Abstract
5′ AMP-activated protein kinase (AMPK) activation may be part of the exercise-induced process that enhances insulin sensitivity. Independent of exercise, acute prior treatment of skeletal muscles isolated from young rats with a pharmacological activator of AMPK, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), causes subsequently improved insulin-stimulated glucose uptake (GU). However, efficacy of a single prior AICAR exposure on insulin-stimulated GU in muscles from old animals has not been studied. The purpose of this study was to determine whether brief, prior exposure to AICAR (3.5 hours before GU assessment) leads to subsequently increased GU in insulin-stimulated skeletal muscles from old rats. Epitrochlearis muscles from 24 month-old male rats were isolated and initially incubated ±AICAR (60 minutes), followed by incubation without AICAR (3 hours), then incubation ±insulin (50 minutes). Muscles were assessed for GU (via 3-O-methyl-[3H]-glucose accumulation) and site-specific phosphorylation of key proteins involved in enhanced GU, including AMPK, Akt, and Akt substrate of 160 kDa (AS160), via western blotting. Prior ex vivo AICAR treatment resulted in greater GU by insulin-stimulated muscles from 24 month-old rats. Prior AICAR treatment also resulted in greater phosphorylation of AMPK (T172) and AS160 (S588, T642 and S704). GLUT4 protein abundance was unaffected by prior AICAR and/or insulin treatment. These findings demonstrate that skeletal muscles from older rats are susceptible to enhanced insulin-stimulated GU after brief activation of AMPK by prior AICAR. Consistent with earlier research using muscles from young rodents, increased phosphorylation of AS160 is implicated in this effect that was not attributable to altered GLUT4 glucose transporter protein abundance.
Keywords: skeletal muscle, AICAR, insulin, AMPK, AS160
Introduction
The prevalence of abnormal glucose homeostasis increases markedly with advancing age in the adult population of the United States. The Center for Disease Control estimates that the prevalence of diabetes among adults in the U.S. increases progressively from 4.0% at ages 18–44 years, to 17.0% at ages 45–64 years, and reaches 25.2% at age 65 or older (Cowie et al. 2009). Furthermore, the estimates for prevalence of prediabetes among U.S. adults are 23.7% at 18–44 years, 40.9% at 45–64, and 48.3% at 65 or older (CDC 2017). Insulin resistance is an essential and primary event in the progression to type 2 diabetes that is evident for decades before the development of insulin deficiency and overt hyperglycemia (Lillioja et al. 1988; Warram et al. 1990). Even in the absence of diabetes, insulin resistance is related to increased risk for many age-related diseases, including hypertension, coronary heart disease, stroke, and certain cancers (Facchini et al. 2001; Haffner 1999; Kumari et al. 2000). Skeletal muscle is the major site for whole body glucose disposal, accounting for up to 85% of the body’s insulin-mediated glucose uptake (DeFronzo et al. 1981). In this context, it is important to understand how to enhance the insulin sensitivity of skeletal muscles in older individuals.
Exercise can lead to improved skeletal muscle insulin sensitivity in both young and older individuals by mechanisms that remain to be completely elucidated (Cartee 2015a; Cartee et al. 2016). It has been suggested that the activation of 5′ AMP-activated protein kinase (AMPK), which is a hallmark of exercise, may be part of the exercise-induced process that produces enhanced insulin sensitivity (Cartee 2015a; Kjobsted et al. 2017; Kjobsted et al. 2016; Kim et al. 2004). Independent of exercise, acute prior treatment of isolated rodent skeletal muscle with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), a pharmacological activator of AMPK, has also been reported to cause subsequently improved insulin-stimulated glucose uptake (Fisher et al. 2002; Iglesias et al. 2002; Kjobsted et al. 2015). Fisher et al. (2002) demonstrated that incubating isolated rat epitrochlearis muscles from young (~6 wk-old) rats with AICAR for 1 h could lead to a subsequent (~3.5 h later) increase in insulin-stimulated glucose uptake (Fisher et al. 2002). A recent study by Kjøbsted et al. (2015) reported similar findings for insulin-stimulated glucose uptake in isolated skeletal muscles from young mice (Kjobsted et al. 2015). The efficacy of a single, brief exposure to prior AICAR on insulin-stimulated glucose uptake in muscles from older animals has not been previously reported. Therefore, our primary aim was to determine whether brief, prior exposure to the AMPK-activator AICAR would lead to subsequently increased glucose uptake in insulin-stimulated skeletal muscles from old rats. The earlier studies that reported the effect of prior AICAR on insulin-stimulated glucose uptake in isolated skeletal muscle from young rodents used a protocol that included both serum and AICAR during the preincubation step (Fisher et al. 2002; Kjobsted et al. 2015). Accordingly, we also assessed the independent and combined effects of prior exposure to serum and/or AICAR on glucose uptake in muscles from old rats.
Survival curves for male Fischer-344 X Brown Norway (FBN) rats compared to survival curves in multiple human populations indicate that the 24 month old rats used in the current study are approximately the equivalent of a person aged ~60 years (Turturro et al. 1999; Weon and Je 2012). Male FBN rats at aged 24–25 months exhibit skeletal muscle insulin resistance in response to physiological level insulin doses compared to 3.5 and 13 month old rats (Cartee et al. 1993).
As a secondary aim, we evaluated the influence of AICAR on the activation of AMPK and several key signaling proteins that are implicated in insulin-stimulated glucose uptake. Insulin leads to activation of the serine/threonine kinase known as Akt via phosphorylation on Ser473 and Thr308, and Akt activation is critical for insulin-stimulated glucose uptake (Kohn et al. 1996; Scheid et al. 2002). Akt substrate of 160 kDa (AS160; also known as TBC1D4) is the Akt substrate that has been most clearly linked to insulin-stimulated glucose uptake (Cartee 2015b). In insulin-stimulated mouse skeletal muscle, Kjøbsted et al. (Kjobsted et al. 2015) found that prior AICAR resulted in greater phosphorylation of AS160 on Ser704 (an AMPK phosphosite) and Thr642 (an Akt phosphosite), but not Ser588 (an Akt phosphosite). Therefore, we also determined the phosphorylation of AS160 on Ser588, Thr642, and Ser704. In addition, we measured the abundance of glucose transporter type 4 (GLUT4), the glucose transporter protein responsible for both insulin- and AICAR-mediated glucose uptake.
Methods
Materials
Serum from male Wistar rats (120–200 g) was purchased from Gemini Bio-Products (West Sacramento, CA). Reagents and apparatus for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). T-PER tissue protein extraction reagent (no. 78510), Bicinchoninic acid protein assay (no. 23225) and Pierce MemCode Reversible Protein Stain Kit (no. 24585) were purchased from ThermoFisher Scientific (Waltham, MA). Anti-phospho-Akt Thr308 (pAktT308; no. 9275), anti-phospho-Akt Ser473 (pAktS473; no. 9272), anti-phospho-AS160 Ser588 (pAS160S588; no. 8730), anti-phospho-AS160 Thr642 (pAS160Thr642, no. 4288), anti-phospho-AMPKα Thr172 (pAMPKT172; no. 2531), anti-Akt (no. 4691), anti-AMPKα (no. 5831), anti-hexokinase II (no. 2867), and anti-rabbit immunoglobulin G horseradish peroxidase conjugate (no. 7074) were from Cell Signaling Technology (Danvers, MA). 5-Aminoimidazole-4-carboxamide-1-β-riboside (AICAR; no. 123040), anti-AS160 (no. ABS54), and anti-GLUT4 (no. CBL243) were from EMD Millipore (Burlington, MA). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Anti-phospho-AS160 Ser704 polyclonal antibody (pAS160S704) was provided by Dr. Makoto Kanzaki at Tohoku University (25). 3-O-methyl-[3H] glucose ([3H]3-MG) was from Sigma-Aldrich, and [14C]mannitol was from PerkinElmer (Waltham, MA). Other reagents were from Sigma-Aldrich (St. Louis, MO) or ThermoFisher (Waltham, MA).
