Abstract
Background
Multimodal treatment of colorectal liver metastases (CRLMs) relies on precise upfront assessment of resectability. Variability in the definition of resectable disease and the importance of early consultation by a liver surgeon have been reported. In this pilot study we investigated the initial resectability assessment and patterns of referral of patients with CRLMs.
Methods
Surgeons and medical oncologists involved in the management of colorectal cancer at 2 academic institutions and affiliated community hospitals were surveyed. Opinions were sought regarding resectability of CRLMs and the type of initial specialty referral (hepatobiliary surgery, medical oncology, palliative care or other) in 6 clinical cases derived from actual cases of successfully performed 1- or 2-stage resection/ablation of hepatic disease. Case scenarios were selected to illustrate critical aspects of assessment of resectability, best therapeutic approaches and specialty referral. Standard statistical analyses were performed.
Results
Of the 75 surgeons contacted, 64 responded (response rate 85%; 372 resectability assessments completed). Hepatic metastases were more often considered resectable by hepatobiliary surgeons than all other respondents (92% v. 57%, p < 0.001). Upfront systemic therapy was most commonly prioritized by surgical oncologists (p = 0.01). Hepatobiliary referral was still considered in 73% of “unresectable” assessments by colorectal surgeons, 59% of those by general surgeons, 57% of those by medical oncologists and 33% of those by surgical oncologists (p = 0.1).
Conclusion
Assessment of resectability varied significantly between specialties, and resectability was often underestimated by nonhepatobiliary surgeons. Hepatobiliary referral was not considered in a substantial proportion of cases erroneously deemed unresectable. These disparities result largely from an imprecise understanding of modern surgical indications for resection of CRLMs.
Abstract
Contexte
Le traitement multimodal des métastases hépatiques du cancer colorectal (MHCR) repose sur une rigoureuse évaluation initiale de la résécabilité. On a fait état de l’imprécision de la définition de résécabilité et de l’importance de demander rapidement une consultation en chirurgie du foie. Au cours de cette étude, nous avons fait le point sur l’évaluation initiale de la résécabilité et sur les types de consultations demandées pour les patients présentant des MHCR.
Méthodes
Nous avons interrogé les chirurgiens et oncologues médicaux responsables de la prise en charge du cancer colorectal dans 2 établissements universitaires et leurs hôpitaux communautaires affiliés. Nous leur avons demandé leur opinion sur la résécabilité des MHCR et le type de consultation demandée initialement (chirurgie hépatobiliaire, oncologie médicale, soins palliatifs ou autres) concernant 6 cas cliniques inspirés de cas réels de résection ou ablation réussie pour maladie hépatique de stade 1 ou 2. Ces scénarios de cas cliniques ont été choisis pour illustrer certains aspects cruciaux de l’évaluation de la résécabilité, des approches thérapeutiques optimales et des demandes de consultation. Des analyses statistiques standards ont été effectuées.
Résultats
Parmi les 75 chirurgiens rejoints, 64 ont répondu (taux de réponse 85 %; 372 évaluations de résécabilité ont été effectuées). Les métastases hépatiques ont été plus souvent jugées résécables par les chirurgiens hépatobiliaires que par tous les autres répondants (92 % c. 57 %, p < 0,001). Un traitement systémique initial a le plus souvent été privilégié par les chirurgiens-oncologues (p = 0,01). Une consultation auprès de spécialistes hépatobiliaires était encore considérée comme nécessaire pour les cas jugés « non résécables » dans une proportion de 73 % par les chirurgiens spécialistes du cancer colorectal, de 59 % par les chirurgiens généraux, de 57 % par les oncologues médicaux et de 33 % par les chirurgiens-oncologues (p = 0,1).
Conclusion
L’évaluation de la résécabilité a significativement varié d’une spécialité à l’autre et la résécabilité a souvent été sous-estimée par les chirurgiens non spécialistes de voies hépatobiliaires. La consultation auprès des spécialistes hépatobiliaires n’a pas été envisagée pour une proportion substantielle de cas jugés à tort non résécables. Ces disparités se soldent en bonne partie d’une mécompréhension des indications actuelles de la chirurgie pour MHCR.
