Abstract
Frailty is prevalent in liver transplant candidates, but little is known of what happens to frailty after liver transplantation. We analyzed data for 214 adult liver transplant recipients who had ≥1 frailty assessment using the Liver Frailty Index (LFI) at 3- (n = 178), 6- (n = 139), or 12- (n = 107) months posttransplant (higher values=more frail). “Frail” and “robust” were defined as LFI ≥4.5 and <3.2. Median pre–liver transplant LFI was 3.7, and was worse at 3 months (3.9; P = .02), similar at 6 months (3.7; P = .07), and improved at 12 months (3.4; P < .001). The percentage who were robust pre-and 3-, 6-, and 12-months posttransplant were 25%, 14%, 28%, and 37%; the percentage frail were 21%, 21%, 10%, and 7%. In univariable analysis, each 0.1 pretransplant LFI point more frail was associated with a decreased odds of being robust at 3-(odds ratio [OR] 0.75), 6-(OR 0.77), and 12-months (OR 0.90) posttransplant (P ≤ .001), which did not change substantially with multivariable adjustment. In conclusion, frailty worsens 3 months posttransplant and improves modestly by 12 months, but fewer than 2 of 5 patients achieve robustness. Pretransplant LFI was a potent predictor of posttransplant robustness. Aggressive interventions aimed at preventing frailty pretransplant are urgently needed to maximize physical health after liver transplantation.
Keywords: clinical research/practice, comorbidities, geriatrics, liver transplantation/hepatology, nutrition, patient characteristics, quality of life (QOL), rehabilitation
1 | INTRODUCTION
Patients with cirrhosis are vulnerable to developing physical frailty that results from muscle wasting and undernutrition—2 conditions that are nearly inseparable from the state of cirrhosis itself.1,2 In theory, liver transplantation should reverse these conditions and therefore, reverse physical frailty. However, no objective data exist on the extent to which—or how rapidly—physical frailty improves after liver transplantation. Such information is crucial to informing discussions with patients and caregivers about what to expect after liver transplantation and guiding prognosis regarding quality of life.
One of the major barriers to investigating recovery from physical frailty after liver transplantation has been the frailty measurement tools themselves.3 While several studies have investigated frailty1,4 or aspects of frailty (eg, cardiopulmonary fitness,5,6 disability,7,8) in the pre–liver transplant setting, these tools have characteristics that have hampered efforts to fully understand if, how, and when frailty reverses after liver transplantation. For example, tools such as the Fried Frailty Index or Activities of Daily Living scale are subjective and scored on a noncontinuous scale, making them insensitive to subtle changes over time; cardiopulmonary exercise testing is technically challenging to administer, limiting the number of patients who are able to undergo repeat posttransplant testing.
Recently, we developed the Liver Frailty Index, a tool comprising 3 easily administered tests—grip strength, chair stands, and balance testing—specifically to measure physical frailty in patients with cirrhosis awaiting liver transplantation.9 It has advantages over other frailty assessment tools that have been studied in cirrhotic patients in that it is entirely performance-based, is scored on a continuous scale (making it suitable for investigating longitudinal changes in frailty posttransplant), and perhaps most importantly, is directed towards dimensions of frailty—malnutrition, muscle wasting, and neuromotor coordination—that are most likely to be impacted by end-stage liver disease.
Armed with this Liver Frailty Index as our objective frailty assessment tool, we aimed to investigate physical frailty after liver transplantation.
2 | METHODS
2.1 | Patient population
We analyzed data from the Functional Assessment in Liver Transplantation (FrAILT) Study; the FrAILT Study protocol has been published.9 Briefly, patients with cirrhosis who were listed and active for liver transplantation at the University of California, San Francisco and seen as outpatients were eligible for enrollment. At enrollment, all participants underwent assessment of frailty pretransplant. For this specific study, inclusion criteria were (1) underwent liver transplantation at the University of California, San Francisco from May 2013 to December 2016 (n = 334), (2) had at least 90 days of follow-up posttransplant (n = 283), and (3) had at least frailty measurement posttransplant (n = 214). Exclusion criteria included those with severe hepatic encephalopathy, as defined by the time to complete a Numbers Connection Test10 of >120 seconds (n = 10 excluded), or those who did not speak English or Spanish (n = 15 excluded), as these reasons may impair the patient’s ability to provide informed consent and complete tests of physical frailty.
2.2 | Study procedures: Measurements of frailty
All patients underwent objective measurement of frailty using the following:
Grip strength11: the average of 3 trials in the subject’s dominant hand using a hand dynamometer, measured in kilograms;
Timed chair stands12: measured as the number of seconds it takes to do 5 chair stands with the subject’s arms folded across the chest;
Balance testing12: measured as the number of seconds that the subject can balance in 3 positions (feet placed side-to-side, semitandem, and tandem) for a maximum of 10 seconds each.
