Abstract
The immune system has evolved to mount an effective defense against pathogens and to minimize deleterious immune-mediated inflammation caused by commensal microorganisms, immune responses against self and environmental antigens, and metabolic inflammatory disorders. Regulatory T (Treg) cell–mediated suppression serves as a vital mechanism of negative regulation of immune-mediated inflammation and features prominently in autoimmune and autoinflammatory disorders, allergy, acute and chronic infections, cancer, and metabolic inflammation. The discovery that Foxp3 is the transcription factor that specifies the Treg cell lineage facilitated recent progress in understanding the biology of regulatory T cells. In this review, we discuss cellular and molecular mechanisms in the differentiation and function of these cells.
Keywords: Foxp3, immune homeostasis, tolerance, autoimmunity
INTRODUCTION
A hallmark of the adaptive immune system is the generation of diverse immune receptors for the anticipated encounter with rapidly evolving pathogens. This powerful strategy for host defense brings considerable challenges, however. Because T cell receptors (TCRs) are selected by highly diverse endogenous ligands, i.e., self-peptide-MHC complexes, potentially pathogenic autoreactive T cells can be generated. The adaptive immune system must refrain from mounting deleterious responses against self, food antigens, commensal microorganisms, and environmental antigens, which may or may not shape the TCR repertoire in the thymus. Furthermore, the restraint of immune responses against pathogens is essential to spare the host from excessive damage to its own tissues.
Negative selection of self-reactive T cells in the thymus leads to elimination or inactivation of self-reactive T cell clones and is likely responsible for neutralization of most high-affinity T cells recognizing self (see for review Reference 1). In the periphery, chronic engagement of TCRs by self antigens induces anergy. Peripheral tolerance is also reinforced by the requirement of simultaneous engagement of TCRs by a cognate peptide-MHC complex and the T cell costimulatory receptor CD28 by CD80 and CD86 (2). CD80 and CD86 are induced on antigen-presenting cells (APCs) upon the activation of innate immune receptors directly in response to microbial or viral products or through sensors of metabolic changes invoked by microorganisms.
Mechanisms of tolerance, operating in a cell-intrinsic manner, and the two-signal requirement for the induction of a productive immune response appear insufficient to counter the threat of immune-mediated pathology without a specialized subset of T cells acting in trans to restrain pathogenic immune responses. The initial evidence in support of thymic generation of cells that can mediate immune tolerance through the suppression of other cells came from neonatal thymectomy experiments performed by Nishizuka, Sakaguchi, and others (3–6) and from studies of transplantation tolerance in chicken-quail chimeras and in mice performed by Le Douarin and colleagues (7). In mice, neonatal thymectomy performed between two and four days of life resulted in T cell–mediated tissue inflammation, which was prevented upon adoptive transfer of thymocytes or splenocytes from adult euthymic mice (3–6). These experiments showed that a T cell subset generated in the mouse thymus after three days of life can prevent autoimmunity.
In another line of experimentation, chicken-quail chimera studies demonstrated that grafted thymic epithelium (TE) is responsible for xenograft tolerance (7). In this experimental system, thymectomized chicken embryos receive TE grafts from quail embryos before hematopoietic colonization of the thymus occurs, resulting in differentiation and selection of recipient (chicken) T cells in response to antigens presented on donor (quail) TE cells. The resulting T cells are immunologically competent—capable of rejecting third-party grafts—but are tolerized against grafts of TE donor (quail) origin. Similar allogeneic TE transplantation experiments in mice also demonstrated that complete clonal deletion of alloreactive (TE donor–reactive) T cells was not necessary for inducing tolerance to allogeneic tissue grafts (8) and implicated a population of thymus-derived cells in suppression of alloreactive T cells. Additional experiments, in which decreasing numbers of graft-tolerized T cells were transferred into athymic nude mice, showed reduced or abrogated tolerance to grafts with diminished cell numbers. These observations suggested that tolerant TE chimeras contain both graft-reactive effector T cells and a less abundant, limiting population of suppressive T cells capable of preventing graft rejection (9). Based on this series of studies, Le Douarin and colleagues concluded that “tolerance to self results at least in part from the interplay between cells potentially harmful for self component and others which exert a strong control on their reactivity. The latter cell type depends upon interactions of thymocytes with the endodermal component of the thymus” (10, p. 49). In addition to these autoimmunity and transplant tolerance studies, other experiments revealed the suppressive function of a subset of CD4+ T cells with an antigen-experienced phenotype; these experiments employed cotransfers of these cells with naive colitogenic CD45RBhighCD4+ T cells into athymic rats or SCID mice (11, 12). The amelioration of colitis observed in these early studies suggested that, in addition to control of immune responses to self and transplantation antigens, suppressive CD4+ T cells might also limit responses to dietary antigens and the gut-resident microbiota.
A culmination of this early work came in 1995, when a subset of CD4+ T cells constitutively expressing high amounts of the interleukin (IL)-2 receptor α-chain (CD25) was identified. These cells, termed regulatory T cells or Treg cells, were highly enriched in suppressor activity (13). Treg cells were able to prevent autoimmunity upon transfer into day-3 thymectomized mice and, in various other experimental models of autoimmunity, inhibit transplant rejection and thwart tumor immunity (reviewed in 14, 15). The presence of a subset of CD25+CD4+ single-positive (SP) thymocytes with suppressive activity in the thymus (6) indicated that CD25+ Treg cells differentiate in the thymus. Although identification of CD25 as a marker of Treg cells allowed for the functional analyses of these cells following their isolation from non-immune animals, its utility was limited because of the upregulation of CD25 in all activated T cells. The inability to discriminate between tolerance- and inflammation-promoting cells during the immune response impeded further understanding of dominant tolerance, especially its mechanistic aspects. Moreover, it was proposed that CD25-expressing Treg cells represented a state of activation of conventional CD4 T cells and that CD25+CD4+ downmodulation of immune responses occurs because of competition for IL-2.
FOXP3 ORCHESTRATES DOMINANT TOLERANCE
The recent insights into the biology of Treg cells were facilitated largely by the identification and study of mutations in the X-chromosome encoded transcription factor Foxp3 in mice and in human IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome patients (16–19). Mice and humans with a loss-of-function mutation in the Foxp3 gene are afflicted with a fatal, early-onset, T cell–dependent, lymphoproliferative, immune-mediated disorder manifested by diabetes, thyroiditis, hemolytic anemia, hyper-IgE syndrome, exfoliative dermatitis, splenomegaly, lymphadenopathy, and cytokine storm (as reviewed in 20). Importantly, the disease affects only hemizygous mutant males and not heterozygous female carriers of Foxp3 mutations. The latter remain healthy because of random X-chromosome inactivation, which ensures that some T cells express a wild-type Foxp3 allele (21). These cells then keep in check pathogenic T cells with a mutant Foxp3 allele, which is consistent with suppression occurring in trans, if one assumes the likely case that Foxp3 mutations do not affect random X-chromosome inactivation in T cells. Indeed, this assumption was confirmed by analysis of Foxp3 reporter mice (22, 23).
On the basis of these considerations, three laboratories assessed the expression of Foxp3 in mouse CD25+CD4+ Treg cells. These studies in mice revealed stable expression of high amounts of Foxp3 in mouse CD25+CD4+ Treg cells, but not in naive CD25−CD4+ T cells or in activated CD4+ T cells (24–26). T cells in Foxp3 mutant mice become activated within a few days of birth, w hereas the numbers of CD25+CD4+ thymocytes are markedly reduced (25, 26). Although these experiments were consistent with the notion that Foxp3 is required for differentiation of Treg cells, early-onset autoimmune disease complicated interpretation of these observations. However, additional evidence of a critical role for Foxp3 in the differentiation of Treg cells came from analysis of CD25+CD4+ T cell populations in the thymus and peripheral lymphoid organs of mixed bone marrow chimeras generated upon transfer of Foxp3-deficient and allelically marked wild-type bone marrow into T cell–deficient mice. The recipient mice were free of lymphoproliferative disease and immune-mediated tissue lesions, and CD25+ Treg cells were generated only from Foxp3-sufficient, but not -deficient precursor cells (26), demonstrating an absolute requirement for Foxp3 in Treg cell differentiation in the thymus. In addition, retroviral transfer of the Foxp3 gene into activated peripheral CD25−CD4+ T cells confers suppressor function and Treg cell surface phenotype (24, 26). In mice, expression of a Foxp3 transgene facilitated suppressor activity of CD8 T cells (25). The level of Foxp3 protein expression in Treg cells is critical for suppressor function, given that experimentally induced reduction in Foxp3 amounts resulted in impaired suppressor function (27). Furthermore, sustained Foxp3 expression in mature Treg cells is necessary for maintenance of the Treg cell phenotype and suppressor function. Cre-mediated ablation of a conditional Foxp3 allele in mature Treg cells results in a loss over time of suppressor function and characteristic Treg cell surface markers and acquisition of effector T cell properties including production of immune response-promoting cytokines IL-2, IL-4, IL-17, and IFN-γ (28). Together, these studies showed that Foxp3 is essential for Treg cell differentiation and suppressor function and defines the Treg cell lineage.
THE INDISPENSABLE ROLE OF TREG CELLS IN IMMUNE HOMEOSTASIS
Analysis of Foxp3 reporter mice expressing GFP or RFP coding sequences under control of the Foxp3 locus revealed that Foxp3 protein expression is limited to a subset of CD4 T cells (22, 29–31). In addition to the CD25hiCD4+ T cell subset, some CD4+ T cells with a low level of CD25 or lacking CD25 express Foxp3 (22). The potent suppressive capacity of both CD25+ and CD25–Foxp3+ T cell subsets supports a dedicated function of Foxp3 in Treg cell differentiation.
Several lines of experimentation provide proof that the lack of Treg cells is the cause of fatal autoimmunity resulting from Foxp3 deficiency. First, adoptive transfers of Treg cells rescue neonatal Foxp3-deficient mice from the disease (26). Second, mice subjected to either the T cell–specific or germ-line ablation of the Foxp3 gene are indistinguishable in the progression and severity of the autoimmune lesions (22). Furthermore, deletion of a conditional Foxp3 allele in thymic epithelial cells or dendritic cells, which shape the repertoire of developing T cell precursors, did not result in any discernible immune dysregulation or alteration in T cell differentiation (32; L.M.Williams & A.Y. Rudensky, unpublished observations). Additionally, ablation of a conditional Foxp3 allele in macrophages using LysM-Cre did not result in changes in the immune status of unchallenged mice, nor did it affect the rate of tumor growth or metastatic burden in an experimental model of transplantable breast carcinoma (P.D. Bos & A.Y. Rudensky, unpublished observations). Thus, the overwhelming evidence from genetic studies indicates that deficiency of Foxp3 within the T cell lineage is the sole cause of the disease observed in Foxp3 mutant mice. During thymic differentiation, negative selection or anergy induction in self-reactive T cells was also unaffected by Foxp3 deficiency (33, 34). Furthermore, Foxp3 ablation did not alter the sensitivity of TCR-triggered activation of naive T cells and its dependence on costimulation (22, 26, 33). Finally, Foxp3 deficiency in effector T cells did not have an effect on levels of cytokine production or clonal expansion in the course of immune responses (22, 26). Thus, these studies prove that the lack of Treg cells is the cause of the disease associated with Foxp3 deficiency (22, 26, 33, 34).
The aforementioned analysis of Foxp3-deficient mice and bone marrow transfer studies demonstrated a requirement for Foxp3 in the differentiation of Treg cells and their critical role early in life for establishing immune homeostasis. A role for Treg cells in adult mice was revealed through the generation and analysis of Foxp3DTR knock-in and Foxp3-DTR BAC transgenic mice in which Treg cells are decorated with the human diphtheria toxin receptor (DTR) (35, 36). Chronic ablation of Treg cells in adult healthy Foxp3DTR mice caused their death within 2–3 weeks from rampant lympho- and myeloproliferative disease, demonstrating that Treg cell–mediated suppression is indispensable for preventing immune pathology throughout the life span of normal mice (35). Although the observed pathology appeared to depend on CD4+ T cells specific for self antigens because simultaneous ablation of CD4+ T cells and Treg cells spared mice from the lympho- and myeloproliferative disease, dendritic cell activation remained (35). This raised the question of whether the disease is driven by the commensal microbiota, the largest source of non-self ligands activating the innate and adaptive immune systems, or whether Treg cells restrain T cells with a diverse self-MHC-restricted TCR repertoire independently of the commensal microbiota. The latter scenario was proven true by a study in germfree Foxp3DTR mice in which similarly explosive lympho- and myeloproliferative disease was observed upon Treg cell ablation (37). These observations showed that Treg cells play a critical role in maintaining immune homeostasis throughout the life span of normal animals and that they are required for restraint of self-MHC-restricted T cells regardless of the presence of the commensal microbiota. We now turn the discussion to the recent advances in mechanistic studies of Foxp3+ Treg cell biology.