Animal treatment
Procedures for animal care were approved by the University of Michigan Committee on Use and Care of Animals. Male Fischer-344 X Brown Norway rats (500–600 g; n = 20) were obtained at 23–24 months of age from the National Institute of Aging (NIA) aging rodent colony. Rats were housed at the University of Michigan for approximately 1 week before experimentation. They were provided with rodent chow (Lab Diet; PMI Nutritional International, Brentwood, MO) and water ad libitum until their food was removed at 1700h the night before the terminal experiment.
Muscle dissection and incubation
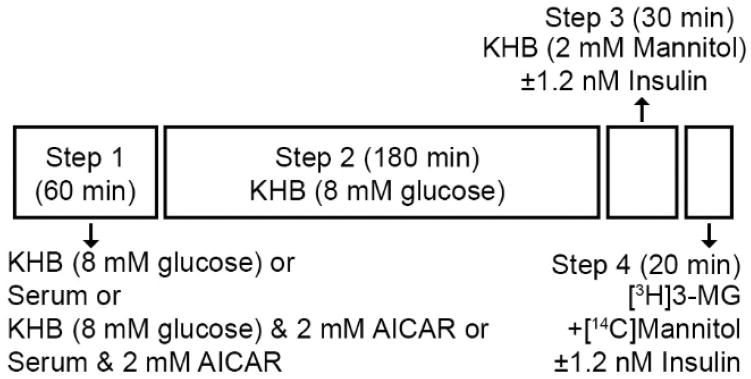
On the morning of the experiment between 0800 and 1000h, rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (50 mg/kg wt). While rats were under deep anesthesia, both epitrochlearis muscles were rapidly removed by dissection, and each epitrochlearis was longitudinally transected with a scalpel into two strips of similar size. Each muscle strip underwent a four-step incubation process (Figure 1). During step 1 (60 min and 35°C), one of the four strips from each rat was incubated with each of the following four solutions (2 ml): KHB+8 mM glucose; KHB+8 mM glucose+2 mM AICAR; serum; or serum+2 mM AICAR. During step 2 (180 min and 35°C), each strip was transferred into a second vial containing KHB+8 mM glucose. During step 3 (30 min and 30°C), all 4 strips from 10 of the rats were incubated without insulin, and all 4 strips from the remaining 10 rats were incubated with a submaximally effective insulin concentration (1.2 nM). During step 4 (20 min and 30°C), muscle strips were incubated in KHB with 8 mM [3H]3-MG (final specific activity of 250 μCi/mmol), 2 mM [14C]mannitol (final specific activity of 50 μCi/mmol) and the same insulin concentration as the previous step.
Figure 1.
Isolated epitrochlearis muscle strips were incubated ex vivo in four steps. During Step 1, each muscle strip from a single animal was incubated with No serum or AICAR (in Krebs-Henseleit Buffer; KHB) with 8 mM glucose, serum alone, KHB with 8 mM glucose and 2 mM AICAR, or serum and 2 mM AICAR for 60 min. During Step 2, muscle strips were incubated in KHB with 8 mM glucose for 180 min. During Step 3, all four muscle strips from 10 rats were incubated in KHB with No Insulin, and all four strips from the remaining 10 rats were incubated with 1.2 nM Insulin for 30 minutes. During Step 4, all muscle strips were incubated in KHB with 2 mM mannitol and radiolabeled 3-O-methyl-D-glucose (3-MG) and the same insulin concentration as Step 3 for 20 minutes.
Muscle lysate preparation
Frozen muscles were weighed and then homogenized in ice-cold lysis buffer (1 ml/muscle strip) using Kimble Kontes Duall Tissue Grinders (ThermoFisher) driven by a Caframo RZR1 stirrer (Georgian Bluffs, Ontario, Canada). The lysis buffer contained T-PER supplemented with 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacedic acid, 2.5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 mM β-glycerophosphate, 1 μg/ml leupeptin, and 1 mM phenylmethyl sulfonyl fluoride.
3-MG uptake
The calculation of [3H]3-MG uptake by skeletal muscle has been described previously (Cartee and Bohn 1995). Briefly, [14C]mannitol counts/min, measured by liquid scintillation counting of aliquots from muscle homogenates, were used to determine extracellular space. The intracellular [3H]3-MG of muscle was calculated as the difference between the total [3H]3-MG in muscle and the [3H]3-MG in the extracellular space.
Western blotting
Western blotting procedures have been described previously (Sharma et al. 2015; Wang et al. 2016). An equal amount of protein of each sample was mixed with 6X Laemmli buffer, boiled for 5 min, and separated using SDS-PAGE (9% resolving gel) before being transferred to polyvinyl difluoride membranes. The MemCode protein stain was used to confirm equal loading (Antharavally et al. 2004; Castorena et al. 2014). Membranes were blocked in 5% bovine serum albumin (BSA) in TBST (Tris-buffered saline, pH 7.5, plus 0.1% Tween-20) for 1 h at room temperature and transferred to 3% BSA-TBST with the appropriate primary antibody overnight at 4°C. Membranes were washed three times for 5 min in TBST and incubated with secondary antibody for 1 h at room temperature. Blots were washed three times for 5 min in TBST and three times for 5 min in TBS (Tris-buffered saline, pH 7.5) and then subjected to enhanced chemiluminescence (Luminata Forte Western horseradish peroxidase substrate no. WBLUF0100; Millipore) to visualize protein bands. Immunoreactive proteins were quantified by densitometry (FluorChemE, ProteinSimple and AlphaViewSA, San Jose, CA). Values are expressed relative to the normalized average of all of the samples on each blot.
Statistical analysis
The initial statistical analysis included the data from all four of the preincubation conditions (No serum or AICAR, serum only, AICAR only, and serum+AICAR). A repeated measures two-way analysis of variance (ANOVA) was run separately within the No Insulin and Insulin groups. In the initial analysis, the effects of serum only and serum+AICAR obscured the effects attributable to only AICAR. Because the primary interest was in the effects of AICAR-induced AMPK activation, we subsequently performed further statistical analysis excluding the data from the serum only or serum+AICAR conditions. For the No serum or AICAR and AICAR only preincubation conditions, a two-way ANOVA was performed (Main Effect of insulin, 0 or 1.2 nM; Main Effect of AICAR, 0 or 2 mM; and insulin x AICAR interaction). The Shapiro-Wilk and Brown-Forsythe tests were used to test for normality and equal variance, respectively. All data passed the equal variance test. Holm-Sidak post hoc analysis was performed when a main effect and/or an interaction effect was/were present. Data lacking normal distribution were mathematically transformed to achieve normality prior to two-way ANOVA being run. Kruskal-Wallis one-way ANOVA on ranks was used if transformation failed to normalize data, and pairwise multiple comparison procedure was performed by the Tukey Test.
