Abstract
Lysosomes perform degradative functions that are important for all cells. However, neurons are particularly dependent on optimal lysosome function due to their extremes of longevity, size and polarity. Axons in particular exemplify the major spatial challenges faced by neurons in the maintenance of lysosome biogenesis and function. What impact does this have on the regulation and functions of lysosomes in axons? This review focuses on the mechanisms whereby axonal lysosome biogenesis, transport and function are adapted to meet neuronal demand. Important features include the dynamic relationship between endosomes, autophagosomes and lysosomes as well as the transport mechanisms that support the movement of lysosome precursors in axons. A picture is emerging wherein intermediates in the lysosome maturation processes that would only exist transiently within the crowded confines of a neuronal cell body are spatially and temporally separated over the extreme distances encountered in axons. Axons may thus offer significant opportunities for the analysis of the mechanisms that control lysosome biogenesis. Insights from the genetics and pathology of human neurodegenerative diseases furthermore emphasize the importance of efficient axonal transport of lysosomes and their precursors.
Introduction
Due to their extremes in size, metabolism and longevity, there are many reasons to expect that neurons would be particularly dependent on the degradative housekeeping functions of lysosomes for the clearance of misfolded proteins and damaged organelles. The first characterizations of lysosome abundance and subcellular localization in neurons were performed in the early 1960s by histochemical methods based on the detection of lysosomal acid phosphatase activity [1]. These efforts detected lysosomes in all neurons and found them to be most prominently concentrated within neuronal cell bodies and proximal regions of dendrites but relatively rare in axons [1]. Shortly thereafter the first clues began to emerge that neuronal lysosome abundance and subcellular localization could be altered in response to neurological disease. More specifically, organelles with lysosome-like morphology were observed to accumulate within swollen neurites surrounding Alzheimer’s disease amyloid plaques in the first electron microscopy study of Alzheimer’s disease brain tissue [2]. The degradative lysosomal nature of such organelles was supported shortly thereafter by the confirmation that the organelles that ultrastructurally resembled lysosomes at amyloid plaques also contained acid phosphatase activity [3]. Other lysosomal enzymes such as cathepsins were also later identified at such sites [4]. Thus, while it has long been known that lysosomes are most abundant within the somatodendritic region of neurons [1,5,6], for nearly as long there have been indications that the subcellular distribution of neuronal lysosomes can change in response to disease states. Nonetheless, the modest steady state abundance of lysosomes in axons of healthy neurons long deterred major investigation of mechanisms controlling axonal lysosome abundance. However, this situation has been changing in recent years as new evidence has emerged concerning axonal lysosomes in health and disease.
Origins of Axonal Lysosomes
The low steady state abundance of lysosomes in axons could in principal arise from: 1) mechanisms that prevent the delivery of lysosomes into axons; and/or 2) the existence of efficient mechanisms that support their rapid transport out of axons. An early observation that pointed to the existence of an efficient clearance mechanism based on retrograde axonal transport came from ultrastructural characterization of the pattern of organelle accumulation following an acute local blockade of axonal transport [7]. While small (50–80 nm diameter) clear vesicles were the major cargo that accumulated on the proximal side of the blockade (reflecting material undergoing anterograde transport), the retrogradely transported material on the distal side of the blockade contained numerous organelles with a multivesicular and multilamellar morphology that is typical of late endosomes and/or lysosomes [7]. Collectively, these observations indicate that while there is not a strong axonal delivery of lysosomes from the cell body, there is a significant retrograde flux of lysosomes and/or closely related late endocytic and autophagosomal organelles that exceeds the minimal expectations drawn from steady state measurements.