These 3 tests were administered by trained study personnel. The Liver Frailty Index was calculated from these 3 tests using the following equation12 (calculator available at: http://liverfrailtyindex.ucsf.edu):
For classifications of frailty, we used previously established cut-offs of the Liver Frailty Index to define “robust” (<3.2), “pre-frail” (between 3.2 and <4.5), and “frail” (≥4.5).9 These cut-offs were selected based on 20th percentile and 80th percentile cut-offs for the Liver Frailty Index in our initial development cohort.9 As a point of clinical reference, the median Liver Frailty Index score of a liver transplant candidate with well-compensated cirrhosis and hepatocellular carcinoma is 3.16, which would be classified as robust.
2.3 | Timing of frailty measurements
Measurements of frailty occurred both before and after liver transplantation in the outpatient clinic setting:
Pretransplant. Due to the unpredictable timing of liver transplantation, patients underwent frailty measurements at every outpatient clinic visit as part of the FrAILT Study. For this particular article, the measurement closest to the date of transplant was used as the “pretransplant” measurement for our analyses.
Posttransplant. Patients underwent repeat measurements of frailty after liver transplantation for up to 1 year. Measurements taken at 3, 6, and 12 months after the date of transplant (±1 month) were used as the posttransplant assessments for our analyses.
2.4 | Additional data collection
Demographic data were extracted from the electronic health record. Patients were considered to have a diagnosis of hypertension or diabetes if this diagnosis was reported in their electronic health record or they were taking medications for either of these diseases (as advancing portal hypertension may affect the manifestation of hypertension or diabetes). Ascites was ascertained from the hepatologists’ recorded physical examination or the management plan and graded as none, mild/moderate, or refractory. Hepatic encephalopathy was determined from the time to complete the Numbers Connection Test10 performed at the time of the frailty measurement, and categorized as none/minimal (<60 seconds) or moderate/severe (≥60 seconds).
Posttransplant outcomes were also extracted from the electronic health record. These outcomes included transplant length of stay, number of days in the intensive care unit (ICU) after transplantation, number of days readmitted within 90 days of transplantation, and death within 90 days of transplantation. Per center protocol, all liver transplant recipients requiring hospitalization within the first 3 months of their transplant are managed at our own center; liver transplant recipients are also required to notify our center of any hospitalization upon admission, facilitating complete data capture of hospitalizations.
2.5 | Statistical analysis
Characteristics were compared between robust, pre-frail, and frail patients using χ2 or Wilcoxon/Kruskal-Wallis tests for categorical and continuous variables, respectively. Liver Frailty Index scores were reported using medians, interquartile ranges (IQR), and standard errors and compared between pre-and posttransplant periods by Wilcoxon signed rank tests to account for paired differences. Logistic regression assessed the odds of being robust at 3-, 6-, and 12-months posttransplant associated with the pretransplant Liver Frailty Index in univariable analysis and then adjusted for covariables that were biologically plausibly associated with frailty, including laboratory Model for End-Stage Liver Disease (MELD) at transplant, recipient age, female sex, and diabetes. Posttransplant outcomes were compared between frail and nonfrail using Wilcoxon rank sum or χ2 tests.
Statistical analyses were performed using Stata (v15, College Station, TX). The Institutional Review Board at the University of California, San Francisco approved this study.
3 | RESULTS
3.1 | Patient characteristics (Table 1)
TABLE 1.
