Abstract
While retrograde neurotrophin signaling has provided an immensely influential paradigm for understanding growth factor signaling in the nervous system, recent studies indicate that growth factors also signal via cell-autonomous, or autocrine, mechanisms. Autocrine signals have been discovered in many neuronal contexts, providing insights into their regulation and function. The growing realization of the importance of cell-autonomous signaling stems from advances in both conditional genetic approaches and in sophisticated analyses of growth factor dynamics, which combine to enable rigorous in vivo dissection of signaling pathways. Here we review recent studies defining autocrine roles for growth factors such as BDNF, and classical morphogens, including Wnts and BMPs, in regulating neuronal development and plasticity. Collectively, these studies highlight an intimate relationship between activity-dependent autocrine signaling and synaptic plasticity, and further suggest a common principle for coordinating paracrine and autocrine signaling in the nervous system.
Introduction
Defining when and where extracellular signals are released is key to understanding how they direct neuronal structure and function. However, the broad expression profiles and pleiotropic phenotypes of evolutionarily-conserved signaling proteins has hindered a detailed view of their in vivo roles. Identifying the cellular requirement for a broadly-expressed secreted protein requires generating mosaic animals where the protein is deleted in only a few cells. Such conditional genetic approaches provide a critical test of signaling directionality in vivo, and have provided evidence for anterograde and retrograde pathways, and increasingly, autocrine pathways as well.
Cell-autonomy of signaling pathways in neurons is consistent with their established autocrine functions in non-neuronal tissues. In general, the extent to which secreted signals spread in vivo is controversial. For example, Wnt family members are palmitoylated and do not freely diffuse [1]. This lipid modification makes them highly hydrophobic and is proposed to promote local action of Wnt family members. Related concerns extend to members of the mammalian neurotrophin family. Brain-Derived Neurotrophic Factor (BDNF) is positively charged at physiological pH (pI > 9) [2], which is predicted to limit its diffusion by promoting electrostatic interactions between BDNF and proteins on the surface of the secreting cell, thus facilitating cell-autonomous signaling. Thus, the biochemical properties of signaling proteins are likely to facilitate highly local, autocrine signaling pathways.
Recent studies have revealed cell-autonomous functions for neurotrophins, including BDNF, as well as classical morphogens, such as Wnts and BMPs. These pathways play widespread roles in the developing and adult nervous system, and regulate processes from neuronal morphology to synaptic plasticity. The diverse contexts in which autocrine signals have been recently discovered begs the question of whether there is an underlying logic to their in vivo functions. Based on the studies reviewed here, we suggest two interrelated possibilities. The first is that the timing of activity-dependent autocrine signals may be particularly important at synapses where the rapid dynamics and highly focal action of an autocrine loop could enable tight coupling of synaptic activity to signal transduction. The second is that autocrine signals may often be activated in response to short-lived paracrine cues in order to sustain or amplify the initial intercellular cue.
Autocrine BDNF signaling takes center stage at the synapse
Retrograde neurotrophin signaling has provided a long-standing model for understanding growth factor signaling in the nervous system (Fig. 1). BDNF was first identified as a target-derived cue required for neuronal survival and is now known to regulate diverse processes including neurogenesis, synaptic development and function [3]. Early hints that BDNF was not exclusively a retrograde cue emerged from classic in vitro studies. Twenty years ago, BDNF was shown to signal in an autocrine fashion to drive maturation of cultured embryonic chick DRG neurons [4], as well as neuronal survival in adult DRGs [5]. In both contexts, BDNF knockdown in isolated neurons in culture inhibited BDNF-dependent signaling, demonstrating that BDNF is capable of signaling cell autonomously [6].
Figure 1.
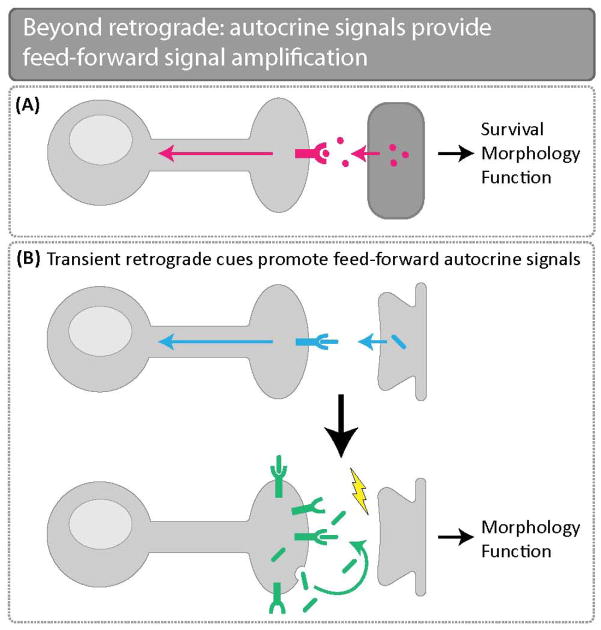
Beyond retrograde: autocrine signals provide feed-forward signal amplification. (A) Target-derived retrograde cues were initially described as essential for neuronal survival, morphology, and function. (B) Recent technological and experimental advances have built upon our understanding of retrograde signaling and have elucidated the hand-off of a transient retrograde cue that initiates downstream autocrine pathways to maintain and amplify signaling critical for neuronal morphology and function.