TREG CELL DIFFERENTIATION IN THE THYMUS: ROLES OF TCR AND OTHER SIGNALS
During thymic differentiation, variations in TCR signaling characteristics such as functional avidity and duration are central determinants of T cell lineage fate determination, informing the CD4 or CD8 T cell choice (38, 39) and the differentiation of specialized T cell subsets, which include NKT cells, CD8αα T cells, and mucosal-associated invariant T cells. These characteristics can also affect the cytokine production bias during T helper differentiation (40, 41). It is not unexpected, then, that a particular requirement for TCR signaling is pivotal for Foxp3 induction and Treg cell lineage commitment. The first indications that Treg cells are exposed to TCR signals of increased strength came from early findings of increased relative expression by Treg cells of CD25, CD5, and cytotoxic T lymphocyte antigen 4 (CTLA-4), which are all induced upon TCR stimulation. CD5 functions as a rheostat that attenuates TCR signaling in a tunable manner through recruitment of the tyrosine phosphatase SHP-1 to its cytoplasmic tail (42, 43). Functional support for Treg cell fate instruction by strong TCR signals came from the observation of increased frequencies of Treg cells in mice with CD5 or SHP-1 deficiency and of associated defects in negative feedback of TCR stimulation (44).
Direct experimental support for the critical role of TCR specificity in thymic Treg (tTreg) cell differentiation came from unexpected findings: Mice that express a TCR transgene specific for myelin basic protein, in the absence of endogenous TCR rearrangements due to RAG deficiency, develop inflammatory lesions in the brain (experimental allergic encephalomyelitis), whereas a subset of T cells expressing endogenous TCRs was able to prevent the disease in RAG-sufficient mice (45, 46). These findings were further extended by observations that to differentiate Treg cells bearing transgene-encoded TCR required coexpression of a cognate ligand for the receptor, encoded by another transgene (47–50). In these studies of TCR transgenic cells, the predominance of negative selection, accompanying the generation of Foxp3-expressing Treg cells (47–49), led to the interpretation that self-reactive Treg cells expressing Foxp3 were conferred a selective survival advantage, whereas without Foxp3 expression, cells receiving equivalent TCR signaling were deleted (51). Consistent with this idea, many prosurvival molecules are highly expressed in Treg cells in a Foxp3-dependent manner.
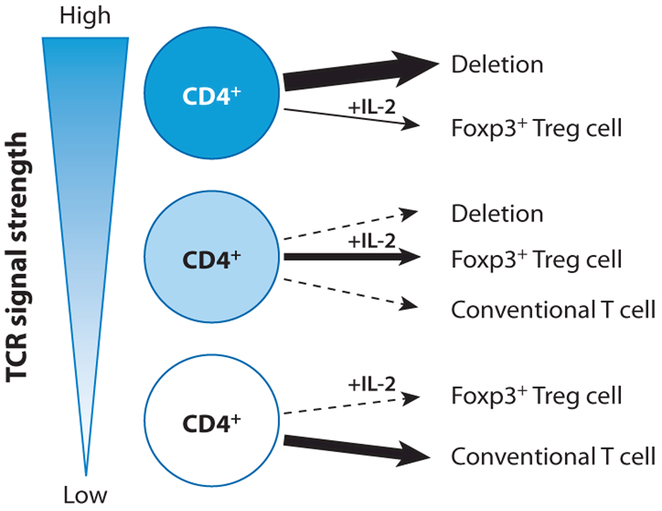
However, a considerable body of recent work points to an instructive role for TCR signaling in Foxp3 induction and Treg cell differentiation. In addition to the aforementioned studies of TCR transgenic mice, sequence analysis of polyclonal TCR repertoires displayed by Treg versus non-Treg cells bearing a single transgene-encoded TCRβ chain showed that Treg TCRα sequences were of broad variety and only partially overlapped with TCRα sequences in non-Treg cells (52–55). Retroviral expression of either Treg or naive CD4+ TCR in effector T cells of a defined specificity for a single foreign antigen demonstrated that Treg TCRs exhibit increased self-reactivity. This self-reactivity manifested in the capacity of effector T cells transfected with Treg TCR for robust expansion and induction of autoimmune disease upon transfer into lymphopenic recipient mice. However, these same pathogenic T cells with Treg TCRs can mount only weak in vitro responses to syngeneic APCs relative to the robust responses these cells can mount against the transgenic TCR-recognized foreign ligand (52). These data indicate that the affinity range of conventional TCRs that recognize foreign antigen during a typical immune response is above the range of affinities of Treg TCRs for self antigens. Therefore, Treg cell selection is likely instructed by TCRs with affinities or avidities for self peptide-MHC ligands in the range between those that mediate positive selection of conventional CD4+ T cells and stronger signals in self-reactive T cells that mediate their negative selection under normal conditions (see Figure 1).
Figure 1.
TCR signal strength instructs CD4+ thymocyte fate and regulatory T cell differentiation. Immature CD4 single-positive (SP) thymocytes receive TCR signals of varied strength via interactions with peptide-MHC on antigen-presenting cells. The strength of TCR signals (or functional avidity, based on a composite of individual peptide-MHC-TCR interaction affinity and peptide-MHC abundance) and their duration determines CD4 SP thymocyte fate. Upon reception of a TCR signal of high strength, most CD4 SP thymocytes undergo programmed cell death. A number of CD4 SP thymocytes receiving TCR signals of intermediate strength are able to escape deletion and are enriched for cells that are instructed to differentiate into Foxp3+ Treg cells. Weight of arrows reflects relative probability of the indicated outcomes.
In support of this hypothesis, partially impaired negative selection that is due to diminished quantities of MHC class II expression in mTEC was accompanied by increased frequencies of Treg cells (56). Similarly, increased negative selection in the absence of TGF-β receptor (TGF-βR) expression on double-positive (DP) and SP thymocytes led to reduced production of Foxp3+ cells in neonates (57). Foxp3 gene expression did not affect the extent or sensitivity of negative selection in thymocytes by high-affinity TCR ligand (33, 34), and furthermore, TCR utilized by Treg cells in Foxp3-sufficient mice are found on activated T cells in Foxp3-deficient mice, a finding that is consistent with their escape from negative selection despite a lack of Foxp3 expression (33). Finally, further support for a role of TCR signaling in this process comes from two experiments: (a) in mice harboring a Foxp3 reporter null allele (Foxp3GFPKO) that was generated upon insertion of a GFP coding sequence into the Foxp3 locus with a concomitant ablation of the Foxp3 protein expression (23) and (b) in mice expressing a truncated Foxp3 protein. In both of these cases, thymocytes expressing these nonfunctional alleles were readily detectable. These cells—considered an equivalent to Treg cell precursors, with self-reactive TCRs—are not deleted but instead, upon maturation, become activated and contribute to pathology in Foxp3-deficient animals.
Initial studies of transgenic mice expressing a single monoclonal TCR originating from Treg cells revealed that, in the absence of endogenous TCR rearrangements, such Treg cell–derived transgenic TCRs support differentiation of very few Foxp3+ Treg cells (58; C.S. Hsieh, J. Marie, J.D. Fontenot, and A.Y. Rudensky, unpublished observations). Recently, however, studies of Treg cell TCR transgenic mice have revealed exquisite TCR-instructed characteristics of the Treg cell population differentiating in the thymus. Experiments by the Hsieh and Lafaille laboratories showed that when the number of precursor cells was dramatically reduced, expression of a single Treg cell–derived transgenic TCR could drive efficient generation of Foxp3+ thymocytes (58, 59). These results imply that severe intraclonal precursor competition restricts the differentiation of numerous Treg cells expressing TCR of identical specificity and, thus, facilitates generation of a broad Treg TCR repertoire in the thymus.
Beyond TCR stimulation, numerous experimental conditions favor induction of Foxp3, including constitutive NF-κB signaling, loss of maintenance DNA methyltransferase activity, deficiency in mTOR or sphingosine-1 phosphate receptor type 1 (S1P1), and reduction of PI3K signaling (60–65). Such evidence supports the idea that particularly tuned TCR signaling and its appropriate coordination with other cell-extrinsic and -intrinsic cues instruct Treg cell differentiation. Besides the TCR, CD28 costimulatory signals also play an essential cell-intrinsic role in the differentiation of thymus-derived Treg cells, with marked decreases in frequencies of Treg cells in both CD28-deficient and CD80-CD86-deficient mice (66, 67). Moreover, the lck-binding domain of the CD28 cytoplasmic tail is required for the induction of Foxp3 (67) and indicates a role for coordinated TCR-CD28 signaling during differentiation of Treg cells in the thymus. Consistent with an important role for TCR and CD28 signaling in Treg cell differentiation, several TCR/CD28-downstream transcription factors, including NFAT, NF-κB, and AP-1, have been implicated in the transcriptional control of Treg cell differentiation. For example, NFAT and AP-1 bind to the Foxp3 promoter (68). Foxo1 and 3 also bind to the Foxp3 promoter and to an intronic regulatory element at the Foxp3 locus, conserved noncoding sequence 2 (Foxp3-CNS2) (69). In addition, CREB-ATF-1 binds Foxp3-CNS2, and both CREB-ATF1 and Foxo enhance expression of a luciferase reporter driven by a Foxp3 promoter in a transient transfection assay (69, 70). Combined Foxo1 and 3 deficiencies result in a severe impairment in Treg cell differentiation in the thymus (see below). Also, targeted ablation or loss-of-function mutations of genes involved in the TCR-dependent NF-κB signaling pathway, including PKCθ, CARMA1, Bcl10, and IκB kinase (IKK) 2, lead to defective Treg cell generation (71–74). Importantly, Alegre and colleagues (75) found that provision of survival signals, through forced expression of Bcl2 or a constitutively active form of STAT5 (STAT5-CA), to CARMA1-deficient T cell precursors failed to rescue the defect in thymic Foxp3 induction and Treg cell differentiation. Intriguingly, CARMA1-deficient peripheral CD4 T cells were able to induce Foxp3, in contrast to CARMA1-deficient thymocytes (74). The observed differential requirement for CARMA1 might be a consequence of iTreg cell generation in response to stronger TCR signals, which would result in quantitative and qualitative differences in downstream effectors of Foxp3 induction that would likely induce apoptosis in thymic precursors. These observations strongly support an instructive role for TCR signaling in Treg cell differentiation.
Although these studies emphasize the importance of NF-κB signaling in tTreg cells, the specific NF-κB family members that are required for Treg cell differentiation and their specific mechanisms of action (i.e., target genes) remain to be elucidated. One study demonstrated that frequencies of Foxp3-positive thymocytes were reduced in mice bearing a mutation in the p105-encoding gene that results in an inability of IKK to phosphorylate cleaved p50 (76). Additionally, it was recently demonstrated that the NF-κB family member c-Rel has a critical cell-intrinsic role in Foxp3 induction (60, 77–80). c-Rel binds to another intronic conserved noncoding element, Foxp3-CNS3. Foxp3-CNS3 is characterized by monomethylation of histone 3 at position K4 (H3K4me1) in Treg precursor cells and, thus, is accessible or poised. Gene-targeting studies showed that this element greatly increases the probability of Foxp3 induction. Further evidence connecting CNS3 and c-Rel functions in Foxp3 comes from comparable defects in Foxp3 induction in the CNS3- and c-Rel-deficient thymocytes observed in wild-type/CNS3 and wild-type/c-Rel−/− mixed bone marrow chimeras (80). Although the molecular mechanism of CNS3- and c-Rel-mediated induction of Foxp3 remains to be elucidated, one possibility is that c-Rel binding to CNS3 initiates chromatin remodeling analogous to the role of c-Rel at the 1L-2 locus following binding to the CD28 response element (81). Another possibility is that c-Rel binding to CNS3 facilitates the formation of the c-Rel enhansosome at the Foxp3 promoter (78). Whether or not the c-Rel enhansosome formation at the Foxp3 promoter is dependent on CNS3 and if CNS3 physically interacts with the promoter have yet to be determined.
In another study, enhancing NF-κB activity via a constitutively active IKKβ transgene in T cells led to increased numbers of Treg cells in the thymus, which further supports the notions that subtle differences downstream of TCR and costimulatory signaling determine Foxp3 induction (60). Interestingly, this study demonstrated c-Rel binding to the CNS2, the regulatory element required for heritable maintenance of Foxp3 expression but dispensable for its induction, suggesting that c-Rel may contribute to the maintenance of Foxp3 expression. Thus, the current data indicate that the pioneer element CNS3, through recruitment of c-Rel, influences the probability of Foxp3 induction within a population of precursor cells by acting as a classical enhancer (82), but outstanding questions remain as to how CNS3 is regulated during differentiation and what signals confer its poised state.
TREG CELL DIFFERENTIATION IN THE THYMUS: THE ROLE OF CYTOKINES
Analysis of TCR sequence repertoires expressed by thymic precursors of Treg and non-Treg cells revealed partial overlap (33). That the same TCR with an increased reactivity for self can be expressed by a Treg cell and a non-Treg cell suggested that TCR signals alone are not sufficient to specify Foxp3 upregulation and Treg cell differentiation. Similarly, a small proportion of thymocytes in TCR transgenic (and RAG-deficient) mice differentiate into Treg cells, whereas the rest become anergic non-Treg cells (48, 50). Additional arguments that more signals are required come from the observation that the generation of Treg cells is delayed in neonatal mice, whereas their immediate precursors (CD25+Foxp3−cells, described below) are present in the thymus immediately after birth (83–85). This early wave of thymocytes is rather enriched in self-reactive TCRs because of a lack of terminal deoxynucleotidyl transferase expression (86), and, therefore, the delay in Treg cell generation results from a paucity of additional signals that are required for Treg cell fate determination.
