Abstract
Study Design
A prospective observational international study.
Objective
The aim of this study was to evaluate outcomes of decompressive surgery in patients with very severe Degenerative Cervical Myelopathy (DCM).
Summary of Background Data
Although decompressive surgery has been evidenced as a safe and effective approach for patients with myelopathic deficiencies, studies have suggested residual disability following treatment in patients with more severe disease presentation.
Methods
Postoperative outcomes of 60 patients with very severe DCM (modified Japanese Orthopaedic Association Score (mJOA) ≤8) were compared to outcomes of 188 patients with severe DCM (mJOA 9—11). Post-imputation follow-up rate was 93.1%. Unadjusted and adjusted analyses were performed using two-way repeated measures of co-variance.
Results
The two cohorts were similar in demographics, length of duration of myelopathy symptoms, source of stenosis and surgical approaches used to decompress the spine. The very severe and severe cohorts differed in preoperative Nurick grades (4.97 vs. 3.91, respectively, P < .0001) and Neck Disability Index (NDI) scores (45.20 vs. 56.21 respectively, P = .0006). There were no differences in Short Form 36 (SF-36v2) Physical (PCS) and Mental (MCS) Component Summary Scores. Both cohorts improved in mJOA, Nurick, NDI, and SF-36v2 PCS and MCS scores. Despite the substantial postoperative improvements, patients in both cohorts had considerable residual symptoms. Two-thirds of the patients in the very severe cohort had severe (mJOA ≤11) or moderate (mJOA ≤ 14) myelopathy symptoms at 24-months follow-up. Longer duration of disease was associated with poorer treatment response.
Conclusion
Decompressive surgery is effective in patients with very severe DCM; however, patients have significant residual symptoms and disability. The very severe subgroup (mJOA ≤8) of patients with DCM represents a distinct group of patients and their different clinical trajectory is important for clinicians and patients to recognize. Duration of symptoms negatively affects chances for recovery. Whenever possible, patients with DCM should be treated before developing very severe symptomatology.
Keywords: Degenerative cervical myelopathy, cervical spondylotic myelopathy, surgical treatment, severe myelopathy
Introduction
Degenerative Cervical Myelopathy (DCM), a degenerative disease of the cervical spine, is the most common cause of spinal dysfunction worldwide. [1,2]Common symptoms include numb or clumsy hands, difficulty walking, sensory changes of the lower extremities, and neck pain.[3] Decompressive surgery has been shown to be safe and effective in the spectrum of disease severity in patients with mild, moderate and severe neurologic impairment.[4],[5] However, studies have suggested residual disability following treatment in patients with more severe disease presentation.[4]
Occasionally, patients present with significant neurologic deficiencies and very severe disease symptoms. In these cases, decompressive surgery is the treatment of choice. Although studies have shown surgery to be safe and effective in the treatment of patients with DCM, there is a paucity in literature concerning the efficacy of surgery in patients with very severe myelopathy. Clinical outcomes among patients with very severe DCM have only been specifically reported in small studies or included without differentiation in larger cohorts. Scardio et al. reported on nine patients with a Nurick grade of 5 or more.[6] Fehlings et al. described outcomes of 83 patients with modified Japanese Orthopaedic Association Score (mJOA) of 11 or less.[4] Acharya et al. described outcomes in 21 patients with Nurick grade 3 or more.[7] Fehlings et al. and Acharya et al. did not differentiate outcomes in patients with very severe preoperative symptomatology.[5,7] The aim of this study was to evaluate and present the outcomes of decompressive surgery in patients with very severe DCM (mJOA≤8) in comparison to patients with severe DCM (mJOA≤11).
Materials and Methods
Patient Population
Data were obtained after a pre-planned merger of two prospective observational studies conducted under the same investigational protocol, the CSM-North America (CSM-NA) study (clinicaltrial.gov NCT00285337) and the CSM-International (CSM-I) study (clinicaltrial.gov NCT00565734). Between December 2005 and January 2011, 757 patients (278 in the CSM-NA and 479 in the CSM-I study) were enrolled at 26 sites.
Key inclusion criteria included age eighteen years or older, symptomatic DCM (secondary to either disc herniation, ossification of the posterior longitudinal ligament (OPLL), ligamentum flavum hypertrophy (HLF), spondylosis, subluxation, congenital stenosis, or a combination of these changes), objective cervical cord compression (determined by magnetic resonance imaging (MRI)), no prior surgical treatment for myelopathy, and the absence of symptomatic lumbar stenosis.
Patients with severe disease were those with preoperative modified Japanese Orthopaedic Association (mJOA) score of 11 or less.[8] We divided this group of patients into two cohorts, those with very severe disease (mJOA score 8 or less) and those with severe disease (mJOA score 9-11, inclusive).[9],[6] Of 254 patients in the sample, one patient died and five patients withdrew their consent prior to the first follow-up visit at six months and were excluded from the study. The follow-up rates among the remaining 248 patients were 86.3%, 82.45% and 76.47% at 6-, 12-, and 24- month follow-up visits, respectively.
Ethical approval was obtained from each site. All participating patients provided verbal and written informed consent.
Surgical Techniques
All participants underwent anterior and/or posterior surgical decompression of the cervical spine. The choice of surgical approach, number of levels decompressed, and the option for instrumentation was left to the discretion of the attending surgeon.
