Abstract
Purpose of Review
Introduction of combination antiretroviral therapy (ART) has increased the life expectancy of patients with HIV infection, allowing them to live longer with this chronic medical condition and consequently experiencing conditions such as cardiovascular diseases (CVDs). Several studies have investigated the increased risk of CVD in people living with HIV (PLWH). However, less is known about the exact mechanisms involved in this increased risk. Also, specific guidelines for management of CVD in PLWH have not been developed yet. In this article, we review the recent literature on the mechanisms involved in pathogenesis of CVD in PLWH, with an emphasis on coronary artery disease (CAD).
Recent Findings
Although initial studies suspected the increased prevalence of traditional CVD risk factors and side effects of ART to be involved in the increased CVD risk in PLWH, recent studies have uncovered the important role of chronic persistent inflammation in this increased risk. In addition, biomarkers of inflammation have been associated with both CVD events and subclinical CAD in this population. Lastly, recent studies and ongoing clinical trials have been investigating medical interventions that aim to reduce inflammation and cardiovascular events.
Summary
Different mechanisms of inflammation have been examined in PLWH, including subclinical viremia, microbial translocation, and coinfection with other pathogens such as cytomegalovirus. Although inflammatory biomarkers have been consistently associated with CVD and subclinical CVD outcomes, their prognostic value is unknown. Recent and ongoing trials are exploring the benefits of anti-inflammatory drugs, statins, and antimicrobial translocation drugs on both inflammation and CVD risk among PLWH.
Keywords: HIV infection, Cardiovascular disease, Inflammation, Coronary artery disease, Biomarkers, Clinical trials
Introduction
HIV infection is a major public health problem, affecting 36.7 million people worldwide in 2015 [1]. With the advent of antiretroviral therapy (ART), the portrait of HIV infection has changed from a fatal disease to a chronic medical condition, especially in countries with wide access to ART [2]. If ART is started early enough, life expectancy of a 20-year-old HIV+ was 69 years in 2006–2007 [3] and can be projected to be even higher now. Thus, people living with HIV (PLWH) now live long enough to face conditions that were traditionally seen in the elderly, such as cardiovascular disease (CVD) [4]. In high-income countries, CVD is the second non-AIDS cause of death in people living with HIV infection, and it is a significant contributor to non-AIDS deaths in developing and sub-Saharan countries [5–7]. Studies have shown that HIV-infected individuals are at higher risk of several cardiovascular abnormalities, such as coronary artery disease (CAD) [8, 9], heart failure and heart failure admission [10], subclinical atherosclerosis of coronary [11–13] and carotid arteries [8, 14, 15], global and regional left ventricular (LV) systolic function [16], LV diastolic dysfunction [16, 17], myocardial fibrosis and steatosis [9, 18], pulmonary hypertension [19], stroke [20], atrial fibrillation [21], and sudden cardiac death [22]. However, the mechanisms contributing to these increased risks are not well understood.
This paper and the companion article published in the same issue of journal [23] review the current state of knowledge about increased risk of CVD in people living with HIV infection with a special focus on possible mechanisms. Although several cardiovascular abnormalities are common in PLWH, CAD is more widely studied. This paper focuses on the risk of CAD in PLWH, and the relationships of HIV infection with other cardiovascular diseases (other than CAD) are summarized in the companion article [23]. The role of chronic inflammation in the pathogenesis of CVD in this population has emerged to be important, especially considering the complex immunological nature of HIV infection that includes both immune depletion and immune activation. Since CAD is more widely studied, the detailed discussion of mechanisms is provided for increased risk of CAD in PLWH. Nonetheless, some of the explained mechanisms can be applied to other CVD as well. This review attempts to focus on virologically suppressed patients on ART; although, this may not be possible for all CVD outcomes, given the paucity of available data in the literature. Therefore, AIDS-related cardiovascular complications are beyond the scope of this paper.
Coronary Artery Disease
Several studies have shown the increased risk of clinical CAD in PLWH [24]. Overall, it is estimated that PLWH are at 1.5- to 2-fold higher risk of CAD compared to individuals without HIV (HIV-) [25]. Therefore, HIV infection could be considered as important as some of the traditional risk factors of CAD, such as diabetes and smoking, may aid in risk stratification and decision making in clinical practice.
Since studying clinical CAD events requires large studies, measures of subclinical atherosclerosis are increasingly used in recent studies as surrogates for subclinical CAD [26]. Specifically, coronary artery calcification (CAC) detected by non-contrast cardiac computed tomography (CT) has been used generally as a proxy measure of subclinical CAD in large cohort studies in general population. Although PLWH have been reported to have increased risk of subclinical atherosclerosis in the carotid artery [8, 14, 15, 27], studies using CAC as a measure of subclinical CAD have been inconclusive [11–13]. However, recent studies using contrast-enhanced coronary CT angiography (CTA) have shown that HIV+ patients have higher risk of subclinical coronary stenosis, presence of any atherosclerotic plaques, and presence of non-calcified plaques [28•].
Pathogenesis
The exact mechanisms for the increased risk of CAD in PLWH are not well understood. Initially, a higher prevalence of traditional risk factors such as smoking, lipid abnormalities, and substance abuse drew a lot of attention [29–31]. Initial research also focused on the effect of ART, particularly because of reported positive associations between lipid dystrophies and some ART medications [32••, 33]. However, more recent studies indicate that chronic inflammation plays a key role in pathogenesis of CAD in PLWH [27]. Here, we briefly review traditional risk factors and ART and then discuss the role of inflammation in more detail.
Traditional Risk Factors
Earlier investigations postulated that the excess in CVD risk among PLWH was due to a higher frequency of traditional risk factors for CVD among PLWH [29–31]. Dyslipidemia, insulin resistance, and fat redistribution (decrease in subcutaneous fat and increase in visceral fat) [6], as well as smoking [34], are more prevalent in PLWH. In Lang et al.’s review of the prevalence of cardiovascular risk factors in PLWH in developed countries and their comparisons with uninfected individuals, smoking, lower high-density lipoprotein cholesterol, higher low-density lipoprotein cholesterol, high triglycerides, hypertension, and cocaine use were listed as traditional risk factors found to be more common among PLWH [7]. In addition to this higher prevalence of traditional risk factors in PLWH, some of these factors may affect HIV+ patients differently and HIV+ patients with the same risk factor profile have higher risk of CVD, compared to HIV- individuals [35•]. For example, prehypertension (blood pressure of 120–139 mmHg) is associated with a larger increase in the risk of MI in HIV+ patients [36]. While all these findings highlight the importance of managing risk factors in PLWH, more recent data shows that the excess risk of CVD observed in this population remains significant even after adjusting for traditional risk factors [37]. Therefore, mechanisms other than traditional risk factors are thought to be involved in pathogenesis of CVD in PLWH.
Antiretroviral Therapy
ART is associated with metabolic abnormalities and therefore is considered as another possible contributing factor for the increased risk in CAD among HIV+ patients. ART is a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a protease inhibitor (PI), or integrase inhibitor, or non-nucleoside reverse transcriptase inhibitor (NNRTI) [2].
Considering the known association of PIs with lipodystrophy [38], several studies investigated the role of PIs in increased risk of CAD. The data collection on adverse events of anti-HIV drugs (D/A/D) study reported an increased risk of CAD with lifetime cumulative exposure to two PIs (indinavir and lopinavir/ritonavir (LPV-r)), but not saquinavir and nelfinavir [39]. A case-control study nested in the French Hospital Database on HIV (FHDH) cohort reported a statistically significant odds ratio of 1.15 (95% CI: 1.06–1.26) for MI associated with every year of exposure to any PI except saquinavir [33]. A meta-analysis of the CVD risk by different classes of ART conducted by Bavinger et al. [32••] estimated a summary relative risk (sRR) of 2.1 (95%CI 1.1–4.3) for MI in patients with recent exposure to PIs based on two observational studies. They also found an increased risk of MI with each additional year of exposure to indinavir (sRR 1.11, 95% CI 1.05–1.17) and lopinavir (sRR 1.22, 95% CI 1.01–1.47).
