Abstract
Background
Norovirus is a leading cause of outbreaks of acute infectious gastroenteritis worldwide, yet its transmissibility within households and associated risk factors remain unknown in developing countries.
Methods
Household, demographic, and clinical data were collected from a semi-urban area in south China where an outbreak occurred in the winter of 2014. Using a Bayesian modeling framework, we assessed the transmissibility and potential risk modifiers in both urban and rural households.
Results
In urban apartment buildings, the secondary attack rates were 84% (95% credible interval [CI]: 60%, 96%) among households of size two and 29% (95% CI: 9.6%, 53%) in larger households. In the rural village, secondary attack rate estimates were lower than the urban setting, 13% (0.51%, 54%) for households of size two and 7.3% (0.38%, 27%) for larger households. Males were 31% (95% CI: 3%, 50%) less susceptible to the disease than female. Water disinfection with chlorine was estimated to reduce environmental risk of infection by 60% (95% CI: 26%, 82%) and case isolation was estimated to reduce person-to-person transmission by 65% (95% CI: 15%, 93%). Nausea and vomiting were not associated with household transmission.
Conclusions
Norovirus is highly contagious within households, in particular in small households in urban communities. Our results suggest that water disinfection and case isolation are associated with reduction of outbreaks in resource-limited communities.
Keywords: Norovirus, Community Outbreak, Secondary Attack Rate, Serial Interval, Intervention Efficacy
INTRODUCTION
Norovirus is the leading cause of acute gastroenteritis outbreaks worldwide, accounting for 18% of diarrheal diseases and about 200,000 deaths each year globally [1–5]. Reinfections by norovirus of different genotypes are also common, especially for children [6, 7]. Norovirus outbreaks are notorious for their explosive dissemination, in which a small number of cases can trigger a large outbreak in a short period [8–10]. Norovirus outbreaks occur in many close-contact settings, including communities, schools, day care centers, hospitals, restaurants, cruises, and passenger aircrafts [11–17]. In a typical outbreak, susceptible individuals may be exposed to either a common environmental source, such as contaminated food or water, infectious individuals, or both. Coexistence of multi-types of sources often complicates the assessment of transmissibility and associated risk factors.
Thus far, most reported norovirus outbreaks with secondary person-to-peron transmission were among non-household settings such as schools and hospitals, and household secondary transmissibility of the virus remains underinvestigated, mostly due to lack of data. The household secondary attack rate is the probability that an infected individual infects a susceptible household member during his or her infectious period. The household secondary attack rate is often approximated by the proportion of infections among all household contacts of an index case, assuming that all secondary cases in the household are infected by the index case, which is hereafter referred to as the crude household secondary attack rate. In a large Swedish community outbreak, investigators were able to trace household members of child cases who were infected in daycare centers and estimated the crude household secondary attack rate at 17% [11]. Given that household is an important venue for transmission of norovirus, the household transmissibility of the virus and relevant risk modifiers warrant careful assessment with rigorous statistical analysis, in particular in resource-limited regions where transmission settings are often complex.
A norovirus outbreak occurred in an underdeveloped semi-urban residential area in Central South China in the winter season of 2014. This outbreak attacked households in both an urban community of apartment buildings and a neighboring rural village, offering a unique opportunity for comparing household transmissibility of norovirus between urban and rural settings. Using the data collected by the local health agency, we employed a Bayesian transmission model to assess the household secondary attack rate and the effects of demographics and intervention measures, including water disinfection and case isolation. This approach takes into consideration co-exposure to environmental and human sources as well as ambiguity among secondary and tertiary cases, so that the household secondary attack rate that measures person-to-person transmissibility could be estimated. In addition, we evaluated between-household transmissibility of the virus at the community level. These data have been previously analyzed using a mathematical simulation model, where household information was ignored [14].