Data Collection and Quality Assurance
For each participant, data were collected on demographics, neurological presentation, medical history, causative pathology, surgical summary, severity, disability, and health-related quality of life. Data were obtained preoperatively and at 6-, 12-, and 24-months postoperatively using electronic case report forms (eCRFs). Adverse events and complications were documented using standardized forms with a predetermined list of anticipated complications as well as an “other” option. Adverse events were adjudicated by a panel of investigators and classified as either related to DCM, related to surgery, or unrelated to either. External monitors performed both on- and off-site monitoring to confirm compliance with study protocol and to ensure the data were authentic, accurate, and complete.
Outcome Measures
Validated functional assessment tools and quality of life questionnaires were used to evaluate preoperative and postoperative status. These included the modified Japanese Orthopaedic Association scale (mJOA),[10] Nurick scale,[11] Neck Disability Index (NDI),[12],[13] and the Short Form-36 version 2 (SF-36v2™).[14] The mJOA and Nurick scales are clinician-administered DCM-specific measures of impairment and disease severity, while the NDI and SF-36v2 are patient-reported questionnaires that evaluate functional disability and overall health status, respectively. The mJOA allocates points between 0 (worst) and 18 (best) based on motor dysfunction of the upper and lower extremities, sensory impairment of the upper extremity, and sphincter dysfunction. The Nurick scale is a six-grade ordinal scale primarily based on gait dysfunction, and ranges from 0 (best) to 5 (worst). The NDI, a modification of the Oswestry Disability Index, is a self-reported, disease-specific, and reliable measure of disability that evaluates performance in ten different categories, including personal care, sleep, and driving; this score ranges from 0 (best) to 100% (worst). The SF-36v2 is a widely-used health status survey that assesses physical component summary (PCS) and mental component summary (MCS); these scores were calculated using the 1998 U.S. norms. The minimum clinically important differences (MCID) have been established for the mJOA (1.1), SF-36v2 PCS (4.1), SF-36v2 MCS (5.7), and NDI (7.5) in a degenerative spine population but not for the Nurick grades.[12],[15],[16],[17]
Analysis
Differences in baseline characteristics and surgical details were compared between the two severity cohorts using t-test and the Fisher’s exact test for quantitative and qualitative variables, respectively. Missing follow-up scores were imputed by a combination of last value carry forward and last value carry backwards approaches. Baseline scores were not used in imputation. After imputation, data were available for 231 (93.1%) patients. Using the imputed data, patient outcomes at 6-, 12- and 24-month follow-up were compared between the severe and very severe cohorts using the general linear model procedure (GLM) available in SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) constructing a two-way repeated measure Analysis of Variance (r-ANOVA) and two-way repeated measures Analysis of Covariance (r-ANCOVA) models. The dependent variables were baseline scores and three (6-, 12-, 24-month) follow-up scores (mJOA, Nurick, NDI, SF-36v2 PCS, and SF36v2 MCS). The r-ANOVA included a repeated visit factor, a cohort factor (severe and very severe), and an interaction term between the visit and the cohort factors. The r-ANOVA is the unadjusted analysis. In the adjusted model (r-ANCOVA), the patient, disease severity, and surgical characteristics were controlled for, including gender, age, logarithm of duration of symptoms, smoking status, years of education, source(s) of stenosis (spondylosis, intervertebral disc, OPLL, HLF, congenital stenosis, subluxation), affected cervical level(s), comorbidities by body system (cardiovascular, respiratory, gastrointestinal, renal, endocrine, psychiatric, rheumatologic and neurological), duration of operation, and surgical approach. Only the covariates with a p-value of less than 0.20 were kept in the model. Post-hoc between-cohort comparisons were adjusted for multiplicity using the Bonferroni correction. The study had 99% power to detect a difference of 1.1 (MCID) and 80% power to detect difference of 0.7 in the mJOA between the two cohorts based on an observed standard deviation (SD) of 1.684. An unadjusted sensitivity analysis was performed using non-imputed data.
All statistical analyses were performed using SAS/STAT version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographics and Patient Characteristics
There were 188 patients in the severe cohort with baseline mJOA score between 9 and 11, and 60 patients in the very severe cohort with baseline mJOA score of 8 or less. The two cohorts were similar in demographics, duration of myelopathy symptoms, and disease characteristics (Table 1). Surgical approaches used to decompress the spine were similar between the two cohorts. The very severe patient cohort had more affected levels compared to the severe patient cohort (4.30 vs. 3.94, P = 0.0671) and longer duration of hospital stay (14.43 vs. 9.66 days, P = 0.0097).
Table 1.
Baseline Demographics by Severity Group.