Over the past 2 decades, improved outcomes after resection of liver-limited colorectal cancer metastases have been attributed mainly to the combination of improved chemotherapy and the ability to completely remove all hepatic disease while leaving sufficient functional liver. The survival benefit of liver resection has now been shown to outweigh factors of adverse tumour biology as well as the burden of hepatic disease (carcinoembryonic antigen level, disease-free interval, node positivity of the primary tumour, size of tumour and number of liver nodules).1 This reality has led to the conclusion that the presence of many factors traditionally considered adverse should not uniformly preclude surgical resection.2 As a result, planning for multimodal curative-intent treatment of colorectal liver metastases (CRLMs) relies largely on the precise and accurate upfront assessment of hepatic resectability.
Important technical advances in liver surgery (e.g., staged resection, hypertrophy-inducing procedures and ablative therapies) combined with novel regional and systemic chemotherapy regimens (and biologic agents) have aggressively expanded both the indications for and the complexity of resection of CRLMs. The assessment of resectability should thus be guided by a precise understanding of surgical hepatic anatomy and techniques as well as the role of preoperative downsizing via conversion therapy.3
Although many uncertainties remain regarding the optimal timing and sequencing of multimodal therapy in a given patient, the central role of surgery is well established. Consequently, the early participation of an experienced liver surgeon in any therapeutic planning has become crucial. 4 Unfortunately, concerns surrounding surgical wait times and treatment delays have also been raised.5 Perhaps even more important, wide variability in definitions of resectable disease and referral patterns for patients with CRLMs have also been reported.6–8
This study serves as a pilot to investigate an initial resectability assessment and specialty referral of patients with CRLMs across multiple oncologic specialties and subspecialties (general surgeons, colorectal surgeons, surgical oncologists and medical oncologists) compared to a panel of hepatobiliary surgeons.
Methods
Participants
Surgeons and medical oncologists involved in the management of colorectal cancer at 2 university-affiliated academic institutions and 2 university-affiliated community hospitals in Canada (in 2 provinces) were invited to participate in this study. We used a Web-based platform (SurveyMonkey [SurveyMonkey Inc.]) to upload and deliver the survey instrument. An invitation email containing a Web link to the online questionnaire was sent to all potential participants. Email reminders were sent 2 weeks and 1 month later to optimize the response rate. Recipients were informed about the voluntary nature of participation, and they had the option to decline at any time before final response submission. Responses were captured anonymously and were stored in a password-protected encrypted environment. Ethics approval from the institutional review board was obtained. No incentives were offered.
Survey instrument
We developed a novel survey tool by consensus. The tool was trialled and edited before distribution. The first component of the survey instrument included 7 questions eliciting information about the surgeon’s institution, type of practice and background training. In the second part of the instrument, opinions were sought regarding resectability of CRLMs and the type of initial specialty referral (hepatobiliary surgery, medical oncology, palliative care or other) in 6 clinical cases.
We selected case scenarios to illustrate critical aspects of assessment of resectability, best therapeutic approaches and specialty referral. These factors were defined by a preliminary consensus meeting of the authors and included distinct aspects of tumour biology (TNM staging of primary colorectal cancer, carcinoembryonic antigen level and disease-free interval), burden and anatomy of hepatic disease (lesion number, size, laterality and vascular invasion as shown on selected cross-sectional liver images), and patient age and comorbidities. Participants were told to assume that all cases had a resected or resectable primary colorectal tumour, a first diagnosis of metastatic disease and no extrahepatic metastases. All clinical information presented in the 6 scenarios was derived from actual cases of 1- or 2-stage resection/ablation of hepatic disease successfully performed by the authors. Participants were requested to confirm the resectability status of each case and provide their chosen service for referral. The survey email letter/introduction to participants outlined the definition of resectability as obtaining R0 margins at the site of all tumour deposits. The participants were blinded to the fact that in all cases the CRLMs had been previously completely resected, with an R0 status.
Statistical analysis
Descriptive summaries included counts and percentages. We compared means using the Student t test, and medians using the Mann–Whitney test. Differences among categorical data were assessed with the Fischer exact test. Statistical analyses were performed with the use of Stata/IC version 12.0 (StataCorp).
