Abstract
Despite the unprecedented tumor regression and long-term survival benefit observed with anti-Programmed Death (PD) (anti-PD-1 or anti-B7-homolog 1 (B7-H1)) therapy in patients with advanced cancers, a large portion of patients do not benefit from such treatment and a fraction of responders relapse. Current efforts to overcome resistance and improve efficacy of anti-PD therapy require a clear understanding of resistance, and should precede current avenues using random combinations with available treatment regimens. Here, we categorized three types of resistance, namely target-missing, primary, and acquired resistance. This categorization requires reliable, accurate tissue sampling and appropriate interpretation of results based on the four classifications of tumor immunity in the microenvironment (TIME). We believe that fundamental understanding of these complex tumor-immune interactions and of the cellular and molecular mechanisms underlying these types of true resistance is the key for targeting the right targets in combination with or beyond anti-PD therapy in the future.
Keywords: B7-H1, PD-1, resistance, cancer, immunotherapy
Anti-Programmed Death (PD) therapy in cancer
Conventional chemotherapy and targeted therapy in cancer are often limited in their efficacy and durability, and are associated with high toxicity. Utilizing a patient’s own immune system to fight against cancer is one solution with the potential to overcome these problems and has been considered an attractive therapeutic approach for decades. However, various immunotherapy approaches based on general enhancement of normal immune mechanisms to fight tumors have faced similar issues as conventional therapies, with limited efficacy (e.g. cancer vaccines) [1], high toxicity (e.g. Interleukin-2 (IL-2) and anti-Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy) [2] and, in the case of targeted therapies, narrow indications (e.g. IL-2 and anti-CTLA-4 therapy for melanoma and chimeric antigen receptor-T (CAR-T) therapy for CD19+ lymphoid leukemia/lymphoma) [2, 3]. Recently, anti-PD therapy has rapidly taken the center stage of cancer treatment. A conceptual breakthrough in understanding the limitations of immune responses to cancer occurred when selective upregulation of B7-H1, and the resulting B7-H1/PD-1-mediated T cell dysfunction in the tumor microenvironment (TME), were found to play major roles in impairing spontaneous immune response and the efficacy of immunotherapy [4, 5], a mechanism termed “adaptive resistance” [6]. Based on these findings, blocking monoclonal antibodies (mAbs) were developed to normalize defective immune responses, rather than over-boosting immune responses, leading to drastic efficacy and durability without serious toxicity in cancer patients. This “normalization” approach has become a major focus in the current development of cancer immunotherapies for solid tumors. Understanding immune responses and immune regulatory mechanisms in the TME, therefore, represents a key future direction to achieve successful outcomes from immunotherapies.
B7-H1 (CD274, PD-L1) was initially discovered by searching for immunoglobulin (Ig)-like molecules that are highly upregulated in human cancer tissues with immune suppressive functions [4]. Subsequently, B7-H1 was found to interact with PD-1 [7] which was shown to be immune inhibitory in an experimental autoimmune disease setting [8]. A series of studies showed that B7-H1 in the TME, especially on cancer cells, could suppress T cell functions [5, 9–11] through adaptive resistance [6, 12]. Blocking the interaction between B7-H1 and PD-1 by either anti-B7-H1 or anti-PD-1 monoclonal antibodies (mAbs) restored T cell activity [10, 13]. In addition to the PD-1/B7-H1 interaction, B7-H1 could also interact with B7-1, and PD-1 could bind PD-L2 (B7-DC); both associations induced inhibitory fuctions [14–16]. However, the roles of these interactions in cancer immunity has not been fully elucidated. Importantly, B7-H1 or PD-1 mAbs which block the B7-H1/PD-1 interaction showed similar clinical activity in many types of human cancers. These data support that the B7-H1/PD-1 interaction is a major contributor to the suppression of tumor-specific T cells. These pre-clinical findings were subsequently translated to the clinic, where mAbs blocking the PD pathway demonstrated durable anti-tumor responses and less immune-related adverse effects than CTLA-4 mAb in cancer patients. The first PD-1 mAb, nivolumab, was approved for the treatment of advanced melanoma in 2014 by the US Food and Drug Administration (FDA). With subsequent approval of multiple mAbs to PD-1 and PD-L1 for more than 10 different cancer indications today, including cancers of the lung, kidney, head and neck, bladder, liver, stomach, colon and others, anti-PD therapy has become the pinnacle of the treatment armamentarium for advanced human cancers.