METHODS
Study subjects
The data were collected by Changsha Municipal Center for Disease Control and Prevention (CDC) in response to an outbreak that occurred near Changsha, the capital city of Hunan Province in Central-South China. Epidemiologic details of this outbreak were described elsewhere [14]. In brief, a few people living in a semi-urban residential area composed of an urban community and a rural village in the southern outskirts of Changsha started to have continual vomiting and diarrhea in late November of 2014. In the urban community lived employees, mostly retired, of a state-run factory and their families. The community shared a deep well (280 meters underground) and pump stations for drinking water with the nearby village. Changsha Municipal CDC was called on 7 December, and a field outbreak investigation was conducted. A probable case was defined by presence of any of the following gastrointestinal symptoms during the outbreak period: vomiting, diarrhea, abdominal distension or appetite loss. Presentation of other symptoms including nausea, stomachache, chills, exhausted, acid reflux, headache, dizziness, and chest tightness was also recorded. Almost all cases had at least two of the above-mentioned symptoms. Feces or vomit samples were collected from six probable cases, and water samples were collected from four households for laboratory testing.
A laboratory-confirmed case was defined by having both clinical symptoms and a sample tested positive for norovirus but negative for other common diarrheal pathogens (Vibrio cholera, rotavirus, adenovirus, among others). Starting from 7 December, newly identified mild cases were isolated at home with instructions on sterilization of utensils and the living environment, while severe cases were isolated at clinics for medical treatment. Daily disinfection of drinking water with a solid form of chlorine was implemented from 9 December until the end of the outbreak. Household sizes as well as ages and genders of household members were collected retrospectively for all households. A household was defined as a family living in an apartment (urban community) or a single house (village). Apartments in the urban community were built in the 1970s and had an average area of 40-50 square meters with independent kitchen and bathroom. Houses in the village were one to two stories with areas between 50-200 square meters. Some residents in the urban community lived in dormitory-like buildings with shared kitchen and bathrooms at each floor. Household information was not available for these residents, and they were therefore excluded from household-related analyses.
Ethics
This effort of outbreak control and investigation was part of Changsha Municipal CDC’s routine responsibility; therefore, institutional review and informed consent were not required for this study.
Statistical analysis
We defined the case with the earliest symptom onset date as the index case in that household. We reported crude secondary attack rates among different groups, and provided confidence intervals by using generalized estimating equations to account for household clustering.
We estimated separately the distribution of serial interval, defined as the time between symptom onsets of a primary case and a secondary case, under two distinct assumptions: [i] in each household, all subsequent cases were infected by the index case, and thus all intervals between the index case and subsequent cases were included in the estimation (liberal); and [ii] only the interval between the index case and the second case contributed to the estimation (conservative). The liberal assumption would yield a relatively long, whereas the conservative assumption would yield a relatively short, estimate for the mean serial interval. We excluded households with multiple index cases, which could result from common environmental exposure, from this estimation. Under each assumption, we fitted gamma, Weibull, and lognormal distributions by the maximum likelihood method, and selected the distribution with the smallest Akaike information criterion. We also extracted the Swedish norovirus outbreak data and performed the same analysis for comparison [11].
To analyze the transmission dynamics of the norovirus outbreak, we need information about the incubation period and the infectious period, as well as the relative infectiousness over the infectious period. The incubation period for norovirus usually varies from one to three days [11, 15, 18–20]. The infectiousness profile of norovirus-infected individuals, however, is not well documented. We assume the infectiousness started on the day of symptom onset. One study reported that the duration of illness was around 2-3 days, but a challenge study suggested that there could be post-symptom shedding of the virus [21]. To accommodate such uncertainty, we assume the infectious period lasts 3-7 days. For sensitivity analyses, we formulated eight settings of the natural history of disease by coupling four scenarios of the relative infectiousness over the infectious period with two plausible distributions of the incubation period (eTable 2). These settings were chosen such that the resulting mean serial intervals are similar to the ones estimated from the data (eAppendix section 1.1).