Insightful dynamic imaging studies performed during the 1990s provided evidence for a process whereby endosomes and autophagosomes that form in the distal region of axons fuse with one another and become acidified by as much as 100 fold as they make their retrograde journey back towards the neuronal cell body [8,9]. This concept was later brought into sharper focus by the live imaging of autophagy proteins tagged with fluorescent proteins which allowed for the direct visualization of the ordered recruitment of specific proteins to growing autophagosomes in distal axons [8,10–13]. In contrast to other cellular contexts where regulation of autophagosome formation is tightly coupled to nutrient availability, autophagosomes form constitutively in axons, a property that appears to be conserved across diverse neuron subtypes and in multiple species [8,11–14]. Cargoes of these axonal autophagosomes included ubiquitinated proteins as well as mitochondrial fragments, thus supporting the idea that their physiological purpose is to clear aging and damaged proteins and organelles from the distal axon [10]. In addition to constitutive autophagy, acutely damaged axonal mitochondria can also trigger their own local engulfment into autophagosomes [15]. While the formation of an autophagosome serves to sequester material destined for degradation, the ultimate disposal of these autophagic cargos is dependent on the ability of autophagosomes to deliver their contents to lysosomes for degradation (Figure 1). Interestingly, rather than achieving such delivery to lysosomes in a single fusion step, axonal autophagosomes first fuse with endosomes to yield a hybrid organelle that is sometime referred to as an amphisome [8,10,16]. Autophagosome-endosome fusion triggers a series of changes. While axonal autophagosomes exhibit limited mobility, following fusion with endosomes, the hybrid organelle undergoes efficient retrograde movement, their lumen becomes progressively more acidified and lysosome markers such as LAMP1 begin to accumulate [8,10,16]. However, in contrast to lysosomes of neuronal cell bodies where levels of cathepsins (major lysosomal proteases) are high, cathepsin levels are much lower within the maturing lysosomes found in axons [17]. This relative deficiency in lysosomal proteases suggests that maximally efficient degradation of axonal lysosome cargos may not occur until arrival into the cell body region.
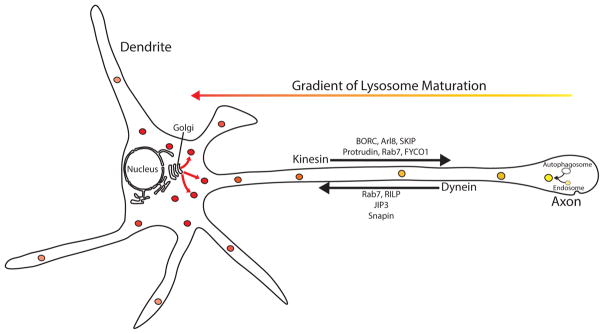
Figure 1.
Schematic diagram summarizing key concepts related to the process of axonal lysosome maturation. Following the fusion of autophagosomes with endosomes in the distal axon, the hybrid organelle begins a coordinated process of retrograde transport and maturation towards a lysosomal identity. Due to the restricted localization of the Golgi apparatus to the somatodendritic compartment, lysosomes within the distal axon are last in line to receive Golgi-derived vesicles containing newly synthesized lysosomal proteins.
Key Questions
The concept that endosomes and autophagosomes fuse within the distal axon and mature towards a lysosomal identity as they make their retrograde journey back towards the neuronal cell body raises numerous important questions. These include:
What controls progressive axonal lysosome acidification in a distal to proximal direction along the length of axons?
To what extent does the process of axonal lysosome maturation resemble endosomal maturation processes that have been well characterized in non-neuronal contexts?
How are lysosomes recognized by the adaptor proteins that couple them to the motors that support their long distance movement?
When in this maturation process does a lysosome become competent to mediate cargo degradation?
Why do neurons transport autophagic and endocytic cargos back the cell body for degradation instead of disposing of them locally?
How does defective axonal lysosome transport contribute to neurological diseases?
While many of these questions remain only incompletely answered, exciting progress has been made on some of these topics. The subsequent sections of this review seek to summarize such advances.