Characteristics of the 214 liver transplant recipients included in this cohort
| Characteristics | All n = 214 | Robust n = 53 (25%) | Pre-frail n = 117 (55%) | Frail n = 44 (21%) | P-value |
|---|---|---|---|---|---|
| Age, y | 62 (56–66) | 63 (59–66) | 62 (57–66) | 60 (55–64) | .08 |
|
| |||||
| Female | 36% | 19% | 39% | 50% | <.01 |
|
| |||||
| Race/ethnicity | |||||
|
| |||||
| Non-Hispanic white | 59% | 70% | 56% | 57% | .15 |
| Black | 4% | 6% | 4% | 0% | |
| Hispanic white | 24% | 11% | 27% | 34% | |
| Asian/Pacific Islander | 8% | 11% | 8% | 5% | |
| Other | 5% | 2% | 6% | 5% | |
|
| |||||
| Cause of liver disease | |||||
|
| |||||
| Chronic hepatitis C | 54% | 53% | 56% | 50% | .69 |
| Alcohol | 13% | 9% | 14% | 14% | |
| Nonalcoholic steatohepatitis | 9% | 8% | 9% | 14% | |
| Autoimmune/cholestatic | 13% | 13% | 14% | 9% | |
| Other | 11% | 17% | 8% | 14% | |
|
| |||||
| Body mass index, kg/m2 | 27 (24–32) | 26 (24–32) | 27 (24–32) | 29 (26–33) | .18 |
|
| |||||
| HCC | 45% | 70% | 42% | 23% | <.01 |
|
| |||||
| Hypertension | 43% | 57% | 40% | 32% | .04 |
|
| |||||
| Diabetes | 25% | 28% | 26% | 20% | .67 |
|
| |||||
| Laboratory MELDNa at frailty measurement | 15 (11–18) | 12 (9–15) | 14 (11–18) | 18 (16–22) | <.001 |
|
| |||||
| Without HCC | 17 (15–21) | 16 (15–19) | 17 (14–22) | 18 (17–24) | .07 |
|
| |||||
| With HCC | 11 (8–14) | 10 (8–13) | 11 (8–14) | 15 (11–18) | .01 |
|
| |||||
| Laboratory MELDNa at transplant | 22 (17–29) | 18 (13–22) | 19 (15–24) | 25 (18–33) | <.01 |
|
| |||||
| Without HCC | 25 (21–32) | 21 (18–30) | 24 (20–32) | 28 (22–39) | .08 |
|
| |||||
| With HCC | 18 (14–22) | 20 (13–25) | 17 (14–20) | 21 (18–28) | .15 |
|
| |||||
| Simultaneous liver/kidney transplant | 7% | 4% | 5% | 16% | .03 |
|
| |||||
| Living donor liver transplant | 19% | 15% | 22% | 16% | .46 |
|
| |||||
| Time from pretransplant frailty measurement to transplant, mo | 2.4 (1.2–4.4) | 2.4 (1.1–4.8) | 2.5 (1.4–5.0) | 1.9 (1.5–3.8) | .23 |
|
| |||||
| Posttransplant follow-up time, mo | 23.2 (15.4–30.0) | 27.3 (19.5–31.6) | 22.6 (15.6–29.2) | 21.9 (12.6–28.4) | .11 |
HCC, hepatocellular carcinoma; MELDNa, Model for End-Stage Liver Disease-Sodium score.
We analyzed data from 214 patients enrolled in the FrAILT Study pretransplant who underwent liver transplantation. Baseline characteristics of the entire cohort are listed in Table 1. Median age was 62 years, 36% were female, 59% were non-Hispanic white, 54% had chronic hepatitis C as their cause of liver disease, and 45% had hepatocellular carcinoma (HCC). Hypertension and diabetes were prevalent in 43% and 25%, respectively. Median (interquartile range [IQR]) laboratory MELD scores at frailty assessment and at transplant for the entire cohort were 15 (11–18) and 20 (15–27). Seven percent underwent simultaneous liver/kidney transplant and 19% underwent living donor liver transplantation. Median (IQR) time from pretransplant frailty measurement to transplant was 2.4 months (1.2–4.4).
Pretransplant, median Liver Frailty Index score was 3.7 (IQR 3.2–4.3; standard error ±0.06). Using previously established cut-offs of <3.2, 3.2–4.4, and ≥4.5 for robust, pre-frail, and frail patients, respectively, 53 (25%) liver transplant recipients were robust, 117 (55%) were pre-frail, and 44 (21%) were frail. Table 1 lists patient characteristics by frailty category. Frail patients (versus pre-frail or robust) were less likely to be male, less likely to be non-Hispanic white, less likely to have chronic hepatitis C, less likely to have HCC, but more likely to have nonalcoholic steatohepatitis. Laboratory MELD scores were higher in frail patients than in pre-frail or robust patients, as were rates of simultaneous liver/kidney transplantation. The median time from pretransplant frailty measurement to liver transplantation was shorter in frail patients at 1.9 months compared to 2.9 months for pre-frail patients and 2.4 months for robust patients.
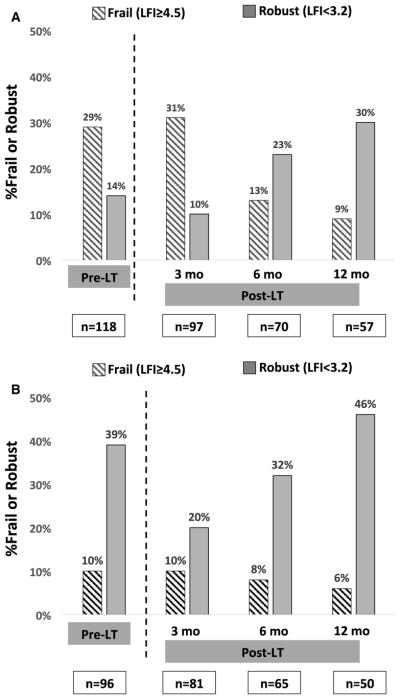
3.2 | Posttransplant frailty measurements (Figure 1)
FIGURE 1.