More recently, autocrine BDNF signaling has been implicated in axon specification in cultured rat hippocampal neurons [7]. Knockdown of BDNF in a small subset of neurons in culture decreased axon formation only in BDNF-deficient neurons, not in their BDNF-expressing neighbors, suggesting cell-autonomous BDNF signaling. Notably, activation of a downstream component of the BDNF pathway cell-autonomously increased both BDNF release and surface levels of its receptor TrkB, arguing for a feed-forward signaling loop that locally amplifies weak or transient signals involved in axon specification [7]. While it is yet unclear if autocrine BDNF signaling regulates axon specification in vivo, the hypothesis that a key function of autocrine loops is to strengthen relatively weaker paracrine signals is an attractive one discussed in more detail below.
A role for autocrine BDNF signaling in synapse formation has been uncovered in an in vitro model of the neurodevelopmental disorder Rett syndrome (RTT) [8]. Methyl CpG Binding Protein (MeCP2) mutations cause RTT [9]; BDNF is relevant to this disorder since BDNF overexpression rescues RTT phenotypes [10,11]. MeCP2 is on the X chromosome, and importantly, X inactivation renders MeCP2 heterozygotes cellular mosaics for MeCP2. This mosaicism raises the intriguing question of whether or not BDNF rescue of MeCP2 phenotypes is cell-autonomous. Co-culture of MeCP2-positive and MeCP2-negative hippocampal neurons revealed evidence for autonomous BDNF signaling. BDNF expression restored normal glutamatergic synapse number only when expressed in MeCP2-negative neurons themselves. Synaptic contact with BDNF-expressing neurons failed to rescue synaptic phenotypes in MeCP2-negative neurons [8]. The strictly cell-autonomous ability of BDNF to rescue synaptic defects in this RTT model has potential implications for dysfunction of circuits mosaic for MeCP2 expression in RTT individuals.
Autocrine BDNF loops have also been discovered in vivo. In the adult mammalian brain, granule cells are continuously generated in the dentate gyrus and their dendrites join pre-existing hippocampal circuits. BDNF/TrkB signaling is required for dendritogenesis of these adult-born neurons [12]. Interestingly, BDNF is proposed to act in an autocrine manner in this context [13]. To rigorously examine the role of a cell-autonomous BDNF pathway, genetic mosaic animals were generated by injecting a Cre and GFP-carrying retrovirus into adult BDNF-floxed mice. Strikingly, BDNF-negative (GFP-positive) neurons had reduced dendrite length and branching, while neighboring BDNF-positive neurons had normal dendrites. Furthermore, the dendrite phenotype of individual BDNF mutant neurons was similar to that observed when BDNF was deleted throughout the forebrain, arguing that BDNF acts primarily in an autocrine manner. Autocrine BDNF signaling was also implicated in activity-dependent plasticity. Exercise-mediated improvements in learning and memory are tied to increased dendrite growth in the hippocampus [3,14,15]. This plasticity requires BDNF expression specifically in adult-born granule cells [13], suggesting that autocrine BDNF signaling is responsible. Together, these findings indicate that autocrine BDNF signaling is necessary for baseline and experience-dependent dendritic growth in adult-born hippocampal neurons.
BDNF has been long implicated in synaptic plasticity [16–18]. Excitingly, studies have recently revealed that post-synaptic plasticity and post-synaptic autocrine BDNF signaling are tightly linked. Dendritic spines are actin-rich, mushroom-shaped structures which serve as postsynaptic specializations of excitatory synapses in the mammalian brain. In response to activity, spines can undergo forms of synaptic strengthening, referred to as structural or functional long-term potentiation (LTP), which are characterized by sustained spine enlargement and increased glutamate sensitivity, respectively. The relationship between BDNF and forms of LTP seen in the CA1 region in the adult hippocampus was recently investigated [19–21]. BDNF/TrkB signaling was found to be selectively required for a specific type of timing-dependent post-synaptic LTP in hippocampal slices [19]. Strikingly, BDNF was found to be released from spines themselves. These data indicate a close relationship between post-synaptic BDNF signaling and this specific form of post-synaptic LTP, suggesting an autocrine BDNF pathway.