Axonal Lysosome Acidification
Several processes are known to contribute to the acidification of lysosomes in non-axonal contexts. The vacuolar ATPase (V-ATPase) is an enormous, multi-subunit molecular machine that uses energy liberated by ATP hydrolysis to transport protons into the lysosome lumen [18]. As the assembly state of the V-ATPase can be regulated to control lysosome acidification in some non-neuronal contexts [18–20], changes in V-ATPase assembly state or composition could potentially coordinate acidification with transport. Additionally, regulation of the abundance and activity of CLCN7, a chloride-proton antiporter that supports lysosome acidification by providing counter ions that balance the positive charge arising from proton accumulation is also a candidate for controlling the efficiency of axonal lysosome acidification [18]. While the existence of lysosomal pH differences along the length of the axon appears to be a neuron-specific problem, it was recently reported that lysosomes found at the cell periphery are less acidic than those at the cell center even in HeLa cells [21]. This spatial pattern of lysosome acidification was elegantly proposed to arise from the fact that more peripheral lysosomes are further from the trans-Golgi network and thus have a lower probability to receive newly synthesized proteins from the biosynthetic pathway [21]. It remains to be determined whether the extent to which the gradient of axonal lysosome acidification reflects spatial constraints of biosynthetic delivery versus more acute regulatory mechanisms arising from control of V-ATPase assembly or regulation of lysosomal chloride channels.
Maturation of Endosomes into Lysosomes
The concept of endosomal maturation wherein endosomes undergo a stereotypic pattern of changes in protein and lipid content is well established from studies in non-neuronal systems [22–24]. It is likely that at least some similar mechanisms are at play in the process whereby endosome to lysosome maturation dynamically occurs in axons and there is a clear need for similarly detailed insights into the molecular mechanisms supporting axonal lysosome maturation. It is also possible that the extreme distances and narrow confines encountered within axons will provide opportunities for identifying intermediates in lysosome formation that are relevant to all cells but which would be difficult to recognize within the more spatially confined environment encountered in other cellular contexts.
Mechanisms Supporting Microtubule-based Transport of Axonal Lysosomes
The microtubule cytoskeleton of axons is polarized such that plus ends point towards the distal ends of axons and minus ends are arrayed towards the cell body [25]. As a result, anterograde axonal transport is kinesin-mediated while retrograde transport depends on the dynein motor [26,27]. In non-neuronal cells, two major mechanisms have been identified for coupling late endosomes and lysosomes to kinesin that depend on the Arl8 and Rab7 small GTPases respectively. Arl8 is recruited to the surface of lysosomes via interactions with the BLOC-one related complex (BORC)[28]. This octameric protein complex was recently reported to furthermore act as a guanine nucleotide exchange factor (GEF) for Arl8 and thus promote the ability of Arl8 to interact with downstream effectors [29]. One such Arl8 effector is SKIP, a protein that in turn interacts directly with kinesin light chain to promote the movement of lysosomes towards the plus ends of microtubules [30]. While most of these Arl8 dependent mechanisms are not neuron specific, Arl8-dependent transport of lysosomes in mammalian axons was recently reported [31]. Interestingly, BORC and Arl8 also support the anterograde transport of presynaptic active zone proteins and synaptic vesicle precursors into axons in C. elegans while lysosomes are not readily detectable in these axons [29,32,33]. These observations suggest that lysosomes and synaptic vesicles share some common machinery in support of their axonal transport. However, it remains unknown how specificity is achieved for the Arl8-mediated transport of lysosomes versus presynaptic proteins.
An alternative mechanism for coupling lysosomes to kinesin involves a series of interactions betweeen protrudin (also known as ZFYVE27), Rab7 and its effector FYCO1 [34]. Protrudin is an ER localized, kinesin 1-interacting protein whose FYVE domain reaches out at endoplasmic reticulum-endolysosome contact sites to interact with phosphatidylinositol-3-phosphate (PI3P) on late endosomes. At such membrane contact sites, kinesin 1 is transferred from protrudin to the endolysosomal Rab7-FYCO1 complex to support plus end directed movement on microtubules [34]. Only limited data is available concerning the physiological function of protrudin in neurons. However, as protrudin was originally identified for its ability to promote neurite outgrowth [35] it is reasonable to consider a similar role in actual axons but more data is required concerning functions in mature neurons. Addressing the question of protrudin functions in axons is of particular interest due to the fact that protrudin mutations cause a form of hereditary spastic paraplegia, a disease that predominantly affects the longest axons of the central nervous system [36].