Most of early clinical trials in melanoma and lung cancer were conducted in all-comer patients, partially due to the technical challenge to define reliable biomarkers to precisely identify patients who will respond to anti-PD therapy. These studies left an impression that only a fraction of cancer patients with solid tumors respond to anti-PD therapy. Despite outstanding response rates and the durability of the response to anti-PD therapy, some responders also relapse over time. Due to lack of reliable biomarkers, much of the current discussion regarding “resistance” to anti-PD therapy is quite confusing. In this opinion article, we will define true resistance and propose the approaches to overcome resistance based on mechanistic insight.
The adaptive resistance hypothesis
The concept of adaptive resistance mediated by the B7-H1/PD-1 pathway as a mechanism of cancer evasion of immunity came to fruition based on the following findings: 1) B7-H1 cell surface protein was upregulated on a large number of human cancer tissues, whereas cultured cancer cell lines were largely negative; 2) in general, normal tissues and cells did not express B7-H1, with the exceptions of tonsil, placenta and a small portion of macrophage-like cells in the liver and lung; 3) B7-H1 on cancer cell lines, as well as normal cells, could be rapidly upregulated by interferon gamma (IFN-γ) in vitro and withdrawal of IFN-γ correlated with the loss of B7-H1[5]; 4) in vitro human tumor/effector T cell co-culture systems and in vivo mouse experiments showed that expression of B7-H1 confered resistance of tumors to T cell-mediated destruction; and 5) the mAb which blocked the interaction of B7-H1/PD-1 eliminated this resistance and enhanced tumor immunity in vitro and in vivo [5]. As IFN-γ is largely produced by tumor antigen-specific T cells as an effector molecule upon recognition of tumor antigens, we subsequently hypothesized that recognition of tumor antigens by T cells may lead to activation of effector T cells and subsequent secretion of IFN-γ, leading to upregulation of B7-H1 on tumor cells. Upregulated B7-H1 binds to PD-1 on T cells adjacent to tumor cells and transmits a co-inhibitory signal to dampen T cell activity, such as induction of apoptosis and exhaustion [5, 17–19]. This hypothesis was further supported by histopathological findings that expression of B7-H1 is largely associated geographically with IFN-γ and tumor infiltrating lymphocytes (TILs) in melanoma [6, 20] and in many other human cancers [21, 22]. Mice deficient in IFN-γ carrying an immunogenic tumor eliminated the upregulation of B7-H1 on tumors [23], further supporting that IFN-γ is a major regulator of B7-H1 expression in vivo.
While antigen-specific T cells are the major source of IFN-γ, NK and NKT cells also secrete IFN-γ. However, the contribution of IFN-γ from NK and NKT cells to the upregulation of B7-H1 in vivo remains to be explored. The contribution of each subset of T cells also remains poorly understood, although CD8+ T cells and CD4+Th1 cells are likely to be the major producers of IFN-γ. In our study, B7-H1 expression is associated with T cell infiltration and forms a scattered but not disseminated pattern in tumor tissues. This “adjacent upregulation” of B7-H1 by IFN-γ may be due to the presence of a large number of IFN-γ receptors on tumor and stromal cells, which would bind IFN-γ rapidly and prevent its disseminated distribution. This could also explain frequent upregulation of B7-H1 on myeloid cells in the TME [9, 24] as well as in lymphoid organs [9] [25], because T cells recognize antigens on professional antigen-presenting myeloid cells and subsequently produce IFN-γ. Therefore, upregulation of B7-H1 on myeloid cells in the TME could be viewed as a result of active antigen recognition and activation of T cells. In addition to IFN-γ, type I IFNs were also shown to upregulate B7-H1 [26]. In this regard, expression of B7-H1 on several normal tissues as well as infected organs could be explained by the presence of IFNs, perhaps due to infection and/or inflammation that trigger the production of IFNs. This may even help explain “autoimmune”-like tissue damage during and after anti-PD therapy because pre-existing and subclinical infection and/or inflammation could potentially be amplified to a pathological level.