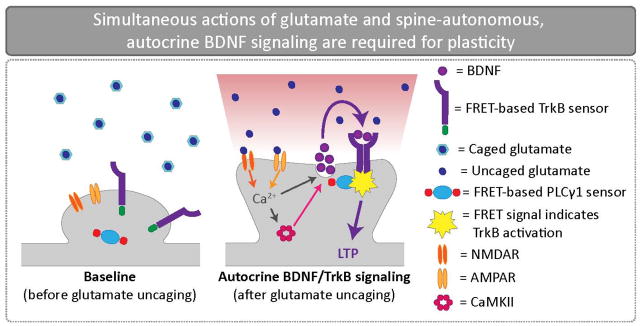
A recent study provides compelling evidence that spine-autonomous BDNF signaling regulates LTP [20]. Using a TrkB FRET-based sensor, these authors demonstrated that focal glutamate uncaging simultaneously triggered spine enlargement and rapid, sustained TrkB activation in individual spines of CA1 pyramidal neurons (Fig. 2) [20]. Surprisingly, the source of BDNF was found to be the spine itself; moreover, BDNF release from the activated spine was activity-dependent and time-locked to glutamate uncaging. These findings indicate that synaptic activity drives highly focal BDNF release which then triggers TrkB activation on the same spine. The coincidence of autocrine BDNF signaling and structural LTP raises the question of whether this BDNF pathway is required for LTP. Accordingly, the authors addressed the involvement of autocrine BDNF-TrkB signaling by generating BDNF mosaics. They utilized a Cre-carrying virus to delete BDNF in small numbers of CA1 neurons in BDNF-floxed mice. In line with an autocrine signal, BDNF was found to be cell-autonomously required for both structural and functional LTP [20].
Figure 2.
Simultaneous actions of glutamate and spine-autonomous, autocrine BDNF signaling are required for plasticity. To visualize rapid, autocrine BDNF signaling at CA1 synapses, a TrkB fluorescence resonance energy transfer (FRET)-based sensor was coupled with glutamate uncaging and two photon imaging. Glutamate uncaging revealed time-locked BDNF release from individual spines and subsequent postsynaptic TrkB activation on the same spine. BDNF release is also dependent upon NMDAR and CaMKII activation. This rapid, autocrine BDNF signaling mechanism is required for both structural and functional plasticity.
How does post-synaptic autocrine BDNF signaling regulate synaptic plasticity? Perhaps not surprisingly, RhoGTPases are involved [21]. Rac1, Cdc42, and RhoA are central regulators of actin dynamics and play key roles in structural and functional LTP in dendritic spines [22,23]. The specific role of autocrine BDNF signaling in RhoGTPase activation was again evaluated by generating mosaic BDNF animals. Using FRET-based sensors to monitor RhoGTPase activation in BDNF mosaic mice, cell-autonomous BDNF signaling was shown to be required for Rac1 and Cdc42 activation in spines, but not for activation of RhoA. These results indicate unexpected specificity in the downstream RhoGTPases activated by autocrine BDNF signaling and link this autocrine mechanism to core components of the molecular machinery underlying synaptic plasticity.
Collectively, these studies demonstrate an intimate relationship between autocrine BDNF signaling and synaptic strengthening in the brain [20,21,24]. Activity-dependent, spine-autonomous BDNF-TrkB signaling is coincident with LTP in CA1 neurons, and moreover, is required for its expression via regulation of Rac1 and Cdc42. Given the unexpected importance of post-synaptic autocrine BDNF signaling for structural and functional LTP, these studies raise questions of the function of the well-established presynaptic BDNF pool [25], as well as the mechanisms by which pre- and post-synaptic BDNF signaling may be integrated.
Retrograde cues pass the baton to autocrine loops
Pioneering studies of target-derived neurotrophin signaling by Viktor Hamburger and Rita Levi-Montalcini in the 1950s established a powerful paradigm for understanding functions of secreted cues in the nervous system [26]. Their studies of Nerve Growth Factor (NGF) demonstrated that it is produced in limiting amounts by target tissues and orchestrates the survival, growth, and branching of innervating neurons. Unexpectedly, recent studies indicate that NGF directs sympathetic axon branching by partnering with two downstream autocrine pathways: Wnt5a and CD40/CD40L [27–29].
NGF signaling has been found to regulate autocrine Wnt5a signaling in sympathetic neurons. The Wnt family member Wnt5a is expressed in sympathetic neurons as axons innervate their targets, and robust Wnt5a expression requires target-derived NGF [27]. Demonstrating a specific role for Wnt5a in sympathetic innervation, loss of Wnt5a in sympathetic neurons results in decreased branching of axons at their targets (Fig. 3A), without accompanying defects in neuronal specification or differentiation [28]. Interestingly, this innervation defect is qualitatively similar to that observed in NGF, Bax double mutant mice [30]. The finding that Wnt5a is required cell-autonomously for sympathetic neuron branching argues for autocrine signaling. Arguing that this autocrine pathway is active at terminals, the non-canonical Wnt receptors Ror1/2 are required locally in sympathetic axons to mediate Wnt5a function [28,31]. These studies argue that an initial target-derived NGF signal triggers feed-forward Wnt5a autocrine signaling, which serves to amplify the initial retrograde cue. Together, these studies indicate that target-derived NGF signaling is at the top of a signaling hierarchy enabling precise control of sympathetic innervation. Transferring an early target-derived signal to cell-autonomous cues may provide strong, sustained activation of downstream pathways essential to growth and branching.
Figure 3.