Among NRTIs, abacavir (ABC) has been implicated in risk of cardiovascular events, but studies have reported conflicting results, especially when comparing cumulative exposure to recent exposure. The D/A/D study found an increased risk of MI associated with recent and cumulative exposure to ABC [39], and a case–control study in a Quebec’s public health insurance database reported increased odds of MI associated with any exposure to ABC [40]. The case–control study nested in FHDH found no association with cumulative use of ABC but found a significant 2-fold increase in risk of MI for recent, short-term (<1 year) exposure to ABC [33]. A study using the Veterans Health Administration’s Clinical Case Registry found a non-significant increased risk of MI and cerebrovascular with cumulative use of abacavir [41], but two other studies conducted on veteran populations found an increased risk of cardiovascular events associated with current or recent exposure to ABC[42, 43].
A meta-analysis by Bavinger et al. [32••] estimated sRR of 1.92 (95% CI 1.5–2.4) for MI in those with recent exposure to ABC. However, this meta-analyses included observational studies (rather than randomized clinical trials), most of which were not designed to examine long-term outcomes and were too short or underpowered to look at cardiovascular risk. Since the study by Bavinger et al., a few observational studies have been published with similar results: (1) a Swiss cohort study found that exposure to ABC during the past 6 to 36 months increased risk, while both current exposure and exposure from more than 3 years ago did not modulate risk [44]; (2) a Kaiser Permanent cohort study found that HIV+ patients who initiated with ABC were over two times as likely to experience CVD events than were patients who initiated without ABC, after adjusting for renal dysfunction or other CVD risk factors[45]; (3) a study of HIV-infected US veterans found that current exposure to abacavir, efavirenz, lamivudine, or zidovudine increased risk of CVD events (OR range 1.40–1.53), and that 5 combinations of ART drugs, all of which included lamivudine, increased risk of having a CVD event [43].
The studies mentioned above support the hypothesis that recent, short-term exposure to ABC may be associated with an increased cardiovascular event risk. In ART-naive patients, significant immune activation and hypersensivity reactions resulted in induced T cell reactivity associated with initiation of abacavir [46], which may be related to increased CVD risk during the first few months of ABC usage [47].
Overall, more studies are required to further elucidate the role of ART in increased risk of CVD in PLWH. Specifically, studies need to be conducted on the mechanisms and timing of the presumable increased risks associated with some medications.
Paradigm Shift: From ART to Other Mechanisms
Despite the initial focus on ART as a possible mechanism for increased CVD risk in PLWH, the results of the Strategies for Management of Antiretroviral Therapy (SMART) study challenged that paradigm [48••]. This study randomized more than 5000 HIV+ participants on stable ART and with CD4 counts >350 cells/μL to either continued ART or interrupted/delayed treatment until CD4 dropped below 250 cells/μL, which is when ART would be resumed. The investigators found that those who interrupted or deferred ART had a 70% increased hazard of CVD compared to those who continued treatment. As reviewed by Siedner [49], other non-randomized studies were also able to demonstrate increased risk of preclinical or clinical CVD among HIV+ patients with lower nadir CD4 counts [50–52]. In addition, recent large cohort studies found increased CVD risk among HIV-infected patients with low CD4 counts and/or current detectable HIV-1 viremia [53, 54]. These studies suggest that the early initiation of ART (consistent with current guidelines recommending to start ART independent of immune status), and sustained ART would lower CVD risk. Recently, a study of more than 4600 HIV+ patients found that initiating ART for patients with CD4 counts >500 cells/μL had less AIDS-related and non-AIDS-related events than those who initiated ART when their CD4 count dropped below 350 cells/μL [55]. Although authors of this study found no significant difference in the risk of CVD between these two groups, the significantly higher risk of other serious events (particularly AIDS-related events) can be considered as a competing risk introducing bias in the estimation of CVD risk. In other words, the risk of CVD becomes relevant mainly when the risk of other AIDS-related events is reduced by ART.
All these findings shifted the paradigm about the role of ART in pathogenesis of CVD and led to the consensus that despite the possible role of ART in pathogenesis of CVD, interruption or delay in ART would be more detrimental. In fact, early ART is arguably the most important step in preventing CVD. There are not enough data from randomized clinical trials on the benefit of intensification of ART in reducing the risk of CVD. One randomized placebo-controlled clinical trial showed that intensification of treatment with raltegravir in virologically suppressed HIV patients on ART did not improve endothelial function, hyperemic velocity, immune activation, or viral load, compared to placebo [56].
Inflammation
The beneficial role of early ART and the increased risk of CVD and other comorbidities associated with ART interruption turned the attention of researchers to other plausible mechanisms, including chronic inflammation [24, 28•], immunosenescence and accelerated aging [57], potential damage from the initial HIV infection, endothelial dysfunction [25, 58], and coinfections with CMV and HCV [59, 60]. Among these, chronic inflammation seems to be a key factor, especially with the increased knowledge of the involvement of general inflammation on CVD [61, 62].
While ART reduces the amount of inflammation, even vi-rologically suppressed patients remain at higher levels of inflammation than HIV− individuals [28•, 63]. The hallmarks of HIV infection in terms of immune activation are depletion and dysfunction of CD4+ T cells and chronic CD8+ T cell activation [64]. As reviewed by Longenecker et al.[65], CD4+ Tcell levels <500 cells/μL, high CD8+ cell counts, and lower CD4+:CD8+ ratio are associated with increased CVD risk. Amplified monocyte/macrophage-mediated inflammation and injury within atherosclerotic lesions due to HIV infection also increases CVD risk. In particular, increased activation of both intermediate/pro-inflammatory CD14+/CD16+ and non- classical/patrolling CD14dim/CD16+ monocytes have been linked to increased CVD in HIV− individuals, after adjusting for traditional risk factors [65].
Mechanisms of Inflammation in HIV
As illustrated in Fig. 1, several mechanisms have been postulated to cause a persistent chronic inflammatory state among PLWH, including subclinical viremia, microbial translocation, and coinfections.
Fig. 1.