We developed an individual-based transmission model to assess the secondary attack rates and associated risk factors (eAppendix section 1). Only individuals with household information were included in this analysis. In this model, the risk of infection for a susceptible individual depends on the timing of symptom onsets of other infected individuals and the mixing venue, i.e., urban vs. rural, and within-household vs. between-household. For the urban community, we assume between-household transmission in the same apartment building but not across buildings. Therefore, the person-to-person transmission hazard of disease from an infected individual i to a susceptible individual j at time t is , where f is the density of the convolution of distributions of the infectiousness profile and the incubation period, and is the symptom onset time for individual i. The baseline transmission hazard from individual i to individual j, , depends on their mixing condition:
Here, are the baseline hazards of disease from human to human under different mixing conditions, and corresponding secondary attack rates are given by , , where is the mean duration of the infectious period.
In addition to infectious human contacts, the model considers an external risk of infection that represents the exposure to contaminated water. Hence, the total hazard of symptom onset at day t, denoted as , is
where measures the susceptibility of individual j, and are the baseline hazards of disease from environment to human for urban and rural settings respectively, and are the effects of water disinfection and case isolation respectively, and is the indicator function. We consider age and sex as potential risk factors for modifying susceptibility .
We implemented the transmission model in a Bayesian framework fitted with a Markov chain Monte Carlo algorithm (eAppendix section 2). Eight cases with symptom onsets during the first four days were considered as initial cases of this outbreak, i.e., they were assumed to be infected by the contaminated environment. To impute missing ages, we set the prior distribution of age to depend on household size. A sensitivity analysis was conducted with a more restrictive definition of probable cases, i.e., the presence of vomiting, nausea, diarrhea, or stomachache, which are the four most common symptoms of norovirus according to the US Centers for Disease Control and Prevention. We assessed goodness-of-fit of the model by comparing the model-predicted epidemic curves to the observed one (eAppendix section 3). To show the potential overall impact of water disinfection on similar outbreaks, we simulated outbreaks based on the estimated model to estimate the number of cases that could have been averted if water disinfection had been implemented on day 5, 7, and 12, compared with no water disinfection. In the simulation, transmission dynamics started from day 5 with initial cases fixed in days 1-4 (similar to the analyses of the observed outbreak), and no case isolation was implemented. All statistical analyses were conducted using R version 3.2.1 and the Rcpp package [22].
RESULTS
In total, 643 residents participated in the outbreak investigation, of whom 159 (25%) were clinically diagnosed as probable norovirus cases. It was previously reported that six human samples and four household water samples, all from the urban community, were tested positive for norovirus GII.17, a subtype that has spread widely in Asian countries since 2012 [23]. We were able to match three individuals to the human samples and two households to the water samples (eAppendix section 4). As the number of human samples is small, all subsequent results are based on probable cases. The outbreak started on 28 November 2014, and the last symptom onset was observed on 10 December 2014, an overall duration of 13 days. A total of 104 participants, including 20 cases, lived in dormitory-like buildings and were excluded from household-related analyses due to the lack of household information. However, these 20 cases were included in the description of the symptom profile of all clinically diagnosed cases.
The demographics of the 539 participants, from 237 households, included in household-related analyses are summarized in Table 1. The majority of households of size two are composed of senior residents ≥50 years old, in particular in the urban community (77%). The overall attack rate among the urban households, 29%, (95% CI: 23%, 35%), was slightly higher than 21% (95% CI: 16%, 27%) in the village. There were 76 households with at least two household members and at least one case. Denoting the first case in each household as the index case and treating all other cases as secondary, the crude household secondary attack rate was 33% (43/131, 95% CI: 25%, 42%). The crude rate was 52% (33/64, 95% CI: 36%, 66%) and 19% (10/67, 95% CI: 7%, 28%) for households in the urban community and the village, respectively. The crude household secondary attack rate was 81% (21/26, 95% CI: 61%, 92%) for households of size 2 and 32% (12/38, 95% CI: 17%, 50%) for larger households in the urban community, and 9% (1/11, 95% CI: 1%, 46%) for households of size 2 and 16% (9/56, 95% CI: 2%, 62%) for larger households in the rural community.