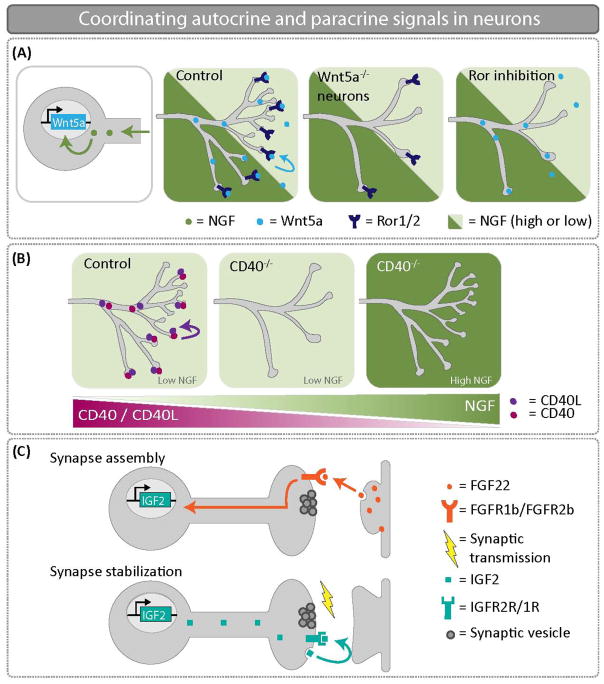
Coordinating autocrine and paracrine signals in neurons. (A) NGF promotes Wnt5a expression in sympathetic neurons. An autocrine Wnt5a-Ror signal is critical to promote axon branching. When Wnt5a is knocked out specifically in sympathetic neurons, there are significant innervation defects at embryonic day 16.5. (B) NGF represses CD40/CD40L expression in sympathetic neurons. Thus, the CD40/CD40L autocrine signaling loop only functions to drive growth and branching in sympathetic neurons where NGF levels are low. In CD40−/− mice at postnatal day 3, low NGF expressing targets are poorly innervated in the absence of CD40/CD40L autocrine signaling. (C) FGF22 is secreted from CA3 dendrites and promotes assembly of the presynaptic DGC terminal. Retrograde transport of FGF22 induces expression of IGF2. Presynaptic localization of IGF2 is necessary for presynaptic stabilization, and IGF2 transport is regulated by activity. Autocrine IGF2 is required to reinforce and stabilize nascent CA3-DGC synapses.
Notably, sympathetic axon branching is not the only process controlled by autocrine Wnt5a signaling. A cell-autonomous Wnt5a pathway has also been implicated in regulating dendrite arbors [32]. Loss of Wnt5a did not lead to differences in initial dendrite arborization of CA1 pyramidal neurons. However, it did result in reduced dendrite arborization in older animals. In fact, dendrite length and branching declined progressively with age, indicating that Wnt5a is required for dendritic arbor maintenance [32]. To test definitively whether Wnt5a is required cell-autonomously, these authors generated mosaic animals by viral delivery of Cre into Wnt5a-floxed animals. Eliminating Wnt5a in isolated neurons results in small dendritic arbors despite the presence of Wnt-expressing neighbors, arguing that it signals cell-autonomously to control maintenance of dendritic arbors. It will be interesting to determine how Wnt5a signaling is initiated in CA1 neurons. The finding that it is selectively required for dendritic arbor maintenance suggests that it might be activated in response to an early transient cue regulating initial arborization.
The role of an autocrine signaling loop consisting of CD40L, a tumor necrosis factor (TNF) family member, and CD40, TNF receptor family member, was also uncovered in sympathetic neurons [29]. Both CD40 and CD40L are expressed in sympathetic neurons as their axons arborize on target tissues. Suggesting a functional requirement in branching, inhibition of CD40/CD40L activity decreased size and complexity of axonal arbors of sympathetic neurons in culture. The requirement for both receptor and ligand in sympathetic neurons argues for an autocrine pathway. How is this pathway coordinated with retrograde NGF signaling? Two experiments shed light on this question. First, expression of CD40 and CD40L is inhibited by NGF. Second, CD40 and CD40L are selectively required for terminal arborization only on low NGF-expressing, but not high NGF-expressing, tissues (Fig. 3B). Together, these findings support the model that autocrine CD40/CD40L signaling promotes branching where NGF signaling alone would be insufficient.
Another instructive example of how early retrograde cues can be translated to autocrine signaling loops comes from an analysis of synapse development. Fibroblast growth factor 22 (FGF22) is a target-derived cue in the mouse hippocampus where it is released from CA3 pyramidal cells and promotes presynaptic organization in dentate granule cells (DGCs) [33,34]. How does retrograde FGF signaling direct synapse development? Insulin-like growth factor 2 (IGF2) was identified in a screen for genes whose expression in DGCs required FGF22 signaling [35], suggesting an IGF signal might be downstream. Genetic studies revealed that while IGF2 is dispensable for initial presynaptic assembly, it is essential for presynaptic stabilization in vitro and in vivo (Fig. 3C), consistent with the idea that IGF2 is downstream of FGF22. Interestingly both IGF22 and IGF receptors localize to presynaptic terminals of DGCs, which argues for a presynaptic and autocrine IGF pathway. Moreover, synaptic activity is required for the presynaptic localization and synaptic stabilizing functions of IGF2, in line with key roles for activity in synaptic strengthening and stabilization [35]. Together, these findings argue that a retrograde FGF pathway regulates synapse assembly and activates a presynaptic IGF loop, which then stabilizes presynaptic terminals. This study illustrates how a hand-off between a retrograde and autocrine pathway couples upstream target-dependent signals to downstream activity-dependent cues required for ongoing synaptic maintenance.
