KEY POINTS
Clear reporting of placebo characteristics in trials is uncommon.
Choice of placebo characteristics can lead to over- or underestimation of intervention benefits and harms within trials.
Over- or underestimation of intervention effect estimates can occur when placebo controls are not adequately matched to experimental interventions, leading to inadequate blinding and expectation bias.
Placebo characteristics should be reported with the same rigour as active interventions.
Researchers now recognize the need to completely describe active interventions within trials.1,2 However, the importance of clearly describing placebo control interventions is not well reported or understood. Placebo controls are interventions used in clinical trials that do not contain the “active” components of the active intervention. The purpose of placebo control interventions is to provide a baseline measure of effectiveness against which the effects of the active interventions can be measured. Yet, placebo control interventions come in many forms, ranging from pills and injections to sham surgery.3–7 The placebo control characteristics chosen by investigators have different effects and can contribute to either an overestimation or underestimation of the benefits or harms of the active intervention.8 Despite this, placebo characteristics are clearly reported in fewer than 10% of drug trials and fewer than 30% of nondrug trials.9 This failure to report placebo characteristics presents a barrier to the interpretation of placebo-controlled trials.
However, the extent to which placebo characteristics lead to mistaken inferences about the benefits and harms of an active intervention cannot be fully understood until proper reporting of placebo characteristics becomes standard practice. Failure to disclose placebo characteristics also makes it difficult to obtain proper informed consent, which is an ethical problem.10
With reference to real examples, we discuss how placebo characteristics may influence the apparent effectiveness of the active intervention and why adequate description of interventions for placebo control interventions is part of the solution.
How can choice of placebo characteristics lead to overestimation of intervention benefits?
Characteristics of placebo control interventions can lead to overestimation of intervention benefits in various ways. One arises when the placebo is not matched in appearance with the active intervention. A 2016 review, which combined data from 36 trials, found that as many as 44% of placebo control interventions were not matched in terms of physical properties.11 Lack of matching can result in inadequate blinding, because it will be easier for trial participants, practitioners and researchers to identify placebos. A 2004 systematic review of 191 trials estimated that only a small proportion (about 7%) of trials test whether blinding has been successful, and when it was tested for, it was rarely successful.12
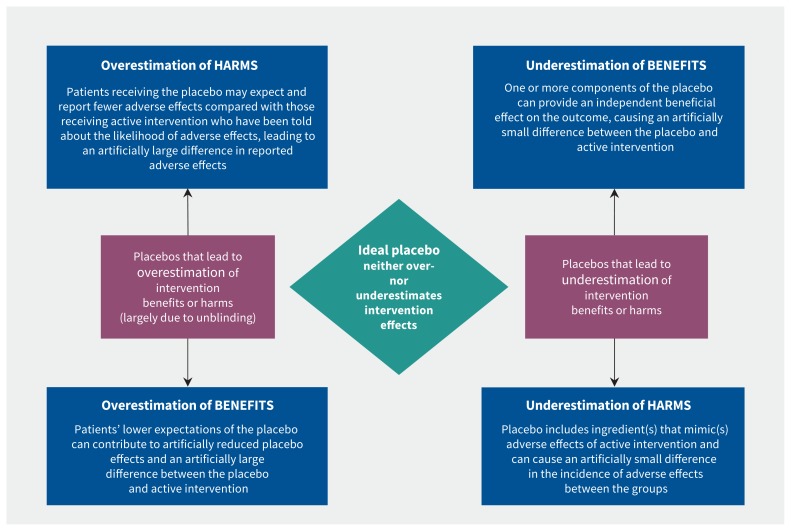
Lack of blinding can lead to exaggerated effect sizes. A systematic review, which included data from 1973 trials, showed that trials with unblinded participants had larger effects than trials with blinded participants and researchers.13 Failure to blind participants adequately seems to affect outcomes because unblinded patients who know they are receiving the active (“real”) intervention are likely to have higher expectations about recovery than in adequately blinded patients who believe they might be getting a placebo. A 2001 systematic review of 45 trials reported that higher expectations of recovery were shown to positively influence patient outcomes.14 In unsuccessfully blinded trials, the apparent intervention effect may be influenced by expectations rather than the active intervention (which is known as “expectation bias,” Figure 1). Blinding will be more easily maintained if the placebo’s visual appearance, taste and smell is the same as that of the active intervention. This type of bias can be estimated properly only if the placebo characteristics are adequately reported.
Figure 1:
How placebo characteristics can cause over- or underestimation of the apparent benefits or harms of an active intervention.