With respect to retrograde axonal transport of lysosomes, several candidate mechanisms have been identified. A complex between Rab7 and Rab-interacting lysosomal protein (RILP) supports the dynein-mediated transport of late endosomes and lysosomes towards the perinuclear region of non-neuronal cells [37–39]. Rab7 has also been implicated in the retrograde axonal transport of endosomes containing activated neurotrophin receptors [40]. However, it remains to be determined to what extent such signaling endosomes are similar to, or distinct from, endosomes containing cargos destined for lysosome-mediated degradation. In addition to serving as a bridge between Rab7 and dynein, RILP also interacts with and stabilizes the assembly of the V-ATPase through interactions with the V1G1 subunit [41]. Through interactions with V1G1, Rab7 and dynein, RILP could potentially provide a mechanistic link between lysosome retrograde axonal transport and acidification. Contributions of Rab7 to axonal transport of lysosomes are of additional interest due to the existence of human Rab7 mutations that cause Charcot-Marie-Tooth disease type 2B (CMT2B), a disease that most prominently impairs long axons of the peripheral nervous system [42].
Meanwhile, mutations in the JNK-interacting protein 3 (JIP3, known as UNC-16 in C. elegans) gene cause the ectopic accumulation of axonal lysosomes in diverse organisms including: C. elegans, zebrafish and mice [43–46]. The work from zebrafish and mice concluded that such axonal accumulations of lysosomes in JIP3 KO axons arise from a defect in lysosome retrograde transport [43,46]. In contrast, an alternative model involving a role for JIP3/UNC-16 in suppressing the export of lysosome proteins from the neuronal cell body was proposed based on experiments in C. elegans [44,45,47]. These studies collectively point strongly to an evolutionarily conserved role for JIP3 in regulating the axonal abundance of lysosomes, but further efforts are required to elucidate the underlying molecular mechanisms.
A third candidate for supporting the retrograde transport of axonal lysosomes is the snapin protein that has been proposed to act as an adaptor between endolysosomes and dynein [48]. In support of a role for snapin in the regulation of neuronal lysosomes, snapin KO mice exhibit multiple lysosome defects in the neuronal cell body compartment [48]. However, since it’s role as an direct adaptor between lysosomes and dynein was originally proposed [48], snapin was independently identified as a component of the BORC complex that supports the Arl8-mediated anterograde axonal transport of lysosomes [28,31]. Furthermore, snapin also has the potential to impact lysosome function as a component of the biogenesis of lysosomes complex 1 (BLOC-1) [49]. It currently remains to be determined to what extent lysosome phenotypes in snapin KO neurons are mediated by BORC, BLOC-1 or dynein dependent mechanisms.
Impaired Retrograde Axonal Transport of Lysosomes in Alzheimer’s Disease
The mechanisms that support retrograde axonal transport and maturation of lysosomes have potentially important disease implications. The longstanding observation that lysosomes ectopically accumulate in swollen neurites surrounding Alzheimer’s disease amyloid plaques [2,50] has prompted studies of underlying mechanisms in mouse models of Alzheimer’s disease. This has led to the conclusion that rather than triggering the anterograde efflux of lysosomes from neuronal cell bodies to the plaque-associated axonal swellings, lysosomes build up at such sites due to a selective blockade in the normal process of retrograde axonal transport and maturation of lysosomes [17]. Evidence that such lysosomes are not innocent bystanders but rather function as important contributors to the amyloidogenic processing of the amyloid precursor protein (APP) comes from the recent discovery that axonal lysosome transport defects in mouse JIP3 KO neurons result in enhanced processing of APP into Aβ peptides [46]. Furthermore, JIP3 haploinsufficiency also results in a dramatic worsening of APP processing and amyloid plaque pathology in a mouse model of Alzheimer’s disease [46]. The concept that retrograde axonal transport of lysosomes is an important negative regulator of APP processing and Alzheimer’s disease pathology is further supported by recent reports that snapin deficiency also exacerbates pathology in a mouse Alzheimer’s disease model [51,52]. Data arising from the JIP3 and snapin studies converge on the role played by lysosomes as sites of degradation for BACE1, the protease that initiates amyloidogenic processing of APP [46,51,52]. It is thought that when the transport and maturation of axonal lysosomes is impaired, lysosome-mediated clearance of BACE1 is slowed and there is greater opportunity for encounters between BACE1 and APP within immature lysosomes. These observations raise questions about how lysosome axonal transport defects arise in human Alzheimer’s disease and whether preventing such disturbances could have therapeutic benefits.