Table 1.
Demographics of households investigated during the norovirus outbreak that occurred from 28 November to 10 December 2014, in Changsha, China.
| Characteristics | Household Size | Category | Urban | Rural | Total |
|---|---|---|---|---|---|
| No. of Households | 149 | 88 | 237 | ||
| No. of individuals | 315 | 224 | 539 | ||
| Age Groupa | 2 | ≤20 | 1 (1%) | 3 (6%) | 4 (3%) |
| 21-50 | 23 (22%) | 16 (33%) | 39 (25%) | ||
| >50 | 82 (77%) | 29 (60%) | 111 (72%) | ||
| >2 | ≤20 | 5 (4%) | 29 (18%) | 34 (12%) | |
| 21-50 | 48 (36%) | 81 (51%) | 129 (44%) | ||
| >50 | 79 (60%) | 50 (31%) | 129 (44%) | ||
| Sex | Female | 148 (47%) | 104 (46%) | 252 (47%) | |
| Male | 167 (53%) | 120 (54%) | 287 (53%) | ||
| No. of probable cases | 91 (29%) | 48 (21%) | 139 (26%) |
Age is missing for 32 individuals in the urban community.
The spectrum of symptoms of all household cases is summarized in eTable 1. Commonly reported symptoms included diarrhea (59%), nausea (48%), stomachache (47%), and vomiting (43%). We also compared the symptom profiles between index cases and subsequent cases in the households. In general, typical symptoms such as diarrhea, nausea, and vomiting were more prevalent in index cases as compared to subsequent cases. However, none of these differences was substantial. To screen symptoms that may modify person-to-person transmissibility, we estimated crude household secondary attack rates in the presence and absence of each symptom in the index cases (Table 2). The crude household secondary attack rates are similar with or without each of the most common norovirus-associated symptoms—nausea, vomiting and diarrhea—in the index cases. As a result, we did not adjust for symptoms in the subsequent transmission analyses.
Table 2.
The crude household secondary attack rates stratified by the presence or absence of a given symptom in the index casesa.
| Symptoms | Presence | Absence |
|---|---|---|
| Diarrhea | 26/90 (29%) | 17/41 (41%) |
| Nausea | 23/69 (33%) | 20/62 (32%) |
| Stomachache | 18/58 (31%) | 25/73 (34%) |
| Vomiting | 27/75 (36%) | 16/56 (29%) |
| Abdominal distension | 15/40 (38%) | 28/91 (31%) |
| Appetite loss | 7/23 (30%) | 36/108 (33%) |
| Chills | 8/19 (42%) | 35/112 (31%) |
| Exhausted | 9/23 (39%) | 34/108 (31%) |
| Acid reflux | 4/4 (100%) | 39/127 (31%) |
| Headache | 0/6 (0%) | 43/125 (34%) |
| Dizziness | 0/2 (0%) | 43/129 (33%) |
The numbers of index cases with or without each symptom are given in eTable 1.
The fitted density functions for the distribution of the serial interval are shown for the Changsha outbreak in Figure 1A and the Swedish outbreak in Figure 1B, together with frequencies of observed serial intervals. Under the liberal assumption that the index case was the infection source for all other cases in each household, we observed 35 serial intervals with an empirical mean of 2.5 days in the Changsha outbreak. The best-fitted parametric distribution was the lognormal distribution, with a mean of 2.4 (95% CI: 1.8, 3.3) days. Under the conservative assumption where serial intervals were restricted to the pairs of the first and second cases, there were 30 observed pairs with an empirical mean of 2.3 days. The best-fit distribution was again the lognormal distribution, with a mean of 2.1 (95% CI: 1.6, 3.0) days. In the Swedish outbreak, the liberal assumption led to 65 observed serial intervals with an empirical mean of 3.6 days and a best-fit gamma distribution with a mean of 3.7 (95% CI: 3.2, 4.1) days. The conservative assumption yielded 39 observed serial intervals with an empirical mean of 3.3 days and a best-fit gamma distribution with a mean of 3.3 (95% CI: 2.7, 3.9) days.