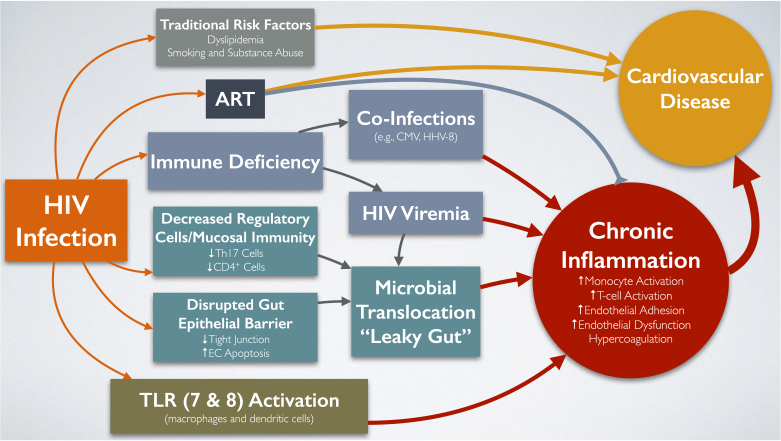
Current paradigm of mechanisms involved in increased risk of cardiovascular disease among people living with HIV. HIV infection results in the emergence of several factors that increase cardiovascular disease (CVD) risk. Traditional CVD risk factors such as dyslipidemia, smoking, high blood pressure, and substance abuse contribute to higher CVD risk in people living with HIV (PLWH). Some antiretroviral therapies (ARTs) have been directly and indirectly (via traditional risk factors) associated with CVD risk, particularly abacavir and protease inhibitors (PIs). Chronic inflammation, in the form of increased monocyte and T cell activation, endothelial adhesion, and dysfunction and hypercoagulation, has been significantly associated with increased CVD in the general population and is more aggravated in PLWH due to (1) HIV-associated immune deficiency leading to coinfections with pathogens such as cytomegalovirus (CMV) and human herpesvirus-8 (HHV-8) that increase chronic inflammation; (2) subclinical viremia also leads to higher inflammation; (3) microbial translocation, or “leaky gut,” characterized by decreased regulatory cells and mucosal immunity (lower Th17 and CD4+ cells), disrupted gut epithelial barrier (less tight junctions and higher endothelial cell (EC) apoptosis), and persistent presence of the HIV virus in the gut; and (4) toll-like receptor (TLR) 7 and 8 activation in macrophages and dendritic cells. Early initiation and sustained use of ART has been shown to decrease chronic inflammation in PLWH
Subclinical Viremia
Even among HIV+ patients on ART, there is low level of viremia (<75 copies/mL)[66]. Hunt et al. [67] demonstrated that although CD8+ T cell activation was highest among HIV+ patients not treated with ART, those undergoing ART still had higher levels than did HIV-negative individuals. In addition, HIV Elite Controllers, who are HIVpositive individuals who maintain undetectable HIV viral loads without ART, also have higher CD8+ T cell activation than do HIV-negative individuals, and their immune activation decreased upon treatment with ART [67]. Therefore, presence of low levels of HIV virus in PLWH appears to be one source of persistent inflammation.
Microbial Translocation
Microbial translocation, or “leaky gut,” occurs when the defense mechanisms of the gastrointestinal tract are impaired, allowing microbial products to traverse [68]. As shown in Fig. 1, in PLWH, microbial translocation is enabled by (1) disrupted decreased tight junctions in gut and increased epithelial cell apoptosis [69]; (2) loss of mucosal immunity, as demonstrated by decreased CD4+ T cells and T helper 17 (Th17) cells in the gut [70, 71]; and (3) presence of the HIV virus itself in the gut, despite ART [72]. In vitro studies have shown that blood lipopolysaccha-ride (LPS) levels, a measure of bacteremia, induces tissue factor (TF, thromboplastin) expression in circulating monocytes, which in turn has been linked to elevated CD8+ T cell activation, D-dimer levels and elevated risk of thrombosis in in vivo studies [73]. Two studies have shown that microbial translocation persists but is attenuated under ART [74] and continues to be a source of inflammation.
Coinfections
There is a high prevalence (>90%) of cytomegalovirus (CMV) infection among PLWH [75], and CMV appears to be independently associated with increased immune response [76]. In a clinical trial, when HIV+ patients with CD <350 cells/μL on ART were treated with valganciclovir, which blocks asymptomatic CMV replication, immune activation significantly decreased compared to placebo and remained at a lower level following treatment (although this level did not get as low as that in HIV- individuals) [77]. In contrast, another trial using valacyclovir, a strong anti-herpes simplex virus (HSV) 1/2 medication with minimal anti-CMV effect, in HIV+ patients on ART, did not show significant difference in immune activation between treatment and placebo arms [78]. These two separate trials suggest that CMV-specific immune activation may play a role in the chronic persistent inflammation and possibly CVD risk in PLWH.
A recent study found that among virologically suppressed HIV+ individuals, coinfection with human herpesvirus 8 (HHV-8), a vasculotropic virus implicated in Kaposi’s sarcoma pathogenesis, was significantly associated with higher levels of inflammatory markers, including high-sensitivity C-reactive protein (CRP), CD4/CD38/HLA-DR and CD8/ CD38/HLA-DR, after adjustment for traditional risk factors, HCV and herpes simplex virus type 2 (HSV-2) infection. However, flow-mediated dilatation and total carotid intima-media thickness did not differ by HHV-8 serostatus [79].
Biomarkers of Inflammation and CVD Risk in the Context of HIV Infection
Biomarkers of inflammation have provided an opportunity to study the underlying mechanisms involved in pathogenesis of CVD. Even after adjusting for traditional risk factors and HIV-related risk factors and despite long-term ART, HIV+ individuals still have moderately elevated levels of inflammatory biomarkers compared to non-HIV-infected individuals [28•, 63]. Among HIV+ patients on ART, baseline levels of interleukin-6 (IL-6), soluble tissue necrosis factor α receptors I and II (sTNFαR-I & sTNFαR-II), soluble CD14 (sCD14), and D-di-mer were all significantly associated with increased mortality and increased risk of MI and stroke [80]. Vos et al. recently published a systematic review of eight studies examining the link between pro-inflammatory markers and CVD (coronary heart disease, MI, stroke, and surrogate markers of CVD such as arterial stiffness and carotid intima-media thickness (CIMT)) among PLWH [81•]. CRP, IL-6 and D-dimer were the most frequently assessed (examined in four out of eight studies); and all three of these markers were associated with an increased CVD risk in three out of four studies, which is consistent with findings from general population-based studies. One study found that higher IL-6, D-dimer, and hsCRP levels were associated with greater risk of fatal CVD and greater risk of all cause mortality beyond 28 days after a non-fatal CVD event [82].sCD14, despite being associated with increased mortality in HIV+ patients [83], was not found to be associated with increased CVD risk in three studies [81•]. A total of 32 other markers were assessed less than three times [81•]. Although studies of inflammatory markers in relation to CIMT were too heterogeneous for a meta-analysis, the literature suggested no association between the markers studied and CIMT [81•].
More recent studies have focused on the relationship between inflammatory markers and subclinical atherosclerosis. In the Multicenter AIDS Cohort Study (MACS), we recently reported that, when compared to HIV- men, HIV+ men had significantly higher levels of IL-6, intercellular adhesion molecular-1 (ICAM-1), CRP, and sTNFr I and II, and higher prevalence of non-calcified plaques determined through CTA imaging. In addition, among HIV+ men, increases in IL-6, ICAM-1, and sTNFαR I and II were associated with increased prevalence of coronary stenosis, and all of these but ICAM-1 were associated with greater coronary artery calcification score. These associations were independent of traditional CVD risk factors and HIV clinical factors [28•]. A recent study from MACS examining the association between biomarkers of monocyte activation and subclinical atherosclerosis found that soluble CD163 (sCD163) and sCD14, both biomarkers of monocyte activation, and monocyte chemoattractant protein 1 (CCL2) were all significantly elevated in HIV+ men undergoing ART and were associated with atherosclerosis. Specifically, higher sCD163 levels were significantly associated with greater prevalence of coronary artery calcium, mixed plaque, and calcified plaque, while higher CCL2 levels were associated with greater extent of non-calcified plaques [84].
However, most of these biomarkers of inflammation are predominantly released outside of the myocardium and may not represent a direct link between HIV infection and CVD. Only a few studies have looked at biomarkers secreted specifically by cardiovascular tissue. However, these biomarkers are not related to inflammation but are related to myocardial stress and are discussed further in the companion article published in the same issue of this journal [23].
Further studies are needed to elucidate the exact role of inflammation in pathogenesis of CAD in HIV-infected patients, as well as in general population, and whether this is a causal relationship or mostly an association highlighting other underlying mechanisms. Nonetheless, biomarkers of inflammation have been consistently related to clinical and subclinical CAD in the context of HIV infection and can shed light on some specific inflammatory pathways involved and their interactions with some other pathways such as coagulation and fibrosis.