| mJOA 9-11 (N=188) | mJOA <=8 (N=60) | P Value | ||
|---|---|---|---|---|
| Gender | Female | 73 (38.83%) | 24 (40.00%) | 0.8803 |
| Age (years) | 59.39 (11.83) | 62.48 (12.26) | 0.0816 | |
| Education | No education | 12 (6.38%) | 5 (8.33%) | 0.7849 |
| < 4 years | 12 (6.38%) | 2 (3.33%) | ||
| 4 to 8 years | 49 (26.06%) | 13 (21.67%) | ||
| 9 to 12 years | 60 (31.91%) | 19 (31.67%) | ||
| 13 years and more | 55 (29.26%) | 21 (35.00%) | ||
| Race | White or Caucasian | 131 (69.68%) | 42 (70.00%) | 0.6784 |
| Black or African | 11 (5.85%) | 6 (10.00%) | ||
| East Asian | 30 (15.96%) | 8 (13.33%) | ||
| Other | 16 (8.51%) | 4 (6.67%) | ||
| Region | Latin America | 24 (12.77%) | 6 (10.00%) | 0.3828 |
| Europe | 21 (11.17%) | 12 (20.00%) | ||
| Asia Pacific | 47 (25.00%) | 13 (21.67%) | ||
| North America | 96 (51.06%) | 29 (48.33%) | ||
| Current Smoker | Yes | 57 (30.32%) | 13 (21.67%) | 0.2489 |
| Comorbidities | ||||
| Any comorbidity | 126 (67.02%) | 43 (71.67%) | 0.5292 | |
| Cardiovascular | 106 (56.38%) | 33 (55.00%) | 0.8820 | |
| Respiratory | 23 (12.23%) | 5 (8.33%) | 0.4890 | |
| Gastroenterologic | 27 (14.36%) | 8 (13.33%) | 1.0000 | |
| Renal | 6 (3.19%) | 5 (8.33%) | 0.1413 | |
| Endocrinologic | 43 (22.87%) | 12 (20.00%) | 0.7231 | |
| Psychiatric | 24 (12.77%) | 5 (8.33%) | 0.4894 | |
| Rheumatologic | 12 (6.38%) | 6 (10.00%) | 0.3917 | |
| Neurologic | 14 (7.45%) | 7 (11.67%) | 0.2974 | |
| Mean Duration of Symptoms (Std. Deviation) | Months | 24.51 (28.37) | 21.63 (37.11) | 0.5832 |
| Source of Stenosis | ||||
| -Spondylosis | 152 (80.85%) | 48 (80.00%) | 0.8533 | |
| -Disk | 131 (69.68%) | 42 (70.00%) | 1.0000 | |
| -Ossification of the Ligament | 45 (23.94%) | 12 (20.00%) | 0.5997 | |
| -Ligament Flavum | 51 (27.13%) | 20 (33.33%) | 0.4124 | |
| -Subluxation | 14 (7.45%) | 3 (5.00%) | 0.7696 | |
| -Congenital Stenosis | 16 (8.51%) | 7 (11.67%) | 0.4510 | |
| -Other | 2 (1.06%) | 3 (5.00%) | 0.0931 | |
| Surgical Approach | Anterior Only | 90 (47.87%) | 24 (40.00%) | 0.5244 |
| Posterior Only | 88 (46.81%) | 32 (53.33%) | ||
| Circumferential (both posterior and anterior) | 10 (5.32%) | 4 (6.67%) | ||
| Posterior Approach | ||||
| Laminoplasty | 34 (18.09%) | 14 (23.33%) | 0.8237 | |
| Laminectomy without Fusion | 8 (4.26%) | 2 (3.33%) | ||
| Laminectomy with Fusion | 56 (29.79%) | 19 (31.67%) | ||
| Mean Levels Operated (Std. Deviation) | Number of Levels | 3.94 (1.31) | 4.30 (1.42) | 0.0671 |
| Operated Level | ||||
| C1 | 6 (3.19%) | 4 (6.67%) | 0.2608 | |
| C2 | 29 (15.43%) | 17 (28.33%) | 0.0349 | |
| C3 | 126 (67.02%) | 42 (70.00%) | 0.7519 | |
| C4 | 152 (80.85%) | 50 (83.33%) | 0.8488 | |
| C5 | 172 (91.49%) | 58 (96.67%) | 0.2555 | |
| C6 | 159 (84.57%) | 52 (86.67%) | 0.8359 | |
| C7 | 96 (51.06%) | 35 (58.33%) | 0.3739 | |
| Duration of Surgery | Minutes | 193.30 (91.41) | 203.82 (89.00) | 0.4356 |
| Length of Hospitalization | Days | 9.66 (9.46) | 14.43 (12.87) | 0.0097 |
Baseline Status
Preoperatively, patients with very severe disease had worse clinical (mJOA and Nurick) and functional status measured by NDI. The average mJOA in the very severe cohort was 6.93 (SD = 1.30, range from3-8); the average mJOA in the severe cohort was 10.22 (SD = 0.77, range from 9-11). There were no differences in health-related quality of life status measured by SF-36v2 PCS and MCS between the cohorts (Table 2).
Table 2.
Baseline Clinical, Functional, and Quality of Life Status.
| Outcome | mJOA 9-11 (N=188) | mJOA <=8 (N=60) | P Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| mJOA | 10.22 | 0.77 | 6.93 | 1.30 | <.0001 |
| Nurick | 3.91 | 0.87 | 4.97 | 1.07 | <.0001 |
| NDI | 45.20 | 20.99 | 56.21 | 19.27 | 0.0006 |
| SF36v2 PCS | 30.54 | 7.84 | 28.96 | 7.61 | 0.1756 |
| SF36v2 MCS | 36.67 | 13.30 | 35.75 | 14.84 | 0.6516 |
Clinical and Patient Outcomes
The mJOA outcomes improved after surgery for both subgroups (P <.0001). The bulk of improvement was obtained by six months postoperatively. While further nominal improvement occurred after six months, it was not significant. (Table 3, Figure 1a). The extent of improvement was larger in the very severe cohort compared to the severe cohort (Follow-up visit*Group Interaction <.0001). However, despite a greater improvement, the average score in very severe patients remained smaller compared to severe patients at all follow-ups. The adjusted analysis confirmed the same pattern (Table 4). Patients with a longer duration of symptoms had poorer treatment response compared to those with a shorter duration of symptoms (P = 0.0059). Further, the effect of duration of symptoms was more pronounced in patients with very severe disease compared to those with severe disease.
