudsen A, et al. HIV infection and arterial inflammation assessed by (18)F-fluorodeoxyglucose (FDG) positron emission tomography (PET): a prospective cross-sectional study. J Nucl Cardiol. 2015;22(2):372–80. doi: 10.1007/s12350-014-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Subramanian S, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Floris-Moore M, et al. Association of HIV viral load with monocyte chemoattractant protein-1 and atherosclerosis burden measured by magnetic resonance imaging. AIDS. 2009;23(8):941–9. doi: 10.1097/QAD.0b013e328329c76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol. 2016;19(2):91–5. doi: 10.1053/j.tvir.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Bernal E, et al. Low prevalence of peripheral arterial disease in HIV-infected patients with multiple cardiovascular risk factors. J Acquir Immune Defic Syndr. 2008;47(1):126–7. doi: 10.1097/QAI.0b013e318157b0b3. [DOI] [PubMed] [Google Scholar]
- 80.Periard D, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46(5):761–7. doi: 10.1086/527564. [DOI] [PubMed] [Google Scholar]
- 81.Johns K, et al. Ankle brachial index screening for occult vascular disease is not useful in HIV-positive patients. AIDS Res Hum Retroviruses. 2010;26(9):955–9. doi: 10.1089/aid.2009.0275. [DOI] [PubMed] [Google Scholar]
- 82.Kwiatkowska W, et al. Peripheral arterial disease and ankle-brachial index abnormalites in young and middle-aged HIV-positive patients in lower Silesia, Poland. PLoS One. 2014;9(12):e113857. doi: 10.1371/journal.pone.0113857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knudsen A, et al. Low prevalence of peripheral arterial disease in a cross-sectional study of Danish HIV-infected patients. Infect Dis (London) 2015;47(11):776–82. doi: 10.3109/23744235.2015.1061204. [DOI] [PubMed] [Google Scholar]
- 84.Pham TV, Torres M. Human immunodeficiency virus infection-related heart disease. Emerg Med Clin North Am. 2015;33(3):613–22. doi: 10.1016/j.emc.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Lind A, et al. Pericardial effusion of HIV-infected patients? Results of a prospective multicenter cohort study in the era of antiretroviral therapy. Eur J Med Res. 2011;16(11):480–3. doi: 10.1186/2047-783X-16-11-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoeper MM, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–22. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 87.Correale M, et al. HIV-associated pulmonary arterial hypertension: from bedside to the future. Eur J Clin Invest. 2015;45(5):515–28. doi: 10.1111/eci.12427. [DOI] [PubMed] [Google Scholar]
- 88.Sitbon O, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177(1):108–13. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 89.Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med. 2010;11(10):620–34. doi: 10.1111/j.1468-1293.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- 90.Pellicelli AM, et al. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology. 2001;52(1):31–41. doi: 10.1177/000331970105200105. [DOI] [PubMed] [Google Scholar]
- 91.Henriques-Forsythe M, Annangi S, Farber HW. Prevalence and hospital discharge status of human immunodeficiency virus-associated pulmonary arterial hypertension in the United States. Pulm Circ. 2015;5(3):506–12. doi: 10.1086/682222. [DOI] [PMC free article] [PubMed] [Google Scholar]