Proportion of patients who were frail or robust at each time point among those without HCC (A) and with HCC (B). HCC, hepatocellular carcinoma; LFI, Liver Frailty Index; LT, liver transplant
Posttransplant frailty measurements were available for 178 recipients at 3 months, 135 recipients at 6 months, and 107 recipients at 12 months. There were no statistically significant differences in the number of posttransplant assessments by pretransplant frailty category (frail, pre-frail, robust; P = .30). Pretransplant, median Liver Frailty Index was 3.7 (IQR 3.2–4.3; standard error ± 0.06). Compared to the scores pretransplant, median Liver Frailty Index scores worsened 3 months posttransplant (3.9 [IQR 3.5–4.4; standard error ± 0.06]; P = .02), were similar 6 months posttransplant (3.7 [IQR 3.2–4.1; standard error ± 0.06]; P = .07), and improved by 12 months posttransplant (3.4 [IQR 3.0–3.9; standard error ± 0.07]; P < .001). The proportion of patients who were robust and frail at each time point by HCC status is shown in Figure 1.
3.3 | Changes in posttransplant frailty measurements
At 3-, 6-, and 12-months posttransplant, median changes in Liver Frailty Index score were 0.09, −0.04, and −0.28 (where a positive value indicates worsening of frailty status). Compared to pretransplant, 59%, 41%, and 32% experienced worsening of their Liver Frailty Index score at 3, 6, and 12 months, respectively.
Table 2 displays pre-and posttransplant frailty categories among the 107 patients with a frailty measurement 12-months posttransplant. Among the 30 patients who were robust pretransplant, 10 (33%) worsened to pre-frail and 1 (4%) worsened to frail. Among the 22 patients who were frail pretransplant, 14 (64%) improved to pre-frail, but only 3 (14%) improved to become robust.
TABLE 2.
Number of liver transplant (LT) recipients categorized by their pre- and 12-month posttransplant frailty categories
| Pretransplant | 12-mo posttransplant | Total | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Robust | % Post-LT robust | Pre-frail | % Post-LT Pre-frail | Frail | % Post-LT frail | ||
| Robust | 19 | 63% | 10 | 33% | 1 | 4% | 30 |
| % Pre-LT Robust | 47% | 17% | 13% | ||||
| Pre-Frail | 18 | 33% | 35 | 63% | 2 | 4% | 55 |
| % Pre-LT Pre-Frail | 45% | 59% | 25% | ||||
| Frail | 3 | 14% | 14 | 63% | 5 | 23% | 22 |
| % Pre-LT Frail | 8% | 24% | 62% | ||||
| Total | 40 | 59 | 8 | 107 | |||
The patients in the white boxes did not experience any change in their frailty category 12-mo posttransplant. The patients in the dark gray–shaded boxes experienced improvement in their frailty status after LT; the patients in the light gray–shaded boxes experienced worsening of their frailty status 12 mo after LT.
In univariable logistic regression, each 0.1 unit worsening of the pretransplant Liver Frailty Index (ie, increase) was associated with a significantly decreased odds of being robust at 3-(odds ratio [OR] 0.75; P < .001), 6-(OR 0.77; P < .001), and 12-months (OR 0.90; P = .001) posttransplant (Table 2). This association remained nearly unchanged even after adjustment for covariables that might be associated with frailty status, including laboratory MELD score at transplant, recipient age, female sex, and diabetes (Table 3). Donor factors—including donor age, donation after cardiac death, or living donor liver transplantation— were not associated with the odds of being robust posttransplant (P > .05).
TABLE 3.