Figure 1.
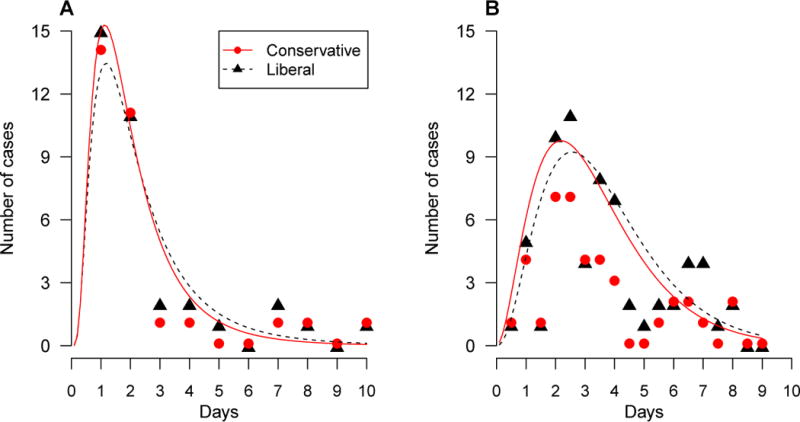
Empirical (dots) and fitted (lines) distributions of serial interval under assumptions about how secondary cases are defined. The liberal assumption (triangle, black, dotted lines) treated all non-index cases as secondary, and the conservative assumption (circle, red, solid lines) treated only the second case after the index case as secondary. Estimation was performed for (A) the Changsha outbreak and (B) the Swedish outbreak.
We then estimated the transmissibility of norovirus stratified by transmission mode (environment-to-person and person-to-person) and mixing setting (urban and rural). We report the analysis under the assumption of a mean serial interval of 2.2 days (short incubation period and short infectious period) as the primary results in Table 3. The daily force of infection from environment to human was 3.2% (95% CI: 2.1%, 4.7%) in the urban community and 2.5% (95% CI: 1.3%, 3.9%) in the rural village. Therefore, on average, about three out of 100 susceptibles were infected via nonspecific environmental exposure per day in the absence of intervention. The baseline household secondary attack rates in the urban community were much higher than those in the rural village (Table 3), where baseline refers to 20-50 year old females without intervention. In the urban community, the baseline household SAR among households of size two was significantly higher than that among larger households, 84% (95% CI: 60%, 96%) vs. 29% (95% CI: 9.6%, 53%). The dependency of household SAR on household size was less obvious in the village, 13% (95% CI: 0.51%, 54%) among households of size 2 and 7.3% (95% CI: 0.38%, 27%) among larger households. The baseline between-household secondary attack rates were 0.63% (95% CI: 0.042%, 2.1%) in the urban community and 0.22% (95% CI: 0.013%, 0.78%) in the village. The results for the other five settings of the natural history of disease were fairly similar to the primary results (eTable 3). As expected, a longer serial interval was associated with a higher household secondary attack rate, as more cases can be attributed to household transmission.
Table 3.
Estimates of baseline transmissibilitya by contact setting for the norovirus outbreak that occurred from 28 November to 10 December 2014, in Changsha, China.
| Parameter | Transmission Mode | Residential Setting | Household Size | Estimate (95% CI) |
|---|---|---|---|---|
| Environmental force of infection | Urban | 3.2% (2.1%, 4.7%) | ||
| Rural | 2.5% (1.3%, 3.9%) | |||
|
| ||||
| SARb | Within-Household | Urban | 2 | 84% (60%, 96%) |
| >2 | 29% (9.6%, 53%) | |||
| Rural | 2 | 13% (0.51%, 54%) | ||
| > 2 | 7.3% (0.38%, 27%) | |||
|
|
||||
| Between-Household | Urban | 0.63% (0.042%, 2.1%) | ||
| Rural | 0.22% (0.013%, 0.78%) | |||
Baseline refers to 20- to 50-year old females without intervention. The mean serial interval is assumed to be 2.2 days.