Lastly, paracrine signaling also activates an autocrine Wnt pathway in the injured CNS [36]. Axon regrowth following injury is very limited, in part due to inhibitory cues in the CNS environment [37]. Surprisingly, fibroblast-derived exosomes strongly stimulate axonal growth on inhibitory CNS substrates. Exosome treatment was found to promote axon outgrowth by relocating Wnt10b to lipid rafts and stimulating autocrine Wnt signaling and mTOR activation in cultured cortical neurons [36]. Arguing that this pathway is active in vivo, applying fibroblast-derived exosomes after an optic nerve injury stimulates robust axon regeneration, which is mediated by Wnt10b. This study demonstrates that an autocrine Wnt10b pathway, which is initially mobilized by a paracrine signal, drives intrinsic pro-growth signaling in the injured CNS. Taken together, these studies suggest a general principle whereby neurons translate weak or transient paracrine signals to autocrine signals to strengthen or sustain the initial cue.
Drosophila step up to the plate to elucidate autocrine signaling mechanisms
As seen thus far, convincing evidence for autocrine directionality of signaling pathways often necessitates genetic approaches to selectively remove gene function in restricted numbers of neurons at defined time points. Such conditional approaches have long been part of the standard genetic toolkit in Drosophila, and facilitate rapid cell-type specific dissection of neuronal signaling pathways. Here we focus on the BMP pathway, which has been recently found to act in an autocrine manner both at the glutamatergic neuromuscular junction (NMJ) and in primary nociceptive neurons.
BMP family members regulate diverse neuronal processes including axon guidance and regeneration in addition to synapse development and function [38–40]. The function of BMP signaling at synapses has been intensively studied at the Drosophila NMJ, where Glass bottom boat (Gbb), a BMP 5–8 homolog, governs NMJ structure and function [41]. Loss of Gbb results in very small NMJs as well as aberrant synapse ultrastructure and markedly reduced neurotransmitter release. Demonstrating that the motoneuron is the signal-receiving cell, selective loss of downstream BMP signaling components, including the R-Smad Mad, in the presynaptic motoneuron mimics gbb null mutants [42].
Where is Gbb released? Importantly, Gbb is released from both pre- and post-synaptic cells, and unexpectedly, these ligand pools are distinct. Rescue experiments indicate that while pre-synaptic Gbb promotes neurotransmitter release, post-synaptic Gbb promotes NMJ growth. Specifically, post-synaptic (muscle-derived) Gbb rescues bouton number in gbb mutants. Yet it does not rescue baseline neurotransmitter release, which is only restored when gbb is reintroduced in the neurons themselves [41,43]. These findings demonstrate that the growth and transmission pools are spatially distinct. Interestingly, they are also temporally separable. While an early, transient pulse of BMP signaling is sufficient for NMJ growth, sustained BMP signaling is required for proper glutamate release [42]. Collectively, these findings argue that an early retrograde signal promotes NMJ growth and ongoing autocrine BMP signaling is required for normal baseline neurotransmitter release (Fig. 4). The temporal relationship between an early retrograde cue and a sustained autocrine signal is reminiscent of studies on mammalian systems (Fig. 2). It will be important to establish whether retrograde pro-growth signaling contributes to the activation of autocrine pro-transmission signaling at the NMJ.
Figure 4.
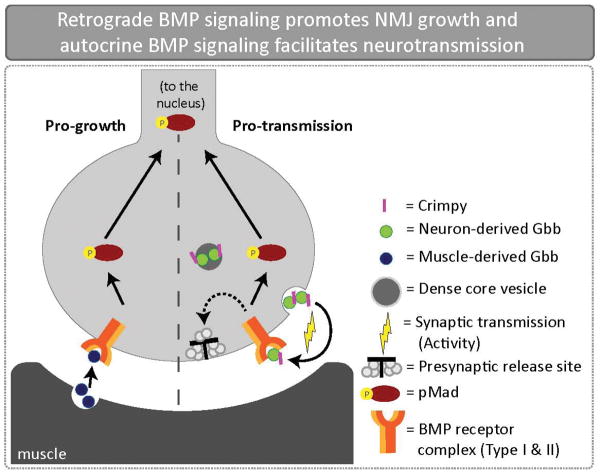
Retrograde BMP signaling promotes NMJ growth and activity-dependent autocrine BMP signaling facilitates neurotransmission in Drosophila. The BMP ligand Gbb is expressed in both the presynaptic motoneuron and postsynaptic muscle. Genetic manipulations of Gbb expression demonstrate that presynaptic Gbb facilitates neural transmission while postsynaptic Gbb regulates NMJ size. Hence, there are two separable pools of Gbb. Crimpy, a single-pass transmembrane protein, physically interacts with neuronal Gbb and traffics delivers it to DCVs for activity-dependent release.