In our opinion, adaptive resistance can be defined by two minimal criteria: 1) a mechanism which is induced by tumor growth and is developed continuously during tumor progression to resist immune destruction; and 2) a mechanism that is confined to the TME. By this definition, adaptive resistance will be not only theoretically significant but also therapeutically useful as overcoming such mechanisms mainly in the TME will elicit focused and therefore powerful immune responses in the TME while also limiting autoimmune toxicity.
Mechanisms of adaptive resistance in human cancer
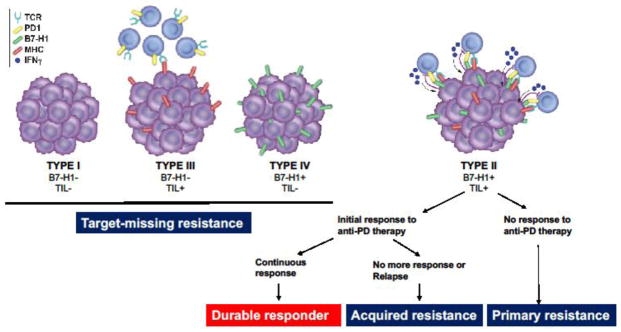
Two essential components of B7-H1/PD-1 pathway-mediated adaptive resistance are: 1) the presence of B7-H1 in the TME; and 2) PD-1+ TILs. Based on this assumption, we categorized the TME into 4 distinct types using surgical specimens of advanced melanoma [6, 12] and lung cancer [21, 22] (Figure 1). This typing method based on tumor immunity in the microenvironment (TIME) was discussed previously [27] and will be briefly summarized here. Tumors with both B7-H1 and TILs (type II) signify the presence of B7-H1/PD-1-mediated adaptive resistance, whereas tumors without either B7-H1 or TILs (type I) indicate no participation of immunity in the TME. Of interest, many tumors are infiltrated with T cells while B7-H1 is not present (type III) and suppression of T cell activity is likely mediated by adaptive resistance mechanisms other than the B7-H1/PD-1 pathway. Finally, there are a significant fraction of patients, especially in non-small cell lung cancer (NSCLC), with B7-H1 expression while TILs are absent (type IV) [21, 22]. The type IV tumors are often associated with several intrinsic genetic alternations such as PTEN loss [28] and epidermal growth factor receptor (EGFR) mutations [29].
Figure 1.
With this molecular categorization, TIME type II patients are predicted to respond to anti-PD therapy while those with other types would not due to lack of one or both key components of the B7-H1/PD-1 pathway. For example, TIME type III patients do not have the expression of B7-H1, although PD-1 may be present on TILs. On the other hand, TIME type IV patients do have B7-H1 while PD-1 is absent due to the absence of TILs. In TIME type I patients, both ligand and receptor are absent. It is important to note that this assessment is based on a single time point; we do not know how much these factors change over time and/or by other therapeutic modelities because many drugs for chemotherapy and targeted therapy are shown to affect immune cell infiltration and activity. These findings emphasize the importance to assess both B7-H1 expression and TILs rather than only using B7-H1 expression for clinical selection of patients who have a high likelihood to respond to anti-PD therapy.
Can we identify patients of TIME type II before anti-PD therapy? B7-H1 expression in the majority of human cancer tissues is heterogeneous: the pattern of expression is typically clustered rather than diffuse within a given tumor [5, 6]. Thus, evaluation of a small amount of tumor tissue via biopsy might miss the B7-H1-positive area. This may be the reason why clinical responses to anti-PD therapy are observed in cases of “B7-H1 negative” tumors. This limitation may be overcome by in vivo imaging with B7-H1 binding agents [30]. In addition, excisional biopsy or surgical tissue resection, to acquire larger tissues if possible, could give a better yield to accurately assess the expression of B7-H1.
As mentioned above, adaptive resistance may include the B7-H1/PD-1 pathway-mediated resistance mechanisms as well as other yet-to-be-discovered mechanisms. While at present, we cannot delineate all mechanisms underlying adaptive resistance, a description based on our current understanding of the B7-H1/PD-1 pathway-mediated adaptive resistance may help us better understand the resistance experienced by cancer patients clinically. These types of resistance include: 1) ”target-missing” resistance; 2) primary resistance, and 3) acquired resistance.