Secondary attack rates measure person-to-person transmissibility.
Estimates for the effects of potential risk factors and the interventions under the primary setting of the natural history of disease are shown in Table 4. We found that males were less susceptible than females with a relative risk of 0.69 (95% CI: 0.50, 0.97). We did not find any association between age and risk of infection. Water disinfection reduced the environmental risk of infection by 60% (95% CI: 26%, 82%). Case isolation reduced the person-to-person transmission by 65% (95% CI: 15%, 93%). These estimates were not sensitive to the assumptions about natural history of disease (eTable 4).
Table 4.
Estimates of covariate effects for the norovirus outbreak that occurred from 28 November to 10 December 2014, in Changsha, China.
| Risk factor | Category | Risk Ratioa (95% CI) |
|---|---|---|
| Sex | Male | 0.69 (0.50, 0.97) |
| Age (years) | ≤20 | 1.2 (0.55, 2.3) |
| 21-50 | Ref | |
| >50 | 1.1 (0.78, 1.7) | |
| Water disinfection | 0.40 (0.18, 0.74) | |
| Case isolation | 0.35 (0.07, 0.85) |
Estimation was based on the assumption that the mean serial interval is 2.2 days.
In Figure 2, we compare model-predicted numbers of symptom onsets on each day conditional on exposure during previous days to the observed onset numbers for overall, urban, and rural households. The mean predicted numbers match, and the 95% uncertainty intervals cover, the observed numbers for all days and for both urban and rural residential settings, indicating adequate goodness-of-fit of our model to the data.
Figure 2.
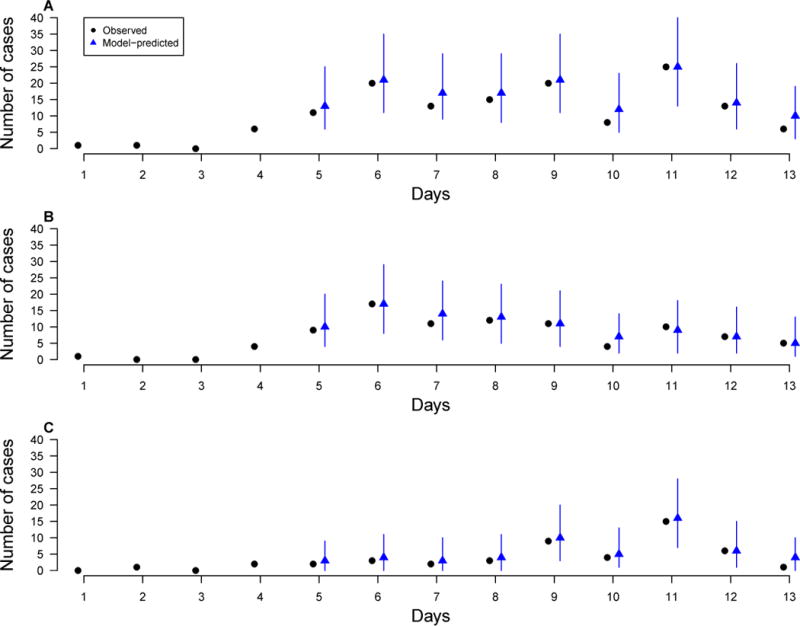
Model-predicted daily numbers of symptom onsets (blue, triangle) versus observed numbers (black, circle) for different contact settings (A) Overall; (B) Urban households; (C) Rural households. The predicted numbers were generated by simulating the epidemics, conditioning on the cases of the first 4 days, for 10000 times, each time using a set of parameters sampled from their posterior distributions. Each blue vertical bar represents the 95% range and the blue dot represents the mean of simulated numbers.