Anti-Inflammatory Medications
Due to the increasing evidence of the role of inflammation in pathogenesis of CVD, several trials have tried to target inflammation. However, there are not many large randomized clinical trials in this field. Moreover, the trials designed to study CVD as the primary outcome are even scarcer. Here, we summarize some of the ongoing studies examining the benefit of anti-inflammatory drugs on CVD risk in HIV-infected individuals.
Antiretroviral Therapy
ART is arguably the most important step in reducing the inflammation in HIV+ patients. As shown in SMART trial, interruptions in ART would enhance the inflammatory state [48••]. Early ART initiation may also decrease the immune activation [85]. Also, small studies have suggested that intensification of ART may decrease T cell activation and D-dimer but did not affect low-level viremia [86]. Large randomized trials are still needed to show the effect of early ART and ART intensification on CVD risk.
Aspirin and Non-steroidal Anti-Inflammatory Drugs
In an uncontrolled trial, 81 mg/day of aspirin for 1 week was shown to decrease platelet activation in both HIV+ and HIV-individuals in response to adenosine diphosphate, collagen, but not arachidonic acid, which remained elevated in HIV-infected group [87]. HIV+ subjects also had increased markers of T cell activation (CD38 and HLA-DR) and monocyte activation (sCD14) when compared to controls at baseline, and these levels decreased after 1 week of aspirin therapy [87]. A more recent double-blinded clinical trial randomized 121 HIV+ patients to receive 100 mg aspirin, 300 mg aspirin, or placebo for 12 weeks and followed them for 4 weeks after treatment was stopped (NCT02155985). Based on the information from clinicaltrials.gov, levels of sCD14 were not significantly different across the treatment arms [88]. Results on secondary outcomes such as IL-6, soluble CD163, D-dimer, T cell or monocyte activation, and flow-mediated dilatation (FMD, a marker of vascular function) have yet to be published [88]. Despite lack of studies directly studying the effect of aspirin in reducing CVD risk in PLWH, it is widely accepted that the same indications for use of aspirin in HIV-uninfected population should apply. However, studies have shown that aspirin is underutilized among PLWH [89, 90]. More studies are needed to develop specific criteria for use of aspirin in this population. Ongoing trials examining the anti-inflammatory effects and cardiovascular benefits of aspirin are presented in Table 1. Some trials are testing the effects of the anticoagulant clopidogrel (NCT02578706, NCT02559414) in comparison to aspirin and the effects of atorvastatin against aspirin (NCT02081638).
Table 1.
Ongoing clinical trials of aspirin and other anti-inflammatory drugs in HIV+ patients to reduce cardiovascular risk
| Drug | Phase | Randomized? | Cardiovascular and/or inflammation outcomes | Size | Status | Identifier |
|---|---|---|---|---|---|---|
| Aspirin | ||||||
| Aspirin (325 mg) | 2 | Y | Cardiovascular endothelial injury and thrombosis | 22 | Terminated | NCT00783614 |
| Inflammation: blood markers of inflammation (not specified further) | ||||||
| Aspirin (1 day 325 mg and followed by 81 mg daily) vs. clopidogrel | 2 | Y | Cardiovascular endothelial function | 50 | Recruiting | NCT02559414 |
| Inflammation: platelet activity, inflammation, immune activity (not specified further) | ||||||
| Aspirin | 1 & 2 | Y | Cardiovascular reactive hyperemia index | 91 | Completed | NCT02401269 |
| Inflammation: TNF-alpha, CRP, IL-6 | ||||||
| Aspirin (81 mg) vs atorvastatin (40 mg) | 2 | Y | Cardiovascular carotid MR! | 120 | Recruiting | NCT02081638 |
| Inflammation: sCD14, IL-6, D-dimer, hsCRP, sCD163, sTF, others T cell activation, (HLA-DR/CD38 coexpression), monocyte activity (CD14+, +CD16+, and CD14varCD16 +, CCR5, TF, CCR2) | ||||||
| Clopidogrel (75 mg) vs. aspirin (81 mg) | 2 | Y | Cardiovascular none | 40 | Recruiting | NCT02578706 |
| Inflammation: sCD14, monocyte activity (CD14, CD16, sCD163, cholesterol uptake), IL-6, D-dimer, sTNFR I/II, platelet activity (light transmission aggregometry, clot formation kinetics, thrombus formation by Badimon chamber | ||||||
| Aspirin (100 and 300 mg) | 2 | Y | Cardiovascular brachial artery FMD | 121 | Completed | NCT02155985 |
| Inflammation: sCD14, sCD163, CD14, CD16, CD69, CD4 + CD38+, CD4 + HLA-DR+, CD8 + CD38+, CD8 + HLA-DR+, PD-1, CTLA-4, IL-6, D-dimer, kynurenine to tryptophan ratio, serum thromboxane b2, 11 -dehydrothromboxane | ||||||
| Other anti-inflammatoiy drills | ||||||
| Methotrexate (5, 10, and 15 mg) | 2 | Y | Cardiovascular brachial artery FMD, diameter, hyperemic flow velocity | 200 | Active, not recruiting | NCT01949116 |
| Inflammation: CD4 cell count, hsCRP, IL-6, sCD163, D-dimer, monocyte activity | ||||||
| Colchicine (0.6 mg) | 2 | Y | Cardiovascular coronary endothelial function, coronary artery cross-sectional artery, brachial artery FMD | 102 | Currently recruiting | NCT02624180 |
| Inflammation: hsCRP, IL-6 | ||||||
| Pentoxifylline (400 mg) | 2 | Y | Cardiovascular brachial artery FMD | 26 | Completed | NCT00796822 |
| Inflammation: activated CD8 cell percentage, CD4 cell count, MCP-1, sVCAM-1, IP-10, MMP-9, TIMP-1, PAI-1 active, hsCRP | ||||||
| Canakinumab (150 mg) | 2 | Y | Cardiovascular number of adverse events, brachial artery FMD, arterial fluorodeoxygenase (FDG) uptake via FDG-PET/CT |
110 | Currently recruiting | NCT02272946 |
| Inflammation: hsCRP, IL-6, CD 163, D-dimer, T cell and monocyte activation, HIV reservoir size. | ||||||
| Anti-fibrosis drugs with anti-inflammatory properties | ||||||
| Losartan (50-100 mg)/raltegravir (400 mg) | 4 | Y | Cardiovascular carotid intima media thickness, levels of metalloproteinases and their inhibitors | 48 | Completed | NCT01529749 |
| Inflammation: lymphatic tissue fibrosis, IL-6, D-dimer, CRP, CD4, viral load, CD4/CD8 ratio | ||||||
| EplCTenone 50 mg | N/a | Y | Cardiovascular: coronary flow reserve measured via canliac PET, myocardial inflammation measured by extracellular volume fraction via cardiac magnetic resonance imaging, coronary plaque measured by coronary CTA, markers of vascular dysfunction |
60 | Currently recruiting | NCT02740179 |
| Inflammation: markers of systemic inflammation, immune activation, and fibrosis (not specified further) | ||||||
Only studies that included inflammatory markers as outcomes are included.
hsCRP highly-sensitive C-reactive protein, CRP C-reactive protein, sCD14soluble CD14, sCD163 soluble CD163, IL-6 interleukin-6, FMD flow-mediated dilatation, sTNFR soluble tumor necrosis factor receptor, TNF tumor necrosis factor, MCP-1 monocyte chemotactic protein 1, sVCAM-1 soluble vascular cell adhesion molecule 1, IP-10 interferon gamma-induced protein 10, MMP-9 matrix metallopeptidase, TIMP-1 TIMP metallopeptidase inhibitor 1, PAI-1 plasminogen activator inhibitor-1, PET positron emission tomography, FDG fluorodeoxygenase, HLA-DR human leukocyte antigen-antigen D-related
Celecoxib is a cyclooxygenase type 2 (COX-2)-selective non-steroidal anti-inflammatory drug (NSAID) that is hypothesized to reduce chronic immune activation and dysfunctional T cells [91]. In a prospective open-label randomized exploratory trial of celecoxib on 31 HIV+ patients, reduced chronic immune activation was seen in the treatment group, as characterized by reduced CD38 density in CD8+ T cells, IgA levels, and a combined score for inflammatory markers. Other T cell-dependent functions were also improved [91]. However, even if larger studies confirm the antiinflammatory effects of celecoxib in PLWH, NSAIDs may not be considered for reducing the risk of CVD anytime soon.