Table 3.
Patient Outcomes by Baseline Severity Group.
| mJOA 9-11 | mJOA <=8 | Difference | Difference | Visit | Group | Visit*Group | ||
|---|---|---|---|---|---|---|---|---|
| Mean (LCL, UCL) | Mean (LCL, UCL) | Mean (LCL, UCL) | ||||||
| mJOA | <.0001 | <.0001 | <.0001 | |||||
| Pre-operative | 10.20 (10.06, 10.33) | 6.83 (6.58, 7.08) | 3.37 (3.08, 3.65) | <.0001 | ||||
| 6 months | 13.33 (12.91, 13.74) | 12.21 (11.44, 12.97) | 1.12 (0.25, 1.99) | 0.0120 | ||||
| 12 months | 13.80 (13.36, 14.25) | 12.70 (11.88, 13.51) | 1.11 (0.18, 2.03) | 0.0197 | ||||
| 24 months | 13.92 (13.46, 14.38) | 12.96 (12.12, 13.80) | 0.95 (−0.01, 1.91) | 0.0514 | ||||
| Nurick | <.0001 | 0.0006 | 0.0256 | |||||
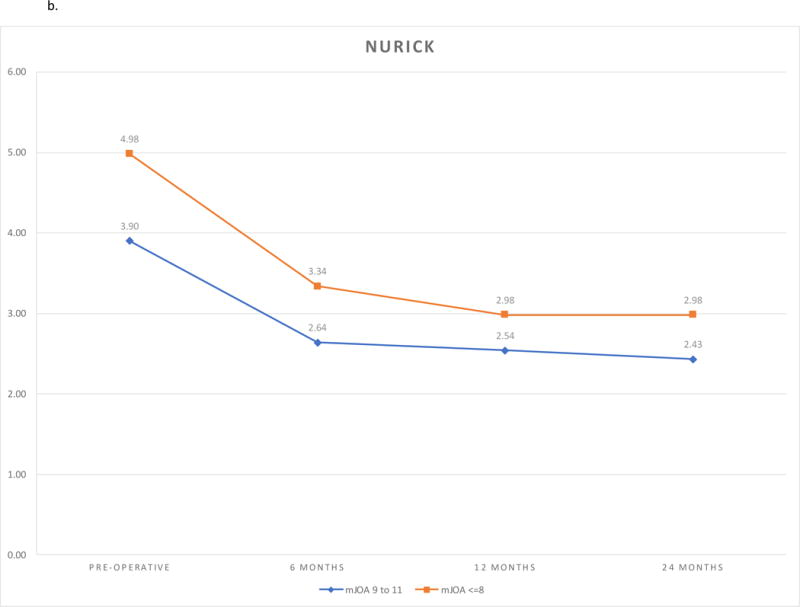
| Pre-operative | 3.90 (3.76, 4.04) | 4.98 (4.73, 5.23) | −1.08 (−1.37, −0.80) | <.0001 | ||||
| 6 months | 2.64 (2.40, 2.88) | 3.34 (2.90, 3.78) | −0.70 (−1.20, −0.20) | 0.0066 | ||||
| 12 months | 2.54 (2.29, 2.79) | 2.98 (2.52, 3.44) | −0.44 (−0.97, 0.09) | 0.1005 | ||||
| 24 months | 2.43 (2.17, 2.69) | 2.98 (2.50, 3.46) | −0.55 (−1.10, 0.00) | 0.0493 | ||||
| NDI | <.0001 | 0.1346 | 0.0243 | |||||
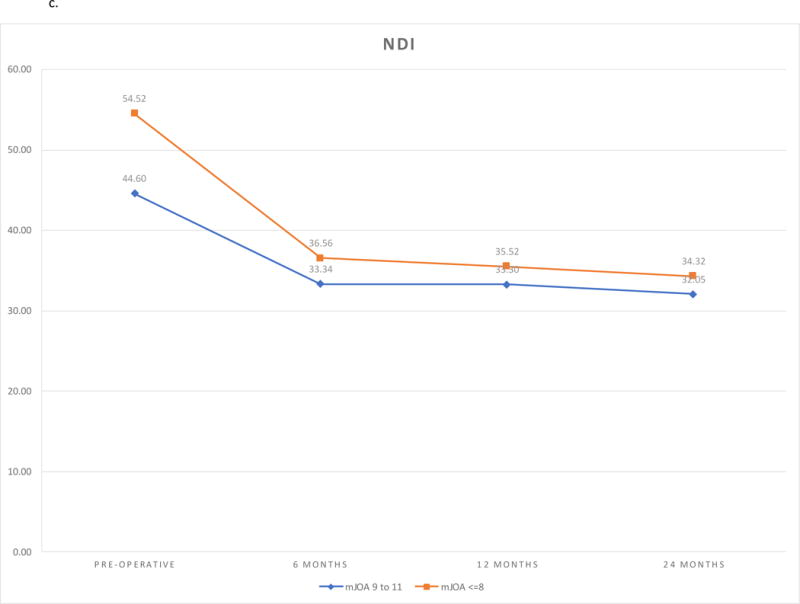
| Pre-operative | 44.60 (41.38, 47.83) | 54.52 (48.77, 60.27) | −9.92 (−16.51, −3.32) | 0.0034 | ||||
| 6 months | 33.34 (30.14, 36.54) | 36.56 (30.85, 42.27) | −3.22 (−9.76, 3.32) | 0.3328 | ||||