In the sensitivity analysis that used a more restrictive definition of probable cases (eTable 5), the within-household secondary attack rates in the urban community decreased from 84% to 58% for households of size two and from 29% to 17% for larger households, likely a result of fewer cases under the new definition. However, estimates for within-household secondary attack rates in the village and between-household rates in urban and rural settings remained similar to the primary results. The estimated effects of age and sex on transmissibility as well as the estimated effectiveness of interventions are also robust to the definition of probable cases (eTable 6).
Among 100 simulated outbreaks using the fitted model in our primary analysis, the median total numbers of cases were 138 (2.5%, 97.5% quantile: 111, 170), 158 (128, 190) and 199 (170, 233) if water disinfection was implemented on day 5, 7 and 12, respectively, compared to 260 (231, 292) for no water disinfection. Even when water disinfection was initiated as late as day 12 (same as the observed outbreak), on average 24% of cases were estimated to have been averted.
DISCUSSION
We provided estimates for the household person-to-person transmissibility of norovirus in both urban and rural settings in China. The crude household secondary attack rate in our study was around 33%, but there was substantial heterogeneity between urban and rural households. The model-estimated household secondary attack rates in the urban community are nearly four times those in the rural village (Table 3), likely due to the more crowded living conditions and hence more intense exposure in the apartment buildings as compared to the rural village. The single-house living style in the rural village is somewhat similar to that in developed countries. The crude household secondary attack rate in the village (19%) is also comparable to 10-20% reported for previous outbreaks in western countries [11, 12, 19, 24]. In the urban community, the secondary attack rate within households of size two was nearly three times that within larger households, 84% vs. 29%. Dependency of the secondary attack rate on household size was also observed in the rural village but to a lesser extent. This negative association of transmissibility with household size is not surprising, because the contact rate between household members generally decreases with household size [25]. Interestingly, the gap in the secondary attack rate between households of 2 people and larger households (mean size is 3.34) implies that the household secondary attack rates is approximately proportional to 1/n2, where n denotes the size, consistent with previous findings about household transmission of influenza [26]. On the other hand, non-causal factors could have contributed to the gaps. For example, case ascertainment could have been better in urban households composed of retired couples who stayed at home more often than other residents.
We estimated that water disinfection was highly effective, reducing the environment risk of infection by 60%. These results were supported by the fact that no new cases were observed two days after the implementation of disinfection. Our simulation study showed that more than 20% cases could have been averted by water disinfection alone despite its late adoption (day 12 of the outbreak), and that early implementation could be even more beneficial. Case isolation was also highly effective, associated with 65% lower person-to-person transmission. Depending on the contact setting, physical isolation of cases may not be necessary. For example, designation of separate toilets for cases and non-cases together with enhanced hand hygiene stopped an outbreak in camps [27]. Using a simulation approach on the same dataset but ignoring clustering structures (buildings and households), Chen et al. found that case isolation alone was not effective, disinfection alone was somewhat effective, and the two combined was most effective in reducing the total attack rate [14]. Those results are not directly comparable to ours as we focused on intervention effectiveness at the individual level and considered clustering patterns.
Unlike outbreaks in camps, schools or health care centers, this outbreak in a residential area of households with a broad age range offered a unique opportunity for studying age effect on norovirus transmission. However, we did not detect any association between age and risk of infection. The lack of age difference, together with the relatively high secondary attack rates, could be partly due to little pre-existing immunity in the population to norovirus GII.17, which was a new genotype at that time. We also found that males were less susceptible than females. This is consistent with a hospital-based study reporting that the total number of infections for females was higher than that for males [28]. A possible explanation is that men were more likely to work outside during the day and hence were less exposed. Another possible reason is that women cared for their sick spouse more frequently. However, there could be other possible explanations for this association.