These essential additional signals for Treg cell differentiation include IL-2 and to a lesser degree two other cytokines, IL-7 and IL-15, acting through the common gamma-chain (γc) cytokine receptors. Mice deficient in IL-2 or IL-2Rα chain have a 50% decrease in the proportion and absolute numbers of Foxp3+ thymocytes, whereas loss of IL-15 or IL-7 alone does not perturb generation of Foxp3+ cells. However, mice with a combined ablation of IL-2, IL-7, and IL-15 are completely devoid of Foxp3+ thymocytes and peripheral Foxp3+ T cells, as are γc cytokine receptor–deficient mice (87–90).
Following the characterization of CD25+Foxp3− precursors of tTreg cells in the neonatal thymus and based on the critical role for IL-2 and also increased TCR signaling strength for tTreg cell differentiation, Lio and Hsieh proposed a two-step model of tTreg cell differentiation (84, 85). The model suggests that a high functional avidity TCR signal results in the upregulation of CD25 and a subsequent increase in the responsiveness of Treg precursor cells to IL-2 signals that facilitate induction of Foxp3 (84, 85). A likely candidate transcription factor for direct regulation of Foxp3 expression is STAT5 because it is activated downstream of IL-2 and other γc cytokine receptors (84). In fact, STAT5 binds to the Foxp3 promoter and also to the Foxp3-CNS2 element. Furthermore, Stat5 conditional allele ablation in DP thymocytes results in a severe reduction in Foxp3+ CD4SP thymocytes, and the few Foxp3+ thymocytes remaining originate from cells that escape STAT5 deletion (91, 92). In complementary gain-of-function studies, expression of a constitutively active STAT5 results in expansion of Treg cells and rescued Treg cell numbers in the absence of IL-2 (84, 93).
Nevertheless, how IL-2 signaling in these immediate Treg cell precursors instructs Foxp3 induction and Treg cell differentiation is not clear. Although there is an important role for IL-2 receptor-STAT5 signaling for the differentiation of Treg cells, it is still unclear if STAT5 (a) directly drives Foxp3 transcription, (b) induces changes in the chromatin characteristics at the Foxp3 locus, or instead (c) promotes survival or expansion of Treg cells and/or their precursors. Considering these possibilities, if STAT5 played an essential nonredundant role in de novo expression of Foxp3, rather than facilitating the survival of Treg cells, then forced expression of a prosurvival molecule such as Bcl2 in STAT5-deficient cells (as in CARMA1-deficient cells; see above) should not rescue the defect in Treg cell numbers. However, expression of a Bcl2 transgene did indeed rescue the differentiation of STAT5-deficient Treg cells, indicating that an important role for IL-2 and STAT5 signaling is to facilitate the survival of differentiating Treg cells (94).
Beyond requirements for TCR and γc cytokine receptors, TGF-βR signaling was suggested to be critical for an early wave of tTreg cell generation. This is especially intriguing considering the established role for TGF-βR signaling in peripheral generation of induced Treg (iTreg) cells (see below) and considerations that TGF-βR-dependent induction of Foxp3 might be restricted to iTreg cell generation. In these studies, ablation of the TGF-βRI subunit in DP thymocytes precipitated a substantial, but only transient, impairment in generation of Foxp3+ Treg cells during the first week of life and was followed by the recovery of Foxp3 thymocyte numbers to levels observed in wild-type mice (95). Earlier reports had failed to observe a defect in tTreg generation in seven-day-old mice lacking TGF-β1 or subjected to TGF-βRII ablation in DP thymocytes (96–98). Based on the more recent neonatal studies and experiments with luciferase reporter assays, it was proposed that TGF-β-induced Smad-mediated activation of the Foxp3 gene through binding of a conserved Smad-NFAT response element (Foxp3-CNS1) in thymocytes is essential for tTreg cell generation (99), which resembles the mechanism of peripheral generation of Foxp3+ Treg cells discussed in detail below. The rebound of tTreg cells was attributed to compensation of TGF-β signaling deficiency by age-dependent increases in IL-2 levels (95). However, there are several alternative explanations for the observed effect of TGF-β on tTreg generation besides those explanations that rely on direct transcriptional control of Foxp3 through Smad and NFAT binding to CNS1. In this regard, TGF-β signaling mediates the survival of tTreg cells or their precursors, when a relatively small population of thymic precursors reaches maturity in the lymphopenic neonatal thymus. Indeed, Li and colleagues (57) recently reported that TGF-β signaling is necessary for inhibiting Bim-dependent apoptosis of self-reactive thymocytes and, therefore, increasing the Treg precursor pool size. We recently demonstrated that Foxp3-CNS1 is dispensable for tTreg cell differentiation but critical for iTreg cell generation in the periphery (80). Together, these studies indicate that in the thymus TGF-β functions to confer survival advantage to Treg precursors, but not to promote Foxp3 expression in thymocytes via Smad binding to Foxp3-CNS1.
PERIPHERAL DIFFERENTIATION OF TREG CELLS: REQUIREMENTS FOR TCR SIGNALING
While the documented vital role of Foxp3-expressing Treg cells is to control autoreactive immune responses and associated lymphoproliferative disease, the question remains as to whether Treg cells that differentiate in the thymus and in the periphery have overlapping or distinct functions. The aforementioned finding that the requirements, i.e., intact CNS1 element, for the induction of Foxp3 in the thymus and the periphery are distinct suggests that the functions of these two Treg cell subsets, tTreg cells and iTreg cells, are also distinct.
The differentiation of tTreg cells in the thymus is promoted by increased affinity interactions with self-peptide-MHC complexes, whereas differentiation of peripheral iTreg cells likely occurs in response to non-self antigens, e.g., allergens, food, and the commensal microbiota (33, 48, 50, 100–102). A requirement for a distinct TCR signal and ligand specificity supporting iTreg cell generation was suggested by an analysis of the TCR repertoire of Foxp3+ T cells generated upon transfer of purified Foxp3− CD4+ T cells into lymphopenic recipients compared with that of cells that remained Foxp3− (103). The resulting TCR repertoires were distinct and only partially overlapping in resemblance to TCR utilization by Foxp3+ Treg cells and Foxp3− non-Treg CD4+ T cells present in unmanipulated mice (52, 103). A direct demonstration of distinct TCR specificities of iTreg cells was provided by a recent finding that colonic Treg cells feature TCRs distinct from those displayed by Treg cells in other tissues or tissue-draining lymph nodes and that a subset of these TCRs recognizes antigens derived from the commensal microbiota (102). TCRs displayed by iTreg cells are likely of high affinity, as suggested by an observation that rare high-affinity antigenic peptides allow for most efficient Foxp3 induction upon stimulation of a cognate transgenic TCR displayed by peripheral CD4+ T cells in comparison with a markedly less efficient iTreg generation by low-affinity peptide variants (104). In addition, induction of Foxp3 was observed upon TCR stimulation under tolerogenic conditions in the presence of nondepleting CD4 blocking antibodies (105). Consistent with the idea of suboptimal T cell activation favoring Foxp3 induction, CTLA-4—dispensable for tTreg cell differentiation—is required for TGF-β-mediated induction of Foxp3 in vitro (106), whereas CD28 cross-linking has the opposite effect (107, 108). Thus, in general, these studies suggest that high-affinity TCR signaling together with suboptimal costimulation (increased CTLA-4 and decreased CD28 signaling) favors Foxp3 induction and iTreg cell generation.
TCR transgenic T cells, which upregulate Foxp3 following oral administration of the cognate antigen, can alleviate antigen-induced airway inflammation (109–111). Along the same lines, chronic systemic administration of low-dose foreign antigen results in induction of antigen-specific iTreg cells in both TCR transgenic and polyclonal T cell populations (101, 112, 113). Insulin mimotope-specific Treg cells induced in such a manner were able to fully protect nonobese diabetic (NOD) mice from diabetes (114). Thus, TCRs that recognize antigens to which an organism is chronically exposed under homeostatic, noninflammatory conditions, such as those derived from the commensal microbiota or environmental antigens, can support differentiation of iTreg cells and shape their distinct TCR repertoire. The unique repertoire of iTreg TCRs indicates their nonredundant, delegated function and further implies that their antigen specificity is a critical determinant of their functional niche.
A ROLE FOR TGF-β AND IL-2 IN PERIPHERAL DIFFERENTIATION OF TREG CELLS
Both in vitro and in vivo studies have demonstrated that, besides strong TCR signaling and suboptimal costimulation, induction of Foxp3 expression in peripheral naive CD4+ T cells is facilitated by high amounts of TGF-β (101, 115–117). TGF-βR signaling appears to be required for most, if not all, induction of Foxp3 in peripheral CD4+ T cells (101, 115–117), with the exception of those CD25+Foxp3−CD4+ T cells that were likely poised to acquire Foxp3 expression in the thymus but failed to receive a sufficient IL-2 signal. These cells, like their aforementioned thymic counterparts, upregulate Foxp3 upon provision of IL-2 with little if any involvement of TGF-β signaling or restimulation through the TCR (118). IL-2 is also required for TGF-β-mediated induction of Foxp3 in peripheral T cells in vitro (119, 120). In addition to potential direct STAT5-dependent activation of the Foxp3 locus and promotion of cell survival and division in the presence of high amounts of TGF-β, IL-2 opposes differentiation of activated CD4+ T cells into T helper 17 (Th17) cells (121). The latter differentiation pathway is favored when TCR and TGF-βR activation in naive CD4+ T cells coincides with IL-6R stimulation (122). Another mechanism by which TGF-β may regulate Treg cell differentiation is through the repression of Gfi-1, a transcriptional repressor that inhibits the differentiation of both iTreg and Th17 cells upon activation of peripheral T cells under Th2 conditions (123).
The induction of Foxp3 upon chronic antigen exposure in vivo also requires TGF-βR signaling and is inversely correlated with cellular proliferation (101). This phenomenon can be explained by a study suggesting that TGF-β cooperates with TCR signals to induce Foxp3 in part by antagonizing cell cycle–dependent recruitment of maintenance DNA methyl-transferase I (Dnmt1) to the Foxp3 locus and thereby opposing its inactivation (62). Therefore, the cytostatic effects of inhibitory signals emanating from CTLA-4 and TGF-βR may be partially responsible for their effects on Foxp3 induction by allowing an active gene state to be established that is associated with the combination of requisite signaling cascades (TCR, IL-2, TGF-βR, etc.) and reduced proliferation. Thus, TGF-β signaling can promote the differentiation of Treg cells through both direct and indirect mechanisms including (a) binding of Smad3 to Foxp3-CNS1 together with NFAT, (b) opposition of Dnmt1 recruitment, and (c) signaling from the TGF-βR for the survival or fitness of tTreg cells or their precursors.
Maizels and colleagues (124) recently provided an example of a key role for TGF-βR signaling in iTreg generation, the putative function of iTreg cells in control of immune activation at mucosal surfaces, and their exploitation by parasitic helminths. These studies demonstrated the existence of a TGF-β mimic produced by the helminth Heligmosomoides polygyrus that induces Foxp3 induction and iTreg cell differentiation in a TGF-βR-dependent, TGF-β-independent manner (124). Therefore, helminths have evolved to exploit host mechanisms for induction of mucosal tolerance dependent on the action of TGF-βR, including the generation of iTreg cells (discussed below), which likely allows chronic H. polygyrus infection in the gastrointestinal tract to be established.
AKT ACTIVATION AND PERIPHERAL DIFFERENTIATION OF TREG CELLS
Additional insights into signal requirements for expression of Foxp3 were provided by studies of a role for Akt activation in iTreg and tTreg cell generation. Early blockade of PI3K signaling through the use of PI3K-mTOR inhibitors after 18 h of stimulation resulted in robust induction of Foxp3 (125), and sustained Akt activation inhibited the stable induction of Foxp3 in peripheral Foxp3− CD4+ T cells (126). A similar trend was observed upon modulation of Akt activity in fetal thymic organ cultures, suggesting that in this regard iTreg and tTreg cell induction is similar (126). Furthermore, researchers reported that TORC2, which phosphorylates (Ser473) and activates Akt, represses Foxp3 induction and that TORC2 deficiency promoted Foxp3 induction under normal activation conditions (61). In addition to activating the Akt-mTOR axis via PI3K downstream of TCR and IL-2R signaling pathways, S1P1 activation of the Akt-mTOR pathway also inhibits Foxp3 induction and differentiation of Treg cells (63). Two recent studies connect these observations to direct transcriptional regulation of Foxp3 by demonstrating that Foxo1 and Foxo3a, which are inactivated by Akt, bind to the Foxp3 promoter and to CNS2 and drive Foxp3 expression (69, 127, 128). However, the relative contribution of Foxo family members to Treg cell survival and fitness versus direct transcriptional activation of the Foxp3 locus during Treg cell differentiation remains unknown. Furthermore, complex PI3K-Akt-mTOR pathways incorporate and converge onto numerous other factors that might affect Treg cell differentiation.
The specific conditions resulting in optimal induction of factors activating Foxp3 expression (e.g., Smad2/3, NFAT, CREB, NF-κB, AP-1, Foxo, RAR, and STAT5) while limiting the activity of Foxp3 inhibitory pathways (i.e., Akt-mTOR) still remain to be fully understood. Nevertheless, it seems that, based on these studies, subtle quantitative and kinetic variations in TCR, costimulatory, and cytokine receptor signaling determine the probability of Foxp3 induction rather than serving as distinct, digital on-and-off switches.