| 12 months | 33.30 (29.91, 36.70) | 35.52 (29.46, 41.58) | −2.22 (−9.16, 4.72) | 0.5294 | ||||
| 24 months | 32.05 (28.59, 35.51) | 34.32 (28.16, 40.48) | −2.27 (−9.34, 4.80) | 0.5273 | ||||
| SF36v2 PCS | <.0001 | 0.4691 | 0.6777 | |||||
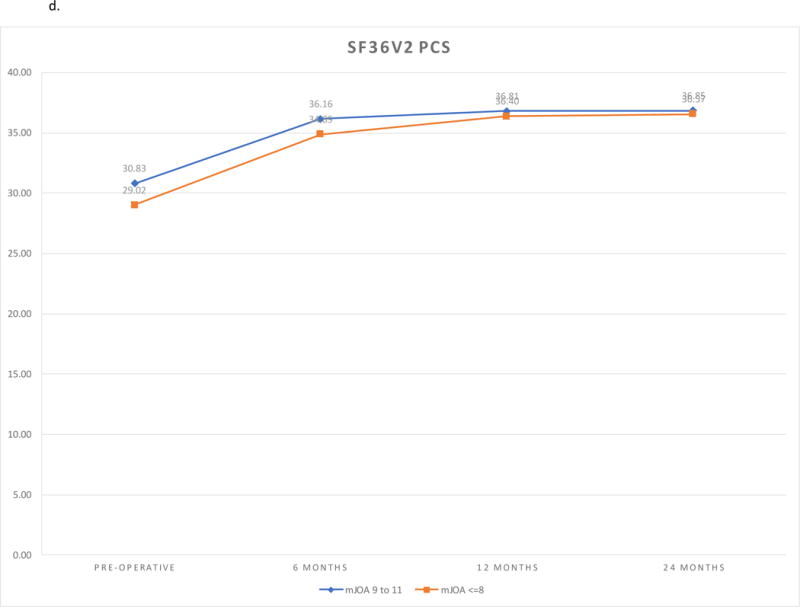
| Pre-operative | 30.83 (29.66, 32.00) | 29.02 (26.86, 31.17) | 1.81 (−0.63, 4.26) | 0.1456 | ||||
| 6 months | 36.16 (34.66, 37.67) | 34.89 (32.11, 37.67) | 1.27 (−1.89, 4.43) | 0.4277 | ||||
| 12 months | 36.81 (35.22, 38.41) | 36.40 (33.46, 39.34) | 0.41 (−2.94, 3.76) | 0.8088 | ||||
| 24 months | 36.85 (35.24, 38.45) | 36.57 (33.61, 39.53) | 0.28 (−3.09, 3.65) | 0.8717 | ||||
| SF36v2 MCS | <.0001 | 0.4793 | 0.6465 | |||||
| Pre-operative | 36.92 (34.88, 38.96) | 36.95 (33.19, 40.71) | −0.031 (−4.307, 4.245) | 0.9886 | ||||
| 6 months | 43.85 (41.78, 45.92) | 45.91 (42.09, 49.73) | −2.06 (−6.406, 2.285) | 0.3511 | ||||
| 12 months | 44.54 (42.43, 46.64) | 45.39 (41.51, 49.27) | −0.852 (−5.268, 3.564) | 0.7041 | ||||
| 24 months | 43.77 (41.69, 45.84) | 46.02 (42.20, 49.85) | −2.259 (−6.606, 2.089) | 0.3070 |
Figure 1.
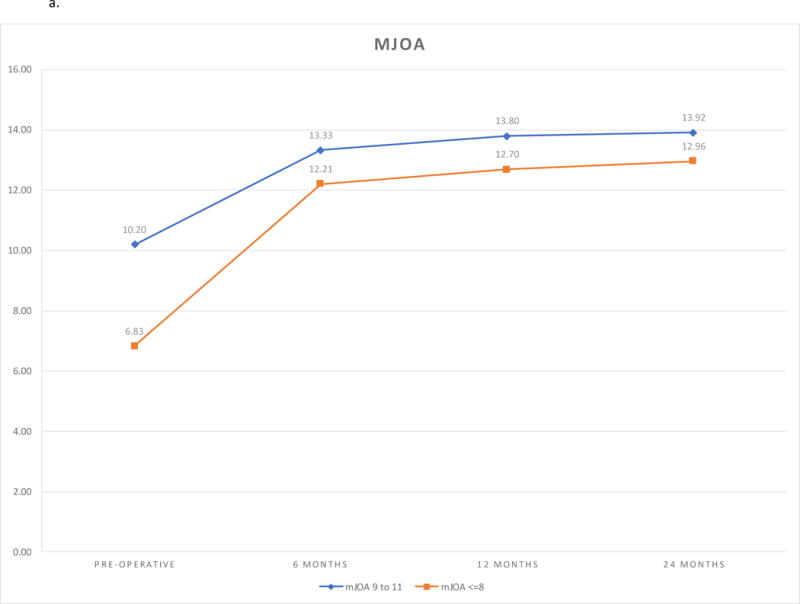
a. mJOA Outcomes by Follow-up and Severity Group.
b. Nurick Outcomes by Follow-up and Severity Group.
c. Neck Disability Index Outcomes by Follow-up and Severity Group.
d. SF36v2 PCS Outcomes by Follow-up and Severity Group.