Results
Of the 75 surgeons invited, 64 responded (response rate 85%). These included 34 nonhepatobiliary surgeons (general surgeons, colorectal surgeons, surgical oncologists), 20 medical oncologists and 10 hepatobiliary surgeons. Surgical oncologists who did not perform liver resection were considered nonhepatobiliary surgeons. All liver surgeons, regardless of type of training, were included in the hepatobiliary surgeon group.
Most respondents (56/64) had completed fellowship training, and almost two-thirds reported that they participated regularly in multidisciplinary tumour boards (40/64) and practised at a university-affiliated teaching hospital (39/64). Colorectal cancer accounted for 25% or more of the clinical practice of two-thirds of the respondents. Most medical oncologists (16/20) and hepatobiliary surgeons (8/10) were employed at a university-affiliated hospital. Fellowship training of the hepatobiliary surgeons varied: hepatobiliary fellowship (6 respondents), liver transplantation (2), surgical oncology (1) and no fellowship (1). Training and practice characteristics of the nonhepatobiliary surgeons are shown in Table 1.
Table 1.
Practice and training characteristics of nonhepatobiliary surgeons
| Characteristic | No. (%) of respondents n = 34 |
|---|---|
| Primary institution | |
| Academic/university-affiliated hospital | 15 (44) |
| Community hospital with residents | 14 (41) |
| Community hospital without residents | 5 (15) |
| Practice type | |
| General surgery | 18 (53) |
| Colorectal surgery | 10 (29) |
| Surgical oncology | 6 (12) |
| Fellowship training | |
| Colorectal surgery | 10 (29) |
| Surgical oncology | 4 (12) |
| Trauma/critical care | 6 (18) |
| Bariatrics, foregut and minimally invasive surgery | 6 (18) |
| Other | 3 (9) |
| No fellowship | 5 (15) |
| % of practice focused on colorectal cancer | |
| < 25 | 20 (59) |
| 25–50 | 4 (12) |
| 51–75 | 8 (24) |
| > 75 | 2 (6) |
| No. of surgeons performing colorectal surgery in institution | |
| ≤ 5 | 18 (53) |
| > 5 | 16 (47) |
| No. of colon cancer cases per year | |
| ≤ 10 | 13 (38) |
| > 10 | 21 (62) |
| No. of rectal cancer cases per year | |
| ≤ 10 | 22 (65) |
| > 10 | 12 (35) |
Most respondents (53/64) reported no difficulties in referring patients with CRLMs. Of the 25 participants from community hospitals, 18 were nonhepatobiliary surgeons, and 20 were located less than 100 km from a referral centre. All 25 of these respondents and 38 of the 39 respondents from university-affiliated hospitals reported wait times for initial specialist consultation of less than 4 weeks.
Reported resectability of colorectal liver metastases
Two participants did not respond to any of the 6 clinical case scenarios and were excluded. A total of 372 resectability assessments from the remaining 62 participants were analyzed. Clinical information provided in the 6 clinical case scenarios is summarized in Table 2.
Table 2.
Summary of clinical information of case scenarios and proportion of respondents who considered the colorectal liver metastases resectable
| Case no. | Patient age, yr/sex | Comorbidities | Primary tumour | Hepatic metastases | Resectable, no. (%) of respondents n = 62 |
|---|---|---|---|---|---|
| 1 | 50/male | Hypertension, dyslipidemia | Rectum pT3N2b | 5 nodules, metachronous | 44 (71) |
| 2 | 64/male | Hypertension, diabetes | Left colon pT3N2b | 3 nodules, synchronous | 47 (76) |
| 3 | 61/male | Osteoarthritis | Rectum pT2N1a | 5 nodules, synchronous | 42 (68) |
| 4 | 6/male | Chronic obstructive pulmonary disease, hypertension, peripheral vascular disease | Right colon pT3N1a | 7 nodules, synchronous | 46 (74) |
| 5 | 61/female | Hypothyroidism, hypertension, diabetes | Right colon pT4aN2a | 7 nodules, synchronous | 18 (29) |
| 6 | 38/male | — | Sigmoid colon pT3N2a | 10 nodules, synchronous | 36 (58) |
An average of 63% (range 29% for case 5 to 76% for case 2) of respondents considered CRLMs resectable (Table 2). The most common factors used by respondents to define resectability were the ability to treat all lesions with a sufficient hepatic remnant (56 respondents [90%]), the disease-free interval (40 [64%]), the absence of extrahepatic metastases (30 [48%]) and the absence of vascular involvement (21 [34%]). More traditional prognostic factors were less often reported as contraindications to resection and included size of the metastatic tumour (8 [13%]), bilobar distribution (4 [6%]) and the inability to achieve a 1-cm margin (3 [5%]).