Results
[3H]3-MG Uptake
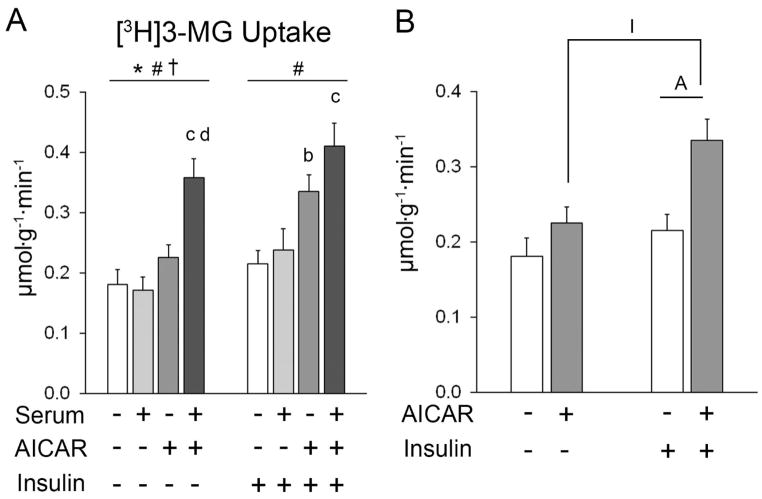
The initial statistical analysis that included all four pre-incubation conditions revealed significant main effects of serum (p<0.05) and AICAR (p<0.001), and an interaction effect (p<0.01) on glucose uptake in muscles incubated without insulin (Figure 2A). Post hoc analysis revealed that glucose uptake for serum+AICAR > serum alone (p<0.001) and for serum+AICAR > AICAR alone (p<0.001). For muscles incubated with insulin, there was a significant main effect of AICAR (p<0.001). Post hoc analysis revealed that glucose uptake for AICAR > No AICAR or serum group (p<0.05) and serum+AICAR > serum alone (p<0.01).
Figure 2.
Glucose uptake of epitrochlearis muscles. A) For muscles incubated without insulin, repeated measures (RM) ANOVA indicated a main effect of serum* (p<0.05), a main effect of AICAR# (p<0.001), and a serum x AICAR interaction effect† (p<0.01). Post hoc analysis for muscles without insulin revealed serum+AICAR>serum alonec (p<0.001) and serum+AICAR>AICAR aloned (p<0.001). For muscles incubated with insulin, RM ANOVA indicated a main effect of AICAR# (p<0.001). Post hoc analysis for muscles with insulin revealed AICAR>KHB (without serum or AICAR)b (p<0.05) and serum+AICAR>serum alonec (p<0.01). B) Two-way ANOVA indicated main effects of insulin (p<0.01) and AICAR (p<0.01). Post hoc analysis revealed AAICAR+insulin>insulin alone (p<0.01) and IAICAR+insulin>AICAR alone (p<0.01). Data are mean ±SEM (n=10/group).
For the secondary analysis that excluded serum conditions, there were significant main effects of insulin (p<0.01) and AICAR (p=0.001) on glucose uptake (Figure 2B). Post hoc analysis revealed that glucose uptake in the AICAR+insulin group exceeded both the insulin alone group (p<0.01) and the AICAR alone group (p<0.01).
AMPK
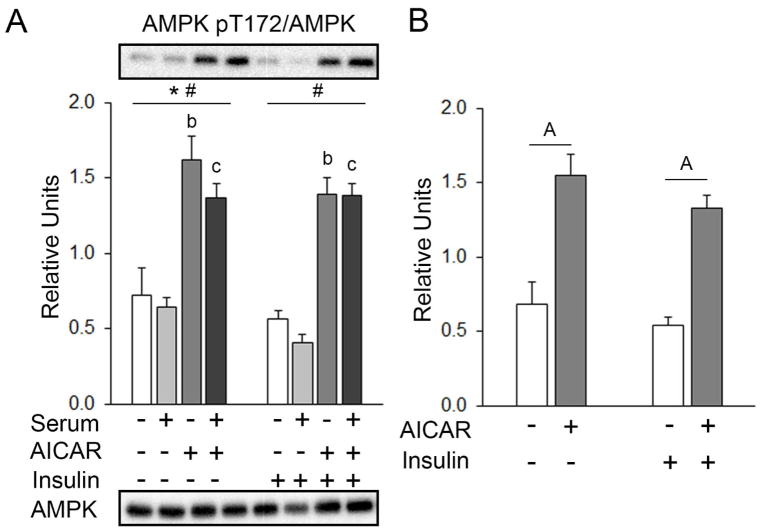
The initial statistical analysis that included all four pre-incubation conditions found that for muscles incubated without insulin, there was a small, but significant main effect of serum on total AMPK abundance (~15%, serum < no serum or AICAR; p<0.01; Table 1A). There was no effect of serum or AICAR in skeletal muscles incubated with insulin. Values for pAMPKT172 are expressed as a ratio of pAMPKT172/total AMPK. For muscles incubated without insulin there were significant main effects of AICAR (p<0.01) and of serum (p<0.05) on pAMPKT172. Post hoc analysis revealed pAMPKT172 with prior AICAR > the No AICAR or serum group (p<0.01). In muscles incubated with insulin, there were no significant effects of AICAR or serum on total AMPK abundance, but there was a significant main effect of AICAR (p<0.001) on pAMPKT172. Post hoc analysis revealed pAMPKT172 with prior serum+AICAR > serum alone (Figure 3A). For the secondary analysis excluding serum effects, there were no significant differences among groups for total AMPK abundance (Table 1B), but there was a significant main effect of AICAR (p<0.001) on pAMPKT172. Post hoc analysis revealed pAMPKT172 with prior AICAR > no insulin or AICAR (p<0.001) or with prior AICAR+insulin > insulin alone (p<0.001; Figure 3B).
Table 1.
Total protein abundance of Akt, AMPK, and AS160 in epitrochlearis muscles. A) For muscles incubated in No Insulin, RM ANOVA indicated a main effect of AICAR* (p<0.05) and an interaction effect‡ (p<0.05) on Akt and a main effect of serum† on AMPK and AS160 (p<0.001). Post hoc analysis revealed Akt with prior serum < No AICAR or seruma (p<0.05) and prior AICAR < No AICAR or serumb (p<0.01). AMPK with prior serum < No AICAR or seruma (p<0.01), and AS160 with prior serum < No AICAR or seruma (p<0.001) and serum+AICAR < AICAR aloned (p<0.001). For muscles incubated in Insulin, RM ANOVA indicated a main effect of serum† (p<0.001) on AS160. Post hoc analysis revealed AS160 with prior serum < No AICAR or seruma (p<0.001). B) Two-way ANOVA indicated a main effect of insulin§ on total AS160. Post hoc analysis revealed AS160 with Insulin alone > No Insulini (p<0.05). Data are presented as Mean±SEM (n=10/group).
| A | Basal | Insulin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Serum | − | + | − | + | − | + | − | + | ||
| AICAR | − | − | + | + | Effects | − | − | + | + | Effects |
| Akt | 1.06±0.06 | 0.97±0.05a | 0.91±0.04b | 0.92±0.02 | *,‡ | 0.99±0.03 | 0.96±0.06 | 1.01±0.06 | 1.17±0.06 | |
| AMPK | 1.12±0.06 | 0.93±0.05a | 1.03±0.05 | 0.91±0.02 | † | 1.03±0.05 | 0.93±0.05 | 1.08±0.05 | 0.98±0.06 | |
| AS160 | 1.11±0.12 | 0.51±0.06a | 1.08±0.13 | 0.59±0.03d | † | 1.90±0.41 | 0.62±0.06a | 1.33±0.14 | 0.85±0.10 | † |
| B | Basal | Insulin | |||
|---|---|---|---|---|---|
|
|
|||||
| AICAR | − | + | − | + | Effects |
| Akt | 1.06±0.06 | 0.91±0.04 | 0.99±0.03 | 1.01±0.06 | |
| AMPK | 1.12±0.06 | 1.03±0.05 | 1.03±0.05 | 1.08±0.05 | |
| AS160 | 1.11±0.12 | 1.08±0.13 | 1.90±0.41I | 1.33±0.14 | § |
Figure 3.
pAMPKT172 in epitrochlearis muscles. A) For muscles incubated without insulin, RM ANOVA indicated a main effect of serum* (p<0.05) and a main effect of AICAR# (p<0.01). Post hoc analysis for muscles incubated without insulin revealed pAMPKT172 with prior AICAR > No AICAR or serumb (p<0.01) and prior AICAR & serum > serum alonec (p<0.01). For muscles incubated with insulin, RM ANOVA indicated a main effect of AICAR# (p<0.001). Post hoc analysis for muscles incubated with insulin revealed pAMPKT172 with prior AICAR > No AICAR or serumb (p<0.001) and serum & AICAR > serum alonec (p<0.001). Representative blots for phospho- and total proteins are shown below the graph. B) Two-way ANOVA indicated a main effect of AICAR (p<0.001). Post hoc analysis revealed pAMPKT172 with Aprior AICAR > No AICAR or serum (p<0.01) and AAICAR+insulin > insulin alone (p<0.001). All data are shown as mean±SEM in the graph (n=10/group).