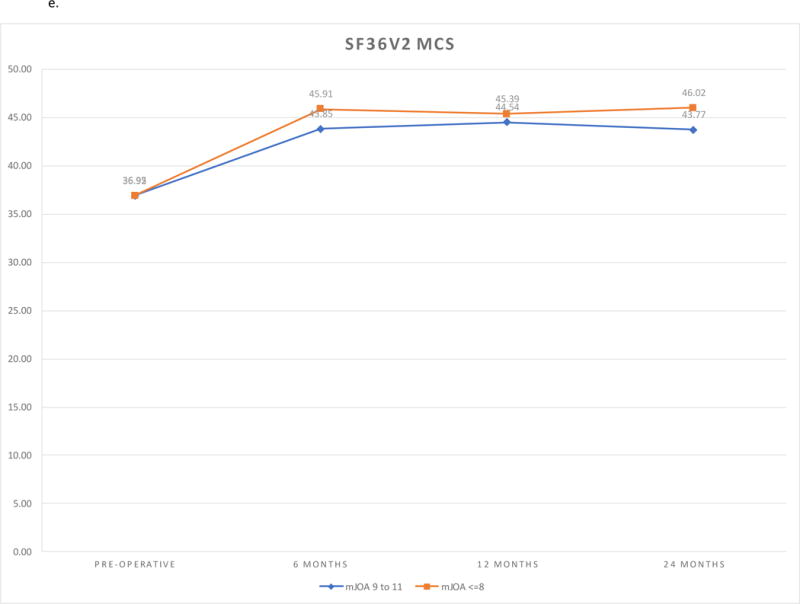
e. SF36v2 MCS Outcomes by Follow-up and Severity Group.
Table 4.
Patient Outcomes by Baseline Severity Group (adjusted).
| mJOA 9-11 | mJOA <=8 | Difference | Difference | Visit | Group | Visit*Group | ||
|---|---|---|---|---|---|---|---|---|
| Mean (LCL, UCL) | Mean (LCL, UCL) | Mean (LCL, UCL) | ||||||
| mJOA | <.0001 | <.0001 | <.0001 | |||||
| Pre-operative | 10.23 (9.56, 10.89) | 6.87 (6.19, 7.56) | 3.35 (3.05, 3.65) | <.0001 | ||||
| 6 months | 11.09 (9.16, 13.02) | 9.91 (7.93, 11.89) | 1.18 (0.31, 2.04) | 0.0081 | ||||
| 12 months | 11.75 (9.69, 13.81) | 10.56 (8.44, 12.67) | 1.19 (0.27, 2.12) | 0.0119 | ||||
| 24 months | 12.65 (10.49, 14.81) | 11.64 (9.43, 13.85) | 1.01 (0.04, 1.98) | 0.0419 | ||||
| Nurick | <.0001 | 0.0012 | 0.0245 | |||||
| Pre-operative | 3.97 (3.57, 4.36) | 5.03 (4.58, 5.48) | −1.06 (−1.35, −0.77) | <.0001 | ||||
| 6 months | 2.79 (2.10, 3.48) | 3.45 (2.67, 4.23) | −0.66 (−1.17, −0.16) | 0.0106 | ||||
| 12 months | 2.35 (1.63, 3.07) | 2.77 (1.96, 3.59) | −0.42 (−0.95, 0.11) | 0.1174 | ||||
| 24 months | 2.28 (1.53, 3.03) | 2.77 (1.92, 3.62) | −0.49 (−1.04, 0.06) | 0.0826 | ||||
| NDI | 0.0195 | 0.0595 | 0.1592 | |||||
| Pre-operative | 56.85 (48.37, 65.33) | 57.04 (66.32, 75.61) | −9.47 (−16.02, −2.92) | 0.0048 | ||||
| 6 months | 42.69 (34.43, 50.94) | 37.77 (46.81, 55.85) | −4.13 (−10.50, 2.25) | 0.2034 | ||||
| 12 months | 40.14 (31.34, 48.94) | 34.42 (44.06, 53.69) | −3.92 (−10.72, 2.88) | 0.2569 | ||||
| 24 months | 39.30 (30.60, 48.00) | 33.66 (43.19, 52.72) | −3.88 (−10.60, 2.84) | 0.2562 | ||||
| SF36v2 PCS | <.0001 | 0.0740 | 0.9718 | |||||
| Pre-operative | 29.02 (26.53, 31.51) | 26.70 (23.43, 29.96) | 2.32 (−0.14, 4.78) | 0.0643 | ||||
| 6 months | 33.01 (29.83, 36.19) | 30.36 (26.19, 34.53) | 2.65 (−0.49, 5.80) | 0.0972 | ||||
| 12 months | 32.47 (29.13, 35.82) | 30.45 (26.06, 34.85) | 2.02 (−1.29, 5.33) | 0.2300 | ||||
| 24 months | 31.77 (28.49, 35.06) | 29.71 (25.39, 34.03) | 2.07 (−1.18, 5.32) | 0.2116 | ||||
| SF36v2 MCS | 0.0001 | 0.4733 | 0.6054 | |||||
| Pre-operative | 33.07 (29.23, 36.91) | 32.93 (28.03, 37.82) | 0.15 (−4.08, 4.37) | 0.946 | ||||
| 6 months | 39.51 (35.60, 43.43) | 41.56 (36.57, 46.55) | −2.04 (−6.35, 2.26) | 0.3508 | ||||
| 12 months | 39.29 (35.33, 43.25) | 40.25 (35.20, 45.30) | −0.97 (−5.32, 3.39) | 0.6627 | ||||
| 24 months | 37.42 (33.59, 41.25) | 39.70 (34.82, 44.59) | −2.28 (−6.49, 1.94) | 0.288 |
The Nurick grades improved after surgery (P <.0001). Most improvement was obtained by twelve months postoperatively. (Table 3, Figure 1b). The extent of improvement was greater in the very severe cohort compared to the severe cohort (Follow-up Visit*Group Interaction P = 0.0256). At 24-months follow-up, Nurick grade was better in the severe cohort compared to the very severe cohort in the unadjusted analysis, but not in the adjusted analysis (Table 4).