“Target-missing” resistance
Target-missing resistance is defined as the status of patients who do not have one or both targets which are necessary for anti-PD therapy in the TME. Cancer patients with TIME type I, III and IV patients would fall into this category and treatment would unlikely benefit these patients. This type of resistance should not be considered as a true drug resistance due to lack of drug targets. Target-missing resistance may account for 60–80% of solid tumors based on data from melanoma and lung cancer [6, 12, 21, 22] and thus represents a major challenge for future cancer immunotherapy. As the B7-H1/PD-1 pathway is essential for the protection of host cells from inflammation-related tissue damage, the absence of B7-H1 in many cancer tissues itself is an interesting scientific issue and will need to be explored at a basic science level. Therefore, it is important to understand intrinsic mechanisms which cause target-missing resistance.
In TIME type I and IV patients, poor immune infiltration may indicate the presence of molecular mechanisms that prevent inflammation from occurring. While these intrinsic inflammation-preventing mechanisms are yet to be discovered, several studies suggest that decreased production of chemokines may partially account for this observation in several animal tumor models [31] and possibly in melanoma, ovarian cancer, and colon cancer tissues [31–33]. Practically, target-missing resistance in TIME could potentially be overcome by various approaches to initiate inflammation and to promote infiltration of TILs. These approaches include the use of anti-CTLA-4 mAb, local radiation, oncolytic viruses, cancer vaccines, DNA damaging agents, epigenetic modifiers, and adoptive cell therapy; indeed, some of these methods have shown promise in animal models and are being tested in clinical trials together with anti-PD therapy [18]. It remains to be seen, however, if these extrinsic inflammation-producing approaches can overcome intrinsic inflammation-preventing mechanisms.
In TIME type III patients, while immune infiltration is successful, it appears that specific mechanisms which are not involved in the PD-pathway prevent effector T cells from functioning normally. Though specific mechanisms underlying type III resistance are yet to be discovered, abnormal functions of effector T cells may not only be limited to the execution of their effector functions but may also include hindrance in proliferation and maturation of effector T cells. While it is obvious that anti-PD therapy is predicted to be ineffective in this type of patient, in our opinion, type III patients could be vigorously tested therapeutically for the agents that rescue T cell activity including those targeting LAG-3, TIM-3, GITR, PD-1H/VISTA if these pathways/molecules are selectively present in the TME. In addition, type III patients may also be excellent candidates for T-cell costimulatory agents which may overcome T cell dysfunction. The manipulation of these pathways includes, but is not limited to, 4-1BB, OX40, CD27, CD28, and CD40, as long as these molecular pathways are found in the type III TIME patient.
Primary resistance
Primary resistance is the status of patients whose tumors express both B7-H1 and TILs in the TME (TIME type II) but do not respond to anti-PD therapy upon initial treatment. Although B7-H1 positivity in tumor tissues has been shown to correlate with higher response rates to anti-PD therapy in melanoma, lung cancer and bladder cancer than B7-H1 negative patients [34–40], these studies did not test for the presence of TILs, especially CD8+ T cells. Therefore, the populations studied also included TIME type IV patients and do not accurately predict real primary resistance [34, 36, 37, 39]. Nevertheless, previous studies using the TIME categorization method showed that there are some TIME type IV patients in more than 250 advanced melanomas analyzed [6, 20] and other types of cancer. This allows for a more accurate estimate of primary resistance in melanoma. Another issue confounding the assessment of the true percentage of patients who encounter primary resistance is related to non-standardized immunohistochemical criteria used to assess biomarker expression across studies. For example, in an earlier study, 80% of patients with advanced melanoma had PD-L1 expression [clone: 22C3 (Dako Agilent Pathology Solutions), definition of PD-L1 positive is PD-L1 expression >1% in tumor cells], yet the response rate to anti-PD-1 therapy (pembrolizumab) was ~31% [34]. In a study testing nivolumab, ~25% of patients were PD-L1 positive [clone: 28-8 (Dako), definition of PD-L1 positive is PD-L1 expression >5% in tumor cells], but overall response was 57% in PD-L1+ patients [41]. Based on these data, an estimated 45–50% of patients have primary resistance to anti-PD therapy. However, this number could also be affected by various factors such as the initial sampling for PD-L1 expression assessment, efficacy of drugs used for study, and accuracy of immunohistochemistry methods.