Akt
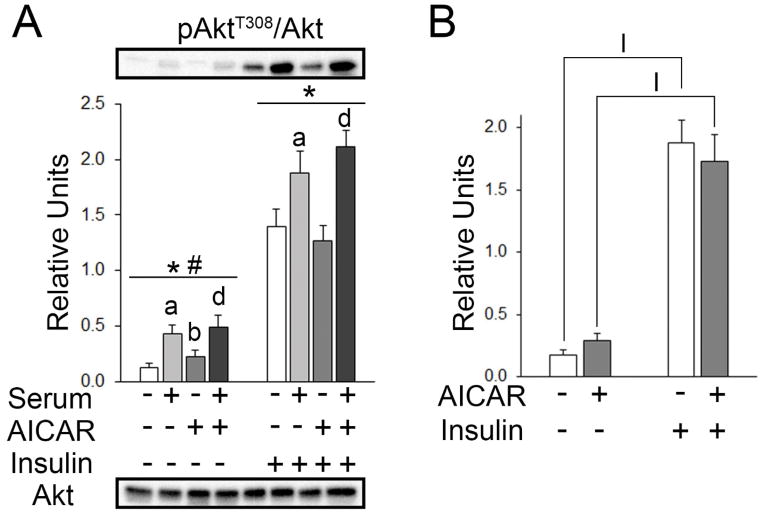
In the initial analysis for all four incubation conditions in muscles without insulin, there was a small but significant effect of AICAR and an interaction effect on total Akt abundance (9–14%, no AICAR > AICAR and serum < no serum; p<0.05; Table 1A). In the initial analysis for muscles incubated with insulin, there were no significant differences among groups in total Akt abundance (Table 1A).
Values for pAkt are expressed as the ratio of pAkt/total Akt. There were significant main effects of serum (p<0.001) and AICAR (p<0.05) on pAktT308 (Figure 4A). Post hoc analysis revealed pAktT308 with prior AICAR > No AICAR or serum (p<0.01), prior serum > No AICAR or serum (p<0.001), and prior serum+AICAR > AICAR alone (p<0.001). For muscles incubated with insulin, there was a significant main effect of serum (p<0.001) on pAktT308 (Figure 4A). Post hoc analysis revealed that pAktT308 values for serum > No AICAR or serum (p<0.05) and prior serum+AICAR > AICAR alone (p<0.01; Figure 4A). In the secondary analysis excluding serum and serum+AICAR, there was a significant main effect of insulin (p<0.001) on pAktT308. Post hoc analysis revealed pAktT308 with insulin > no insulin (p<0.001) and AICAR+insulin>AICAR alone (p<0.001; Figure 4B).
Figure 4.
pAktT308 in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001) and a main effect of AICAR# (p<0.05). Post hoc analysis revealed pAktT308 with prior serum > No AICAR or seruma (p<0.001), prior AICAR > No AICAR or serumb (p<0.01), and prior serum & AICAR > AICAR aloned (p<0.001). For muscles incubated with insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001). Post hoc analysis revealed pAktT308 with prior serum > No AICAR or seruma (p<0.05) and prior serum & AICAR > AICAR aloned (p<0.01). B) Two-way ANOVA indicated a main effect of insulin (p<0.001). Post hoc analysis revealed pAktT308 with Iinsulin > No AICAR or serum (p<0.001), and IAICAR+insulin > AICAR alone (p<0.001).
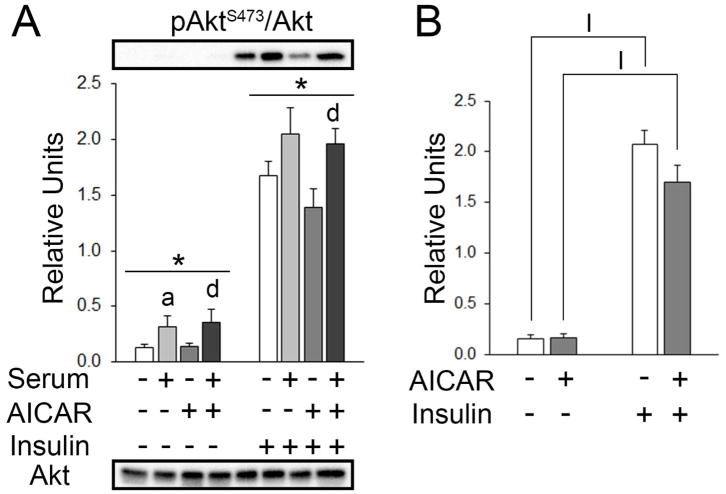
There was a significant main effect of serum on pAktS473 (p<0.05; Figure 5A). Post hoc analysis revealed pAktS473 with serum > no AICAR or serum (p<0.05) and serum+AICAR > AICAR alone (p<0.05; Figure 5A). For muscles incubated with insulin, there was also a significant main effect of serum on pAkt S473 (p<0.001; Figure 5A). Post hoc analysis revealed pAktS473 with prior serum+AICAR > AICAR alone (p<0.05; Figure 5A). In the secondary analysis excluding serum and serum+AICAR, there was a significant main effect of insulin (p<0.001) on pAktS473. Post hoc analysis revealed pAktS473 values with insulin > without AICAR or insulin (p<0.001) and insulin+AICAR > AICAR alone (p<0.001; Figure 5B).
Figure 5.
pAktS473 in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.05). Post hoc analysis revealed pAktS473 with serum > KHBa (p<0.05) and serum & AICAR > AICAR aloned (p<0.05). For muscles incubated with insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001). Post hoc analysis revealed pAktS473 with serum & AICAR > AICAR aloned (p<0.05). B) Two-way ANOVA indicated a main effect of insulin (p<0.001). Post hoc analysis revealed pAktS473 with Iinsulin > No AICAR or serum (p<0.001) and IAICAR+insulin > AICAR (p<0.001). All data are shown as mean±SEM in the graph with panel A containing representative blots for phosphoproteins above the bars and total protein below the bottom graph (n=10/group).
AS160
For the initial statistical analysis on muscles incubated without insulin, there was a main effect of serum on total AS160 abundance (~50% decrease with serum, serum < no serum; p<0.001; Table 1A). For muscles incubated with insulin, there was a main effect of serum on total AS160 abundance (60–70%, serum < no serum; p<0.001; Table 1A). Values for pAS160 are expressed as the ratio of pAS160/total AS160.