SITES OF PERIPHERAL TREG CELL GENERATION
Distinct signaling and TCR specificity requirements—in particular, strong TCR and suboptimal costimulatory signals and TGF-β—for iTreg cell generation do not indicate that all chronic exposure of a peripheral T cell to a cognate self or foreign antigen would result in generation of iTreg cells. Indeed, it appears that most of the peripheral Treg cell pool in younger animals originates in the thymus, as indicated by considerable overlaps between TCR repertoires displayed by thymic and peripheral Foxp3+ cells and by thymic and peripheral Foxp3−CD4+ T cells, respectively, but not between corresponding Foxp3+ and Foxp3− cell subsets (33). In addition, in TCRαβ BDC2.5 transgenic mice, both thymic and peripheral Foxp3+CD4+ T cells expressed a highly similar repertoire of endogenous TCRα chains, which served as unique tags of individual T cell clones. Effector T cells expressing the BDC2.5 TCR specific for chromogranin A cause aggressive diabetes in the absence of Treg cells. The generation of Foxp3+ cells in BDC2.5 TCR transgenic mice requires endogenous TCRa chain rearrangement. Analysis of TCRα chain utilization revealed that both thymic and peripheral Foxp3− non-Treg cells expressed TCRs distinct from Foxp3+ Treg cells (129). The intriguing observation in this study was that BDC2.5 TCR-expressing Treg cell clones and non-Treg cell clones present in the pancreas and pancreatic lymph nodes (and therefore exposed to chronic stimulation by self antigen) were distinct and that their endogenous TCRα chain usage reflected their thymic origin (129). Together, these results indicate that most Treg cells present in the periphery are of thymic origin and that iTreg cell generation has specific prerequisites, exceeding that of the chronic exposure of BDC2.5 TCR-expressing T cells to their cognate antigen.
The data discussed so far indicate that TCR-dependent, sustained expression of high levels of Foxp3 in iTreg cells is influenced by a particular mode of TCR signaling, by kinetics of cell proliferation, and through synergy with other signals, such as TGF-β and IL-2. These prerequisites imply that iTreg cell differentiation is limited to particular environments or tissues. The appropriate factors, together with constitutive exposure to antigens derived from the commensal microbiota and to environmental and food antigens, are all present at mucosal tissues that serve as environmental interfaces. Indeed, the gut-associated lymphoid tissues (GALT) or Peyer’s patches serve as unique environments favoring iTreg cell generation. The capacity of these tissues to support iTreg cell generation is in part attributable to CD103+ dendritic cells present in the GALT or the gut-draining mesenteric lymph nodes, which under homeostatic conditions can induce Foxp3 expression in peripheral naive CD4+ T cells through presentation of antigen together with production of TGF-β and retinoic acid (108, 130–132). Whereas TGF-β acts directly, the mechanism by which retinoic acid enhances Foxp3 induction may be predominantly through curtailing production of IFN-γ, IL-4, and IL-21 by bystander CD44hi effector memory CD4+ T cells (133). Retinoic acid also exerts direct effects on iTreg cell differentiation (134, 135), and the relative contribution of these cell-intrinsic and -extrinsic pathways in vivo is difficult to assess. RAR binds to CNS1 and can activate Foxp3 gene expression (136). Interestingly, although retinoic acid augments Foxp3 induction, it inhibits expression of IL-10, indicating that at least some differentiation cues for iTreg cells and IL-10-producing Tr1 cells are distinct and that these two immunoregulatory cell subsets may represent competing and alternative cell lineages (137).
Thus, the GALT and mesenteric lymph nodes represent tissues rich in retinoic acid and conducive to the induction of Foxp3 in response to chronic antigen exposure under tolerogenic conditions (101, 109, 112, 138, 139). Further evidence for the generation of a distinct population of iTreg cells in the gut comes from studies revealing a distinct TCR repertoire among Treg cells present in the mesenteric lymph nodes and in the colon compared with Treg cells from other lymphoid compartments (102, 103), likely reflecting the efficient generation of iTreg cells in response to distinct gut-associated antigens such as those derived from food and the commensal microbiota. Indeed, the numbers of Foxp3+ Treg cells are diminished in the colon of germ-free animals, and their colonization with particular members of the commensal microbiota, i.e., a cocktail of several Clostridium species, results in a marked increase in their numbers (140). In agreement with these results, the aforementioned analysis of TCR specificity of colonic Treg cells identified a subset of iTreg cells specific for antigens encoded by specific commensal microorganisms (102). However, the function of these normal, polyclonal iTreg cells has yet to be explored in unmanipulated mice. Given their prevalence at mucosal tissues and their known and presumed TCR specificity, iTreg cells may control immune responses to innocuous antigens and prevent allergic-type inflammation. Further support of these ideas comes from studies using Rag-1-deficient T-B monoclonal mice sufficient or deficient in Foxp3. In these experiments, TCR transgenic iTreg cells were sufficient to establish mucosal tolerance and control allergic inflammation induced by the model antigen recognized by the TCR (109). However, it is as yet unclear what the relative contribution of iTreg cells is to the mature peripheral Treg cell population or if the control of allergic responses and mucosal immune homeostasis represents a nonredundant function of iTreg cells for which tTreg cells cannot substitute. Further studies using genetic models comparing selective impairment of tTreg and iTreg cell generation will be important for dissecting the functional niches of these cells.
FOXP3-DEPENDENT TREG CELL TRANSCRIPTIONAL AND FUNCTIONAL PROGRAMS
The central role of Foxp3 in Treg cell identity and function raised the question regarding how much Foxp3 directly affects the transcriptional signature of Treg cells. Analysis of gene expression profiles in Foxp3+ and Foxp3− T cells generated under different conditions (141, 142) suggested that the distinct Foxp3-independent features of the Treg cell transcriptional program precede or are established in parallel with the Foxp3-dependent transcriptional program, with contributions from IL-2R-, TCR-, and TGF-βR-associated responses. A direct investigation of the contribution of Foxp3 to transcriptional and functional features of Treg cells was made possible through the analysis of aforementioned mice harboring a Foxp3 reporter null allele (Foxp3GFPKO) (23) or a truncated version of the Foxp3 protein lacking the DNA-binding domain tagged with GFP (Foxp3ƊeGFP) (31). Cells expressing these alleles exhibit some of the phenotypic and molecular characteristics of Foxp3+ Treg cells, including (a) an inability to proliferate and produce IL-2 in response to TCR stimulation, (b) expression of low amounts of IL-7Rα chain, and (c) elevated amounts of CD25, CTLA-4, and GITR, albeit at significantly lower levels compared with Treg cells. However, unlike Treg cells, GFP+ Foxp3GFPKO T cells produce the Th2 and Th17 cytokines IL-4 and IL-17, and the block in their autonomous proliferative activity is reduced, as their in vitro proliferation can be readily restored by limited TCR/CD28 costimulation. The Foxp3-mediated repression of IL-17, a characteristic cytokine ofTh17 cells, is likely due to a modulation of transcriptional activity of the orphan nuclear receptors RORγ and RORα through direct interaction with Foxp3 (143), with RORγ serving as a key Th17 lineage–specifying factor (144). In addition, the GFP+Foxp3GFPKO T cell population was quiescent, whereas Foxp3+ Treg cell subsets exhibit impressive proliferative activity in vivo in the absence of inflammation. Most importantly, GFP+ Foxp3GFPKO T cells were completely devoid of suppressor activity (23). Similar results were obtained by Chatila and colleagues (31), although notable differences in the phenotype of T cells expressing the dysfunctional Foxp3 allele were observed in the latter study, most likely because of the presence of the Foxp3 protein lacking the DNA-binding domain yet capable of protein-protein interactions (31), in contrast to a complete Foxp3 ablation in the former study (23). Together these studies showed that Foxp3 is absolutely required for the suppressor function, proliferative potential, and metabolic fitness of Treg cells.
In addition, Foxp3 prevents differentiation of Treg precursor cells into effector T cell lineages. Characteristically, Foxp3 amplifies and stabilizes expression of a number of genes transiently upregulated in activated nonregulatory T cells. Many products of these Foxp3-dependent amplified or stabilized genes in Treg cells (such as CTLA-4, IL-10, IL-10ra, CD5, Fasl) act in a cell-intrinsic manner to limit activation of conventional T cells and serve as negative feedback regulators. Indeed, we propose that Foxp3 co-opts these pathways for implementation of negative regulation of immune responses in trans. At the same time, Foxp3 enforces repression of the immune response–promoting genes normally induced in naive and effector T cells upon TCR stimulation. Thus, Foxp3 controls Treg cell differentiation by potentiating or consolidating the beneficial features and at the same time correcting the disadvantageous features of precursor cells.
The analyses of the Foxp3-dependent transcriptional program invite the question of how many genes are directly regulated by Foxp3. Initial studies of Foxp3 target genes were undertaken using a combination of chromatin immunoprecipitation (ChIP) with mouse genome tiling array or promoter array analyses (145, 146). Recent in-depth exploration of Foxp3 target genes using ChIP combined with high-throughput sequencing (ChIP-Seq) confirmed these earlier studies and expanded the number of genes bound by Foxp3 in Treg cells (147; R.M. Samstein, A. Aarvey, S.Z. Josefowicz, and A.Y. Rudensky, manuscript in preparation). A cross-comparison of the data sets of Foxp3-bound genes and genes differentially expressed in Foxp3+ Treg cells versus GFP+Foxp3GFPKO T cells revealed that approximately 20–30% of Foxp3-dependent genes are directly regulated by Foxp3 (145; R.M. Samstein, A. Arvey, S.Z. Josefowicz, and A.Y. Rudensky, manuscript in preparation). The direct Foxp3 target genes include numerous sequence-specific transcription factors and microRNAs (miRNAs), playing important roles in Treg cell biology and contributing to differential mRNA and protein expression in Treg cells (145). The analysis of Foxp3-binding genes also showed that in contrast to the early notion that Foxp3 acts as a transcriptional repressor (148–151), more Foxp3-bound genes are upregulated than repressed in Treg cells (145). Thus, Foxp3 acts as both transcriptional activator and repressor. Furthermore, Foxp3 binding correlates with marked enrichment in permissive (H3K4me3) and inhibitory (H3K27me3) histone modifications associated with its binding sites in activated and repressed genes, respectively (145). These results suggest that Foxp3 may mediate its regulatory function through association with, or recruitment of, chromatin-modifying enzymes, and they also establish the possibility of a unique Foxp3-dependent chromatin landscape and heritable transcriptional program during Treg cell differentiation and cell division. Indeed, these features appear to be Foxp3 dependent because ablation of a conditional Foxp3 allele in mature Treg cells results in a loss of characteristic gene expression and suppressor function and the acquisition of effector T cell function (28). Thus, the heritable maintenance of a developmentally established Treg cell transcriptional and functional program requires continuous expression of Foxp3. One recent study demonstrated a similar requirement for Pax5 for the maintenance of the B cell lineage identity (152). Additionally, the continuous expression of the transcription factor PU.1 is required for the maintenance of cell type–specific regulatory chromatin characteristics following macrophage and B cell differentiation (153, 154). These observations suggest that sustained expression of lineage-specifying transcription factors is likely a common feature of late cellular differentiation required for the maintenance of a given cell identity in the progeny of dividing differentiated cells.
FOXP3 AND ITS INTERACTION PARTNERS
Co-immunoprecipitation studies revealed interactions of several transcription factors, including IRF4 and RORγ, with Foxp3 (143, 155). RORγ and Foxp3 are co-expressed in a subset of human and mouse CD4+ T cells in intestinal lamina propria. Such co-expression likely occurs during a transient uncommitted stage of T cell activation in the presence of varied amounts of TGF-β and inflammatory cytokines—IL-6, IL-21, and IL-1—during which RORγ and Foxp3 instruct opposing Th17 or Treg cell fates (143). In addition, it is likely that RORγ can be stably expressed in some Foxp3+ T cells in the gut, however its role in this context remains unknown.
These observations emphasized the importance of understanding the composition of Foxp3 transcriptional complexes and different modalities afforded by Foxp3-interacting partners. Recent mass-spectrometric analysis of Foxp3 protein complexes isolated by immunoprecipitation revealed numerous regulators of gene expression, including factors involved in chromatin remodeling (BRG1, Ku70/Ku80, and MBD3), acetyltransferase TIP60 and histone deacetylase HDAC7, and sequence-specific transcription factors (156). Investigators also proposed that the recruitment of TIP60 into Foxp3 transcriptional complexes results in Foxp3 acetylation, whereas HDAC7 deacetylates Foxp3, and that the ensuing changes in the Foxp3 acetylation state modulate Foxp3 activity in a manner analogous to c-myc (157, 158). Among sequence-specific transcription factors serving as Foxp3-interaction partners, NFAT and Runx1 were proposed to be indispensable for establishing Treg cell transcriptional and functional programs (159, 160). The molecular details of NFAT-Foxp3 interactions were initially provided by crystallographic analysis of tertiary complexes of DNA and DNA-binding domains of NFAT and Foxp2, a close relative of Foxp3 (159), and subsequently of similar ternary complexes with the Foxp3 forkhead domain (161). DNA template-dependent interactions of Foxp3 with NFAT are thought to prevent formation of NFAT-AP-1 complexes, required for the expression of immune response–promoting genes in effector T cells, thereby ensuring their repression in Treg cells. NFAT-Foxp3 cooperation can drive the genomic program required for Treg cell differentiation and function (159). In addition, Foxp3 may inhibit AP-1 function through direct association with the activated AP-1 protein (162). Site-directed mutagenesis of predicted NFAT interaction sites in the DNA-binding domain of the Foxp3 protein resulted in a loss of its ability to impose the Treg gene signature and suppressor function (159). A similar loss of function was observed upon introduction of mutations disrupting Foxp3 interactions with Runx1, initially identified in a yeast two-hybrid screen (160). As a note of caution, a considerable caveat to the site-mutagenesis approach is that introduced mutations might lead to a loss of interacting partners in addition to those under study. Indeed, our studies have shown that even in the absence of functional Runx1-CBFβ complexes, Foxp3 can confer Treg cell–specific gene expression and suppressive capacity and that the moderate decline in suppressor function of Treg cells lacking Runx1/CBFβ is largely due to progressively diminishing Foxp3 expression (163). Considering the complexity of the Foxp3 interactome, further biochemical, genetic, and functional studies of the components of Foxp3 transcriptional complexes are warranted.