Unadjusted and adjusted associations between pretransplant Liver Frailty Index and the odds of being robust at 3-, 6-, and 12-months posttransplant
| Variables | Odds ratio (95% confidence interval) for being robust after liver transplant for the pretransplant Liver Frailty Index, per 0.1-unit increase P-value |
||
|---|---|---|---|
|
| |||
| At 3 mo | At 6 mo | At 12 mo | |
| Univariable | |||
|
| |||
| No adjustment | 0.75 (0.67–0.84) | 0.77 (0.70–0.85) | 0.90 (0.85–0.96) |
| <.001 | <.001 | .001 | |
|
| |||
| Multivariable | |||
|
| |||
| + laboratory MELD at transplant | 0.74 (0.66–0.83) | 0.76 (0.68–0.84) | 0.90 (0.85–0.96) |
| <.001 | <.001 | .001 | |
|
| |||
| + laboratory MELD | 0.74 (0.66–0.83) | 0.76 (0.68–0.84) | 0.91 (0.85–0.96) |
| + age at transplant | <.001 | <.001 | .001 |
|
| |||
| + laboratory MELD | 0.74 (0.66–0.84) | 0.77 (0.69–0.85) | 0.91 (0.86–0.97) |
| + age at transplant | <.001 | <.001 | .002 |
| + female sex | |||
|
| |||
| + laboratory MELD | 0.74 (0.66–0.84) | 0.77 (0.69–0.86) | 0.91 (0.86–0.96) |
| + age at transplant | <.001 | <.001 | .002 |
| + female sex | |||
| + diabetes | |||
MELD, Model for End-Stage Liver Disease.
3.4 | Posttransplant outcomes (Table 4)
TABLE 4.
Posttransplant outcomes in liver transplant recipients categorized by their frailty status by their pretransplant Liver Frailty Index
| Posttransplant outcome | Non-frail n = 170 (79%) | Frail n = 44 (21%) | P-value |
|---|---|---|---|
| Transplant LOS | 7 (5–11) | 9 (7–14) | .004 |
| ICU days during transplant hospitalization | 2 (1–3) | 3 (2–5) | .06 |
| Nonhome discharge | 7 (4%) | 4 (9%) | .19 |
| Additional hospital days after transplant, excluding transplant LOS | |||
| 3 mo | 0 (0–3) | 2 (0–9) | .03 |
| 6 mo | 0 (0–5) | 3 (0–17) | .08 |
| 12 mo | 0 (0–6) | 0 (0–5) | .84 |
| Death within 12 mo of transplant | 4 (2%) | 1 (2%) | .94 |
| Death at any time posttransplant | 7 (4%) | 5 (11%) | .06 |
Median (interquartile range) or n (%). Outcomes that are statistically significantly different between the 2 groups are indicated in bold font. ICU, intensive care unit; LOS, length of stay.
For the entire cohort, median (IQR) length of stay for the transplant hospitalization was 7 days (6–11) and the number of days in the ICU was 2 days (1–3). The median (IQR) number of readmission inpatient days within the first 3 months after transplantation was 5 days (3–10). Patients who were frail pretransplant experienced significantly higher transplant length of stay compared to nonfrail patients (9 vs 7 days; P = .004) and hospitalized days within 3 months of transplant (2 vs 0 days; P = .03). There was a trend toward greater ICU days among the frail versus nonfrail patients (3 vs 2 days; P = .06). The proportion of patients who were discharged to an institution after their acute stay (eg, rehabilitation facility, skilled nursing home) was similar in frail versus nonfrail patients (9% vs 4%; P = .19). There was a trend toward a greater median number of hospitalized days during the 3-to 6-month time period following liver transplantation between frail versus nonfrail patients (3 vs 0 days; P = .08), but no significant difference in hospitalized days during the 6–12- month posttransplant time period. Rates of death within 12 months posttransplant were similar between frail and nonfrail patients (2% vs 2%; P = .94). There was a strong trend toward increased rates of death overall among frail versus nonfrail patients (11% vs. 4%; P = .06).
4 | DISCUSSION
Care of patients with cirrhosis awaiting liver transplantation has traditionally focused on management of portal hypertensive manifestations (eg, ascites, hepatic encephalopathy, and gastroesophageal varices).13–15 The “miracle” of liver transplantation is that, by resolving the portal hypertension, these manifestations disappear—nearly immediately upon receiving a new liver. However, cirrhosis also exacts its toll on patients through progressive physical frailty that results from chronic protein synthetic dysfunction, undernutrition, physical inactivity, and cachexia. While physical frailty prior to liver transplantation has, to date, been well-characterized, 1,2 what happens to physical frailty after liver transplantation has not.
In this article, we sought to address this knowledge gap in a cohort of liver transplant candidates who were characterized by their physical frailty status as outpatients prior to liver transplantation. While the majority of liver transplant recipients experienced some degree of improvement in their physical frailty, this improvement occurred gradually, with overall worsening of frailty status at 3 months and incremental improvement at 12 months. Perhaps more importantly, fewer than 40% of liver transplant recipients achieved “robustness” by 1 year. Of these individuals who were robust at 1 year, two thirds were already robust, and one third were pre-frail prior to liver transplantation. The association between pretransplant frailty status and posttransplant frailty status was statistically significant, and the magnitude of the effect clinically substantial, which persisted despite adjustment for a number of pretransplant variables that we believed might impact the posttransplant frailty phenotype.