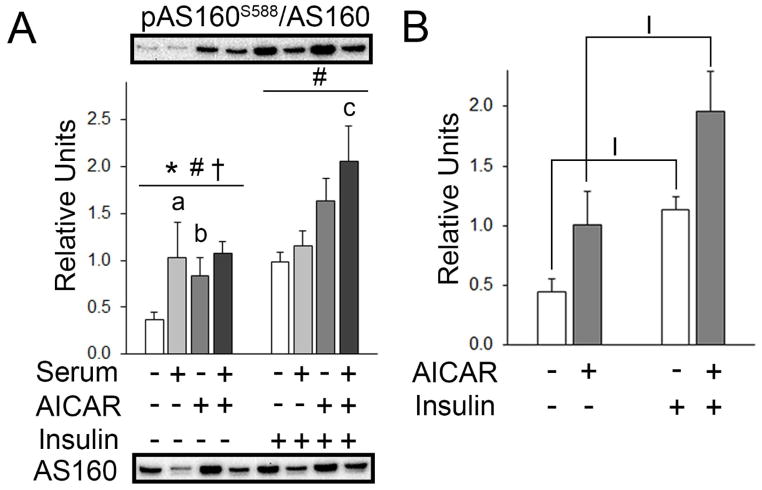
There were significant main effects of serum (p<0.001) and AICAR (p<0.01), and an interaction effect (p<0.01) on pAS160S588. Post hoc analysis revealed pAS160S588 with prior AICAR > No AICAR or serum (p<0.001) and prior serum > No AICAR or serum (p<0.001). For muscles incubated with insulin, there was a significant main effect of AICAR with insulin (p<0.05). Post hoc analysis revealed pAS160S588 with prior serum+AICAR > serum alone (p<0.05; Figure 6A). Secondary analysis for pAS160S588 without serum or serum+AICAR used the Kruskal—Wallis one-way ANOVA and Tukey post hoc tests because transformations failed to normalize the data. This analysis indicated that insulin led to greater pAS160S588 with insulin > no AICAR or insulin (p<0.05) and AICAR+insulin>AICAR alone (p<0.05; Figure 6B).
Figure 6.
pAS160S588 in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001), AICAR# (p<0.01), and a serum x AICAR interaction effect† (p<0.01). Post hoc analysis revealed pAS160S588 with prior serum > No AICAR or seruma (p<0.001) and prior AICAR > No AICAR or serumb (p<0.001). For muscles incubated with insulin, RM 2-way ANOVA indicated a main effect of AICAR# (p<0.05). Post hoc analysis revealed pAS160S588 with prior serum & AICAR > serum alonec (p<0.05). B) In the secondary analysis, Kruskal-Wallis one-way ANOVA on ranks and subsequent pairwise multiple comparison using Tukey Test revealed pAS160S588 with Iinsulin > No AICAR or serum (p<0.05) and IAICAR+insulin > AICAR alone (p<0.05). All data are shown as mean±SEM. The graph in panel A contains representative blots of phosphoproteins above the bars and total protein below the graph (n=10/group).
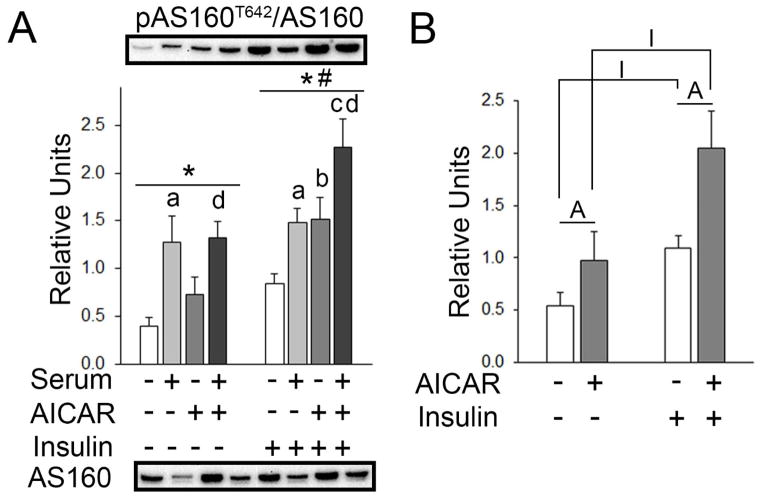
For muscles incubated without insulin, a significant main effect of serum (p<0.001) was present on pAS160T642. Post hoc analysis revealed pAS160T642 with prior serum > No AICAR or serum (p<0.001) and serum+AICAR > AICAR alone (p<0.05). For muscles incubated with insulin, there were significant main effects of serum (p=0.001) and AICAR (p<0.01) on pAS160T642 (Figure 7A). Post hoc analysis revealed pAS160T642 with prior AICAR > No AICAR or serum (p<0.05), prior serum > No AICAR or serum (p<0.05), prior serum+AICAR > serum (p<0.05), and prior serum+AICAR > AICAR alone (p<0.05; Figure 7A). Secondary analysis using two-way ANOVA found there were significant main effects of insulin (p<0.001) and AICAR on pAS160T642. Post hoc analysis revealed pAS160T642 was increased by AICAR either in the absence (p<0.05) or presence of insulin (p<0.05), and also increased by insulin either in the absence (p<0.01) or presence of AICAR (p<0.01; Figure 7B).
Figure 7.
pAS160T642 in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001). Post hoc analysis revealed pAS160T642 with prior serum > No AICAR or seruma (p<0.001) and serum+AICAR > AICAR aloned (p<0.05). For muscles incubated with insulin, RM 2-way ANOVA indicated a main effect of serum* (p<0.001) and a main effect of AICAR# (p<0.01). Post hoc analysis revealed pAS160T642 with prior serum > no AICAR or seruma (p<0.05), prior AICAR > No AICAR or serumb (p<0.05), prior AICAR+serum > serumc (p<0.05), and prior AICAR+serum > AICAR aloned (p<0.05). B) Two-way ANOVA indicated a main effect of insulin (p<0.001) and a main effect of AICAR (p<0.01). Post hoc analysis revealed pAS160T642 with prior AAICAR > No AICAR or serum (p<0.05), Iinsulin > No AICAR or serum (p<0.01), AAICAR+insulin > insulin alone (p<0.05), and IAICAR+insulin > AICAR alone (p<0.001). All data are shown as mean±SEM. The graph in panel A contains representative blots of phosphoproteins above the bars and total protein below the graph (n=10/group).
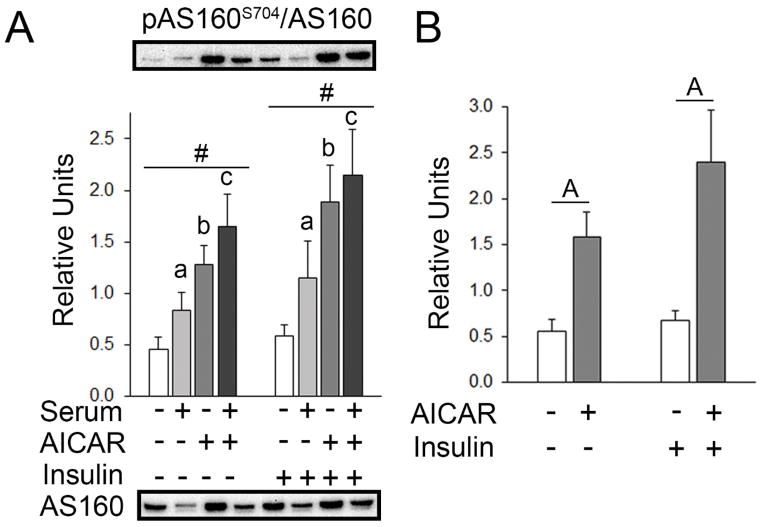
For muscles incubated without insulin, a main effect of AICAR was present on pAS160S704 (p=0.01). Post hoc analysis revealed pAS160S704 with prior serum > KHB (p<0.05), prior AICAR > No AICAR or serum (p<0.01), and prior serum+AICAR > serum alone (p<0.05; Figure 8A). For muscles incubated with insulin, a main effect of AICAR on pAS160S704 was present (p<0.01). Post hoc analysis revealed pAS160S704 with prior serum > the No AICAR or serum (p<0.05), prior AICAR > No AICAR or serum (p=0.001), and prior serum+AICAR > serum alone (p<0.05; Figure 8A). Secondary analysis indicated a main effect of AICAR was present on pAS160S704 (p<0.001). Post hoc analysis revealed that pAS160S704 with AICAR alone > No insulin or AICAR (p<0.001) and with AICAR+insulin > AICAR alone (p<0.001; Figure 8B).