We have just started to explore the mechanisms of primary resistance to anti-PD therapy. Currently we do not know how much PD-L1 expression in the TME is required for anti-PD therapy to work. As the level of PD-L1 expression in TIME type II patients may impact the strength of T cell-mediated inflammation, a quantitative method will need to be developed, preferentially by in vivo imaging, to accurately predict the relationship between PD-L1 expression and the efficacy of anti-PD therapy. In addition to the expression of PD-L1 on cancer cells, it is also important to assess the expression of essential molecules for antigen processing and presentation such as MHC class I/II, TAP-1/2 and others. The deficiency of these molecules has been shown to affect T cell-mediated recognition in the effector phase of the immune response, and therefore, may be critical for primary resistance to anti-PD therapy.
While the presence of TILs is essential to define primary resistance, it is still unclear what the key cellular and molecular components are that dictate the primary resistance. The presence of CD8+ and type 1 helper T cells (Th1) in the TME is more consistently associated with better prognosis [42–45] and is likely to be an essential cellular component to prevent primary resistance. However, the roles of other TIL components including subsets of CD4+ T cells, natural killer (NK) cells, natural killer T (NKT) cells, B cells, regulatory T cells (Treg) and γδ T cells are less clear in the TME. In addition, the presence of Treg and myeloid derived suppressor cells (MDSC) may also contribute to primary resistance. A comprehensive cellular and molecular analysis of TIME type II patient tumors should help us identify the patients at highest risk for primary resistance.
Acquired resistance
Acquried resistence to anti-PD therapy is defined as the recurrence of tumors following clear signs of clinical responses (partial or complete response). In advanced melanoma, 25–35% of initial responders to pembrolizumab relapsed within 2–3 years of follow-up [46]. In NSCLC, 34–37% of initial responders to nivolumab relapsed in a 2-year follow-up [47]. Can we say these patients have acquired resistance to anti-PD therapy? It is possible to re-induce a response in patients with acquired resistance; it was shown that 30–50% of relapsed patients who stopped the drugs after a positive clinical response responded to anti-PD therapy upon re-initiation [48]. While we expect more data to confirm relapse rates in long-term follow-up studies, an evaluation of B7-H1 expression and TILs needs to be conducted. If TIME remains type II in these relapsed patients, this may explain the re-induction effect. The underlying mechanism of this re-induction effect is unclear but it is possible that these patients may undergo transient down-regulation of B7-H1 and/or loss of T cell infiltration in the TME after initial anti-PD therapy. Many recurrent lesions are new and not always the primary lesions at the starting point of treatment [48, 49], possibly indicating that T cell immunity may ignore small tumors due to lack of sufficient antigenic stimulation [50]. If patients who do not respond to reinduction of anti-PD therapy despite the fact that they are type II TIME, it may be a true acquired resistance to anti-PD therapy.
Acquired resistance to anti-PD therapy drugs is largely a clinical definition: underlying mechanisms have not fully been elucidated to define real acquired resistance. Our understanding of the cause and effect, as well as the necessary cellular and molecular components of acquired resistance, is rudimentary. There is no obvious biomarker to predict or determine the development of acquired resistance and it is unknown if this resistance is caused by the complete loss of B7-H1 and/or the absence of TILs. Nevertheless, several studies indicate that down-regulation of tumor antigen presentation, possibly via genetic deletions, genetic mutation, or epigenetic changes affecting the antigens themselves or antigen-presentation machinery, may contribute to acquired resistance [46, 51]. Alterations of other immune modulatory molecules and intracellular pathways including JAK1 and JAK2, STAT1, and TIM-3 were also found to correlate with relapse [46, 52] [53, 54]. These studies provide initial evidence that acquired resistance may involve substantial alteration and evolution of cancer cells and immune cells in the TME.
Concluding Remarks
The discovery of the B7-H1/PD-1 pathway and elucidation of its role in adaptive resistance and its application to cancer medicine have revived cancer immunology and immunotherapy and set a new benchmark for future advances to further improve the treatment of cancer. Normalization of defective immune responses in the TME, while preserving homeostasis of the immune response systemically thus represents a major conceptual progress. The catagories and classifications of highly heterogeneic TMEs in this article is an initial attempt to understand the mechanisms of adaptive resistance and to highlight the major effort required to continue to evolve this area of research in the future.