In order to understand these data from the patients’ perspective, we offer additional information to interpret the actual values of the Liver Frailty Index. The median Liver Frailty Index score for patients listed with HCC and a low Model for End-Stage Liver Disease-Sodium score (which we set at ≤12) in this cohort—whom we believed were the “healthiest” patients based on our clinical experience—was 3.16 (95% confidence interval, 2.79–3.51). Furthermore, in a small sample of healthy community-dwelling adults with a mean age of 59 years, close to the age of our cohort, average Liver Frailty Index score was 2.4. Therefore, we believe that the cut-point of 3.2 that we used to define “robust” is a reasonable cut-point that is consistent with the level of physical function that patients would desire to achieve after liver transplantation. Using a distribution-based method to establish clinically important differences16—where minimally and moderately clinically important differences are defined as 0.2 or 0.5 multiplied by the baseline standard deviation (0.92 in our cohort)—a minimally significant difference in the Liver Frailty Index score is 0.18 and a moderately clinically important difference is 0.46. With these numbers in mind, the change in the median Liver Frailty Index score from 3.7 to 3.4 at 12 months after liver transplantation, while minimally clinically important, cannot be interpreted as substantial. While we did not directly measure health-related quality of life (HRQoL) using standardized HRQoL instruments in this study, we have previously demonstrated that the Liver Frailty Index is strongly correlated with Activities of Daily Living and Instrumental Activities of Daily Living, both of which are crucial components of HRQoL.9
This is the first study in the published domain to focus on the clinical frailty phenotype as an outcome after liver transplantation, rather than simply as a predictor. However, it is not the first to study physical frailty after solid organ transplantation. In kidney transplantation, kidney transplant recipients experienced a significant improvement in overall rates of frailty, as measured by the Fried Frailty Index,11 by 3 months after transplant surgery (as compared to their frailty score at the time of kidney transplantation). 17 Among 13 frail (again, by the Fried Frailty Index11) patients with heart failure, nearly all (n = 12) experienced reversal of their frailty after heart transplantation at a median of 6 months after surgery, although data regarding the extent of their improvement (ie, whether they became robust) are lacking.18 While kidney and heart transplant surgery may be associated with less morbidity than liver transplant surgery, our data in liver transplant recipients stand in stark contrast to these other solid organ transplant recipients, highlighting the need for organ-specific efforts to improve frailty both pre-and posttransplantation.
There are undoubtedly a number of factors that contribute to persistent frailty after liver transplantation. Pretransplant frailty results from not only cirrhosis itself, but also from age-and comorbidity-associated diseases that will not reverse with a new liver. Liver transplant surgery is a major stressor on the body that consumes an enormous amount of physiologic reserve, transiently worsening frailty despite reversal of the liver failure and portal hypertension. Complications are inevitable; in a recent multicenter study including nearly 7500 liver transplant recipients, one half of patients experienced a serious posttransplant complication requiring surgical, radiological, or endoscopic intervention within 1 year of transplantation.19 Donor quality can further impact these factors, either by slowing the reversal of the liver failure or increasing the risk of surgical complications. Mechanistically, ammonia contributes substantially to pretransplant muscle wasting, a major component of physical frailty, increasing vulnerability to posttransplant stressors, 20 but what biological factors, including sarcopenia, contribute to worsening physical frailty posttransplant remains unknown. Although quantifying the contribution of each of these factors is beyond the scope of this initial descriptive study, they represent important avenues for future research.
Before we discuss the clinical implications of these data, it is important for us to acknowledge the limitations of this study. First, the Liver Frailty Index has not been validated as a tool to measure frailty after liver transplantation. Consisting of grip strength, chair stands, and balance testing—all 3 of which have been shown to be clinically important components of frailty and/or functional impairment in the general population in both surgical and nonsurgical settings12,21— we believe that it is reasonable to use the Liver Frailty Index as a patient-oriented outcome for liver transplant recipients. Second, not all patients had measurements of frailty at all time-points after transplantation, with only 50% having a 12-month posttransplant frailty assessment. However, median pretransplant Liver Frailty Index scores among patients who were missing a measurement at 3-, 6-, and 12-months posttransplant were 3.7, 3.8, and 3.7, similar to the median pretransplant value (3.7) for the overall cohort, reducing the likelihood that there was bias (in those who had available posttransplant frailty measurements). Survival rates were also similar between frail and nonfrail patients, also reducing the likelihood that survival bias influenced our results. Lastly, the timing of the pretransplant frailty measurement (during the outpatient clinic visit) prior to transplant varied from patient to patient. There were logistical reasons for this, most obviously that the timing of liver transplantation was unpredictable. However, we believe that analyses using these outpatient measurements allow for greater implications for clinical decision-making, rather than from an assessment on the day of transplant, when the decision to proceed with transplantation has already been made. As frailty typically worsens over time on the waitlist,2 the outpatient measurement of frailty several months before liver transplantation is likely an underestimation of the degree of frailty at the time of transplant surgery, which would further decrease the already modest rates of improvement that we observed.