In addition to the abovementioned examples pertaining to Alzheimer’s disease, axonal lysosomes were also observed in the developmental context of mouse motor neuron axon pruning [53]. This observation suggests the putative existence of novel signaling mechanisms that redistribute lysosomes into axons.
A Model for the Coordinated Biogenesis and Transport of Axonal Lysosomes
In summary, evidence for the existence of distinct pools of lysosomes and/or their precursors in neuronal axons has raised questions about the origin and functions of such organelles. If fully functional lysosomes were freely exchanging between the neuronal cell body and axons then lysosomes in both compartments should be indistinguishable from one another. However, the immature nature of axonal lysosomes (as judged by their minimal hydrolase content) implies either a distinct mechanism for the de novo assembly of lysosomes in axons or that lysosomes are modified in axons following their delivery from the soma.
A model (Figure 1) that proposes a solution to this problem is that lysosome precursors including late endosomes and autophagosomes form and fuse with one another in the distal axon before beginning a process of maturation into lysosomes that is coupled with their retrograde transport toward the neuronal cell body. However, the extreme spatial separation imposed by axon length places these newly formed lysosome precursors in the distal axon at a significant disadvantage for the receipt of newly synthesized luminal hydrolases that must be delivered from the neuronal cell body. As axons do not contain rough ER or Golgi apparatus, they are completely dependent on the distant cell body for receipt of lysosomal hydrolases. The extreme length and small diameter of axons means that there is a much greater probability for vesicles containing newly synthesized lysosome proteins to fuse with late endosomes/lysosomes in the neuronal cell body or even proximal axon than to pass by such organelles in favor of lysosomes in the distal axon. However, as immature lysosomes are transported from the distal axon towards the cell body, opportunities for them to encounter Golgi-derived vesicles increase. In this model, robust retrograde transport of maturing lysosomes combined with competition for encounters with anterograde vesicles delivering of newly synthesized lysosomal proteins helps to explain the observed gradient of axonal lysosome maturation. Such a model is also consistent with a recent report from non-neuronal cells that identified distance from the Golgi as a factor explaining a spatial gradient of both acidification and protease activity [21]. The model outlined above provides a foundation for future studies to elucidate the mechanisms that coordinate axonal lysosome transport and maturation. These are important problems to address for the understanding of neuronal cell biology and neurodegenerative disease mechanisms
Text Box 1. The lysosome family tree.
While lysosomes mediate the degradation of cargos delivered by the endocytic and autophagic pathways, they are in turn highly connected via membrane traffic with other cellular compartments. This can present challenges for the investigation of lysosome biogenesis and function as proteins that ultimately function in the lysosome must first be delivered via the secretory and endo-lysosomal pathways and are thus not absolutely limited to being present in just lysosomes. Likewise, lysosomes receive cargos for degradation via fusion with endosomes and autophagosomes. Such interconnectedness between lysosomes and upstream organelles of the secretory and endocytic pathways generates challenges for the identification of lysosomes and illustrates the importance of recognizing that lysosomes do not function in isolation but rather as part of a dynamic pathway where lysosome function is dependent on interactions with multiple upstream compartments [54].
Text Box 2. A field guide for the identification of lysosomes.
The catabolic functions of lysosomes are supported by >200 proteins that include ~60 distinct enzymes that function optimally within the acidic environment of the lysosomal lumen, the V-ATPase that is responsible for lysosome acidification, ion channels and transporters within the limiting membrane of lysosomes that support the efflux from lysosomes of the cellular building blocks that are liberated by lysosome mediated degradation, small GTPases and their associated regulatory proteins and effectors that support endo-lysosomal membrane traffic as well as multiple proteins whose precise functions remain to be identified [55].
In light of this complexity, the identification of lysosomes and their relationship to other organelles in the autophagic, endocytic and secretory pathways should ideally be established by multiple assays. Key approaches that are commonly used in the identification of lysosomes include:
The use of fluorescent dyes (e.g. Lysotracker or cresyl violet [56]) that selectively accumulate in the acidic environment (pH<5) of the lysosome lumen.