Highlights.
Recently, anti-PD therapy has taken center stage for the treatment of cancer, especially for solid tumors
While anti-PD therapy is highly effective in some patients (response), others do not respond (resistance).
A proper understanding of the tumor micro-environment, both in terms of PD-L1/B7-H1 expression and of immune infiltration, is critical to separate true primary resistance from “target-missing” resistance
Additionally, acquired resistance may occur in some relapsing patients, although its causes and markers remain ill-defined
Clear definitions and mechanistic understanding of these distinct resistances will be critical to improve the efficacy of cancer immunotherapy
Outstanding Questions Box.
What have we learned from the success of anti-PD therapy?
Why are some patients affected while others are not?
How to identify the patients in which therapy will be effective and ineffective?
How to best test new approaches to improve cancer therapy?
Acknowledgments
We apologize for not being able to cite all significant publications due to page limits. We thank Beth Cadugan for editing the manuscript. This study was financially supported by NIH grants P50 CA196530, P30 CA16359 and an endowment from United Technologies Corporation.
Footnotes
Conflict of interest:
Dr. Tae Kon Kim declares no competing interest. Dr. Roy Herbst is a consultant for AstraZeneca, Merck, Genentech/Roche, Lilly, Bristol-Myers Squibb, and a scientific advisor of NextCure. Dr. Lieping Chen is a scientific advisor for Pfizer, NextCure, GenomiCare and Vcanbio and has sponsored research funding from Boehringer Ingelheim, Pfizer and NextCure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wong KK, et al. Advances in Therapeutic Cancer Vaccines. Adv Immunol. 2016;130:191–249. doi: 10.1016/bs.ai.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat Rev Clin Oncol. 2014;11(11):630–2. doi: 10.1038/nrclinonc.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 5.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 6.Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4(127):127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Curiel TJ, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 10.Wong RM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–34. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 11.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer--response. Clin Cancer Res. 2013;19(19):5542. doi: 10.1158/1078-0432.CCR-13-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu K, et al. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–75. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 15.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obeid JM, et al. PD-L1, PD-L2 and PD-1 expression in metastatic melanoma: Correlation with tumor-infiltrating immune cells and clinical outcome. Oncoimmunology. 2016;5(11):e1235107. doi: 10.1080/2162402X.2016.1235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schalper KA, et al. Differential Expression and Significance of PD-L1, IDO-1, and B7-H4 in Human Lung Cancer. Clin Cancer Res. 2017;23(2):370–378. doi: 10.1158/1078-0432.CCR-16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velcheti V, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94(1):107–16. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spranger S, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, et al. The expression profiles and regulation of PD-L1 in tumor-induced myeloid-derived suppressor cells. Oncoimmunology. 2016;5(12):e1247135. doi: 10.1080/2162402X.2016.1247135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima F, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110(1):180–5. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreiner B, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155(1–2):172–82. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Chen L. Classification of Advanced Human Cancers Based on Tumor Immunity in the MicroEnvironment (TIME) for Cancer Immunotherapy. JAMA Oncol. 2016;2(11):1403–1404. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–8. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 29.Akbay EA, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maute RL, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci U S A. 2015;112(47):E6506–14. doi: 10.1073/pnas.1519623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 32.Peng D, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–53. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagarsheth N, et al. PRC2 Epigenetically Silences Th1-Type Chemokines to Suppress Effector T-Cell Trafficking in Colon Cancer. Cancer Res. 2016;76(2):275–82. doi: 10.1158/0008-5472.CAN-15-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 35.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reck M, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 38.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 39.Bellmunt J, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powles T, et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mlecnik B, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44(3):698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 43.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 44.Pages F, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 45.Pages F, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29(8):1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 46.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horn L, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057) J Clin Oncol. 2017 doi: 10.1200/JCO.2017.74.3062. JCO2017743062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipson EJ, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19(2):462–8. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gettinger S, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L. Immunological ignorance of silent antigens as an explanation of tumor evasion. Immunol Today. 1998;19(1):27–30. doi: 10.1016/s0167-5699(97)01180-8. [DOI] [PubMed] [Google Scholar]
- 51.DuPage M, et al. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482(7385):405–9. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benci JL, et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–1554 e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koyama S, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roh W, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. 2017;9(379) doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]