Even if a placebo appears identical to the active intervention, the trial can still become unblinded if the placebo does not produce the same adverse reactions as the active intervention. For example, a 2009 systematic review of 143 trials found that a common adverse reaction to tricyclic antidepressants is a dry mouth.15 Patients in trials of tricyclic antidepressants who get a dry mouth might correctly suspect that they are taking the active intervention and develop a positive expectation about recovery. Those who do not experience a dry mouth could correctly suspect that they are taking a placebo, possibly generating negative expectations about recovery, leading to reporting — or even having — worse outcomes. To prevent this expectation bias from arising, some trials use active placebos. Active placebos are not to be confused with active interventions; they are placebos that contain ingredients that cause some of the adverse effects of the active intervention. Active placebos are more difficult to distinguish from the active intervention, leading to more successful blinding, less expectation bias and a more accurate (usually smaller) estimate of apparent benefit of the active interventions. A 2004 systematic review (nine studies) found that trials of antidepressants that used active placebos as controls showed a smaller drug–placebo difference than trials that used standard placebos.8
By improving the success of blinding and reducing expectation bias, using active placebos leads to smaller but more accurate observed benefits of active interventions compared with placebo. However, the presence of characteristics that are intended to mimic harmful adverse effects raises ethical issues that have been largely ignored because of the failure to report placebo characteristics.
The use of bulking agents in placebo controls to replace the characteristic ingredient of the active intervention may also produce exaggerated effect sizes. For example, a 1990 randomized controlled trial (RCT) of megestrol acetate as an intervention for cancer-related anorexia showed an unexpected benefit in reducing gastrointestinal symptoms compared with a lactose placebo.16 However, lactose intolerance is a common adverse effect of many interventions for cancer.17 The adverse reactions to the lactose placebo included gastrointestinal symptoms, which may have caused patients taking the lactose placebo to have negative effects, leading in turn to a possibly exaggerated benefit of megestrol acetate.
How can choice of placebo characteristics lead to underestimation of intervention benefits?
When placebo controls contain characteristics that can positively affect the target outcome, the apparent effect size of the active intervention will be underestimated (Figure 1). For example, olive oil was used previously in placebo controls for cholesterol-lowering drugs;18 however, it was discovered subsequently that olive oil has cholesterol-lowering properties of its own, rendering its use as a placebo control inappropriate. Such difficulties can also occur with more complex placebo controls. For example, some investigators have argued that the effects of psychotherapeutic interventions are difficult to disentangle from placebo effects,19 and sham surgery involves the administration of analgesia and an incision — both of which can have therapeutic effects.20 In some instances, these could augment the effect of the placebo.21 In an even more complex example, sham acupuncture contains features such as acupressure and longer consultations. Making sham acupuncture more similar to real acupuncture is likely to improve the success of blinding. But the increased success of blinding may come at the cost of including what may be considered to be nonplacebo elements. In fact, acupressure was shown in a 2008 systematic review of 10 RCTs to reduce nausea22 and in a 2018 systematic review of 32 RCTs to improve sleep quality.23 Importantly, the effects of these placebo characteristics go beyond the general effects of being in a trial (Hawthorne effects), which affect all trial participants.24 In trials where adequate placebo controls are difficult to construct, established treatment controls could be employed (perhaps in addition to the placebo control).25
How can choice of placebo characteristics lead to underestimation of intervention harms?
Although using active placebos can improve the success of blinding, if the placebo characteristics are not chosen carefully, a placebo can contribute to underestimation of the harms for an active intervention. If a characteristic was introduced into a placebo to mimic an adverse event, there will be no (or minimal) differences between the groups in the occurrence of that adverse event by design. For example, in trials of oseltamivir, the placebo contained dehydrocholic acid and dibasic calcium phosphate dehydrate, presumably to mimic the bitter taste of the active intervention (oseltamivir powder) and maintain blinding.26 However, dehydrocholic acid can cause gastrointestinal symptoms, as can oseltamivir.27 Although there was an increased risk of gastrointestinal upset (nausea and vomiting) in the group taking oseltamivir compared with the placebo group, this was probably an underestimate of the true incidence of the harms caused by the intervention because the placebo contained an ingredient that can cause the same adverse reactions as the active intervention.
How can choice of placebo characteristics lead to overestimation of intervention harms?
Unblinded patients who know they are taking the placebo, which they believe to be harmless, will not have expectations about the harms of the intervention. Unblinded patients within the same trial who know they are taking the active intervention and have been informed about potential harms could experience negative placebo (nocebo) effects. We are unaware of any examples of harm overestimation because of choice of placebos; however, there is evidence that nocebo effects can be produced by what patients are told to expect. In one RCT that compared treatment with acetylsalicylic acid and with sulfinpyrazone in patients with unstable angina, participants were randomly assigned to receive a statement outlining possible gastrointestinal adverse effects of the drug or to not receive the statement. Receiving the additional information about adverse effects resulted in a sixfold increase in the number of participants who withdrew from the trial (across both intervention and placebo groups) because of subjective minor gastrointestinal symptoms.28
How can the reporting of placebo characteristics in trials be improved?