Figure 8.
pAS160S704 in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of AICAR# (p<0.05). Post hoc analysis revealed pAS160S704 with prior serum > No AICAR or seruma (p<0.05), prior AICAR > No AICAR or serumb (p<0.01), and prior serum+AICAR > serum alonec (p<0.05). For muscles incubated with insulin, RM 2-way ANOVA indicated a main effect of AICAR# (p<0.05). Similar to the results without insulin, post hoc analysis for muscles incubated with insulin revealed pAS160S704 with prior serum > No AICAR or seruma (p<0.05), prior AICAR > No AICAR or serumb (p<0.01), and prior serum & AICAR > serum alonec (p<0.05). B) Two-way ANOVA indicated a main effect of AICAR (p<0.001). Post hoc analysis revealed pAS160S704 with prior AAICAR > No AICAR or serum (p<0.001) and AAICAR+insulin > insulin alone (p<0.001). All data are shown as mean±SEM. The graph in panel A contains representative blots of phosphoproteins above the bars and total protein below the graph (n=10/group).
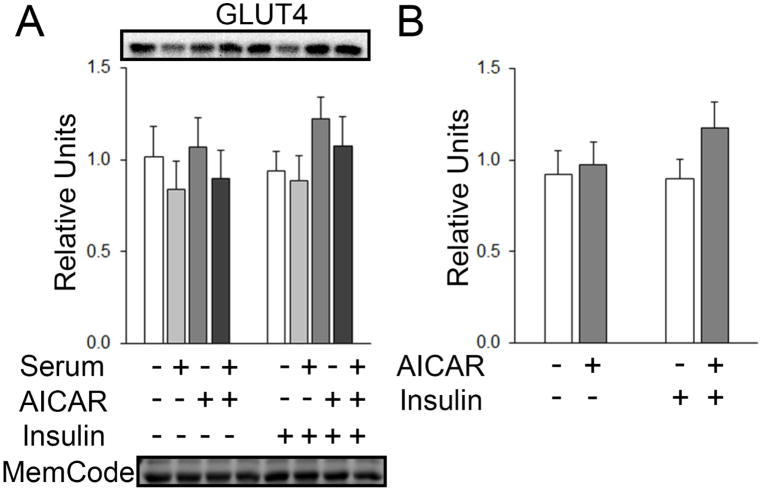
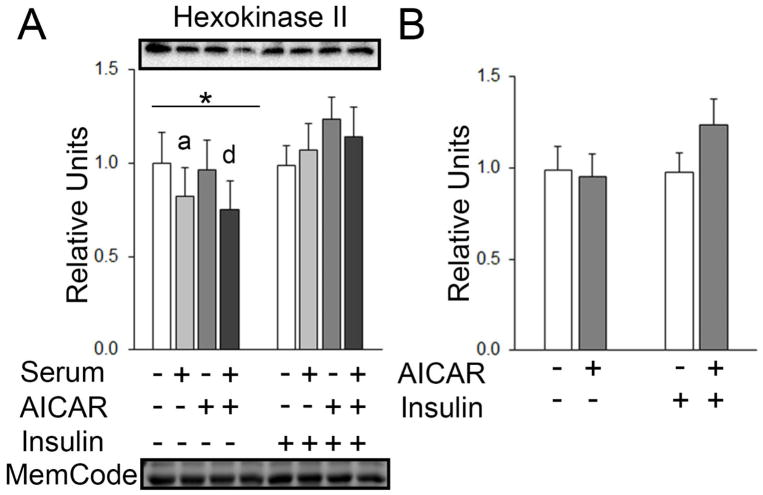
GLUT4 and Hexokinase II
In the initial statistical analysis for muscles treated without and with serum, there was no main effect of serum or AICAR on GLUT4 (Figure 9A). In the secondary analysis, there was no main effect of insulin or AICAR on GLUT4 (Figure 9B). There was no main effect of serum or AICAR on hexokinase II (HKII) with insulin, but for muscles incubated without insulin, there was a significant main effect of serum on HKII expression (p<0.01; Figure 10A). Post hoc analysis indicated HKII with serum < No AICAR or serum (p<0.05) and serum+AICAR < AICAR alone (p<0.01; Figure 10A). There was no main effect of insulin or AICAR on hexokinase II expression (Figure 10B).
Figure 9.
GLUT4 in epitrochlearis muscles. A) RM 2-way ANOVA and B) two-way ANOVA did not indicate any main effect of AICAR or insulin on GLUT4 expression. All data are shown as mean±SEM. All data are shown as mean±SEM in the graph with representative blots above the bars and loading control below the bottom graph (n=10/group). MemCode, a protein stain used to confirm efficacy of transfer before the blocking step, was used as a loading control by quantitating the band at molecular weight 45 kD (Antharavally et al. 2004; Castorena et al. 2014).
Figure 10.
HKII in epitrochlearis muscles. A) For muscles incubated without insulin, RM 2-way ANOVA indicated a main effect of serum* (p=0.001). Post hoc analysis revealed depressed HKII with prior serum < No AICAR or seruma (p<0.05) and prior serum+AICAR < AICAR aloned (p<0.01). No effects were observed in muscles incubated with insulin. B) Two-way ANOVA did not indicate any main effect of AICAR or insulin on HKII expression. All data are shown as mean±SEM in the graph with representative blots above the bars and loading control below the bottom graph (n=10/group). MemCode, a protein stain used to confirm efficacy of transfer before the blocking step, was used as a loading control by quantitating the band at molecular weight 45 kD (Antharavally et al. 2004; Castorena et al. 2014).
Discussion
The primary aim of the current study was to determine whether a single brief, prior exposure to the AMPK activator AICAR would lead to subsequently increased glucose uptake in insulin-stimulated skeletal muscles from old rats. Accordingly, the most important finding in this study of old rats was that prior AICAR treatment resulted in enhanced glucose uptake in muscles that were subsequently stimulated with insulin compared to muscles without prior AICAR treatment. Prior serum incubation was not essential for this effect, i.e., it was found with both the initial statistical analysis that included the serum-treated muscles and the secondary statistical analysis that eliminated the serum-treated conditions. Prior AICAR treatment led to greater pAMPKT172, pAS160S704 and pAS160T642 without altering pAktS473, pAktT308 or the abundance of GLUT4 or HKII. In addition, prior serum+AICAR treatment, but not serum or AICAR alone, led to greater glucose uptake only in the absence of insulin. The effects of AICAR and/or serum on glucose uptake were not attributable to increases in GLUT4 or HKII protein abundance.
The design for the current study examining male 24 month old rats was primarily based on the protocol developed in the original study by Fisher et al. (2002) who evaluated young (120–140 g, likely ~6 week old) male rats. The current finding that a single, brief prior exposure to AICAR treatment increased insulin-stimulated glucose uptake by isolated muscles is consistent with two earlier studies that evaluated isolated muscles from young animals (Fisher et al. 2002; Kjobsted et al. 2015). Similar to the current study, Fisher et al. (2002) reported that in young rats, insulin-stimulated glucose uptake by isolated epitrochlearis muscles 3.5 h after incubation with rat serum+AICAR exceeded values for muscles incubated in serum without AICAR or for muscles that were incubated with AICAR alone. Kjøbsted et al. (2015) evaluated the effects of prior AICAR and serum on subsequent glucose uptake by isolated muscles from female mice. The mice were ~4 months of age when studied (Rasmus Kjøbsted, personal communication). Similar to the current study, they reported that glucose uptake by insulin-stimulated extensor digitorum longus (EDL) muscles was greater in muscles after exposure to human serum+AICAR versus muscles incubated with only serum (Kjobsted et al. 2015). In one of their four experiments, prior serum+AICAR compared to controls also resulted in a small, but significant increase in EDL glucose uptake in the absence of insulin, and there was a non-significant trend for an increase in their other three experiments (Kjobsted et al. 2015). Although it is uncertain if muscles from old female rats would respond similarly to the old male rats in the current study, the results from Kjøbsted et al. (2015) indicate that the enhanced insulin-stimulated glucose uptake after AICAR treatment can also occur in females (Kjobsted et al. 2015).