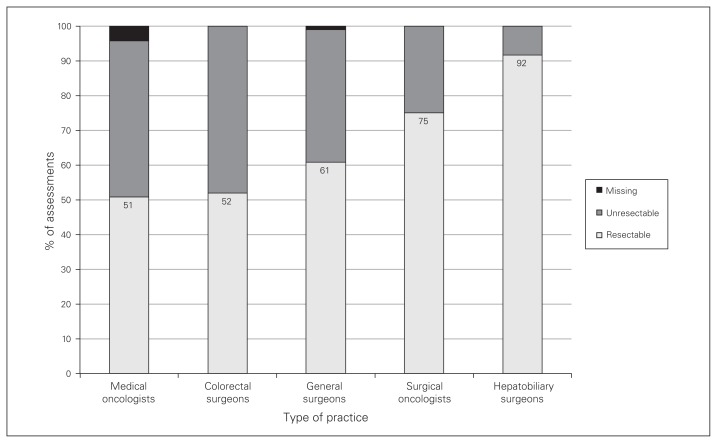
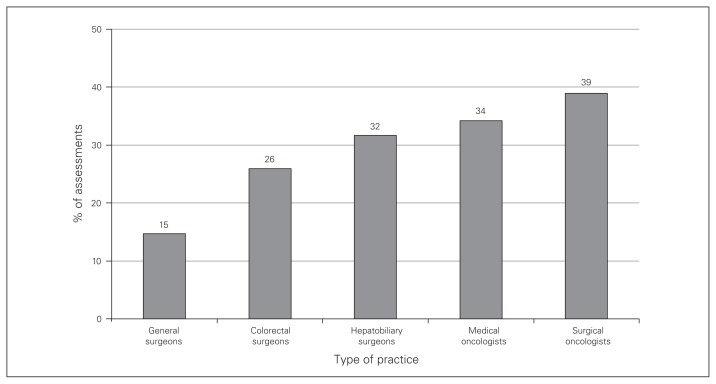
Hepatobiliary surgeons considered CRLMs to be resectable in 92% of assessments, compared to 57% of assessments for all other specialists combined (p < 0.001) (Fig. 1). Hepatobiliary surgeons reported an unresectable scenario in 5 assessments: 3 for case 5 and 2 for case 6; factors cited for these assessments were distribution of disease and lack of viable liver remnant. Initial (upfront) referral for systemic therapy was prioritized on average for 28% of assessments, most commonly by surgical oncologists (Fig. 2). When the CRLMs were deemed unresectable, hepatobiliary referral was still considered in 73% of the assessments by colorectal surgeons, 59% of those by general surgeons, 57% of those by medical oncologists and 33% of those by surgical oncologists (p = 0.1). All remaining CRLMs considered unresectable by the diagnosing physician would be referred directly for systemic therapy. Exclusive palliative supportive care or “no treatment” was not recommended in any initial assessment.
Fig. 1.
Proportion of assessments in which colorectal liver metastases were considered resectable according to respondents’ type of practice (p < 0.001).
Fig. 2.
Proportion of assessments in which patient would be referred for upfront systemic therapy according to respondents’ type of practice (p = 0.01).
Discussion
In our study, all 10 hepatobiliary surgeons agreed with resection of CRLMs in 4 of the 6 case scenarios, and only 3 and 2 did not consider resection feasible in scenarios 5 and 6, respectively. All of our case scenarios included detailed individualized clinical information, no patient received downsizing chemotherapy, and scenarios 5 and 6 were considered resectable by the smallest proportions of respondents. We conclude that providing detailed clinical and imaging information results in a less controversial oncologic scenario, increasing the reliability and clinical relevance of the resectability assessment.
A lack of agreement among hepatobiliary surgeons regarding the resectability of CRLMs has been previously reported. Mohammad and colleagues9 described poor agreement in 4 of 8 case scenarios presented to 20 liver surgeons. However, all scenarios presented in that study provided the same clinical history of preoperative downsizing chemotherapy for each case.