The presence of lysosomal enzymes. While a relatively small number of cathepsins are commonly investigated, multiple types of lysosomal enzymes should be analyzed before making broad conclusions about lysosome function.
Assays for assessing the activity of specific lysosome enzymes in living cells based on fluorescent substrates that get trapped in lysosomes following their cleavage by specific enzymes (e.g. Cresyl violet conjugated cathepsin substrates) or which become fluorescent following proteolytic processing [e.g. dequenching of DQ-BSA fluorescence [54]].
Accumulation of endocytic cargos such as fluorescently labeled dextrans.
Presence of abundant highly glycosylated integral membrane proteins such as lysosome associated membrane protein 1 (LAMP1) that participate in cholesterol export from lysosomes and are also thought to protect lysosome membranes from damage by lysosome hydrolases [57].
Transmission electron microscopy analysis of ultrastructural morphology. Key features include: multi-vesicular and multi-lamellar appearance along with electron density arising from osmiophilic properties [58].
Note that while each of these assays has value in the identification of lysosomes in intact cells, many of these assays will also label earlier steps in the endo-lysosomal pathway to varying degrees. It is therefore always important to interpret observations in the context of a dynamic endo-lysosomal degradative pathway.
Highlights.
Lysosomes degrade diverse cargos that are captured within endosomes and autophagosomes.
Lysosome abundance in axons is limited by efficient retrograde transport mechanisms.
Impaired retrograde transport of axonal lysosomes may confer Alzheimer’s disease risk.
Hydrolase delivery to axonal lysosomes is likely to be limited by their distance from the Golgi.
Acknowledgments
I am thankful for helpful feedback from Swetha Gowrishankar and Agnes Roczniak-Ferguson. Grants from the NIH (GM105718 and AG047270) provided financial support for my research focused on lysosome regulatory mechanisms. I have no competing financial interests to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Becker NH, Goldfischer S, Shin WY, Novikoff AB. The localization of enzyme activities in the rat brain. J Biophys Biochem Cytol. 1960;8:649–663. doi: 10.1083/jcb.8.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terry RD, Gonatas NK, Weiss M. Ultrastructural Studies in Alzheimer’s Presenile Dementia. Am J Pathol. 1964;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Terry RD. Fine structural localization of acid phosphatase in senile plaques in Alzheimer’s presenile dementia. Acta Neuropathol. 1967;8:276–284. doi: 10.1007/BF00688828. [DOI] [PubMed] [Google Scholar]
- 4.Cataldo AM, Nixon RA. Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc Natl Acad Sci U S A. 1990;87:3861–3865. doi: 10.1073/pnas.87.10.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parton RG, Simons K, Dotti CG. Axonal and dendritic endocytic pathways in cultured neurons. J Cell Biol. 1992;119:123–137. doi: 10.1083/jcb.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorenstein C, Bundman MC, Lew PJ, Olds JL, Ribak CE. Dendritic transport. I. Colchicine stimulates the transport of lysosomal enzymes from cell bodies to dendrites. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1985;5:2009–2017. doi: 10.1523/JNEUROSCI.05-08-02009.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukita S, Ishikawa H. The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J Cell Biol. 1980;84:513–530. doi: 10.1083/jcb.84.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol. 1993;121:305–315. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc Natl Acad Sci U S A. 1995;92:3156–3160. doi: 10.1073/pnas.92.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. An elegant imaging study that demonstrates the coordinated process of axonal autophagosome formation, transport and maturation towards a lysosomal identity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30:71–85. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernandez-Diaz S, Swerts J, Schoovaerts N, Vilain S, Gounko NV, Vints K, et al. A LRRK2-Dependent EndophilinA Phosphoswitch Is Critical for Macroautophagy at Presynaptic Terminals. Neuron. 2016;92:829–844. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Stavoe AK, Hill SE, Hall DH, Colon-Ramos DA. KIF1A/UNC-104 Transports ATG-9 to Regulate Neurodevelopment and Autophagy at Synapses. Dev Cell. 2016;38:171–185. doi: 10.1016/j.devcel.