ROLE OF MIRNA IN FOXP3+ TREG CELLS
Foxp3 can control the differentiation and function of Treg cells directly and indirectly by changing expression of various regulators of genes expression including miRNAs (145), small untranslated RNA species that have been well recognized for their roles in organ development, cellular differentiation, homeostasis, and function through target mRNA degradation or translational interference (164). Recent studies suggest that miRNA-dependent post-translational regulation is playing a prominent role in the Foxp3-dependent Treg genomic program (165–168). Ablation of either Dicer or Drosha, two RNase III enzymes critical for the generation of mature miRNAs in Treg cells, results in a fatal autoimmunity indistinguishable from that in Treg cell–deficient mice (166–168). The suppressor capacity of Dicer-deficient Treg cells is reduced under noninflammatory conditions but is lost entirely in inflammatory settings. These findings implicate miRNAs as key guardians of a stable Treg cell suppressor program under inflammatory conditions. Finally, similar to its role in T and B cells, the miRNA pathway facilitates survival and proliferative potential of Treg cell lineages (166).
Catastrophic consequences and the development of systemic disease phenotypes observed in Treg cell–specific Dicer and Drosha ablation studies raised a question as to the identity of specific miRNAs regulating distinct aspects of Treg cell biology. Differential miRNA expression in Foxp3+ Treg cells was first demonstrated by Cobb and colleagues (165). Furthermore, many of Treg cell–specific miRNAs are expressed in Treg cells in a Foxp3-dependent manner (145, 146, 169). We have previously demonstrated that, albeit dispensable for Treg cell differentiation and suppressor function, Foxp3-driven miR-155 upregulation is critical for heightened responsiveness of Treg cells to their key survival and growth factor, IL-2, through targeting SOCS1, a negative regulator of IL-2R signaling, and for Treg cell maintenance in a competitive environment (169).
The role of miRNA in controlling Treg cell suppressor capacity was first demonstrated by the study of miR-142-3p in Treg cells (170). The authors suggested that a high level of cAMP expression in Treg cells is conditional upon low amounts of miR-142-3p and that forced expression of this miRNA in Treg cells attenuates their ability to suppress T cell proliferation in vitro. However, the striking loss of suppressor function observed in Dicer- or Drosha-deficient Treg cells is most likely due to the loss of miRNAs that are overrepresented in these cells (166, 167). Recently, we demonstrated that miR-146a, another miRNA prevalently expressed in Treg cells, is indispensable for suppression mediated by Treg cells in vivo. Excessive activation of STAT1 in Treg cells is kept in check by miR-146a to ensure efficient control of spontaneous IFN-γ-dependent Th1-mediated immunopathology and to prevent deviation of activated Treg cells into IFN-γ-producing Th1-like cells (171). Thus, miR-146a plays a pivotal role not only in regulating Treg cell suppression function but also in maintaining Treg cell identity in response to dynamically changing inflammatory environments.
Together, these findings provide the first few examples of a single miRNA controlling defined Treg cell function, consistent with the aforementioned notion that distinct facets of Treg cell biology are regulated by different miRNAs.
STABILITY AND REGULATION OF FOXP3 EXPRESSION
Given the central role that Foxp3 plays in maintaining the Treg cell transcriptional program and cellular phenotype, maintenance of Foxp3 expression is central to Treg cell lineage stability. Foxp3 represses production of proinflammatory cytokines, including IL-2, TNF-α, IFN-γ, IL-17, and IL-4, by Treg cells (23, 27, 31). The latter feature is particularly important because Treg cells bear TCRs with an increased affinity for self-peptide-MHC complexes and, therefore, have the potential to mount autoimmune responses (48, 50, 52, 55). Indeed, in diseased Foxp3-deficient mice, activated but not naive T cells utilize TCRs normally expressed by Treg cells in healthy mice. This observation is consistent with the idea that in the absence of Foxp3 a significant number of self-reactive T cell precursors, which would normally differentiate into Treg cells, do not undergo deletion or functional inactivation and instead give rise to activated effector T cells that likely contribute to immune-mediated inflammation (33).
In agreement with these results, ablation of a conditional Foxp3 allele in mature Treg cells leads to eventual loss of the developmentally established Foxp3-dependent functional and transcriptional program (28). Therefore, continuous expression of Foxp3 is required for the maintenance of the Treg cell transcriptional program and suppressor function and for the restraint of conventional activated T cell–associated effector function (including expression of the aforementioned cytokines). In addition, experimental perturbation of the amount of Foxp3 in Treg cells results in immunopathology and elevated Th2 cytokine production by these cells, further highlighting the potential hazards of unstable or diminished Foxp3 expression (27). Thus, high levels of sustained Foxp3 expression define the identity and function of Treg cells and prevent their diversion into potentially pathogenic self-reactive effector T cells.
These results suggest that the stability of Foxp3 expression or lack thereof under basal and inflammatory conditions is an important determinant of immune homeostasis. Foxp3 stability is also of immediate relevance to the more general question of whether Treg cells represent a distinct cell lineage characterized by a stable and heritable cell fate or a transient meta-stable activation state maintained through chronic stimulation of IL-2R and TCRs.
Recent studies present a complicated picture of Treg cell stability. One study indicates that relatively few Treg cells exhibit unstable expression of Foxp3 and that detectable former Treg cells are derived from a very rare subpopulation, which is preferentially enriched following lymphopenia-driven expansion (172). An intriguing possibility is that this rare population, Foxp3+CD25− cells, is enriched for cells that transiently upregulate expression of Foxp3 during their differentiation into alternative T helper lineages, a feature consistent with the lack of a stable Foxp3-dependent transcriptional program. Several other reports suggest that Foxp3 expression in Treg cells is unstable under basal or inflammatory conditions. In this regard, loss of Foxp3 expression was observed in Treg cells following exposure to TNF-α, IL-6, or OX40 (173–177). Most of these studies relied on isolation of Foxp3+ Treg cells, followed by their in vitro stimulation in the presence of proinflammatory cytokines, e.g., IL-6, or their adoptive transfer into lymphopenic or lymphoreplete recipients. However, the potential outgrowth of a few contaminating Foxp3-negative cells and the stress associated with cell purification procedures and culture may account for the outcomes of these experiments. Alternatively, these particular culture conditions may be peculiar in facilitating conversion of Foxp3+ Treg cells to effector cells. Regardless, the study of Treg cell stability in vivo under relevant physiological conditions is of critical importance.
The results of these studies and the idea of plasticity of Treg cell fate received further support from a recent study suggesting that the instability of Foxp3 expression in Treg cells yields a population of ex-Treg cells that mediate autoimmune pathology under certain conditions. To genetically mark cells that express Foxp3 at some point in their history and to trace the fate of these cells, these investigators employed a Cre recombinase encoded by a Foxp3 BAC transgene, together with a Cre-mediated recombination reporter allele of the ubiquitously expressed ROSA26 locus harboring a targeted insertion of a loxP site-flanked STOP cassette followed by a DNA sequence encoding YFP (178). These studies, utilizing genetic tagging of Treg cells by a constitutive Foxp3 BAC-driven Cre, demonstrated loss of Foxp3 expression in the autoimmune microenvironment after crossing the reporter mice to NOD mice and upon transfer of Foxp3+ reporter cells into NOD mice. However, this result does not preclude the possibility that committed Treg cells stably express Foxp3 and represent a faithfully committed cell lineage.
Indeed, in vivo evaluation of the stability of Foxp3 expression in a temporal manner using Foxp3eGFP-Cre-ERT2 x R26Y mice, which allowed for noninvasive inducible labeling of Foxp3-expressing cells and tracking their fate in vivo, demonstrated that the Treg cell population exhibits remarkably stable expression of Foxp3 under basal physiologic conditions. Stability of Foxp3 expression was also maintained following various challenges, including radiation-induced lymphopenia, Th1 cytokine–induced inflammatory response, and acute bacterial infection (179). The observed stability of the Treg cell lineage implies the existence of dedicated mechanisms that underlie stably maintained expression of Foxp3. One such putative mechanism involves binding of Foxp3 in a complex with Runx1-CBFβ to CNS2, a proximal conserved noncoding DNA element within the Foxp3 locus (see below) (80). This binding, conditional upon demethylation of a CpG island within CNS2, affords heritable maintenance of the active state of the Foxp3 locus in the progeny of dividing Treg cells likely via yet-to-be-understood epigenetic mechanisms (65, 80).
The Foxp3 basal promoter has relatively weak transactivation activity as observed in luciferase reporter assays (70, 99). Therefore, Foxp3 gene expression is heavily reliant on the activity of other proximal regulatory DNA elements. The previously discussed CNS1, which contains binding sites for NFAT, RAR/RXR, and TGF-β-activated Smad3, is critical for the induction of Foxp3 in peripheral naive CD4+ T cells (180). In addition to activation of CNS1, other Foxp3 regulatory DNA elements are likely to impact the chromatin state, thereby promoting accessibility of the Foxp3 locus and recruitment of activating factors to increase the probability of Foxp3 induction. Of course, chromatin modifications associated with active gene states (i.e., H3K4me3 and histone acetylation) at CNS1 and the Foxp3 promoter will coincide with or directly precede Foxp3 expression (68, 70, 99, 125). However, because acquisition of an activated chromatin state at the Foxp3 promoter and at CNS1 is coincident with Foxp3 expression, it seemed unlikely that these regulatory DNA elements would act as the early mediators of chromatin remodeling and de novo activation of the locus. Instead, a distinct regulatory DNA element might act earlier than CNS1 and the Foxp3 promoter in locus activation through the reception of appropriate signals. Indeed, CNS3 is characterized by chromatin modifications associated with distal regulatory DNA elements, including H3K4me1 and H3K27Ac, and accessibility, not only in Treg cells actively expressing Foxp3, but also in both thymic and peripheral Treg cell precursors, thereby implicating CNS3 in early regulation of Foxp3 locus activation. Consistent with this, CNS3 controls the probability of Foxp3 expression among a population of precursors cells (rather than the level of Foxp3 expression on a per cell level) and is bound by the NF-κB family member, c-Rel (80). Thus, CNS3 represents a developmentally poised regulatory DNA element that functions early in Foxp3 locus activation, likely before the other regulatory DNA elements, i.e., Foxp3 promoter, CNS1, and CNS2. Future studies will identify the mechanisms and kinetics of regulatory DNA element function throughout the events of Foxp3 induction and stable high-level expression in Treg cells. It will also be exciting to identify additional regulatory DNA elements, transcription factors, and chromatin modifying and remodeling complexes and reveal their concerted function with the Foxp3 promoter, CNS1, CNS2, and CNS3 to promote and propagate the activated chromatin state, which allows for the induction and maintenance of Foxp3 expression.
Given the impressive stability of Foxp3 in differentiated Treg cells, it is interesting to consider the molecular mechanisms employed by Treg cells to confer it. Several studies have pointed to CpG dinucleotide demethylation at the Foxp3 promoter and at CNS2 as an important requirement of stable Foxp3 expression. Demethylation of these methylcytosines at the Foxp3 regulatory DNA correlates with stable Foxp3 expression in ex vivo isolated human and mouse Treg cells. Intriguingly, these elements remain heavily methylated in in vitro–generated iTreg cells, which do not stably express Foxp3 (65, 181, 182). Methylated CpG motifs could function in repression or aborted activation of the Foxp3 locus in iTreg cells through a combination of both shrouding transcription factor–binding motifs on the proximal DNA and the direct recruitment of repressive chromatin machinery. These pathways are likely active at the CNS2 element, and transcription factors that mediate stable heritable maintenance of Foxp3 expression might bind to CNS2 in a demethylation-dependent manner.
Indeed, CNS2 binds Foxp3/Runx1/CBFβ protein complexes, and this binding is dependent on demethylation of CNS2 CpG dinucleotides (80). This Foxp3 complex binding at CNS2 was therefore absent in in vitro-generated iTreg cells, which maintain high levels of CpG methylation at CNS2. Additionally, CREB/ATF, NF-κB, and Ets-1 bind to CNS2 in a demethylation-dependent manner as well (183). Therefore, unstable expression of Foxp3 in in vitro iTreg cells may be the result of a failure to engage the CNS2 demethylation-dependent Foxp3 autoregulatory loop as well as other transcription factors. In support of this idea, both Foxp3 protein and Runx1/CBFβ are required for maintenance of high levels of Foxp3 expression in mature Treg cells (23, 27, 163, 184–186). In this putative autoregulatory loop, Foxp3 protein complexes, acquired by the progeny of dividing mature Treg cells, bind to the demethylated CNS2 to maintain faithful expression of Foxp3 and, therefore, Treg cell lineage stability. A bistable feed-forward autoregulatory loop could explain the remarkable stability of Foxp3 expression observed in mature Treg cells (179).