While retrograde BMP signaling has been intensively studied, the pre-synaptic ligand pool is less well described. Insight into trafficking and release of pre-synaptic BMP came from analysis of Crimpy, a cargo receptor for Gbb in motoneurons [44,45]. Crimpy is a transmembrane protein that binds Gbb and delivers it to dense core vesicles (DCVs) for activity-dependent release from presynaptic terminals. Consistent with Crimpy-mediated Gbb localization to DCVs, Gbb release from presynaptic terminals depends on both synaptic activity and Crimpy [45]. Supporting a role for autocrine BMP signaling in neurotransmitter release, selective loss of the presynaptic ligand disrupts Ca2+ channel localization and synaptic vesicle clustering (Hoover and Broihier, unpublished). DCV exocytosis is known to require sustained Ca2+ increase in presynaptic terminals and occurs in response to prolonged activity [46]. Thus, Gbb release from DCVs represents an appealing mechanism to strengthen or reinforce highly active terminals.
These findings are consistent with studies reporting both DCV localization and activity-dependent release of other TGFβ family members [47,48]. Excitingly, they are also in line with recent studies demonstrating that temporal dynamics of BMP/TGFβ activation are critical for signaling. Specifically, pulsed release of BMP/TGFβ is essential for high levels of signal transduction in multiple settings [49,50]. It will be critical to investigate if the timing of presynaptic BMP release is required to organize presynaptic terminals.
Functions for autocrine BMP signaling in Drosophila likely extend beyond the NMJ and include sensory neuron plasticity [51]. BMP signaling in primary nociceptive neurons is necessary for thermal allodynia, or increased sensitivity to an innocuous stimulus following UV-induced injury [52]. Notably, while loss of BMP signaling impairs nociceptive sensitization, BMP overexpression in nociceptors is sufficient to induce sensitization in uninjured animals, demonstrating that BMP is critical for this form of synaptic plasticity. Because both the BMP ligand and receptors are required in primary nociceptors, which have non-overlapping territories on the body wall, BMP signaling is proposed to function in an autocrine manner [51]. It will be interesting to determine if this pathway is required for changes in synaptic strength between the nociceptive neurons and the first-order neurons in the nociceptive circuit [53]. Together, these studies argue that autocrine BMP signaling regulates synaptic development and plasticity in motor and sensory neurons in Drosophila. Interestingly, an autocrine TGFβ pathway has been recently reported to control axon and dendrite growth in midbrain dopaminergic neurons [54], indicating that autocrine mechanisms of BMP/TGFβ superfamily pathways are evolutionarily conserved.
Conclusions
Defining signal-sending and signal-receiving cells has been complicated by the widespread expression profiles of conserved signaling cues as well as the pleiotropic nature of their loss-of-function phenotypes. Advances in analyzing growth factor localization and release as well as techniques for genetic mosaic analysis have recently enabled the dissection of signaling pathway requirements in vivo. While cell non-autonomous paracrine signaling is no doubt pervasive, emerging evidence indicates that cell-autonomous autocrine signaling also serves widespread neuronal functions. Specifically, autocrine systems regulate axon and dendrite development and maintenance as well as synapse structure, function, and plasticity. In general, it appears that autocrine signaling loops are prevalent at synapses where activity-dependent autocrine signaling may permit highly focal, rapid signaling in response to Ca2+ influx. In addition, bursts of paracrine signaling may frequently activate sustained autocrine signaling loops in order to strengthen or maintain the initial intercellular cue. As additional autocrine signals are identified, it will be interesting to determine if they lend further support to these ideas and whether novel themes emerge.
Highlights.