Steroids and Other Anti-Inflammatory Drugs
A randomized clinical trial of prednisolone (5 mg/day) did not show any significant effect on the primary endpoint of HIV disease progression to AIDS but found significant decrease in immune activation and increase in CD4+ counts and viral load [92]. A selected list of the studies using anti-inflammatory medications, including other classes of medications with anti-inflammatory properties, which are designed to study CVD, is provided in Table 1.
Methotrexate has been shown to have anti-inflammatory effects, and low doses (10–25 mg) have been used to treat rheumatoid arthritis (RA) for the past two decades. A phase I trial in HIV patients has been completed (NCT00000834), and a phase II trial of methotrexate in HIV patients is currently ongoing (NCT01949116). A phase II trial comparing antiplatelet activity of clopidogrel versus aspirin (NCT02559414) is currently ongoing and aims to measure inflammatory markers, immune activity, and endothelial function as secondary outcomes. Other drugs currently being tested in phase II trials are canakinumab, an anti-IL1 β antibody (NCT02272946); colchicine, an anti-inflammatory drug used to treat arthritis (NCT02624180); and pentoxifylline, an anti-inflammatory drug used to treat leg pain due in patients with leg thrombosis (NCT00796822).
Statins
Statins are lipid-lowering medications widely used for CVD prevention and are believed to have antiinflammatory effects independent of their lipidlowering properties [93]. The Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial was a randomized, double-blind, placebo-controlled 96-week study designed to examine the effects of rosuvastatin (10 mg daily) on markers of CVD risk in HIV+ patients on ART [94]. After 24 weeks of rosuvastatin, there was a significant decrease in plasma levels of sCD14 and in proportions of tissue factor (TF)-positive patrolling monocytes in rosuvastatin arm compared to placebo [94]. More recently, SATURN investigators found less progression of common carotid artery intima-media thickness in HIV patients assigned to statin compared to placebo [95]. Several trials examining the anti-inflammatory effects and cardiovascular benefits of statins are currently ongoing for drugs such as atorvastatin, pravastatin, lov-astatin, and rosuvastatin (Table 2).
Table 2.
Ongoing clinical trials of statins and anti-microbial translocation drugs in HIV+ patients to examine anti-inflammatory effects and/or cardiovascular benefits
| Drug | Phase | Randomized? | Cardiovascular and/or inflammation outcomes* | Size | Status | Identifier |
|---|---|---|---|---|---|---|
| Statins | ||||||
| Atorvastatin | n/a | Y | Cardiovascular: coronary and aortic plaque inflammation, plaque progression, endothelial function Inflammation: immune function, CRP |
40 | Completed | NCT00965185 |
| Atorvastatin | 4 | N | Cardiovascular: none specified Inflammation: peripheral blood monocytes, T cell markers |
15 | Active, not recruiting | NCT01263938 |
| Lovastatin | 2 | Y | Cardiovascular: none specified Inflammation: T cell activity (CD4, CD8), CD38, HLA-DR, LFA-1 ICAM-1 |
112 | Completed | NCT00721305 |
| Rosuvastatin calcium | 0 | Y | Cardiovascular: cEMT, FMD Inflammation: hsCRP | 30 | Completed | NCT01881971 |
| Atorvastatin | 4 | Y | Cardiovascular: none specified Inflammation: peripheral blood monocytes, T cell, and CSF activation markers |
30 | Recruiting | NCT01600170 |
| Pravastatin sodium vs efavirenz/emtricitabine/tenofovir disoproxil fumarate | 2 | Y | Cardiovascular: brachial artery FMD Inflammation: IL-6, hs-CRP, D-dimer | 0 | Withdrawn | NCT01515813 |
| Atorvastatin | 2 | Y | Cardiovascular: none specified Inflammation: markers of inflammation (not specified further) |
9 | Completed | NCT003 67458 |
| Atorvastatin | 4 | Y | Inflammation: IL-6, antiretroviral resistance test | 60 | Recruiting | NCT02577042 |
| Rosuvastatin | 2 & 3 | N | Cardiovascular: brachial artery FMD, Inflammation: T cell CD38 and CD69, monocyte CD16 and CD69, hsCRP |
7 | Terminated | NCT00986999 |
| Pitavastatin | 4 | Y | Cardiovascular: any major adverse cardiovascular events, evidence of non-calcified coronary atherosclerotic plaque, volume of NCP at study entry and change in NCP over 2 years, progression of NCP, number of high risk plaque features Inflammation: inflammatory markers (not specified further) |
6500 | Recruiting | NCT02344290 |
| Atorvastatin | 2 | Y | Cardiovascular: none specified Inflammation: IL-6, CD4+ T cell activation, D-dimer, CD8+ T cell activation, MCP-1, EP-10, CD40L, sCD14, P-selectin, sCD163 |
98 | Completed | NCT01351025 |
| Atorvastatin | 3 | Y | Cardiovascular: none specified Inflammation: percentage change in immune activation levels after 12 weeks of atorvastatin 80 mg daily |
30 | Completed | NCT01766076 |
| Probiotics | ||||||
| Probiotics | n/a | N | CD4+/CD38-/HLA-DR, CD8+/CD38+/HLA-DR + |CD4 recovery | 30 | Unknown status | NCT02164344 |
| Probiotic | n/a | N | LPS, sCD14, change in inflammation around the gut measured with PET/MR scans of the abdomen, hsCRP, cytokines |
45 | Completed | NCT02764684 |
| Probiotics | n/a | Y | LPS, cytokines and macrophage activation, lymphocyte activation markers, viral load, CD4 T cell count |
0 | Withdrawn | NCT01492803 |
| Multi-strain probiotic | 2 | Y | Measures of microbial translocation, markers of immune activation, immune reconstitution in ART-treated patients |
100 | Unknown status | NCT0143 9841 |
| VSL#3® probiotic | n/a | N | sCD14, sCD163, IL-6, D-dimer, plasma intestinal fatty acid biding protein, CD4+ T cell counts | 23 | Completed | NCT02448238 |
| Probiotic | 2 | Y | LBP, sCD14, interferon gamma, TNF, IL-6, CRP, D-dimer, fibrinogen, CD4 -l- lymphocyte count, CD 8+, and HIV viral load |
44 | Active, not recruiting | NCT01908049 |
| Visbiome | 2 | Y | LPS, sCD14, IL-6, D-dimer, CRP, gut immune cell counts (including CD4 T cell subsets), intestinal permeability (Lac/Mac ratio), gut HIV DNA levels |
40 | Recruiting | NCT02441244 |
| Other Drugs Targeting Microbial Translocation | ||||||
| Lubiprostone | 2 | Y | CD8+ T cell activation, CD8+ T cell activation, CD4+ T cell activation, levels of systemic inflammation, plasma HIV-1 RNA, peripheral CD4+, Actl Antibody |
20 | Active, not recruiting | NCT01839734 |
| Rifaximin | 2 | Y | CD8+ T cell activation, D-dimer, IL-6, LPS, hsCRP, sCD14, Peripheral B7hi CD4+ T cell, %CD3 8+ of CD4+, %CD38+ of CD8+, %Ki67+ ofCD4+, %Ki67+ of CD8+, %HLA-DR+/CD38+ of CD4+, CD38+ of CD8+ mean fluorescence intensity |
73 | Completed | NCT01466595 |
| Teduglutide | 2 | Y | Cardiovascular: arterial target to background ratio of 18-FDG-PET uptake, CD4+ T cell percentage, plaque volume on cardiac computed tomography angiography Inflammation: sCD14, sCD163, intestinal fatty acid binding protein, LPS |
60 | Recruiting | NCT02431325 |
| Fluarix vs pneumovax | Y | CD4+ T cell activity, IL-6, TNF, sCD14, sCD163, CD3, CD4, CD8, CD38, DR, levels of HIV specific immune response |
56 | Recruiting | NCT02707692 | |
| Isotretinoin | 2 | Y | CD8+ T cell activation, markers of gut microbial translocation, markers of systemic inflammation and coagulation, sCD163, CD4+ T cell count, Thl7 and Treg frequency |
76 | Active, not recruiting | NCTO1969058 |
*Only studies that included inflammatory markers as outcomes are included.