The ideal placebo control in a trial is one that provides an accurate baseline against which the effects of the active intervention can be gauged. Such a placebo control will help maintain successful blinding and will not contribute to overestimation or underestimation of the benefits or harms of the active intervention. Even a small deviation from the ideal can have an important effect on the interpretation of the active intervention’s benefits or harms.
Finding the ideal placebo control is difficult and may be impossible for some complex interventions. However, the extent to which placebo characteristics can influence estimates of effect size for an active intervention varies. For example, a placebo control that is unlikely to enable the trial to remain blinded may be more serious in trials with outcomes that are placebo responsive such as pain,29 and a placebo control that contains an ingredient to mimic the adverse effect of an experimental drug could be more pertinent in trials where the benefit-to-harm ratio for the intervention is uncertain.26 Reporting of placebo characteristics should also include aspects such as adherence to the placebo,30 because differing levels of adherence between the groups can be another source of bias.
Adequate reporting of placebo characteristics allows investigators to justify the choice of placebo characteristics, enables trial participants to make truly informed decisions about consent, and assists users of the trial results to evaluate how close the placebo approaches the ideal and interpret effects accordingly. Aided by development of the Template for Intervention Description and Replication (TIDieR) checklist, adequate description of active interventions has become increasingly common.31 However, the international group of experts and stakeholders who developed TIDieR neither focused on nor provided examples of how or why placebo controls should be described. We encourage investigators to adequately describe placebo controls in trial reports because this will enable more informed critical appraisal of placebo-controlled trials and systematic reviews of such trials. We encourage the use of TIDieR as a basis for this. Table 1 lists some of the items from the TIDieR checklist and provides examples of why these details can be important for placebo controls.
Table 1:
Examples of some items in the Template for Intervention Description and Replication checklist31 and their importance for placebo controls
| Checklist item | Example of why this detail is important |
|---|---|
| Why (what is the rationale, theory or goal of the elements essential to the intervention) | To determine the extent to which actual placebos deviate from the ideal, we need to know what the placebo control is designed to control for. In a drug trial, this will typically be the patented chemical(s) in the formulation. For complex interventions, such as exercise and acupuncture, this can be more difficult but is nevertheless important to articulate. |
|
What is in the placebo control The colour, size and shape of the placebo can have direct effects on the outcome. Procedures that involve additional liquids or food could influence the outcomes unless these are shared in control and experimental groups. |
|
|
Who delivered the placebo control Clinician style and communication manner can affect outcomes. |
In general, clinicians who are women are perceived as more empathic than those who are men, and this can affect outcomes, especially if the type of provider is not similarly distributed between intervention and control groups.21,33 |
|
When and how much The number of placebo formulations and the type of placebo can affect outcomes. |
Conclusion
Describing placebo characteristics enhances appraisal of intervention effects and supports ethical trial conduct. The ideal placebo control in a trial is one that helps to maintain successful blinding but does not contribute to over- or underestimation of the benefits or harms of the active intervention. We have shown that through inadequate blinding or the effects of placebo characteristics, the choice of placebo control can lead to overestimation or underestimation of intervention effects. Because the requirement of informed consent demands that patients know what they agree to when they enrol in a trial, disclosure of placebo characteristics is also required from an ethical perspective. Therefore, we suggest that trial registry entries, protocols and reports contain complete descriptions of all placebo characteristics using the TIDieR checklist as a guide, that journal editors require this and that future reporting guidelines for interventions emphasize the need to completely describe active interventions and placebo controls.