Activity-dependent autocrine signals play critical roles in synaptic plasticity
Transient retrograde signals can activate sustained autocrine signals
Drosophila is an ideal model system to investigate mechanisms of autocrine signaling
Acknowledgments
Work in the Broihier lab is supported by NIH RO1 NS095895. The authors thank Pola Philippidou and members of the Broihier lab for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 2.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Poo M-M. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 4.Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992;9:139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- 5.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 6.Davies AM, Wright EM. Neurotrophic factors. Neurotrophin autocrine loops. Curr Biol. 1995;5:723–726. doi: 10.1016/s0960-9822(95)00144-8. [DOI] [PubMed] [Google Scholar]
- 7.Cheng P-L, Song A-H, Wong Y-H, Wang S, Zhang X, Poo M-M. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci US A. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampathkumar C, Wu Y-J, Vadhvani M, Trimbuch T, Eickholt B, Rosenmund C. Loss of MeCP2 disrupts cell autonomous and autocrine BDNF signaling in mouse glutamatergic neurons. Elife. 2016;5:214. doi: 10.7554/eLife.19374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 10.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci US A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **13.Wang L, Chang X, She L, Xu D, Huang W, Poo M-M. Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci. 2015;35:8384–8393. doi: 10.1523/JNEUROSCI.4682-14.2015. Generation of mosaic animals through retrovirus-mediated Cre recombination in adult BDNFfl/fl mice to sparsely knockout BDNF permitted analysis of autocrine BDNF signaling in adult-born granule cells. Importantly, this study demonstrated that TrkB is activated in an autocrine manner to promote dendrite growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobilo T, Liu Q-R, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafenêtre P, Leske O, Ma-Högemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R. Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci. 2010;3:34. doi: 10.3389/neuro.08.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottmann K, Mittmann T, Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- 17.Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka J-I, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GCR, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelmann E, Cepeda-Prado E, Franck M, Lichtenecker P, Brigadski T, Lessmann V. Theta Burst Firing Recruits BDNF Release and Signaling in Postsynaptic CA1 Neurons in Spike-Timing-Dependent LTP. Neuron. 2015;86:1041–1054. doi: 10.1016/j.neuron.2015.04.007. [DOI] [PubMed] [Google Scholar]
- **20.Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature. 2016;538:99–103. doi: 10.1038/nature19766. Using an innovative combination of two-photon microscopy and fluorescent reporters, this paper visualized BDNF release and TrkB activation in single dendritic spines during plasticity. This approach revealed that BDNF is rapidly released from a stimulated spine and activates TrkB receptors on the same spine from which it originated. This autocrine BDNF signaling in dendritic spines is responsible for both structural and functional plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature. 2016;538:104–108. doi: 10.1038/nature19784. The specific role of autocrine BDNF signaling in RhoGTPase activation was evaluated. Using FRET-based sensors to monitor RhoGTPase activation in BDNF mosaic mice, cell-autonomous BDNF signaling was shown to be required for Rac1 and Cdc42 activation but not for activation of RhoA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 24.Hedrick NG, Yasuda R. Regulation of Rho GTPase proteins during spine structural plasticity for the control of local dendritic plasticity. Curr Opin Neurobiol. 2017;45:193–201. doi: 10.1016/j.conb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R, Gundelfinger ED, Kojima M, Nestel S, Frotscher M, et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol. 2012;196:775–788. doi: 10.1083/jcb.201201038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamburger V. The history of the discovery of the nerve growth factor. J Neurobiol. 1993;24:893–897. doi: 10.1002/neu.480240702. [DOI] [PubMed] [Google Scholar]
- 27.Bodmer D, Levine-Wilkinson S, Richmond A, Hirsh S, Kuruvilla R. Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J Neurosci. 2009;29:7569–7581. doi: 10.1523/JNEUROSCI.1445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu YK, Collins SE, Ho H-YH, Zhao H, Kuruvilla R. An autocrine Wnt5a-Ror signaling loop mediates sympathetic target innervation. Dev Biol. 2013;377:79–89. doi: 10.1016/j.ydbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.McWilliams TG, Howard L, Wyatt S, Davies AM. Regulation of Autocrine Signaling in Subsets of Sympathetic Neurons Has Regional Effects on Tissue Innervation. Cell Rep. 2015;10:1443–1449. doi: 10.1016/j.celrep.2015.02.016. NGF negatively regulates CD40/CD40L levels in sympathetic neurons during axon outgrowth. In tissues where NGF expression is low, CD40/CD40L is expressed and promotes axon growth and target innervation via an autocrine mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci. 2004;24:743–751. doi: 10.1523/JNEUROSCI.4523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho H-YH, Susman MW, Bikoff JB, Ryu YK, Jonas AM, Hu L, Kuruvilla R, Greenberg ME. Wnt5a-Ror-Dishevelled signaling constitutes a core developmental pathway that controls tissue morphogenesis. Proc Natl Acad Sci US A. 2012;109:4044–4051. doi: 10.1073/pnas.1200421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Chen C-M, Orefice LL, Chiu S-L, LeGates TA, Hattar S, Huganir RL, Zhao H, Xu B, Kuruvilla R. Wnt5a is essential for hippocampal dendritic maintenance and spatial learning and memory in adult mice. Proc Natl Acad Sci US A. 2017;114:E619–E628. doi: 10.1073/pnas.1615792114. Wnt5a knockout mice exhibit decreased synaptic plasticity, attrition of CA1 dendritic arbors, and spatial learning and memory deficits that progress with age. Genetic mosaics were utilized to rescue Wnt5a expression in CA1 neurons in CaMKII-Wnt5afl/fl mice. Remarkably, restoration of Wnt5a expression in CA1 in 3-month-old mice rescued dendritic architecture. Thus, autocrine Wnt5a signaling in CA1 pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–787. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terauchi A, Timmons KM, Kikuma K, Pechmann Y, Kneussel M, Umemori H. Selective synaptic targeting of the excitatory and inhibitory presynaptic organizers FGF22 and FGF7. J Cell Sci. 2015;128:281–292. doi: 10.1242/jcs.158337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **35.Terauchi A, Johnson-Venkatesh EM, Bullock B, Lehtinen MK, Umemori H. Retrograde fibroblast growth factor 22 (FGF22) signaling regulates insulin-like growth factor 2 (IGF2) expression for activity-dependent synapse stabilization in the mammalian brain. Elife. 2016;5:545. doi: 10.7554/eLife.12151. Target-derived FGF22 is initially required for synapse assembly and induces IGF2 expression in the presynaptic cell. IGF2 transport to the terminal is activity-dependent and is necessary for synapse stabilization. IGF2 is released and acts in an autocrine manner through IGF receptors to promote synapse stabilization. This IGF2 autocrine loop that is stage and cell-type specific. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, D’Onofrio P, Choi B, Ellabban A, Nickerson PEB, et al. Exosomes Mediate Mobilization of Autocrine Wnt10b to Promote Axonal Regeneration in the Injured CNS. Cell Rep. 2017;20:99–111. doi: 10.1016/j.celrep.2017.06.009. Target-derived FGF22 is initially required for synapse assembly and induces IGF2 expression in the presynaptic cell. IGF2 transport to the terminal is activity-dependent and is necessary for synapse stabilization. IGF2 is released and acts in an autocrine manner through IGF receptors to promote synapse stabilization. This IGF2 autocrine loop that is stage and cell-type specific. [DOI] [PubMed] [Google Scholar]
- 37.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayat V, Jaiswal M, Bellen HJ. The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr Opin Neurobiol. 2011;21:182–188. doi: 10.1016/j.conb.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong J, Zou H. BMP signaling in axon regeneration. Curr Opin Neurobiol. 2014;27:127–134. doi: 10.1016/j.conb.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yam PT, Charron F. Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol. 2013;23:965–973. doi: 10.1016/j.conb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 41.McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 42.Berke B, Wittnam J, McNeill E, Van Vactor DL, Keshishian H. Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity. J Neurosci. 2013;33:17937–17950. doi: 10.1523/JNEUROSCI.6075-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James RE, Broihier HT. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development. 2011;138:3273–3286. doi: 10.1242/dev.066142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.James RE, Hoover KM, Bulgari D, McLaughlin CN, Wilson CG, Wharton KA, Levitan ES, Broihier HT. Crimpy enables discrimination of presynaptic and postsynaptic pools of a BMP at the Drosophila neuromuscular junction. Dev Cell. 2014;31:586–598. doi: 10.1016/j.devcel.2014.10.006. BMP release occurs both pre- and postsynaptically at the Drosophila NMJ; yet, it was unclear how these ligand pools are discerned by the motoneuron. This paper demonstrates that presynaptic BMP is regulated by a novel sorting receptor Crimpy. Crimpy, in addition to activity, are required for presynaptic release of the BMP ligand, Gbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol. 2010;22:496–505. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redhai S, Hellberg JEEU, Wainwright M, Perera SW, Castellanos F, Kroeger B, Gandy C, Leiblich A, Corrigan L, Hilton T, et al. Regulation of Dense-Core Granule Replenishment by Autocrine BMP Signalling in Drosophila Secondary Cells. PLoS Genet. 2016;12:e1006366. doi: 10.1371/journal.pgen.1006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacmann A, Hess D, Gohla G, Roussa E, Krieglstein K. Activity-dependent release of transforming growth factor-beta in a neuronal network in vitro. Neuroscience. 2007;150:647–657. doi: 10.1016/j.neuroscience.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 49.Sorre B, Warmflash A, Brivanlou AH, Siggia ED. Encoding of temporal signals by the TGF-β pathway and implications for embryonic patterning. Dev Cell. 2014;30:334–342. doi: 10.1016/j.devcel.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahal GR, Pradhan SJ, Bates EA. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development. 2017;144:2771–2783. doi: 10.1242/dev.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Follansbee TL, Gjelsvik KJ, Brann CL, McParland AL, Longhurst CA, Galko MJ, Ganter GK. Drosophila Nociceptive Sensitization Requires BMP Signaling via the Canonical SMAD Pathway. J Neurosci. 2017;37:8524–8533. doi: 10.1523/JNEUROSCI.3458-16.2017. BMP release occurs both pre- and postsynaptically at the Drosophila NMJ; yet, it was unclear how these ligand pools are discerned by the motoneuron. This paper demonstrates that presynaptic BMP is regulated by a novel sorting receptor Crimpy. Crimpy, in addition to activity, are required for presynaptic release of the BMP ligand, Gbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohyama T, Schneider-Mizell CM, Fetter RD, Aleman JV, Franconville R, Rivera-Alba M, Mensh BD, Branson KM, Simpson JH, Truman JW, et al. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]
- 54.Luo SX, Timbang L, Kim J-I, Shang Y, Sandoval K, Tang AA, Whistler JL, Ding JB, Huang EJ. TGF-β Signaling in Dopaminergic Neurons Regulates Dendritic Growth, Excitatory-Inhibitory Synaptic Balance, and Reversal Learning. Cell Rep. 2016;17:3233–3245. doi: 10.1016/j.celrep.2016.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]