Abbreviations: highly-sensitive C-reactive protein, hsCRP; C-reactive protein, CRP; soluble CD14, sCD14; soluble CD163, sCD163; interleukin-6, IL-6; Flow-mediated dilatation, FMD; soluble tumor necrosis factor receptor, sTNFR; tumor necrosis factor, TNF; monocyte chemotactic protein 1,MCP-1; soluble vascular cell adhesion molecule 1, sVCAM-1; interferon gamma-induced protein 10, IP-10; Matrix metallopeptidase, MMP-9; TIMP metallopeptidase inhibitor 1, TIMP-1; Plasminogen activator inhibitor-1, PAI-1; Positron emission tomography, PET; Fluorodeoxygenase, FDG; human leukocyte antigen-antigen D related, HLA-DR
Antimicrobial Translocation
Multiple ongoing trials are currently investigating the effects of probiotics on microbial translocation and immune activation markers in the context of HIV infection (Table 2). Other ongoing studies that specifically target microbial translocation include drugs such as rifaximin, sevelamer, mesalamine, visbiome, teduglutide, fluarix, and isotretinoin (Table 2). It is worth noting that the anti-inflammatory properties of many anti-inflammatory medications such as statins is likely mediated through mechanisms not related to gut integrity or microbial translocation. For example, rosuvastatin was found to reduce intestinal fatty acid-binding protein, a marker of enterocyte death, but not zonulin-1, a marker of gut epithelial cell function [96]. Therefore, by targeting a different pathway, AMT medications may provide an incremental value when added to other anti-inflammatory medications.
Clinical Considerations in Managing Cardiovascular Disease Risk in HIV-Infected Patients
As our knowledge of the increased risk of cardiovascular diseases in HIV-infected patients increases, there is a growing need for incorporating this knowledge into clinical practice. There are no available guidelines specific to PLWH; however, the scientific community is reaching some consensus in particular areas based on the available body of evidence. Here, we summarize some of the important considerations in clinical management of HIV infected patients.
Risk stratification
With 1.5- to 2-fold increase in the risk of CAD, 1.8 times higher risk of HF, and 6-fold increase in risk of myocardial fibrosis, HIV infection should be considered as an important risk factor for CVD and this should be taken into account in our risk stratification scores. The increased risks of atherosclerosis in FRAM study [8] and increased risk of MI in Veterans [37] associated with HIV infection were similar to that of diabetes mellitus. This suggests that HIV infection should potentially be considered as a CAD equivalent. Furthermore, as mentioned above, traditional risk factors such as high blood pressure may have a different impact on HIV+ than HIV- individuals. This could affect the utility of general risk prediction models and may result in underestimation of CVD risk in PLWH [97]. A newly developed score developed for HIV+ patients, the DAD score, has added length of ART treatment and the exposure to certain drugs (abacavir, indinavir, lopinavir) to traditional risk factors. Three studies have compared Framingham risk scores (FRS) to the DAD score [98–100], as well as to the FRS with aggravating factors [99], Systematic Coronary Risk Evaluation (SCORE)[100], and Prospective Cardiovascular Munster (PROCAM) score [99, 100] in HIV-infected individuals. Although results from these studies were inconsistent due to differences in study population characteristics, all three studies found fair to good agreement (range 32.5 to 77.4%) among these scores but mostly low (range −0.045 to 0.533) kappa values. All three studies found the DAD score to be the most accurate. However, the DAD score has not been validated in studies with long-term outcomes. A large risk estimation study of more than 11,000 PLWH followed for myocardial infarction found that inclusion of HIV-specific factors did not improve the performance of cardiovascular risk estimation equations. More studies are needed to devise appropriate management guidelines and develop risk prediction models that capture more HIV-specific risk factors.
Clinical considerations regarding ART
Despite controversies around some of the medications used in ART, controlling HIV infection with early ART remain the most important therapeutic goal. However, the risk of CVD and other chronic diseases should be taken into account in making decisions about ART. If possible, it is wise to avoid protease inhibitors that are associated with lipid abnormalities, such as lopinavir/ ritonavir. In patients who are already virologically suppressed on these PIs and have lipid abnormalities, consider switching to integrase inhibitors or NNRTIs—taking into careful consideration any potential drug interactions and/or resistance patterns in the individual patient per current ART guidelines. Also, given the controversies around abacavir, this drug should be used cautiously in those with higher CVD risk. In particular, given that the significant initial immune activation associated with abacavir increases the risk [46, 47], it is recommended to avoid abacavir in treatment naive patients with significant CVD risk factors.
Management of traditional risk factors
We recommend intensive management of traditional risk factors. This includes lifestyle modifications (such as diet and physical activity), smoking cessation, and monitoring and treatment of high blood glucose and high blood pressure. Given that microbial translocation or “leaky gut” syndrome is considered an important contributor to the inflammatory state in these patients, diet is of particular importance [101]. Although no current nutritional guidelines exist for PLWH, in HIV-uninfected patients with CVD, there is increasing data suggesting the beneficial effect of some dietary modifications, such as plant-based or vegetarian diets, in decreasing the endothelial damage and inflammation [102–104]. More studies are needed to identify ideal nutritional guidelines and assess the clinical implications of these types of more intensive lifestyle intervention strategies in PLWH. Also, as mentioned above, even mild elevation in blood pressure (prehypertension) is associated with increased CVD risk in HIV+ population. Therefore, both prehypertension and clinical hypertension should be aggressively managed in these patients. It is also recommended to check fasting blood glucose and hemoglobin A1C at baseline, 1–3 months after initiation of a new regimen, and every 3–6 months thereafter.
Treatment of dyslipidemia
Serum lipid panel is recommended to be monitored regularly in PLWH, particularly at baseline and after initiation of a new regimen. Guidelines for management of dyslipidemia in HIV+ patients published in 2003[105] recommend following NCEP ATP III guideline with particular attention to drug interactions and virologic control of HIV infection. These guidelines recommend that, when medication becomes necessary, statins are recommended for elevated LDL cholesterol levels or elevated non-HDL cholesterol in patients with triglyceride level of 200–500 mg/ dL, and fenofibrate and gemfibrozil are recommended for those with triglyceride levels greater than 500 mg/dL. Fibrates are a good choice due to lack of significant interaction with ART medications. When statins are indicated, generally atorvastatin or rosuvastatin can be considered, except in those on lopinavir/ritonavir for whom pitavastatin is the statin of choice (due to least drug interaction). The National Lipid Association (NLA) recommendations for patient-centered management of dyslipidemia in 2015[106] states that HIV patients with dyslipidemia should be treated similarly to the general population, but that HIV should be considered as an additional major risk factor for atherosclerotic cardiovascular disease in their risk stratification guidelines. However, some studies have suggested that these guidelines may need to be modified for HIV-infected population. For example, Zanni et al. showed that based on 2013 ACC/AHA guidelines, statin therapy was not recommended for more than 74% of HIV+ participants with high-risk coronary plaque on CT angiography [107]. Nonetheless, as concluded by the NLA guidelines [106], there has not been enough research to develop comprehensive data-driven guidelines and validated risk stratification strategies for HIV patients.