While the functions of Foxp3 are clearly shared between humans and mice, there are some apparent differences in the control of FOXP3 expression in activated human T cells (187). Although stable, high-level expression of Foxp3 is unique to Treg cells in both species, after stimulation human T cells transiently induce FOXP3 expression (49, 188–191). This relatively low-level FOXP3 expression in activated human T cells requires TGF-β produced by activated T cells or present in serum, and it does not result in acquisition of Treg cell phenotype and suppressor activity (27, 191). Additionally, this FOXP3 expression in conventional human T cells in the presence of high amounts of TGF-β does not confer suppressor function (189, 192). We suggest that low-level FOXP3 expression upon activation of conventional human T cells and unstable expression of Foxp3 in iTreg cells generated in vitro are the results of a lack of engagement of the CNS2 regulatory DNA element due to its methylated and repressed state. In agreement with this hypothesis, knockdown, pharmacologic inhibition, or ablation of the Dnmtl gene and resultant CpG motif demethylation increases both the induction and, critically, the stability of Foxp3 expression (62, 65, 70). However, the mechanisms responsible for establishing the appropriate active chromatin features and for initiating the demethylation of CNS2, as well as the Foxp3 protein complex–dependent propagation of these states for heritable maintenance of Foxp3 expression in dividing Treg cells, remain poorly understood. Further mechanistic studies will considerably advance our understanding of Foxp3 induction and Treg cell biology.
MOLECULAR MECHANISMS OF TREG CELL–MEDIATED SUPPRESSION
Despite the rapidly accumulating knowledge of Treg cell involvement in immune regulation, our understanding of molecular mechanisms of suppression is still limited. Transcriptional profiling of Treg cells versus naive or activated T cells revealed a substantial number of genes, including cell-surface molecules and secreted proteins, that could potentially function as suppression molecules in Treg cell–mediated immune regulation (193–196).
Several cell-surface molecules were proposed to play a role as mediators of Treg cell–mediated suppression; CD25, a subunit of IL-2 receptor (IL-2R) and the original Treg cell marker, is upregulated on effector T cells and is constitutively expressed at a high level on Treg cells. While indispensable in Treg cell homeostasis (87, 197), high-level IL-2R expression on Treg cells could deprive effector T cells of IL-2 and inhibit their proliferation (198). Similarly, another well-known Treg cell–specific surface molecule, CTLA-4, is implicated in Treg cell–mediated suppression function, in addition to its important cell-intrinsic role in limiting responses of activated T cells (199). Building on earlier reports using cell transfer and antibody blockade (200–202), a role of CTLA-4 in Treg cells has been recently reassessed in genetic studies, in which the mice with a selective loss of CTLA-4 in Treg cells were analyzed (203, 204). In these studies, selective CTLA-4 deficiency resulted in greatly increased numbers and activation of Treg cells under inflammatory conditions, yet the suppressive activity of CTLA-4-deficient Treg cells was impaired. It was suggested that in BALB/c mice, known for their susceptibility to various immune-mediated disorders, the reduced suppression capacity of CTLA-4-deficient Treg cells is due to their inability to downregulate CD80 and CD86 via trans-endocytosis (203, 205). These results were consistent with a pronounced expansion and activation of dendritic cells observed early upon acute Treg cell ablation (35). However, Treg cell–specific ablation of CTLA-4 on a less autoimmune-prone genetic background of C57Bl/6 mice did not cause an increase in dendritic cell numbers or CD80/CD86 expression, yet Treg cell suppressor capacity was partially impaired and their TCR repertoire altered (Y. Rubtsov, A. Patterson, R.M. Samstein, A.Y. Rudensky, A. Sharp, manuscript in preparation). Thus, CTLA-4 function in facilitating Treg cell suppression and its contribution to the overall control of immune homeostasis by Treg cells can be significantly modified by the genetic background.
Other cell-surface molecules such as CD39 and CD73, two ectoenzymes highly expressed on Treg cells, have also recently been shown to take part in Treg cell–mediated suppression by facilitating the elaboration of adenosine and the extrusion of cAMP (206–209). As a consequence, adenosine signaling initiated by Treg cells not only directly inhibits the proliferation of effector T cells, but also negatively impacts the function of dendritic cells. As Treg cells were suggested to play a pivotal role in controlling the priming of effector cells through APCs, there are also other molecules that Treg cells might use to control APC function. For instance, LAG-3, a CD4 homolog that exhibits a high binding affinity with MHC class II, is suggested to be required for maximal suppressive activity of both natural and induced Treg cells (210). Engagement of a MHC molecule on immature dendritic cells through LAG-3 could lead to the inhibition of their maturation and costimulatory capacity (211). Moreover, the dendritic cell–dependent Treg cell–mediated immune regulation is further supported by a recent discovery of a novel Ig family member, TIGIT, that is expressed, like CTLA-4, at a high level on Treg cells and on activated conventional T cells. Upon Treg cell interactions with dendritic cells, TIGIT appears to induce production of immunosuppressive cytokines IL-10 and TGF-β by dendritic cells (212). Finally, long-lasting Treg cell interactions with dendritic cells were convincingly documented by recent studies employing intravital microscopy (213, 214). These interactions are facilitated by a neuronal guidance protein neuropilin-1, which is highly expressed by most Treg cells, and neuropilin-1 blockade or ablation interferes with suppression mediated by Treg cells (215, 216). However, rather than serving as an effector molecule, neuropilin-1 likely facilitates interactions of Treg cells with their targets.
TNF receptor family members such as GITR are important players in controlling Treg cell function (193, 194). In addition to elevated expression on Treg cells, GITR is upregulated on activated effector T cells, where—along with other TNFR family members OX40, 4-1BB, and TNFRII—it serves as a potent costimulatory and survival molecule. It seems more likely that engagement of GITR on the surface of effector T cells enhances their responses, effectively releasing these cells from Treg cell–mediated suppression (195, 196). Consistent with these results, GITR deficiency does not result in noticeable aberrations in immune homeostasis or tolerance (217), yet GITR serves as a potential target for cancer immunotherapy.
In addition to functionally important transmembrane molecules, several secreted proteins identified in gene expression studies have been implicated in Treg cell–mediated suppression, including IL-10, IL-35, granzyme B, IL-9, and TGF-β (reviewed in 218, 219). Selective ablation of IL-10 in Foxp3+ Treg cells revealed that IL-10 production by Treg cells is essential for keeping the immune response in check at environmental interfaces such as colon and lungs (220). Similarly, another immunosuppressive cytokine secreted by Treg cells, IL-35, has also been suggested to maintain immunological tolerance in the gut. In the absence of IL-12p35 or Ebi3, two of the major components of IL-35, Treg cells adoptively transferred into lymphopenic hosts cannot control homeostatic proliferation, which ultimately leads to the development of inflammatory bowel disease (221). Furthermore, TGF-β produced by Treg cells can suppress Th1 responses (222).
Cytolysis of target cells as a means for Treg cell–mediated suppression was first suggested by the finding that, in human Treg cells, granzyme A can be induced by a combination of CD3 and CD46 stimulation, resulting in the induction of apoptosis in activated target cells (223). Later, several groups identified granzyme B (but not granzyme A) as being highly upregulated in mouse Treg cells. Upon activation, Treg cells can kill either responder T cells or APCs in a granzyme B–dependent manner in vitro (224, 225). These findings have been further confirmed by the in vivo studies in which granzyme B has been critical in maintaining Treg cell–dependent long-lived skin graft tolerance as well as in Treg cell–mediated suppression of tumor clearance (226, 227).
TREG CELL–MEDIATED SUPPRESSION OF DISTINCT CLASSES OF THE IMMUNE RESPONSE
It is important to note that none of the aforementioned mechanisms of suppression can singly account for Treg cell–mediated control of immunity. Moreover, the Foxp3-dependent suppressor program implemented by Treg cells keeps in check various types of effector immune responses to self antigens and pathogens. However, it was not clear originally whether Treg cells implement a universal hard-wired program to limit different types of immunity or modular programs of suppression tailored to inhibit a particular class of the immune response. In the past few years, mounting experimental evidence has suggested that distinct suppressor mechanisms prominently feature in particular tissue and inflammatory settings (see Figure 2). For example, the analysis of IRF4, a transcription factor required for differentiation of Th2 effector cells, demonstrated that in regulatory T cells IRF4 forms complexes with Foxp3 and in a cooperative manner regulates a set of genes that endow Treg cells with the ability to suppress Th2 responses. Ablation of a conditional Irf4 allele in Treg cells resulted in a selective dysregulation of unprovoked pathogenic Th2 responses, i.e., increased production of Th2 cytokines, IL-4-dependent Ig isotype production, and pronounced plasma cell infiltration (155).
Figure 2.
Regulatory T (Treg) cell heterogeneity and suppression of distinct classes of the immune response. Treg cells generated in the thymus or extrathymically can further specialize through upregulation or activation of transcription factors in response to different environmental stimuli. These environmental response factors can cooperate with Foxp3 to confer to Treg cells a transient or lasting cell state, enabling their tailored function under particular environmental or inflammatory conditions; for example, STAT3 activation in response to IL-10 leads to the generation of pSTAT3+ Treg cells capable of suppressing Th17 responses, or activation of STAT1 in response to IFN-γ or other STAT1-signaling cytokines leads to generation of Tbet+ Treg cells. In a given tissue, Treg cells, upon instruction by the tissue environment, induce expression of tissue-specific transcription factors whose cooperation with Foxp3 results in a distinct tissue-specific Treg cell transcriptional signature and function, and also supports Treg cell subset homeostasis.
Similarly, another study has shown that the expression of T-bet, a key transcription factor in Th1 effector cell differentiation, in Treg cells enables them through the expression of CXCR3 to migrate, proliferate, and accumulate at the sites of Th1 responses (228). Although not clearly altering Treg cell suppressor activity, T-bet deficiency in Treg cells leads to a failure in regulation of Th1, but not Th2 or Th17, responses. Consistent with these studies, selective ablation of STAT3, a transcription factor required for Th17 induction, in Treg cells results in uncontrolled Th17-dependent pathology (229). Finally, as demonstrated by two recent studies, in Treg cells the expression of Bcl6, a transcription factor pivotal for the development of T follicular helper cells, is essential for Treg cell–mediated regulation of germinal center responses, likely through the induction of CXCR5 (230, 231). Whether Th lineage–specific transcription factors allow Treg cells (a) to be equipped with selective migration properties, (b) to strengthen certain suppression capacities specialized for efficiently controlling the corresponding type of immune responses, or (c) to do both remains to be further explored. Nonetheless, these findings clearly suggest the possibility that transcription factors required for different Th lineage development play an equally important role in Treg cell homeostasis and function in the presence of similar inflammatory cues. Moreover, as mentioned above, miR-146a-deficient Treg cells fail to control Th1 responses, likely due to unrestrained expression and activation of Stat1. Similarly, excessive Stat1 activation in SOCS1-deficient Treg cells also leads to dysregulated Th1 pathology (171).
These findings support the aforementioned concept of symmetry in the integration of environmental cues by Treg cells and immune effector cells. However, in the earlier studies, it was the lack of Th lineage–specific transcription factors Bcl6, Stat3, IRF4, and Stat1/T-bet in Treg cells that resulted in impaired suppression of T follicular helper, Th17, Th2, and Th1 responses, respectively. In contrast, the aforementioned studies of a role of miR-146a and SOCS1 showed that unrestrained activation of Stat1 in Treg cells leads to immunopathology. The observations that both the lack of Stat1 and unrestrained Stat1 activation resulted in a breakdown of immunologic tolerance implied a general scheme in which specialized Treg cell suppression programs are established in dynamically changing inflammatory environments by maintaining an optimal threshold of activation of transcription factors downstream of cytokine receptors crucial for the corresponding type of immune responses.
To extend this further, one could envision that other cell-/tissue-specific genomic programs orchestrated by the corresponding transcription factors might also facilitate the ability of tissue-resident Treg cell subpopulations to maintain tissue homeostasis (see Figure 2). First experimental evidence in support of this notion was provided by the study demonstrating that Treg cells isolated from adipose tissue exhibit a distinct gene expression signature when compared with those isolated from secondary lymphoid tissues (232). A role for Treg cells in opposing metabolic inflammation in fat tissue was also suggested by another group (233). Furthermore, a recent study by Mathis, Benoist, and coworkers demonstrated that high amounts of peroxisome proliferator activated receptor gamma (PPARγ), a transcription factor essential for adipocyte differentiation, is a unique distinguishing feature of fat Treg cells and that PPARγ plays a central and important role in homeostasis of fat Treg cells and possible function (D. Mathis & C. Benoist, personal communication). These observations raise two questions: Is the principle that a corresponding tissue-specific transcription factor(s) controls the numbers and functions of Treg cells present in a given nonlymphoid tissue similarly applicable to other tissues? And is this principle also applicable to a tumor environment?