Lastly, this study was underpowered to detect differences in 12-month posttransplant mortality, as this was a rare event. There is a large body of high-quality evidence supporting the prognostic value of physical function on longer-term functional recovery as well as intermediate-and long-term all-cause mortality in large population-based studies.22–25 Based on these data in nontransplant populations, it is possible that persistent functional impairment after liver transplantation will also impact intermediate and long-term outcomes in this population. This hypothesis is supported by the strong trend in the association between pretransplant frailty and overall posttransplant mortality, although median posttransplant follow-up time was only 2 years. Our data provide justification for larger-scale (ie, multicenter), longer-term studies that are adequately powered to evaluate the association between physical frailty and short-and long-term posttransplant mortality.
So how can we incorporate these data into our clinical practice? First, it is important to point out that we measured pretransplant physical frailty in the outpatient setting. As such, these data may not be applicable to those initially evaluated in the inpatient setting. However, this was intentional, as we aimed to capture the patients’ underlying physiologic reserve, which may not reverse after liver transplantation, rather than simply the effects of acute hepatic decompensation that are more likely to substantially and rapidly reverse with a new liver, as we have previously conceptualized.26 This allows the opportunity for greater impact on patient care, through 2 specific ways:
-
Inform. These data are critical to setting reasonable expectations for our patients and their caregivers regarding the timing and degree of physical recovery after liver transplantation. Those who are frail prior to transplant should be prepared to expect that they have a very small likelihood of achieving “robustness,” although many can expect to improve their physical function at least modestly. Those who are pre-frail prior to transplant may stand the most to gain, as ≈40% may become robust at 1 year. Our analyses enable clinicians to expand the conversation about what to expect after liver transplantation beyond survival alone to physical function that is both patient centered27 and potential modifiable.
Our data also offer critical information for those who are robust prior to liver transplantation, who are traditionally considered to be excellent surgical candidates. These patients should be informed that while the majority maintain their high level of function prior to liver transplantation, as many as one third experience worsening of their physical function 12 months after surgery. Particularly for a robust patient with HCC, for whom alternative therapies to prolong survival may be available, such as chemoembolization plus ablation or hepatic resection, our data may influence a patient’s decision to pursue liver transplantation.
Intervene. By demonstrating that pretransplant frailty status is a potent predictor of posttransplant frailty status, our data provide objective evidence in support of incorporating prehabilitation programs for all liver transplant candidates. One of the advantages of using this 3-component Liver Frailty Index as the pretransplant predictor is that the prehabilitation program can be tailored to individual deficits (ie, weak grip strength [as a marker of nutritional status], slow chair stands [as a marker of lower extremity strength], or impaired balance [as a marker of neuromotor function]). Furthermore, the intensity of the intervention can be tailored to the degree of pretransplant frailty: whereas a robust patient may only need a single session to provide strategies on preventing functional decline, a frail patient may require structured physical therapy sessions and intensive nutritional intervention with the goal of reversal.
In conclusion, using a liver-specific metric of physical frailty in liver transplant patients, we present exploratory data on physical frailty after liver transplantation, a patient-oriented outcome, that begins to advance the literature beyond survival alone to a broader spectrum of health outcomes including functional limitations and disability that have been identified as crucial to living “well” with chronic illness by the Institute of Medicine.28 Our data allow the transplant community to study the liver transplant experience from the viewpoint of the patient, answering key questions that patients and families also care about, such as “Given my personal characteristics, conditions, and preferences, what should I expect will happen to me?”27 Furthermore, this line of research acknowledges that liver transplantation, while a cure for cirrhosis and liver cancer, is associated with a number of other conditions—one of which may be persistent functional impairment—that may be as important if not more important than survival to some if not most patients. While few (7%) remained frail at 1 year following transplant surgery, only 2 out of 5 achieved robustness, raising an important question about what we in the transplant community—and our patients—hope to achieve with liver transplantation. Is the goal to “return to normal function,” or is it enough that liver transplant recipients are no longer frail? At the very least, our data provide strong motivation to establish programs dedicated not only to prehabilitating patients prior to liver transplantation but also engaging patients through interventions aimed at accelerating physical recovery afterwards. The next critical step is to integrate a metrics of frailty in liver transplant practice, laying the foundation for future frailty-focused interventions.