There were several differences with regard to effects of serum and/or AICAR on glucose uptake among the current study and the previous ones. The current study found that without insulin, prior serum+AICAR led to subsequently enhanced glucose uptake in muscle from old rats whereas Fisher et al. found glucose uptake was not different in muscles incubated without insulin after serum+AICAR treatment versus no serum and AICAR (Fisher et al. 2002). In the current study, prior AICAR incubation without serum effectively increased insulin-stimulated glucose uptake by epitrochlearis muscles from old rats, whereas Fisher et al. found that prior AICAR incubation without serum did not enhance insulin-stimulated glucose uptake by epitrochlearis muscles from young rats (Fisher et al. 2002). The current study versus the study by Fisher et al. was identical with regard to species, sex, use of epitrochlearis muscle, incubation durations, AICAR concentration, and glucose analog, but they differed with regard to the age and strain of the rats and insulin concentration (200 versus 30 μU/ml) (Fisher et al. 2002). The current study and the study by Kjøbsted et al. differed with regard to species, sex, muscles, source of serum (rat versus human), AICAR concentration (2 versus 1 mM), duration of incubation after AICAR exposure (3.5 versus 6.5 h), feeding status of their animals (fasted versus fed), and glucose analog (3-MG versus 2-deoxyglucose). In addition, they did not report the independent effects of either serum or AICAR and did not test if serum was required for the effect of prior AICAR treatment on insulin-stimulated glucose uptake by EDL muscles from young mice (Kjobsted et al. 2015). Thus, three studies have evaluated the effects of prior AICAR treatment on insulin-stimulated glucose uptake by isolated rodent muscles and found that prior AICAR consistently leads to a subsequent increase in insulin sensitivity in rat epitrochlearis and mouse EDL. However, these studies have varied with regard to the effects of prior serum and/or AICAR incubation on subsequent insulin-independent glucose uptake and with regard to the serum being necessary for the effect of prior AICAR on insulin-stimulated glucose uptake. The reasons for differing results among these studies are unclear, including why prior AICAR treatment in the absence of serum resulted in greater glucose uptake in insulin-stimulated muscles in the current study. It seems possible that the use of substantially older animals in the current study may be particularly relevant for the different results compared to the findings of the previous studies with much younger animals.
With or without serum, prior AICAR treatment resulted in greater pAMPKT172 in muscles that were subsequently incubated either with or without insulin. These results correspond with the observations by Kjøbsted et al. (Kjobsted et al. 2015) in isolated mouse EDL muscles several hours after incubation with serum+AICAR. In the current study, the magnitude of the AICAR-induced increase in pAMPKT172 was similar for muscles incubated 1) with or without serum and 2) with or without insulin. However, insulin-independent glucose uptake was increased only for the muscles after prior incubation with serum+AICAR. AS160 phosphorylation on both Thr642 and Ser588 has been linked to enhanced glucose uptake, and phosphorylation on both sites was increased in the serum+AICAR muscles compared to muscles that were incubated in the absence of either serum or AICAR. However, in the absence of insulin, prior incubation with serum alone induced a similar increase in phosphorylation on both sites compared to serum+AICAR without causing increased insulin-independent glucose uptake. In the presence or absence of insulin, pAS160S704 was also increased with prior serum+AICAR compared to serum alone, but it was not greater than AICAR alone, which did not increase insulin-independent glucose uptake. Taken together, these results indicate that neither enhanced AMPK nor AS160 phosphorylation is sufficient to explain the influence of prior serum+AICAR on insulin-independent glucose uptake.
We also probed the effects of prior AICAR treatment on Akt and other phosphorylation sites on AS160 to gain insights into the observed influence of AICAR on subsequent insulin-stimulated glucose uptake. Incubation of skeletal muscle with AICAR mimics several effects of exercise, including both AMPK activation and a subsequent increase in insulin-stimulated glucose uptake several hours after exercise cessation. It has been suggested that prior exercise and prior AICAR treatment may share some of the same mechanisms to induce improved insulin sensitivity (Kjobsted et al. 2015). Substantial evidence indicates that increased insulin-stimulated glucose uptake after acute exercise is not secondary to enhanced insulin signaling at proximal steps including activation of Akt (Cartee 2015a; Castorena et al. 2014; Funai et al. 2010; Funai et al. 2009; Schweitzer et al. 2012). However, a number of studies have found greater phosphorylation of AS160 on Thr642 and/or Ser588, key phosphosites for insulin-stimulated GLUT4 translocation, concomitant with improved muscle insulin sensitivity (Cartee 2015a). Kjøbsted et al. (2015) recently demonstrated that prior AICAR treatment can elevate Thr642 phosphorylation in mouse skeletal muscle. Furthermore, they found that prior AICAR resulted in greater phosphorylation on Ser704, an AMPK phosphosite. Importantly, they reported that effects of prior AICAR on pSer704, pThr642 and insulin-stimulated glucose uptake were eliminated in muscles of mice with genetically induced AMPK deficiency. They also discovered that mutating AS160 Ser704 to Ala704 prevented AS160 phosphorylation on this AMPK-phosphosite and resulted in substantially lower insulin-stimulated pThr642, strongly suggesting that pSer704 favors greater pThr642 (Kjobsted et al. 2015). Therefore, we evaluated the influence of prior AICAR on AS160 phosphorylation. Analysis that excluded the serum-treated conditions in the current study clearly demonstrated that prior AICAR-treatment increased AS160 phosphorylation on both Ser704 and Thr642, either in the absence or presence of insulin. When the serum-treated conditions were also included in the statistical analysis, AS160 phosphorylation on all three sites was increased by AICAR-treatment in the insulin-stimulated muscles, and prior AICAR also induced elevated pSer588 and pS704 in muscles incubated without insulin. In the insulin-stimulated muscles, there was an effect of prior serum-treatment on Akt phosphorylation on both Ser473 and Thr308, but there was no effect of AICAR on Akt phosphorylation on either site. These results in response to prior AICAR-treatment are similar to the previously reported observation that prior acute exercise does not amplify proximal insulin signaling steps (Castorena et al. 2014).
TBC1D1 is an AS160 analog that can be phosphorylated on Ser231 by AMPK and on Thr590 by Akt (Cartee 2015b). However, greater TBC1D1 phosphorylation on these sites does not appear to be sufficient for greater insulin-stimulated glucose uptake after prior AICAR treatment (Kjobsted et al. 2015). Furthermore, either prior exercise or prior electrically stimulated muscle contraction leading to subsequently increased insulin-stimulated glucose uptake does not lead to a subsequent increase in TBC1D1 phosphorylation on these sites (Kjobsted et al. 2015; Pehmoller et al. 2012).
Although AICAR has long been known to enter cells and activate AMPK (Corton et al. 1995), AICAR also has biological actions that occur separate from AMPK activation (Rao et al. 2016). However, the effects of prior AICAR treatment on glucose uptake in insulin-stimulated skeletal muscle observed in the current study seem likely to be AMPK-dependent given that AICAR effects on insulin-mediated glucose uptake were reported to be abolished in multiple genetic mouse models with deficient AMPK activity (Kjobsted et al. 2015).