Conclusions
PLWH are at higher risk of clinical and subclinical CAD. Among different mechanisms suggested for this increased risk, chronic persistent inflammation appears to play an important role. Possible mechanisms for this inflammation include subclinical viremia, microbial translocation, and coinfection with other pathogens such as cytomegalovirus. Although inflammatory biomarkers have been consistently associated with increased CAD risk, their independent prognostic value is unknown. We recommend early ART and intensive management of traditional risk factors in HIV+ patients. Also, HIV infection should be considered as an important risk factor for CAD in risk estimations. Recent and ongoing trials are exploring the benefits of anti-inflammatory drugs, statins, and antimicrobial translocation drugs on both inflammation and CVD risk among PLWH.
Acknowledgements
The authors would like to thank Dr. Farin Kamangar, Dr. Sonia Singh, and Dr. Gillian Buckley for their valuable contributions to this paper. This project is supported by National Institutes of Health/National Heart, Lung, and Blood Institute (NHLBI) grant 5K23HL128164A. The senior author (HB) has also received NIH LRP grant and the first author (JCAL) is supported by NIH/NIGMS T32 Training Grant GM067587–09.
Footnotes
Compliance with Ethical Standards
Conflict of Interest John Charles A. Lacson, Revery P. Barnes, and Hossein Bahrami declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Global Health Observatory data on HIV/AIDS [http://www.who. int/gho/hiv/en/]
- 2.Vella S, Schwartlander B, Sow SP, Eholie SP, Murphy RL: The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS (London, England) 2012, 26(10): 1231–1241. [DOI] [PubMed] [Google Scholar]
- 3.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009;95(14):1193–202. [DOI] [PubMed] [Google Scholar]
- 5.Farahani M, Mulinder H, Farahani A, Marlink R: Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2016. [DOI] [PubMed] [Google Scholar]
- 6.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. [DOI] [PubMed] [Google Scholar]
- 7.Lang S, Boccara F, Mary-Krause M, Cohen A. Epidemiology of coronary heart disease in HIV-infected versus uninfected individuals in developed countries. Arch Cardiovasc Dis. 2015;108(3): 206–15. [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS (London, England). 2009;23(14):1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–22. [DOI] [PubMed] [Google Scholar]
- 10.Bahrami H, Fonarow G, Heidenreich P: Heart failure admission in HIV-infected patients. J Am Coll Cardiol 2014, 63(12_S). [Google Scholar]
- 11.Talwani R, Falusi OM, de Mendes Leon CF, et al. Electron beam computed tomography for assessment of coronary artery disease in HIV-infected men receiving antiretroviral therapy. J Acquir Immune Defic Syndr (1999). 2002;30(2):191–5. [DOI] [PubMed] [Google Scholar]
- 12.Acevedo M, Sprecher DL, Calabrese L, et al. Pilot study of coronary atherosclerotic risk and plaque burden in HIV patients: ‘a call for cardiovascular prevention’. Atherosclerosis. 2002;163(2):349–54. [DOI] [PubMed] [Google Scholar]
- 13.Fitch KV, Lo J, Abbara S, et al. Increased coronary artery calcium score and noncalcified plaque among HIV-infected men: relationship to metabolic syndrome and cardiac risk parameters. J Acquir Immune Defic Syndr (1999). 2010;55(4):495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS (London, England). 2007;21(9):1137–45. [DOI] [PubMed] [Google Scholar]
- 15.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–8. [DOI] [PubMed] [Google Scholar]
- 16.Lipshultz SE, Miller TL, Wilkinson JD, et al. Cardiac effects in perinatally HIV-infected and HIV-exposed but uninfected children and adolescents: a view from the United States of America. J Int AIDS Soc. 2013;16:18597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontes-Carvalho R, Mancio J, Marcos A, et al. HIV patients have impaired diastolic function that is not aggravated by anti-retroviral treatment. Cardiovasc Drugs Ther. 2015;29(1):31–9. [DOI] [PubMed] [Google Scholar]
- 18.Hsue PY, Tawakol A. Inflammation and fibrosis in HIV: getting to the heart of the matter. Circ Cardiovasc Imaging. 2016;9(3): e004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnett CF, Hsue PY. Human immunodeficiency virus-associated pulmonary arterial hypertension. Clin Chest Med. 2013;34(2):283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu JC, Li Y, Marcus GM, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol. 2013;61(22):2288–95. [DOI] [PubMed] [Google Scholar]
- 22.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes RP, Lacson JC, Bahrami H: HIV infection and risk of cardiovascular diseases beyond coronary artery disease. Current atherosclerosis reports 2017;19(5). doi: 10.1007/s11883-017-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205 suppl 3:S355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS (London, England). 2009;23(9):1059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–45. [DOI] [PubMed] [Google Scholar]
- 27.Kingsley LA, Cuervo-Rojas J, Munoz A, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS (London, England). 2008;22(13):1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.•.Bahrami H, Budoff M, Haberlen SA, et al. : Inflammatory markers associated with subclinical coronary artery disease: the Multicenter AIDS Cohort Study. J Am Heart Assoc 2016, 5(6). This study is the largest study of subclinical CAD using CT angiography and showed the relationship of inflammatory markers with subclinical CAD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. results from the DAD study. AIDS (London, England). 2003;17(8):1179–93. [DOI] [PubMed] [Google Scholar]
- 30.Krishnaswamy G, Chi DS, Kelley JL, Sarubbi F, Smith JK, Peiris A. The cardiovascular and metabolic complications of HIV infection. Cardiol Rev. 2000;8(5):260–8. [DOI] [PubMed] [Google Scholar]
- 31.Ssinabulya I, Kayima J, Longenecker C, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One. 2014;9(2):e89537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.••.Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PloS One. 2013;8(3):e59551 Performed a meta-analysis of the effects of different ART drugs on CVD risk and found that PIs and recent exposure to abacavir increases CVD risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immune deficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170(14):1228–38. [DOI] [PubMed] [Google Scholar]
- 34.Petrosillo N, Cicalini S. Smoking and HIV: time for a change? BMC Med. 2013;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.•.Paisible AL, Chang CC, So-Armah KA, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial Infarction. J Acquir Immune Defic Syndr (1999). 2015;68(2):209–16. This study found that when comparing between HIV+ and uninfected individuals of the same cardiovascular risk factor profiles, HIV+ individuals remain at higher risk for CVD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armah KA, Chang CC, Baker JV, et al. Prehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and -uninfected veterans. Clin Infect Dis. 2014;58(1): 121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flint OP, Noor MA, Hruz PW, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201(3):318–30. [DOI] [PubMed] [Google Scholar]
- 40.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. 2011. J Acquir Immune Defic Syndr (1999). 1999;57(3):245–53. [DOI] [PubMed] [Google Scholar]
- 41.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis. 2011;53(1):84–91. [DOI] [PubMed] [Google Scholar]
- 42.Choi AI, Vittinghoff E, Deeks SG, Weekley CC, Li Y, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS (London, England). 2011;25(10):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai M, Joyce V, Bendavid E, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis. 2015;61(3):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young J, Xiao Y, Moodie EE, et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV cohort study. J Acquir Immune Defic Syndr (1999). 2015;69(4):413–21. [DOI] [PubMed] [Google Scholar]
- 45.Marcus JL, Neugebauer RS, Leyden WA, et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr (1999). 2016;71(4):413–9. [DOI] [PubMed] [Google Scholar]
- 46.Adam J, Wuillemin N, Watkins S, et al. Abacavir induced T cell reactivity from drug naive individuals shares features of allo- immune responses. PLoS One. 2014;9(4):e95339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chini M, Tsoupras AB, Mangafas N, et al. Effects of highly active antiretroviral therapy on platelet activating factor metabolism in naive HIV-infected patients: ii) study of the abacavir/lamivudine/ efavirenz HAART regimen. Int J Immunopathol Pharmacol. 2012;25(1):247–58. [DOI] [PubMed] [Google Scholar]