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maday S, Holzbaur EL. Compartment-Specific Regulation of Autophagy in Primary Neurons. J Neurosci. 2016;36:5933–5945. doi: 10.1523/JNEUROSCI.4401-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J Cell Biol. 2015;209:377–386. doi: 10.1083/jcb.201412046. This study provides strong support for the idea that autophagosomes must fuse with late endosomes before engaging in robust retrograde transport back towards the neuronal cell body. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Gowrishankar S, Yuan P, Wu Y, Schrag M, Paradise S, Grutzendler J, De Camilli P, Ferguson SM. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc Natl Acad Sci U S A. 2015;112:E3699–3708. doi: 10.1073/pnas.1510329112. This paper supports a link between amyloid plaques and the selective defects in axonal lysosome transport and maturation. It furthermore identifies a relationship between neuronal lysosome subcellular position and luminal protease content wherein lysosomes in the neuronal cell body contain significantly higher levels of such proteins than lysosomes found in neuronal projections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 19.McGuire C, Cotter K, Stransky L, Forgac M. Regulation of V-ATPase assembly and function of V-ATPases in tumor cell invasiveness. Biochim Biophys Acta. 2016;1857:1213–1218. doi: 10.1016/j.bbabio.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman N, Ramos-Espiritu L, Milner TA, Buck J, Levin LR. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol. 2016;148:325–339. doi: 10.1085/jgp.201611606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Johnson DE, Ostrowski P, Jaumouille V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. This study makes the striking observation that distance from the Golgi may explain a gradient of lysosome acidification and protease content in non-neuronal cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 24.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A. Identification of the switch in early-to-late endosome transition. Cell. 2010;141:497–508. doi: 10.1016/j.cell.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twelvetrees AE, Pernigo S, Sanger A, Guedes-Dias P, Schiavo G, Steiner RA, Dodding MP, Holzbaur EL. The Dynamic Localization of Cytoplasmic Dynein in Neurons Is Driven by Kinesin-1. Neuron. 2016;90:1000–1015. doi: 10.1016/j.neuron.2016.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur EL. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell. 2015;33:176–188. doi: 10.1016/j.devcel.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niwa S, Tao L, Lu SY, Liew GM, Feng W, Nachury MV, Shen K. BORC Regulates the Axonal Transport of Synaptic Vesicle Precursors by Activating ARL-8. Curr Biol. 2017 doi: 10.1016/j.cub.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosa-Ferreira C, Munro S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev Cell. 2011;21:1171–1178. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Farias GG, Guardia CM, De Pace R, Britt DJ, Bonifacino JS. BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc Natl Acad Sci U S A. 2017;114:E2955–E2964. doi: 10.1073/pnas.1616363114. This study contributes to understanding of lysosome distribution in neurons and the role played by BORC-Arl8 in promoting delivery into axons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu YE, Huo L, Maeder CI, Feng W, Shen K. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron. 2013;78:994–1011. doi: 10.1016/j.neuron.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klassen MP, Wu YE, Maeder CI, Nakae I, Cueva JG, Lehrman EK, Tada M, Gengyo-Ando K, Wang GJ, Goodman M, et al. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron. 2010;66:710–723. doi: 10.1016/j.neuron.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raiborg C, Wenzel EM, Pedersen NM, Olsvik H, Schink KO, Schultz SW, Vietri M, Nisi V, Bucci C, Brech A, et al. Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth. Nature. 2015;520:234–238. doi: 10.1038/nature14359. [DOI] [PubMed] [Google Scholar]
- 35.Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818–821. doi: 10.1126/science.1134027. [DOI] [PubMed] [Google Scholar]