Footnotes
Competing interests: Tammy Hoffmann is an author of the Template for Invention and Replication checklist. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Both authors contributed to the conception and design of the work, drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Hoffmann TC, Erueti C, Glasziou PP. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ 2013;347:f3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howick J. The relativity of placebos: defending a modified version of Grunbaum’s scheme. Synthese 2017;194:1363–96. [Google Scholar]
- 3.Blackwell B, Bloomfield SS, Buncher CR. Demonstration to medical students of placebo responses and non-drug factors. Lancet 1972;1:1279–82. [DOI] [PubMed] [Google Scholar]
- 4.de Craen AJ, Roos PJ, Leonard de Vries A, et al. Effect of colour of drugs: systematic review of perceived effect of drugs and of their effectiveness. BMJ 1996;313:1624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wartolowska K, Judge A, Collins G, et al. Use of placebo controls in the evaluation of surgery: systematic review. BMJ 2014;348:g3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckalew LW, Ross S. Relationship of perceptual characteristics to efficacy of placebos. Psychol Rep 1981;49:955–61. [DOI] [PubMed] [Google Scholar]
- 7.Kaptchuk TJ, Stason WB, Davis RB, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 2006;332:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moncrieff J. A comparison of antidepressant trials using active and inert placebos. Int J Methods Psychiatr Res 2003;12:117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golomb BA, Erickson LC, Koperski S, et al. What’s in placebos: Who knows? Analysis of randomized, controlled trials. Ann Intern Med 2010;153:532–5. [DOI] [PubMed] [Google Scholar]
- 10.Blease CR, Bishop FL, Kaptchuk TJ. Informed consent and clinical trials: where is the placebo effect? BMJ 2017;356:j463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bello S, Wei M, Hilden J, et al. The matching quality of experimental and control interventions in blinded pharmacological randomised clinical trials: a methodological systematic review. BMC Med Res Methodol 2016;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fergusson D, Glass KC, Waring D, et al. Turning a blind eye: the success of blinding reported in a random sample of randomised, placebo controlled trials. BMJ 2004;328:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savovic J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429–38. [DOI] [PubMed] [Google Scholar]
- 14.Mondloch MV, Cole DC, Frank JW. Does how you do depend on how you think you’ll do? A systematic review of the evidence for a relation between patients’ recovery expectations and health outcomes. CMAJ 2001;165:174–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf 2009;32:1041–56. [DOI] [PubMed] [Google Scholar]
- 16.Loprinzi CL, Ellison NM, Schaid DJ, et al. Controlled trial of megestrol acetate for the treatment of cancer anorexia and cachexia. J Natl Cancer Inst 1990;82:1127–32. [DOI] [PubMed] [Google Scholar]
- 17.Osterlund P, Ruotsalainen T, Peuhkuri K, et al. Lactose intolerance associated with adjuvant 5-fluorouracil-based chemotherapy for colorectal cancer. Clin Gastroenterol Hepatol 2004;2:696–703. [DOI] [PubMed] [Google Scholar]
- 18.Golomb BA. Paradox of placebo effect. Nature 1995;375:530. [DOI] [PubMed] [Google Scholar]
- 19.Kirsch I, Wampold B, Kelley JM. Controlling for the placebo effect in psychotherapy: Noble quest or tilting at windmills? Psychol Conscious 2016;3:121–31. [Google Scholar]
- 20.Sinno H, Prakash S. Complements and the wound healing cascade: an updated review. Plast Surg Int 2013;2013:146764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howick J, Moscrop A, Mebius A, et al. Effects of empathic and positive communication in healthcare consultations: a systematic review and meta-analysis. J R Soc Med 2018;111:240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Dodd M, Dibble S, et al. Review of acupressure studies for chemotherapy-induced nausea and vomiting control. J Pain Symptom Manage 2008;36:529–44. [DOI] [PubMed] [Google Scholar]
- 23.Waits A, Tang YR, Cheng HM, et al. Acupressure effect on sleep quality: a systematic review and meta-analysis. Sleep Med Rev 2018;37:24–34. [DOI] [PubMed] [Google Scholar]
- 24.Benedetti F, Carlino E, Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurology 2016;15:736–47. [DOI] [PubMed] [Google Scholar]
- 25.Howick J. Reviewing the unsubstantiated claims for the methodological superiority of ‘placebo’ over ‘active’ controlled trials: reply to open peer commentaries. Am J Bioeth 2009;9:W5–7. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson T, Jones M, Doshi P, et al. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 2014;348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014;(4):CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers MG, Cairns JA, Singer J. The consent form as a possible cause of side effects. Clin Pharmacol Ther 1987;42:250–3. [DOI] [PubMed] [Google Scholar]
- 29.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev 2010;(1):CD003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stetler C. Adherence, expectations and the placebo response: why is good adherence to an inert treatment beneficial? Psychol Health 2014;29:127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 32.Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med 2010;152:797–803. [DOI] [PubMed] [Google Scholar]
- 33.Di Blasi Z, Harkness E, Ernst E, et al. Influence of context effects on health outcomes: a systematic review. Lancet 2001;357:757–62. [DOI] [PubMed] [Google Scholar]
- 34.Blackman K, Brown SGA, Wilkes GJ. Plasma alkalinization for tricyclic antidepressant toxicity: a systematic review. Emerg Med (Fremantle) 2001;13:204–10. [DOI] [PubMed] [Google Scholar]