At baseline, functional limitations measured by NDI scores were worse in the very severe cohort compared to the severe cohort (P = 0.0034). NDI scores improved after surgery in both cohorts (<.0001). The extent of improvement was greater in the very severe cohort compared to the severe cohort (Follow-up Visit*Cohort Interaction P = 0.0243). There were no differences in the score between the cohorts at any follow-up. (Figure 1c)
SF36v2 PCS scores and SF36v2 MCS scores improved in both cohorts (P <.0001). There were no differences between the cohorts at baseline or any of the follow-up visits. The findings remained the same after adjustment for covariates. Sensitivity analysis using non-imputed data did not differ from the findings of the imputed analysis. (Figures 1d, 1e)
At 24-months follow-up, 35.85% patients in the very severe cohort had mild disease severity, 30.19% had moderate disease severity, and 33.96% still had severe disease. For the patients in the severe disease cohort, 44.38%, 33.71% and 21.91% had mild, moderate and severe symptoms at 24-months follow-up, respectively (P = 0.2177).
In the very severe cohort, one patient (1.89%) had worsening symptoms and one patient (1.89%) did not improve. The remaining 51 patients (96.23%) improved for at least one point on the mJOA scale (range 1-14 points). Of those, 6 patients (11.32%) were symptom-free. In the severe cohort, 14 (7.87%) experienced worsening symptoms, 11 (6.18%) remained the same, and the remaining 153 patients (85.96%) improved between 1-9 points. Of those, 23 (12.92%) were symptom-free.
Complications
Six patients (3.19%) from the severe cohort and 3 patients (5.0%) from the very severe cohort required subsequent surgery (P = 0.4552). Fifty patients (26.60%) from the severe cohort and 12 (20%) patients in the very severe cohort had one or more complications. The most common complications were dysphagia (6.05%) and superficial infection (3.7%). (Table 5).
Table 5.
Complications by Severity Group.
| mJOA 9-11 | mJOA <= 8 | |
|---|---|---|
| Pseudarthrosis | 3 | 0 |
| Hardware Failure | 2 | 1 |
| C5 Radiculopathy | 3 | 1 |
| Adjacent Segment Degeneration | 3 | 0 |
| Dural Tear | 7 | 0 |
| Deep Infection | 1 | 0 |
| DVT | 1 | 0 |
| Superficial Infection | 6 | 3 |
| Dysphagia | 13 | 2 |
| Dysphonia | 3 | 0 |
| New Radiculopathy {not C5} | 1 | 0 |
| Postoperative Kyphosis | 3 | 1 |
| Cardiopulmonary Event | 1 | 0 |
| Relevant Bleeding | 2 | 2 |
| Thromboembolism | 0 | 1 |
| Instrumentation Malposition/Migration | 4 | 1 |
| Neck/Arm Pain | 12 | 5 |
| Surgical Wound Problems {e.g. hematoma, dehiscence} | 2 | 0 |
| Other | 9 | 2 |
Discussion
Patients with very severe degenerative cervical myelopathy (mJOA≤8) benefit from surgical intervention. There were improvements in clinical, functional and health-related quality of life outcomes. These improvements are achieved at six months and sustained at two years, postoperatively. The extent of improvement in clinical and functional outcomes in patients with very severe disease exceeds the improvement in patients with severe disease. However, despite greater improvement, the follow-up mJOA and Nurick scores remained significantly lower in the very severe patients due to significantly lower pre-operative scores. Very severe patients attained similar NDI functional outcomes and SF-36v2 quality of life outcomes as did the patients with severe disease. While the extent of improvement is substantial, the patients with very severe disease endure significant residual symptoms and disability. Surgical treatment, while the most effective treatment modality, is not the cure of advanced DCM. In our study, at two years postoperative, approximately one third of the patients with very severe disease still had severe symptoms, one third had moderate symptoms, and one third had mild disease. Only about one in 10 patients were symptom-free. The average NDI was above 30 at all follow-ups. This underlines the challenges and limitations in the treatment of advanced DCM.
The very severe subcohort (mJOA≤8) of patients with DCM represents a distinct cohort of patients and their different clinical trajectory is important for clinicians and patients to recognize. The very severe patients utilize more treatment resources and have more residual symptomatology compared to severe patients. The economic impact of resource utilization and disability should be considered when prioritizing treatment.
Although there was a high rate of complications associated with surgical intervention, these appear to be transitory and with no significant impact on patient outcomes. There was no evidence of differences in complication rates between patients with very severe and those with severe disease.
There are important limitations to this study. First, this evaluation was based on a non-randomized comparison study. Although extensive statistical adjustments were performed, there may have been confounding covariates unaccounted for in our statistical models. However, the authors believe it to be unlikely that the unadjusted confounding may affect any of the major conclusions of this study. While the follow-up rate was high, outcome data from some patients were missing and were accounted for by imputation. A sensitivity analysis was performed to address this limitation.