Disagreement regarding resectability of CRLMs between specialties has also been previously reported. In a virtual board meeting with 30 cases presented to 11 surgeons and 10 medical oncologists, surgeons considered CRLMs resectable more often than did medical oncologists.6 Medical oncologists were more likely than surgeons to indicate preoperative chemotherapy, mostly in cases deemed unresectable. Interestingly, the proportion of cases considered unresectable and the indication for preoperative chemotherapy by both surgeons and medical oncologists decreased after an educational intervention outlining the latest resectability criteria. In our study, significant variation in assessment of CRLM resectability was observed among medical oncologists and different surgical subspecialists. Interpretation of this finding in light of the reasons for unresectability reported by each specialty revealed that, as resectability rates decreased, there was an increased cumulative reliance on adverse prognostic factors (e.g., disease-free interval, carcinoembryonic antigen level, and number and size of nodules) as contraindications to surgery. Accordingly, hepatobiliary surgeons more often restricted resectability criteria to technical concepts and yielded the highest proportion of “resectable” assessments (92%). Therefore, we can infer that much of the disagreement about resectability results from misinformation about what factors constitute absolute contraindications to resection. Since defining resectability is central in multimodal treatment planning, and since this relies on a profound and detailed understanding of surgical anatomy/technique, it is reasonable to conclude that an experienced liver surgeon should be the primary clinician responsible for this assessment.
Surgical oncologists considered the indication of upfront chemotherapy more frequently (39% of assessments) than other specialists. This finding is particularly interesting given that, although only 25% of surgical oncologists’ assessments were “unresectable,” medical oncologists considered upfront chemotherapy just 33% of the time despite deeming almost 50% of cases as “unresectable.” This is likely explained by the fact that, even after assessing a case as unresectable, medical oncologists would still commonly refer patients to hepatobiliary surgeons before indicating upfront chemotherapy. The substantial proportion of cases deemed unresectable that would not referred to hepatobiliary surgeons is concerning, particularly when considering that all our case scenarios were actual patients with successfully resected/ablated R0 CRLMs. This may be partly explained by a preference for upfront systemic therapy, even in the setting of resectable disease, by surgical oncologists.10 Even in this situation, however, initial assessment and close monitoring by a liver surgeon is advisable to define the best timing for surgery, prevent chemotherapy-associated morbidity after liver resection and minimize the occurrence of “vanishing” liver metastases. Even though no patients in this study were referred directly to exclusive supportive care, concern remains about missing the opportunity to adequately treat resectable cases. In a retrospective study of 110 palliative cases of colorectal cancer, the cases of 53 patients with liver-only metastases had not been reviewed by a liver surgeon.7 On specialist review, 33 cases were considered potentially resectable, with a high level of interobserver agreement.
Limitations
The first limitation of this pilot study is the modest population size, notably in the hepatobiliary surgeon group. A broader population and larger number of hepatobiliary surgeons would allow greater applicability and offer improved external validity. Nonetheless, we feel that this sample is representative of the greater national experience, and it is possible that local factors such as availability and wait times for specialist consultation may have influenced referral patterns. It is interesting to note, however, that minimal relevance was attributed by most respondents to distance or wait times for access to specialist consultation. In addition, most respondents reported a distance of less than 100 km from a referral centre, and, consequently, the true impact on those negotiating the longest distance to access specialist care was not addressed. This allowed us to focus our inferences about referral decision-making on more idealized clinical grounds. Second, although the truth regarding participants’ opinions about hypothetical scenarios is always difficult to ascertain, stated preference methods have been increasingly used and validated in health care studies.11–15 Careful inclusion of pertinent clinical and imaging information to compose accurate case scenarios, as well as use of dichotomous questions, were prioritized in the survey instrument design in an ex ante approach to minimize hypothetical bias. However, this pilot study provides insight into factors required to optimize the survey. Subsequent studies will include scenarios deemed unresectable by consensus for optimized calibration, as well as clinically relevant information used in the decision-making process, such as future liver remnant volume and synthetic function.