Acknowledgments
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: K24DK101828 and P30DK026743; National Institute on Aging, Grant/Award Number: K23AG048337 and P30AG044281; American College of Gastroenterology, Grant: Junior Faculty Career Development Award
This study was funded by an American College of Gastroenterology Junior Faculty Development Award (Lai), P30AG044281 (UCSF Older Americans Independence Center; Lai), K23AG048337 (Paul B. Beeson Career Development Award in Aging Research; Lai), K24DK101828 (Segev), and P30 DK026743 (UCSF Liver Center; Lai/Dodge). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations
- FrAILT
Functional Assessment in Liver Transplantation
- HCC
hepatocellular carcinoma
- ICU
intensive care unit
- IQR
interquartile range
- LFI
Liver Frailty Index
- MELD
Model for End-Stage Liver Disease
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
AUTHOR CONTRIBUTIONS
Lai: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision. Segev: Analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content. McCulloch: Study design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Covinsky: Study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content. Dodge: Study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content. Feng: Study concept and design; interpretation of data; critical revision of the manuscript for important intellectual content.
References
- 1.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14(8):1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai JC, Dodge JL, Sen S, Covinsky K, Feng S. Functional decline in patients with cirrhosis awaiting liver transplantation: results from the Functional Assessment in Liver Transplantation (FrAILT) study. Hepatology. 2016;63:574–580. doi: 10.1002/hep.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC. Editorial: advancing adoption of frailty to improve the care of patients with cirrhosis: time for a consensus on a frailty index. Am J Gastroenterol. 2016;111(12):1776–1777. doi: 10.1038/ajg.2016.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn MA, Josbeno DA, Tevar AD, et al. Frailty as tested by gait speed is an independent risk factor for cirrhosis complications that require hospitalization. Am J Gastroenterol. 2016;111(12):1768–1775. doi: 10.1038/ajg.2016.336. [DOI] [PubMed] [Google Scholar]
- 5.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16(12):1373–1378. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 6.Bernal W, Martin-Mateos R, Lipcsey M, et al. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl. 2013;20(1):54–62. doi: 10.1002/lt.23766. [DOI] [PubMed] [Google Scholar]
- 7.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62(2):584–590. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samoylova ML, Covinsky KE, Haftek M, Kuo S, Roberts JP, Lai JC. Disability in patients with end-stage liver disease: results from the functional assessment in liver transplantation study. Liver Transpl. 2017;23(3):292–298. doi: 10.1002/lt.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology. 2017;66(2):564–574. doi: 10.1002/hep.29219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissenborn K, Rückert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol. 1998;28(4):646–653. doi: 10.1016/s0168-8278(98)80289-4. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 14.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2016;65(1):310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 16.Lemieux J, Beaton DE, Hogg-Johnson S, Bordeleau LJ, Goodwin PJ. Three methods for minimally important difference: no relationship was found with the net proportion of patients improving. J Clin Epidemiol. 2007;60(5):448–455. doi: 10.1016/j.jclinepi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Isaacs K, Darko L, et al. Changes in frailty after kidney transplantation. J Am Geriatr Soc. 2015;63(10):2152–2157. doi: 10.1111/jgs.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha SR, Hannu MK, Newton PJ, et al. Reversibility of frailty after bridge-to- transplant ventricular assist device implantation or heart transplantation. Transplant Direct. 2017;3(7):e167–e168. doi: 10.1097/TXD.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller X, Marcon F, Sapisochin G, et al. Defining benchmarks in liver transplantation: a multicenter outcome analysis determining best achievable results. Ann Surg. 2018;267(3):419–425. doi: 10.1097/SLA.0000000000002477. [DOI] [PubMed] [Google Scholar]
- 20.Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB- mediated mechanism. Proc Natl Acad Sci USA. 2013;110(45):18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56(12):2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavasini R, Guralnik J, Brown JC, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 2016;14(1):1–9. doi: 10.1186/s12916-016-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. doi: 10.1001/jama.295.7.801. [DOI] [PubMed] [Google Scholar]
- 26.Lai JC. A framework to determine when liver transplantation is futile. Clin Liv Dis. 2016;8(6):137–139. doi: 10.1002/cld.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. [Accessed February 18, 2018];Patient-Centered Outcomes Research Definition: Consensus Definition. https://www.pcori.org/establishing-definition-patient-centered-outcomes-research. Published March 2, 2012.
- 28. [Accessed December 16, 2017];Living well with chronic illness. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2012/Living-Well-with-Chronic-Illness/livingwell_chronicillness_reportbrief.pdf. Published January 25, 2012.