- 48.••.Strategies for Management of Antiretroviral Therapy Study G et al. CD4+ count-guided interruption of antiretroviral treatment. New England J Med. 2006;355(22):2283–96. This study found that those who delayed or interrupted ART were at higher risk of CVD than those under continuous ART. [DOI] [PubMed] [Google Scholar]
- 49.Siedner MJ. START or SMART? Timing of antiretroviral therapy initiation and cardiovascular risk for people with human immunodeficiency virus infection. Open Forum Infect Dis. 2016;3(1): ofw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang S, Mary-Krause M, Simon A, et al. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55(4):600–7. [DOI] [PubMed] [Google Scholar]
- 51.Post WS, Budoff M, Kingsley L, et al. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160(7):458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr (1999). 2014;65(2): 160–6. [DOI] [PubMed] [Google Scholar]
- 53.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr (1999). 2010;55(5):615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51(4):435–47. [DOI] [PubMed] [Google Scholar]
- 55.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatano H, Scherzer R, Wu Y, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. J Acquir Immune Defic Syndr. 2012;61(3):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24(4):501–6. [DOI] [PubMed] [Google Scholar]
- 58.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV- infected patients. AIDS (London, England). 2009;23(15):2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freiberg MS, Chang C-CH, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circulation: Cardiovasc Quality Outcomes. 2011;4(4):425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11(7): 462–8. [DOI] [PubMed] [Google Scholar]
- 61.Ridker PM. Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res. 2014;114(4):594–5. [DOI] [PubMed] [Google Scholar]
- 62.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. [DOI] [PubMed] [Google Scholar]
- 63.Neuhaus J, Jacobs DR Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS (London, England). 2008;22(4):439–46. [DOI] [PubMed] [Google Scholar]
- 65.Longenecker CT, Sullivan C, Baker JV. Immune activation and cardiovascular disease in chronic HIV infection. Curr Opin HIV AIDS. 2016;11(2):216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21(1):6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Epple HJ, Schneider T, Troeger H, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58(2):220–7. [DOI] [PubMed] [Google Scholar]
- 70.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S, Snider JJ, Gill MJ. Cytomegalovirus disease in HIV infection: twenty years of a regional population’s experience. Clin Infect Dis. 2006;42(12):1808–9. [DOI] [PubMed] [Google Scholar]
- 76.Freeman ML, Lederman MM, Gianella S. Partners in crime: the role of CMV in immune dysregulation and clinical outcome during HIV infection. Curr HIV/AIDS Rep. 2016;13(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203(10):1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi TJ, Walmsley S, Szadkowski L, et al. A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis. 2013;57(9): 1331–8. [DOI] [PubMed] [Google Scholar]
- 79.Masia M, Robledano C, Tabla V, et al. Coinfection with human herpesvirus 8 is associated with persistent inflammation and immune activation in virologically suppressed HIV-infected patients. PloS One. 2014;9(8):e105442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non- AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.•.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE: Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PloS One 2016, 11(1): e0147484 Vos et al. conducted a systematic review and metaanalysis to examine the association between different biomarkers of inflammation and CVD risk and carotid intima- media thickness and found that CRP, IL-6, and D-dimer were positively associated with CVD risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nordell AD, McKenna M, Borges AH, et al. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3(3):e000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J infect Dis. 2011;203(6):780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211(8):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krebs SJ, Ananworanich J. Immune activation during acute HIV infection and the impact of early antiretroviral therapy. Curr Opin HIV AIDS. 2016;11(2):163–72. [DOI] [PubMed] [Google Scholar]
- 86.Hatano H, Strain MC, Scherzer R, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis. 2013;208(9):1436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Brien M, Montenont E, Hu L, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndrom (1999). 2013;63(3):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Modulation of immune activation by aspirin [https://clinicaltrials.gov/ct2/show/results/NCT02155985]
- 89.Burkholder GA, Tamhane AR, Salinas JL, et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55(11):1550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suchindran S, Regan S, Meigs JB, Grinspoon SK, Triant VA. Aspirin use for primary and secondary prevention in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients. Open Forum Infect Dis. 2014;1(3):ofu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettersen FO, Torheim EA, Dahm AE, et al. An exploratory trial of cyclooxygenase type 2 inhibitor in HIV-1 infection: downreg- ulated immune activation and improved T cell-dependent vaccine responses. J Virol. 2011;85(13):6557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kasang C, Kalluvya S, Majinge C, et al. Effects of prednisolone on disease progression in antiretroviral-untreated HIV infection: a 2-year randomized, double-blind placebo-controlled clinical trial. PloS One. 2016;11(1):e0146678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977–87. [DOI] [PubMed] [Google Scholar]
- 94.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4): 588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Longenecker CT, Sattar A, Gilkeson R, McComsey GA: Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS (London, England) 2016, 30(14):2195–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Funderburg NT, Boucher M, Sattar A, et al. Rosuvastatin decreases intestinal fatty acid binding protein (I-FABP), but does not alter zonulin or lipopolysaccharide binding protein (LBP) levels, in HIV-infected subjects on antiretroviral therapy. Pathog Immun. 2016;1(1):118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Friis-Moller N, Ryom L, Smith C, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. [DOI] [PubMed] [Google Scholar]
- 98.Markowicz S, Delforge M, Necsoi C, De Wit S. Cardiovascular risk evaluation of HIV-positive patients in a case-control study: comparison of the D:A:D and Framingham equations. J Int AIDS Soc. 2014;17(4 Suppl 3):19515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nery MW, Martelli CM, Silveira EA, et al. Cardiovascular risk assessment: a comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. ScientificWorldJournal. 2013;2013:969281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pirš M, Jug B, Erzen B, et al. Cardiovascular risk assessment in HIV-infected male patients: a comparison of Framingham, SCORE, PROCAM and DAD risk equations. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23(3):43–7. [DOI] [PubMed] [Google Scholar]
- 101.Douek D HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15(4):114–7. [PubMed] [Google Scholar]
- 102.Tuso P, Stoll SR, Li WW. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm J. 2015;19(1):62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Esselstyn CB Jr, Gendy G, Doyle J, Golubic M, Roizen MF. A way to reverse CAD? J Fam Pract. 2014;63(7):356–364b. [PubMed] [Google Scholar]
- 104.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7. [DOI] [PubMed] [Google Scholar]
- 105.Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37(5):613–27. [DOI] [PubMed] [Google Scholar]
- 106.Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipido. 2015;9(6 Suppl):S1–122.e121. [DOI] [PubMed] [Google Scholar]
- 107.Zanni MV, Fitch KV, Feldpausch M, et al. American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV- infected patients with/without subclinical high-risk coronary plaque. AIDS (London, England) 2014. 2013;28(14):2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