- 36.Fink JK. Hereditary spastic paraplegia: clinico-pathologic features and emerging molecular mechanisms. Acta Neuropathol. 2013;126:307–328. doi: 10.1007/s00401-013-1115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansson M, Rocha N, Zwart W, Jordens I, Janssen L, Kuijl C, Olkkonen VM, Neefjes J. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol. 2007;176:459–471. doi: 10.1083/jcb.200606077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantalupo G, Alifano P, Roberti V, Bruni CB, Bucci C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, Janssen H, Wubbolts R, Neefjes J. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 40.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 41.De Luca M, Cogli L, Progida C, Nisi V, Pascolutti R, Sigismund S, Di Fiore PP, Bucci C. RILP regulates vacuolar ATPase through interaction with the V1G1 subunit. J Cell Sci. 2014;127:2697–2708. doi: 10.1242/jcs.142604. [DOI] [PubMed] [Google Scholar]
- 42.Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drerup CM, Nechiporuk AV. JNK-interacting protein 3 mediates the retrograde transport of activated c-Jun N-terminal kinase and lysosomes. PLoS Genet. 2013;9:e1003303. doi: 10.1371/journal.pgen.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards SL, Morrison LM, Yorks RM, Hoover CM, Boominathan S, Miller KG. UNC-16 (JIP3) Acts Through Synapse-Assembly Proteins to Inhibit the Active Transport of Cell Soma Organelles to Caenorhabditis elegans Motor Neuron Axons. Genetics. 2015;201:117–141. doi: 10.1534/genetics.115.177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards SL, Yu SC, Hoover CM, Phillips BC, Richmond JE, Miller KG. An Organelle Gatekeeper Function for Caenorhabditis elegans UNC-16 (JIP3) at the Axon Initial Segment. Genetics. 2013;194:143–161. doi: 10.1534/genetics.112.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Gowrishankar S, Wu Y, Ferguson SM. Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. J Cell Biol. 2017;216:3291–3305. doi: 10.1083/jcb.201612148. This research establishes both a critical role for JIP3 in the axonal transport and maturation of lysosomes as well as the importance of such transport in protecting against amyloid plaque pathology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choudhary B, Kamak M, Ratnakaran N, Kumar J, Awasthi A, Li C, Nguyen K, Matsumoto K, Hisamoto N, Koushika SP. UNC-16/JIP3 regulates early events in synaptic vesicle protein trafficking via LRK-1/LRRK2 and AP complexes. PLoS Genet. 2017;13:e1007100. doi: 10.1371/journal.pgen.1007100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Q, Lu L, Tian JH, Zhu YB, Qiao H, Sheng ZH. Snapin-regulated late endosomal transport is critical for efficient autophagy-lysosomal function in neurons. Neuron. 2010;68:73–86. doi: 10.1016/j.neuron.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 50.Cataldo AM, Hamilton DJ, Nixon RA. Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res. 1994;640:68–80. doi: 10.1016/0006-8993(94)91858-9. [DOI] [PubMed] [Google Scholar]
- 51.Tammineni P, Ye X, Feng T, Aikal D, Cai Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. Elife. 2017:6. doi: 10.7554/eLife.21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye X, Feng T, Tammineni P, Chang Q, Jeong YY, Margolis DJ, Cai H, Kusnecov A, Cai Q. Regulation of Synaptic Amyloid-beta Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer’s Disease. J Neurosci. 2017;37:2639–2655. doi: 10.1523/JNEUROSCI.2851-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song JW, Misgeld T, Kang H, Knecht S, Lu J, Cao Y, Cotman SL, Bishop DL, Lichtman JW. Lysosomal activity associated with developmental axon pruning. J Neurosci. 2008;28:8993–9001. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Bright NA, Davis LJ, Luzio JP. Endolysosomes Are the Principal Intracellular Sites of Acid Hydrolase Activity. Curr Biol. 2016;26:2233–2245. doi: 10.1016/j.cub.2016.06.046. Of interest due to the detailed elucidation of relationships between dynamic endolysosomal membrane traffic and degradative activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballabio A. The awesome lysosome. EMBO Mol Med. 2016;8:73–76. doi: 10.15252/emmm.201505966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ostrowski PP, Fairn GD, Grinstein S, Johnson DE. Cresyl violet: a superior fluorescent lysosomal marker. Traffic. 2016;17:1313–1321. doi: 10.1111/tra.12447. [DOI] [PubMed] [Google Scholar]
- 57.Li J, Pfeffer SR. Lysosomal membrane glycoproteins bind cholesterol and contribute to lysosomal cholesterol export. Elife. 2016:5. doi: 10.7554/eLife.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb Perspect Biol. 2014;6:a016857. doi: 10.1101/cshperspect.a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]