Sustained or repeated intermittent AMPK activation by AICAR can lead to increased GLUT4 expression in skeletal muscle of young rats (Holloszy 2011). Qiang et al. (2007) reported that daily injections of AICAR for one week could also increase GLUT4 protein abundance and both basal and insulin-stimulated glucose uptake in skeletal muscle of 24 month-old rats. Neither Akt nor AS160 phosphorylation were reported in this earlier study (Qiang et al. 2007). In contrast, none of the observed increases in glucose uptake in the current study were attributable to greater GLUT4 protein abundance. These results suggest that the effects of acute prior serum and/or AICAR on glucose uptake, both with and without insulin, were likely related to differences in GLUT4 translocation. Although there is evidence that AMPK activation by AICAR can induce increased HKII expression in skeletal muscle (Ojuka et al. 2000; Stoppani et al. 2002), prior AICAR treatment did not alter HKII protein abundance in this study, and HKII would not influence the uptake of the non-metabolizable glucose analog used to assess glucose uptake in these experiments.
In conclusion, previous research has demonstrated that older rats are able to increase insulin-stimulated glucose uptake by skeletal muscle after acute exercise (Cartee et al. 1993; Xiao et al. 2013). However, because some older individuals may have an impaired capacity to perform exercise, it would be useful to identify alternative approaches to induce some of the important health-related benefits of exercise, including increased insulin sensitivity. The current study offers novel evidence that a brief prior exposure to AICAR, a pharmacological activator of AMPK, can increase glucose uptake in insulin-stimulated isolated skeletal muscles from old rats. The results also provide insights into potential mechanisms for this outcome. Our working hypothesis is that the mechanism in the muscles from older rats involves the AMPK-AS160 signaling axis that was identified by Kjøbsted et al. (2015) in their study of the effects of prior AICAR on insulin-stimulated glucose uptake in isolated mouse skeletal muscle (Kjobsted et al. 2015).
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health to GDC (R01 AG10026) and from the National Institute on Aging (Training Grant 000114).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GDC conception and design of research; KO and EBA performed experiments; KO analyzed data; KO and GDC interpreted results of experiments; KO and GDC drafted manuscript; KO, EBA, and GDC edited and revised manuscript; GDC approved final version of manuscript; KO prepared figures.
Ethics approval
All procedures performed with the rats were approved by the Institutional Animal Care and Use Committee at the University of Michigan and are in accordance with all standards set forth by federal, state, and local authorities.
Contributor Information
Kentaro Oki, Muscle Biology Laboratory – School of Kinesiology, 401 Washtenaw Ave, University of Michigan, Ann Arbor, MI, USA.
Edward B. Arias, Muscle Biology Laboratory – School of Kinesiology, 401 Washtenaw Ave, University of Michigan, Ann Arbor, MI, USA
Makoto Kanzaki, Graduate School of Biomedical Engineering, Tohoku University, Sendai, Japan.
Gregory D. Cartee, Muscle Biology Laboratory – School of Kinesiology, 401 Washtenaw Ave, University of Michigan, Ann Arbor, MI, USA. Department of Molecular and Integrative Physiology and The Institute of Gerontology, University of Michigan, Ann Arbor, MI, USA
References
- Antharavally BS, Carter B, Bell PA, Krishna Mallia A. A high-affinity reversible protein stain for Western blots. Anal Biochem. 2004;329(2):276–280. doi: 10.1016/j.ab.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab. 2015a;309(12):E949–959. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD. Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia. 2015b;58(1):19–30. doi: 10.1007/s00125-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;2685(Pt 1):E902–909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol (1985) 1993;75(2):972–978. doi: 10.1152/jappl.1993.75.2.972. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23(6):1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes. 2014;63(7):2297–2308. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National Diabetes Statistics Report. 2017. [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86(8):3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282(1):E18–23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298(5):E999–1010. doi: 10.1152/ajpendo.00758.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297(1):E242–251. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol. 1999;84(1A):11J–14J. doi: 10.1016/s0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol. 2011;1(2):921–940. doi: 10.1002/cphy.c100052. [DOI] [PubMed] [Google Scholar]
- Iglesias MA, Ye JM, Frangioudakis G, Saha AK, Tomas E, Ruderman NB, Cooney GJ, Kraegen EW. AICAR administration causes an apparent enhancement of muscle and liver insulin action in insulin-resistant high-fat-fed rats. Diabetes. 2002;51(10):2886–2894. doi: 10.2337/diabetes.51.10.2886. [DOI] [PubMed] [Google Scholar]
- Kim J, Solis RS, Arias EB, Cartee GD. Postcontraction insulin sensitivity: relationship with contraction protocol, glycogen concentration, and 5′ AMP-activated protein kinase phosphorylation. J Appl Physiol (1985) 2004;96(2):575–583. doi: 10.1152/japplphysiol.00909.2003. [DOI] [PubMed] [Google Scholar]
- Kjobsted R, Munk-Hansen N, Birk JB, Foretz M, Viollet B, Bjornholm M, Zierath JR, Treebak JT, Wojtaszewski JF. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes. 2017;66(3):598–612. doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- Kjobsted R, Pedersen AJ, Hingst JR, Sabaratnam R, Birk JB, Kristensen JM, Hojlund K, Wojtaszewski JF. Intact Regulation of the AMPK Signaling Network in Response to Exercise and Insulin in Skeletal Muscle of Male Patients With Type 2 Diabetes: Illumination of AMPK Activation in Recovery From Exercise. Diabetes. 2016;65(5):1219–1230. doi: 10.2337/db15-1034. [DOI] [PubMed] [Google Scholar]
- Kjobsted R, Treebak JT, Fentz J, Lantier L, Viollet B, Birk JB, Schjerling P, Bjornholm M, Zierath JR, Wojtaszewski JF. Prior AICAR stimulation increases insulin sensitivity in mouse skeletal muscle in an AMPK-dependent manner. Diabetes. 2015;64(6):2042–2055. doi: 10.2337/db14-1402. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271(49):31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Kumari M, Brunner E, Fuhrer R. Minireview: mechanisms by which the metabolic syndrome and diabetes impair memory. J Gerontol A Biol Sci Med Sci. 2000;55(5):B228–232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action. Longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318(19):1217–1225. doi: 10.1056/NEJM198805123181901. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT-4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol (1985) 2000;88(3):1072–1075. doi: 10.1152/jappl.2000.88.3.1072. [DOI] [PubMed] [Google Scholar]
- Pehmoller C, Brandt N, Birk JB, Hoeg LD, Sjoberg KA, Goodyear LJ, Kiens B, Richter EA, Wojtaszewski JF. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes. 2012;61(11):2743–2752. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39(4):535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- Rao E, Zhang Y, Li Q, Hao J, Egilmez NK, Suttles J, Li B. AMPK-dependent and independent effects of AICAR and compound C on T-cell responses. Oncotarget. 2016;7(23):33783–33795. doi: 10.18632/oncotarget.9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22(17):6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. J Appl Physiol (1985) 2012;113(12):1852–1861. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Wang H, Arias EB, Castorena CM, Cartee GD. Mechanisms for independent and combined effects of calorie restriction and acute exercise on insulin-stimulated glucose uptake by skeletal muscle of old rats. Am J Physiol Endocrinol Metab. 2015;308(7):E603–612. doi: 10.1152/ajpendo.00618.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppani J, Hildebrandt AL, Sakamoto K, Cameron-Smith D, Goodyear LJ, Neufer PD. AMP-activated protein kinase activates transcription of the UCP3 and HKII genes in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(6):E1239–1248. doi: 10.1152/ajpendo.00278.2002. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54(11):B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Wang H, Sharma N, Arias EB, Cartee GD. Insulin Signaling and Glucose Uptake in the Soleus Muscle of 30-Month-Old Rats After Calorie Restriction With or Without Acute Exercise. J Gerontol A Biol Sci Med Sci. 2016;71(3):323–332. doi: 10.1093/gerona/glv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113(12):909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- Weon BM, Je JH. Trends in scale and shape of survival curves. Sci Rep. 2012;2:504. doi: 10.1038/srep00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Sharma N, Arias EB, Castorena CM, Cartee GD. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. Age (Dordr) 2013;35(3):573–582. doi: 10.1007/s11357-012-9383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]