Conclusion
The results of this pilot study addressing the management of CRLMs suggest that the assessment of CRLM resectability varied significantly between different specialties. The definition of resectability was often underestimated by medical oncologists and nonhepatobiliary surgeons. Although these findings highlight the importance of early engagement of liver surgeons within the multidisciplinary team, this did not take place in a substantial proportion of cases erroneously deemed unresectable by the diagnosing physician. These disparities result largely from an imprecise understanding of the latest surgical indications for resection. Multidisciplinary collaborations through tumour board meetings and educational events to disseminate updated evidence may assist in optimizing specialist referral patterns. Further studies should be conducted to validate the survey tool and deepen our understanding of the true impact of the initial assessment and referral of patients with CRLMs.
Footnotes
This manuscript was presented as an oral presentation at the Digestive Disease Week/Society for Surgery of the Alimentary Tract meeting, May 21–24, 2016, San Diego.
Competing interests: None declared.
Contributors: J.-M. Aubin, A. Bressan, S. Grondin, A. MacLean, G. Kaplan and C. Ball designed the study. J.-M. Aubin, S. Grondin, S. Gregg, P. Tang, G. Kaplan and C. Ball acquired the data, which J.-M. Aubin, A. Bressan, S. Grondin, E. Dixon, G. Kaplan, G. Martel and C. Ball analyzed. J.-M. Aubin, A. Bressan, S. Grondin, E. Dixon, G. Kaplan and C. Ball wrote the article, which all authors reviewed and approved for publication.
References
- 1.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–8. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 3.Lam VW, Spiro C, Laurence JM, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol. 2012;19:1292–301. doi: 10.1245/s10434-011-2061-0. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelica MI, Kemeny NE. Metastatic colorectal cancer to the liver: involve the surgeon early and often. Ann Surg Oncol. 2015;22:2104–6. doi: 10.1245/s10434-015-4456-9. [DOI] [PubMed] [Google Scholar]
- 5.Leal JN, Bressan AK, Vachharajani N, et al. Time-to-surgery and survival outcomes in resectable colorectal liver metastases: a multi-institutional evaluation. J Am Coll Surg. 2016;222:766–79. doi: 10.1016/j.jamcollsurg.2016.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homayounfar K, Bleckmann A, Helms HJ, et al. Discrepancies between medical oncologists and surgeons in assessment of resectability and indication for chemotherapy in patients with colorectal liver metastases. Br J Surg. 2014;101:550–7. doi: 10.1002/bjs.9436. [DOI] [PubMed] [Google Scholar]
- 7.Jones RP, Vauthey JN, Adam R, et al. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg. 2012;99:1263–9. doi: 10.1002/bjs.8835. [DOI] [PubMed] [Google Scholar]
- 8.Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg. 2010;97:1110–8. doi: 10.1002/bjs.7032. [DOI] [PubMed] [Google Scholar]
- 9.Mohammad WM, Martel G, Mimeault R, et al. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB (Oxford) 2012;14:291–7. doi: 10.1111/j.1477-2574.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan H, Bridges JF, Cosgrove DP, et al. Treating patients with colon cancer liver metastasis: a nationwide analysis of therapeutic decision making. Ann Surg Oncol. 2012;19:3668–76. doi: 10.1245/s10434-012-2564-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bipat S, van Leeuwen MS, Ijzermans JN, et al. Imaging and treatment of patients with colorectal liver metastases in the Netherlands: a survey. Neth J Med. 2006;64:147–51. [PubMed] [Google Scholar]
- 12.Krell RW, Reames BN, Hendren S, et al. Surgical referral for colorectal liver metastases: a population-based survey. Ann Surg Oncol. 2015;22:2179–94. doi: 10.1245/s10434-014-4318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan H, Bridges JF, Schulick RD, et al. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29:619–25. doi: 10.1200/JCO.2010.30.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan H, Segev DL, Bridges JF, et al. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol. 2013;20:448–56. doi: 10.1245/s10434-012-2619-5. [DOI] [PubMed] [Google Scholar]
- 15.Timmermans DR, Gooszen AW, Geelkerken RH, et al. Analysis of the variety in surgeons’ decision strategies for the management of left colonic emergencies. Med Care. 1997;35:701–13. doi: 10.1097/00005650-199707000-00004. [DOI] [PubMed] [Google Scholar]