CONCLUDING REMARKS
Recent progress in dissecting molecular mechanisms of differentiation and function of Treg cells opens new avenues for analyses of heterogeneity within regulatory T cells and their adaptation to and stability in diverse inflammatory and tissue environments. The potential roles of Treg cells in the regulation of nonimmune tissues and organs and the involvement of these cells in responses to stress, injury, and cellular transformation are under investigation. We expect this work to yield novel insights into integration of immune and nonimmune mechanisms of homeostasis.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
NOTE ADDED IN PROOF
In our overview of early studies of regulatory T cells, we inadvertently omitted a reference to an important paper by Waldmann and coauthors (234). This study showed that, upon treatment with a nondepleting CD4 antibody, CD4+ T cells stimulated with alloantigens acquired a nonresponsive state and were capable of transferring tolerance to the same alloantigen to third-party recipients. A recent follow-up study showed that the tolerance observed in this model was dependent on generation of Foxp3-expressing Treg cells (235).
LITERATURE CITED
- 1.Starr TK, Jameson SC, Hogquist KA 2003. Positive and negative selection of T cells. Annu. Rev. Immunol 21:139–76 [DOI] [PubMed] [Google Scholar]
- 2.Lenschow DJ, Walunas TL, Bluestone JA 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol 14:233–58 [DOI] [PubMed] [Google Scholar]
- 3.Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM 1995. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J. Immunol 154:6602–113 [PubMed] [Google Scholar]
- 4.Nishizuka Y, Sakakura T 1969. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science 166:753–55 [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Takahashi T, Nishizuka Y 1982. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J. Exp. Med 156:1565–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asano M, Toda M, Sakaguchi N, Sakaguchi S 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med 184:387–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin NM 1987. Tolerance induced by thymic epithelial grafts in birds. Science 237:1032–35 [DOI] [PubMed] [Google Scholar]
- 8.Salaun J, Bandeira A, Khazaal I, Calman F, Coltey M, et al. 1990. Thymic epithelium tolerizes for histocompatibility antigens. Science 247:1471–74 [DOI] [PubMed] [Google Scholar]
- 9.Modigliani Y, Thomas-Vaslin V, Bandeira A, Coltey M, Le Douarin NM, et al. 1995. Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype. Proc. Natl. Acad. Sci. USA 92:7555–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, et al. 1996. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol. Rev 149:35–53 [DOI] [PubMed] [Google Scholar]
- 11.Powrie F, Mason D 1990. OX-22highCD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J. Exp. Med 172:1701–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD 1993. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med 178:237–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol 155:1151–64 [PubMed] [Google Scholar]
- 14.Shevach EM 2000. Regulatory T cells in autoimmunity. Annu. Rev. Immunol 18:423–49 [DOI] [PubMed] [Google Scholar]
- 15.Fehervari Z, Sakaguchi S 2004. Development and function of CD25+CD4+ regulatory T cells. Curr. Opin. Immunol 16:203–8 [DOI] [PubMed] [Google Scholar]
- 16.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, et al. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet 27:68–73 [DOI] [PubMed] [Google Scholar]
- 17.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, et al. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic deregulation syndrome. J. Clin. Investig 106:R75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, et al. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet 27:20–21 [DOI] [PubMed] [Google Scholar]
- 19.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, et al. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet 27:18–20 [DOI] [PubMed] [Google Scholar]
- 20.Gambineri E, Torgerson TR, Ochs HD 2003. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol 15:430–35 [DOI] [PubMed] [Google Scholar]
- 21.Godfrey VL, Rouse BT, Wilkinson JE 1994. Transplantation of T cell-mediated, lymphoreticular disease from the scurfy (sf) mouse. Am. J. Pathol 145:281–86 [PMC free article] [PubMed] [Google Scholar]
- 22.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY 2005. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22:329–41 [DOI] [PubMed] [Google Scholar]
- 23.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, et al. 2007. Foxp3-dependent programme of regulatory T-cell differentiation. Nature 445:771–75 [DOI] [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057–61 [DOI] [PubMed] [Google Scholar]
- 25.Khattri R, Cox T, Yasayko SA, Ramsdell F 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol 4:337–42 [DOI] [PubMed] [Google Scholar]
- 26.Fontenot JD, Gavin MA, Rudensky AY 2003. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol 4:330–36 [DOI] [PubMed] [Google Scholar]
- 27.Wan YY, Flavell RA 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445:766–70 [DOI] [PubMed] [Google Scholar]
- 28.Williams LM, Rudensky AY 2007. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol 8:277–84 [DOI] [PubMed] [Google Scholar]
- 29.Wan YY, Flavell RA 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA 102:5126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–38 [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, et al. 2007. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol 8:359–68 [DOI] [PubMed] [Google Scholar]
- 32.Liston A, Farr AG, Chen Z, Benoist C, Mathis D, et al. 2007. Lack of Foxp3 function and expression in the thymic epithelium. J. Exp. Med 204:475–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh CS,Zheng Y, Liang Y, Fontenot JD, Rudensky AY 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol 7:401–10 [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, Benoist C, Mathis D 2005. How defects in central tolerance impinge on a deficiency in regulatory T cells. Proc. Natl. Acad. Sci. USA 102:14735–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JM, Rasmussen JP, Rudensky AY 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol 8:191–97 [DOI] [PubMed] [Google Scholar]
- 36.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, et al. 2007. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med 204:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY 2010. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J. Exp. Med 207:2323–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer A, Adoro S, Park JH 2008. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol 8:788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Germain RN 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol 2:309–22 [DOI] [PubMed] [Google Scholar]
- 40.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM 2008. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat. Med 14:337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, et al. 2011. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med 208:1279–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, et al. 2001. Fine tuning of TCR signaling by CD5. J. Immunol 166:5464–72 [DOI] [PubMed] [Google Scholar]
- 43.Wong P, Barton GM, Forbush KA, Rudensky AY 2001. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J. Exp. Med 193:1179–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter JD, Calabrese GM, Naganuma M, Lorenz U 2005. Deficiency of the Src homology region 2 domain-containing phosphatase 1 (SHP-1) causes enrichment of CD4+CD25+ regulatory T cells. J. Immunol 174:6627–38 [DOI] [PubMed] [Google Scholar]
- 45.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S 1994. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell 78:399–408 [DOI] [PubMed] [Google Scholar]
- 46.Olivares-Villagómez D, Wang Y, Lafaille JJ 1998. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med 188:1883–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, et al. 2002. Generation of CD4+ CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J. Immunol 168:4399–405 [DOI] [PubMed] [Google Scholar]
- 48.Apostolou I, Sarukhan A, Klein L, von Boehmer H 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol 3:756–63 [DOI] [PubMed] [Google Scholar]
- 49.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK 2003. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med 198:249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, et al. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol 2:301–6 [DOI] [PubMed] [Google Scholar]
- 51.van Santen HM, Benoist C, Mathis D 2004. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J. Exp. Med 200:1221–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY 2004. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity 21:267–77 [DOI] [PubMed] [Google Scholar]
- 53.Pacholczyk R, Kern J 2008. The T-cell receptor repertoire of regulatory T cells. Immunology 125:450–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong J, Obst R, Correia-Neves M, Losyev G,Mathis D, Benoist C 2007. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J. Immunol 178:7032–41 [DOI] [PubMed] [Google Scholar]
- 55.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L 2006. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 25:249–59 [DOI] [PubMed] [Google Scholar]
- 56.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L 2010. Autonomous role of medullary thymic epithelial cells in central CD4+ T cell tolerance. Nat. Immunol 11:512–19 [DOI] [PubMed] [Google Scholar]
- 57.Ouyang W, Beckett O, Ma Q, Li MO 2010. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32:642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, et al. 2009. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol 10:610–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung MW, Shen S, Lafaille JJ 2009. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. J. Exp. Med 206:2121–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Long M, Park SG, Strickland I, Hayden MS, Ghosh S 2009. Nuclear factor-κB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 31:921–31 [DOI] [PubMed] [Google Scholar]
- 61.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, et al. 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30:832–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Josefowicz SZ, Wilson CB, Rudensky AY 2009. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol 182:6648–52 [DOI] [PubMed] [Google Scholar]
- 63.Liu G, Burns S, Huang G, Boyd K, Proia RL, et al. 2009. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol 10:769–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patton DT, Garden OA, Pearce WP, Clough LE, Monk CR, et al. 2006. Cutting edge: the phosphoinositide 3-kinase p110δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol 177:6598–602 [DOI] [PubMed] [Google Scholar]
- 65.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, et al. 2008. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol 38:1654–63 [DOI] [PubMed] [Google Scholar]
- 66.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, et al. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431–40 [DOI] [PubMed] [Google Scholar]
- 67.Tai X, Cowan M, Feigenbaum L, Singer A 2005. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol 6:152–62 [DOI] [PubMed] [Google Scholar]
- 68.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, et al. 2006. Molecular mechanisms underlying FOXP3 induction in human T cells. J. Immunol 176:3593–602 [DOI] [PubMed] [Google Scholar]
- 69.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO 2010. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat. Immunol 11:618–27 [DOI] [PubMed] [Google Scholar]
- 70.Kim HP, Leonard WJ 2007. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J. Exp. Med 204:1543–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, et al. 2004. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc. Natl. Acad. Sci. USA 101:4566–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta S,Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z 2008. Differential requirement of PKC-θ in the development and function of natural regulatory T cells. Mol. Immunol 46:213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medoff BD, Sandall BP, Landry A, Nagahama K, Mizoguchi A, et al. 2009. Differential requirement for CARMA1 in agonist-selected T-cell development. Eur. J. Immunol 39:78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintial R, et al. 2009. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol 7:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molinero LL, Yang J, Gajewski T, Abraham C, Farrar MA, Alegre ML 2009. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J. Immunol 182:6736–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sriskantharajah S, Belich MP, Papoutsopoulou S, Janzen J, Tybulewicz V, et al. 2009. Proteolysis of NF-κB1 p105 is essential for T cell antigen receptor-induced proliferation. Nat. Immunol 10:38–47 [DOI] [PubMed] [Google Scholar]
- 77.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, et al. 2009. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med 206:3001–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, et al. 2009. Development of Foxp3+ regulatory T cells is driven by the c-Rel enhanceosome. Immunity 31:932–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visekruna A, Huber M, Hellhund A, Bothur E, Reinhard K, et al. 2010. c-Rel is crucial for the induction of Foxp3+ regulatory CD4+ T cells but not T(H)17 cells. Eur. J. Immunol 40:671–76 [DOI] [PubMed] [Google Scholar]
- 80.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463:808–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rao S, Gerondakis S, Woltring D, Shannon MF 2003. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J. Immunol 170:3724–31 [DOI] [PubMed] [Google Scholar]
- 82.Weintraub H 1988. Formation of stable transcription complexes as assayed by analysis of individual templates. Proc. Natl. Acad. Sci. USA 85:5819–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fontenot JD, Dooley JL, Farr AG, Rudensky AY 2005. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med 202:901–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, et al. 2008. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28:112–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lio CW, Hsieh CS 2008. A two-step process for thymic regulatory T cell development. Immunity 28:100–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gavin MA, Bevan MJ 1995. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity 3:793–800 [DOI] [PubMed] [Google Scholar]
- 87.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol 6:1142–51 [DOI] [PubMed] [Google Scholar]
- 88.Burchill MA, Yang J, Vang KB, Farrar MA 2007. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett 114:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malek TR 2008. The biology of interleukin-2. Annu. Rev. Immunol 26:453–79 [DOI] [PubMed] [Google Scholar]
- 90.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA 2008. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol 181:3285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA 2007. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol 178:280–90 [DOI] [PubMed] [Google Scholar]
- 92.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, et al. 2007. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109:4368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, et al. 2003. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol 171:5853–64 [DOI] [PubMed] [Google Scholar]
- 94.Malin S,McManus S, Cobaleda C, Novatchkova M, Delogu A, et al. 2010. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat. Immunol 11:171–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W 2008. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol 9:632–40 [DOI] [PubMed] [Google Scholar]
- 96.Marie JC, Letterio JJ, Gavin M, Rudensky AY 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med 201:1061–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li MO, Sanjabi S, Flavell RA 2006. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25:455–71 [DOI] [PubMed] [Google Scholar]
- 98.Marie JC, Liggitt D, Rudensky AY 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25:441–54 [DOI] [PubMed] [Google Scholar]
- 99.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol 9:194–202 [DOI] [PubMed] [Google Scholar]
- 100.Hsieh CS, Rudensky AY 2005. The role of TCR specificity in naturally arising CD25+CD4+ regulatory T cell biology. Curr. Top. Microbiol. Immunol 293:25–42 [DOI] [PubMed] [Google Scholar]
- 101.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol 6:1219–27 [DOI] [PubMed] [Google Scholar]
- 102.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, et al. 2011. Peripheral education of the immune system by colonic commensal microbiota. Nature 478:250–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lathrop SK, Santacruz NA, Pham D, Luo J, Hsieh CS 2008. Antigen-specific peripheral shaping of the natural regulatory T cell population. J. Exp. Med 205:3105–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gottschalk RA, Corse E, Allison JP 2010. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med 207:1701–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waldmann H, Adams E, Cobbold S 2008. Reprogramming the immune system: co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immunol. Rev 223:361–70 [DOI] [PubMed] [Google Scholar]
- 106.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA 2006. TGF-β requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J. Immunol 176:3321–29 [DOI] [PubMed] [Google Scholar]
- 107.Kim JM, Rudensky A 2006. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev 212:86–98 [DOI] [PubMed] [Google Scholar]
- 108.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ 2007. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J. Exp. Med 204:1765–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ 2008. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity 29:114–26 [DOI] [PubMed] [Google Scholar]
- 110.Curotto de Lafaille MA, Lafaille JJ 2009. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30:626–35 [DOI] [PubMed] [Google Scholar]
- 111.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA 2005. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Investig 115:1923–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kretschmer K, Heng TS, von Boehmer H 2006. De novo production of antigen-specific suppressor cells in vivo. Nat. Protoc 1:653–61 [DOI] [PubMed] [Google Scholar]