In conclusion, decompressive surgery is effective in patients with very severe DCM, however, patients have significant residual symptoms and disability. Duration of symptoms negatively affects chances for recovery. Patients with DCM should be monitored accordingly and treated before developing very severe symptomatology.
Acknowledgments
The authors thank Tamara Kopjar for technical assistance in preparing this article. Michael Fehlings wishes to acknowledge the support of the Halbert Chair in Neural Repair and Regeneration.
A Clinical and Translational Science Award (CTSA) grant from the National Center for Advancing Translational Sciences (NCATS) awarded for Frontiers: The Heartland Institute for Clinical and Translational Research #TL1TR000120, AOSpine International, and AOSpine North America funds were received in support of this work.
Relevant financial activities outside the submitted work: consultancy, grants, stocks, patents, royalties, travel/accommodations/meeting expenses.
References
- 1.Karadimas SK, Erwin WM, Ely CG, Dettori JR, Fehlings MG. Pathophysiology and Natural History of Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2013;38:S21–36. doi: 10.1097/BRS.0b013e3182a7f2c3. [DOI] [PubMed] [Google Scholar]
- 2.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976) 2015;40:E675–93. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Tetreault LA, Massicotte EM, Arnold PM, Skelly AC, Brodt ED, et al. Differential Diagnosis for Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976) 2013;38:S78–88. doi: 10.1097/BRS.0b013e3182a7eb06. [DOI] [PubMed] [Google Scholar]
- 4.Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651–8. doi: 10.2106/JBJS.L.00589. [DOI] [PubMed] [Google Scholar]
- 5.Fehlings MG, Ibrahim A, Tetreault L, Albanese V, Alvarado M, Arnold P, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976) 2015;40:1322–8. doi: 10.1097/BRS.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 6.Scardino FB, Rocha LP, Barcelos ACES, Rotta JM, Botelho RV. Is there a benefit to operating on patients (bedridden or in wheelchairs) with advanced stage cervical spondylotic myelopathy? Eur Spine J. 2010;19:699–705. doi: 10.1007/s00586-009-1267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acharya S, Srivastava A, Virmani S, Tandon R. Resolution of Physical Signs and Recovery in Severe Cervical Spondylotic Myelopathy After Cervical Laminoplasty. Spine (Phila Pa 1976) 2010;35:E1083–7. doi: 10.1097/BRS.0b013e3181df1a8e. [DOI] [PubMed] [Google Scholar]
- 8.Tetreault L, Kopjar B, Nouri A, Arnold P, Barbagallo G, Bartels R, Qiang Z, Singh A, Zileli M, Vaccaro A, Fehlings MG. The modified Japanese Orthopaedic Association scale: establishing criteria for mild, moderate and severe impairment in patients with degenerative cervical myelopathy. Eur Spine J. 2017;26(1):78–84. doi: 10.1007/s00586-016-4660-8. [DOI] [PubMed] [Google Scholar]
- 9.Revanappa KK, Rajshekhar V. Comparison of Nurick grading system and modified Japanese Orthopaedic Association scoring system in evaluation of patients with cervical spondylotic myelopathy. Eur Spine J. 2011;20:1545–51. doi: 10.1007/s00586-011-1773-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–95. doi: 10.1097/00002517-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101–8. doi: 10.1093/brain/95.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Carreon LY, Glassman SD, Campbell MJ, Anderson PA. Neck Disability Index, short form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J. 2010;10:469–74. doi: 10.1016/j.spinee.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–15. [PubMed] [Google Scholar]
- 14.The MOS 36-Item Short-Form Health Survey (SF-36): II Psychometric and clinical tests of validity in measuring physical and mental health constructs. doi: 10.1097/00005650-199303000-00006. http://www-ncbi-nlm-nih-gov.offcampus.lib.washington.edu/pubmed?myncbishare=uwonline&term=8450681 (accessed December 17, 2015) [DOI] [PubMed]
- 15.Tetreault L, Nouri A, Kopjar B, Côté P, Fehlings MG. The Minimum Clinically Important Difference of the Modified Japanese Orthopaedic Association Scale in Patients with Degenerative Cervical Myelopathy. Spine (Phila Pa 1976) 2015;40:1653–9. doi: 10.1097/BRS.0000000000001127. [DOI] [PubMed] [Google Scholar]
- 16.Auffinger BM, Lall RR, Dahdaleh NS, Wong AP, Lam SK, Koski T, et al. Measuring surgical outcomes in cervical spondylotic myelopathy patients undergoing anterior cervical discectomy and fusion: assessment of minimum clinically important difference. PLoS One. 2013;8:e67408. doi: 10.1371/journal.pone.0067408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young BA, Walker MJ, Strunce JB, Boyles RE, Whitman JM, Childs JD. Responsiveness of the Neck Disability Index in patients with mechanical neck disorders. Spine J. 2009;9:802–8. doi: 10.1016/j.spinee.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Tetreault LA, Côté P, Kopjar B, Arnold P, Fehlings MG. A clinical prediction model to assess surgical outcome in patients with cervical spondylotic myelopathy: internal and external validations using the prospective multicenter AOSpine North American and international datasets of 743 patients. Spine J. 2015;15:388–97. doi: 10.1016/j.spinee.2014.12.145. [DOI] [PubMed] [Google Scholar]