- 113.Verginis P, McLaughlin KA, Wucherpfennig KW, von Boehmer H, Apostolou I 2008. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc. Natl. Acad. Sci. USA 105:3479–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Daniel C, Weigmann B, Bronson R, von Boehmer H 2011. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med 208:1501–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al. 2003. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med 198:1875–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Selvaraj RK, Geiger TL 2007. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. J. Immunol 179:1390. [PubMed] [Google Scholar]
- 117.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA 2004. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J. Immunol 172:5213–21 [DOI] [PubMed] [Google Scholar]
- 118.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K 2010. Identification of an immediate Foxp3- precursor to Foxp3+ regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J. Exp. Med 207:1393–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM 2007. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J. Immunol 178:4022–26 [DOI] [PubMed] [Google Scholar]
- 120.Horwitz DA, Zheng SG, Wang J, Gray JD 2008. Critical role of IL-2 and TGF-β in generation, function and stabilization of Foxp3+CD4+ Treg. Eur. J. Immunol 38:912–15 [DOI] [PubMed] [Google Scholar]
- 121.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26:371–81 [DOI] [PubMed] [Google Scholar]
- 122.Bettelli E, Korn T, Oukka M, Kuchroo VK 2008. Induction and effector functions of T(H)17 cells. Nature 453:1051–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, et al. 2009. Down-regulation of Gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J. Exp. Med 206:329–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, et al. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J. Exp. Med 207:2331–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, et al. 2008. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 105:7797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Haxhinasto S, Mathis D, Benoist C 2008. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med 205:565–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harada Y, Harada Y, Elly C, Ying G, Paik JH, et al. 2010. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J. Exp. Med 207:1381–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, et al. 2010. Foxo transcription factors control regulatory T cell development and function. Immunity 33:890–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wong J, Mathis D, Benoist C 2007. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J. Exp. Med 204:2039–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, et al. 2005. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med 202:1051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, et al. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med 204:1757–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, et al. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med 204:1775–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, et al. 2008. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi cells. Immunity 29:758–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xiao S, Jin H, Korn T, Liu SM, Oukka M, et al. 2008. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol 181:2277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, et al. 2008. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111:1013–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W 2010. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 33:313–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT 2009. Contrasting roles for all-trans retinoic acid in TGF-β-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J. Exp. Med 206:343–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, et al. 2008. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29:637–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Belkaid Y, Oldenhove G 2008. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity 29:362–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, et al. 2006. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int. Immunol 18:1197–209 [DOI] [PubMed] [Google Scholar]
- 142.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, et al. 2007. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity 27:786–800 [DOI] [PubMed] [Google Scholar]
- 143.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, et al. 2008. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORγt function. Nature 453:236–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, et al. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–33 [DOI] [PubMed] [Google Scholar]
- 145.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY 2007. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 445:936–40 [DOI] [PubMed] [Google Scholar]
- 146.Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, et al. 2007. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature 445:931–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Birzele F, Fauti T, Stahl H, Lenter MC, Simon E, et al. 2011. Next-generation insights into regulatory T cells: expression profiling and FoxP3 occupancy in human. Nucleic Acids Res 39:7946–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF 2001. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J. Biol. Chem 276:37672–79 [DOI] [PubMed] [Google Scholar]
- 149.Bettelli E, Dastrange M, Oukka M 2005. Foxp3 interacts with nuclear factor of activated T cells and NF-κB to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl. Acad. Sci. USA 102:5138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grant C, Oh U, Fugo K, Takenouchi N, Griffith C, et al. 2006. Foxp3 represses retroviral transcription by targeting both NF-κB and CREB pathways. PLoS Pathog 2:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, et al. 2006. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J. Immunol 177:3133–42 [DOI] [PubMed] [Google Scholar]
- 152.Cobaleda C, Jochum W, Busslinger M. 2007. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature 449:473–77 [DOI] [PubMed] [Google Scholar]
- 153.Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, et al. 2010. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32:317–28 [DOI] [PubMed] [Google Scholar]
- 154.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, et al. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38:576–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, et al. 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 458:351–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li B, Saouaf SJ, Samanta A, Shen Y, Hancock WW, Greene MI. 2007. Biochemistry and therapeutic implications of mechanisms involved in FOXP3 activity in immune suppression. Curr. Opin. Immunol 19:583–88 [DOI] [PubMed] [Google Scholar]
- 157.Li B, Samanta A, Song X, Iacono KT, Bembas K, et al. 2007. FOXP3 interactions with histone acetyltrans-ferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA 104:4571–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Patel JH, Du Y, Ard PG, Phillips C, Carella B, et al. 2004. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol. Cell. Biol 24:10826–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, et al. 2006. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell 126:375–87 [DOI] [PubMed] [Google Scholar]
- 160.Ono M, Yaguchi H, Ohkura N, Kitabayashi I, Nagamura Y, et al. 2007. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature 446:685–89 [DOI] [PubMed] [Google Scholar]
- 161.Bandukwala HS, Wu Y, Feuerer M, Chen Y, Barboza B, et al. 2011. Structure of a domain-swapped FOXP3 dimer on DNA and its function in regulatory T cells. Immunity 34:479–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suwanvanichkij V 2008. Displacement and disease: the Shan exodus and infectious disease implications for Thailand. Confl. Health 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rudra D, Egawa T, Chong MM, Treuting P, Littman DR, Rudensky AY. 2009. Runx-CBFβ complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat. Immunol 10:1170–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–97 [DOI] [PubMed] [Google Scholar]
- 165.Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, et al. 2006A role for Dicer in immune regulation. J. Exp. Med 203:2519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. 2008. Dicer-dependent microRNA path-way safeguards regulatory T cell function. J. Exp. Med 205:1993–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. 2008. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J. Exp. Med 205:2005–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS,et al. 2008. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J. Exp. Med 205:1983–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, et al. 2009. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang B, Zhao J, Lei Z, Shen S, Li D, et al. 2009. miR-142-3p restricts cAMP production in CD4+ CD25− T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep 10:180–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, et al. 2010. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142:914–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Komatsu N, Mariotti Ferrandiz ME, Wang Y, Malissen B, Waldmann H, Hori S 2009. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc. Natl. Acad. Sci. USA 106:1903–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, et al. 2008. DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol 38:3274–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xu L, Kitani A, Fuss I, Strober W 2007. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-β. J. Immunol 178:6725–29 [DOI] [PubMed] [Google Scholar]
- 175.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, et al. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vu MD, Xiao X, Gao W, Degauque N, Chen M, et al. 2007. OX40 costimulation turns off Foxp3+ Tregs. Blood 110:2501–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.So T, Croft M 2007. Cutting edge: OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol 179:1427–30 [DOI] [PubMed] [Google Scholar]
- 178.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, et al. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol 10:1000–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, et al. 2010. Stability of the regulatory T cell lineage in vivo. Science 329:1667–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Josefowicz SZ, Niec R, Kim HY, Treuting P, Chinen T,et al. 2012. Extrathymically generated regulatory T cells control mucosal Th2 inflammation. Nature. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, et al. 2007. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3+ conventional T cells. Eur. J. Immunol 37:2378–89 [DOI] [PubMed] [Google Scholar]
- 182.Floess S, Freyer J, Siewert C, Baron U, Olek S, et al. 2007. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 5:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Polansky JK, Schreiber L, Thelemann C, Ludwig L, Krüger M, et al. 2010Methylationmatters: binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J. Mol. Med 88:1029–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bruno L, Mazzarella L, Hoogenkamp M, Hertweck A, Cobb BS, et al. 2009. Runx proteins regulate Foxp3 expression. J. Exp. Med 206:2329–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kitoh A, Ono M, Naoe Y, Ohkura N, Yamaguchi T, et al. 2009. Indispensable role of the Runx1-Cbfβ transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity 31:609–20 [DOI] [PubMed] [Google Scholar]
- 186.Klunker S, Chong MM, Mantel PY, Palomares O, Bassin C, et al. 2009. Transcription factors RUNX1 and RUNX3 in the induction and suppressive function of Foxp3+ inducible regulatory T cells. J. Exp. Med 206:2701–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ziegler SF 2006. FOXP3: of mice and men. Annu. Rev. Immunol 24:209–26 [DOI] [PubMed] [Google Scholar]
- 188.Allan SE, Passerini L, Bacchetta R, Crellin N, Dai M, et al. 2005. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Investig 115:3276–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, et al. 2006. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl. Acad. Sci. USA 103:6659–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, et al. 2005. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol 66:13–20 [DOI] [PubMed] [Google Scholar]
- 191.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE 2007Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol 37:129–38 [DOI] [PubMed] [Google Scholar]
- 192.Shevach EM, Tran DQ, Davidson TS, Andersson J.2008The critical contribution of TGF-β to the induction of Foxp3 expression and regulatory T cell function. Eur. J. Immunol 38:915–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM,et al. 2002CD4+CD25+ immunoregulatory T cells:gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16:311–23 [DOI] [PubMed] [Google Scholar]
- 194.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S.2002Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol 3:135–42 [DOI] [PubMed] [Google Scholar]
- 195.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, et al. 2004. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur. J. Immunol 34:613–22 [DOI] [PubMed] [Google Scholar]
- 196.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, et al. 2004. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol 173:5008–20 [DOI] [PubMed] [Google Scholar]
- 197.Malek TR, Yu A, Zhu L, Matsutani T, Adeegbe D, Bayer AL. 2008. IL-2 family of cytokines in T regulatory cell development and homeostasis. J. Clin. Immunol 28:635–39 [DOI] [PubMed] [Google Scholar]
- 198.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol 8:1353–62 [DOI] [PubMed] [Google Scholar]
- 199.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J. Immunol 163:1128–31 [PubMed] [Google Scholar]
- 200.Read S, Malmstrom V, Powrie F. 2000. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med 192:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, et al. 2006. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol 177:4376–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, et al. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med 192:303–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, et al. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271–75 [DOI] [PubMed] [Google Scholar]
- 204.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, et al. 2009. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med 206:421–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, et al. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332:600–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. 2006. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol 177:6780–86 [DOI] [PubMed] [Google Scholar]
- 207.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, et al. 2007. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J. Exp. Med 204:1303–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, et al. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110:1225–32 [DOI] [PubMed] [Google Scholar]
- 209.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med 204:1257–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, et al. 2004. Role ofLAG-3 inregulatory T cells. Immunity 21:503–13 [DOI] [PubMed] [Google Scholar]
- 211.Liang B, Workman C, Lee J, Chew C, Dale BM, et al. 2008. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J. Immunol 180:5916–26 [DOI] [PubMed] [Google Scholar]
- 212.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, et al. 2009. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol 10:48–57 [DOI] [PubMed] [Google Scholar]
- 213.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, et al. 2006. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat. Immunol 7:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, et al. 2006. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J. Exp. Med 203:505–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. 2008. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28:402–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Solomon BD, Mueller C, Chae WJ, Alabanza LM, Bynoe MS. 2011. Neuropilin-1 attenuates autoreactivity in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108:2040–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. 2002. Role of GITR in activation response of T lymphocytes. Blood 100:350–52 [DOI] [PubMed] [Google Scholar]
- 218.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev 182:58–67 [DOI] [PubMed] [Google Scholar]
- 219.von Boehmer H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol 6:338–44 [DOI] [PubMed] [Google Scholar]
- 220.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castell L, et al. 2008. IL-10 produced by regulatory T cells contributes to their suppressor function by limiting inflammation at environmental interfaces. Immunity 28:546–58 [DOI] [PubMed] [Google Scholar]
- 221.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, et al. 2007. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450:566–69 [DOI] [PubMed] [Google Scholar]
- 222.Li MO, Wan YY, Flavell RA 2007. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26:579–91 [DOI] [PubMed] [Google Scholar]
- 223.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ 2004. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 21:589–601 [DOI] [PubMed] [Google Scholar]
- 224.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ 2005. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol 174:1783–86 [DOI] [PubMed] [Google Scholar]
- 225.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM 2006. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood 107:3925–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, et al. 2008. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J. Immunol 181:4752–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, et al. 2007. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity 27:635–46 [DOI] [PubMed] [Google Scholar]
- 228.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol 10:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, et al. 2009. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326:986–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ,et al. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med 17:983–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, et al. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med 17:975–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Feuerer M, Hill JA, Mathis D, Benoist C 2009. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat. Immunol 10:689–95 [DOI] [PubMed] [Google Scholar]
- 233.Winer S, Chan Y, Paltser G, Truong D, Tsui H, et al. 2009. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med 15:921–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, et al. 1993. “Infectious” transplantation tolerance. Science 259:974–77 [DOI] [PubMed] [Google Scholar]
- 235.Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, et al. 2011. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J. Exp. Med 208:2043–53 [DOI] [PMC free article] [PubMed] [Google Scholar]