We estimated the mean serial interval as 2.2-2.5 days, which is shorter than 3.6 days reported by the Swedish study [11]. A couple of reasons may explain the difference. The distribution of serial interval depends on the distribution of the incubation period and the infectiousness profile, both of which likely differ between the two studies due to potential differences in viral subtype, hygienic habits, and contact pattern within households, analogous to other infectious diseases [26, 29, 30]. In addition, household members in our study were exposed to environmental risks due to contaminated water supply, which was not the case in the Swedish study [11, 14]. The existence of environmental exposure will contract the serial interval.
The infectious period in our modeling analyses was set to be around a week, but it could be longer. Previous norovirus outbreak studies in nursing homes and long-term care facilities reported that viral shedding periods could be 3 weeks or even longer [31, 32]. In addition, human challenge studies suggested that norovirus has a low infectious dose (between 18 to 2800 viral particles) [33, 34]. However, whether these observations imply an infectious period much longer than a week is unclear. In one study that showed prolonged shedding periods, a statistically inferred transmission tree of 17 infections covered a time span of only 2 weeks, similar to our study [35]. In addition, our study population is younger and likely healthier than nursing home residents in previous studies. Finally, given the short outbreak period (13 days) and serial intervals (≤10 days) in our study, the assumption of a longer infectious period will not qualitatively change our estimates of secondary attack rates.
Our analyses have a few limitations. First, household information was missing for around one-sixth of individuals, who were consequently excluded from all household-related analyses. However, the missing data occurred mostly in dormitory-like buildings, which is not a typical residential environment for households, and will not affect the reliability and generalizability of our estimates for the household secondary attack rate. Second, most cases were clinically diagnosed, and most asymptomatic individuals were not laboratory-tested. Therefore, asymptomatic infections were not considered in our analyses. However, previous studies indicated that up to 30% of norovirus infections could be asymptomatic and that asymptomatically infected individuals could have comparable fecal shedding levels of the virus to that of symptomatic cases [32, 36]. The contribution of asymptomatic infections to the transmission dynamics of norovirus was found to be negligible in nosocomial settings [35], but their role in the household setting is not clear and warrants future investigation. Our secondary attack rate estimates should be interpreted as rates of disease rather than infection. Finally, the rural village is physically close to the urban community in our study, and thus our estimated secondary attack rate for the rural household may not be generalizable to all rural villages in China, especially those in remote areas with more limited resources.
In China, there are still many old communities with deteriorating living conditions and rusted water distribution systems, which are the legacy of historical state-owned factories [37]. In these communities, many households are composed of senior couples who were former employees of these factories. While age may not be a risk factor, these small households constitute a subpopulation vulnerable to norovirus, given the high transmissibility according to our analysis. Globally, it was estimated that at least 1.8 billion people worldwide use drinking water sources that are fecally contaminated [38]. The epidemiological parameters estimated from our study could be used to inform future studies of intervention strategies in fields or in silicon for norovirus and comparable water-borne pathogens in resource-limited communities.
Supplementary Material
Acknowledgments
The authors thank all study participants for providing the data and field investigators for collecting the data.
SOURCES OF FINANCIAL SUPPORT
The results reported herein correspond to specific aims of grant R37-AI032042 to M. Elizabeth Halloran from the National Institute of Allergy and Infectious Diseases and grant U54-GM111274 to M. Elizabeth Halloran from the National Institute of General Medical Sciences.
Footnotes
POTENTIAL CONFLICTS OF INTEREST
All authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
DATA AND CODE AVAILABILITY
Data and code are available upon request.
References
- 1.Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361(18):1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–30. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Ryan M, Riera-Montes M, Lopman B. Norovirus in Latin America: Systematic Review and Meta-analysis. Pediatr Infect Dis J. 2017;36(2):127–34. doi: 10.1097/INF.0000000000001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, et al. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012;140(7):1161–72. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito M, Goel-Apaza S, Espetia S, Velasquez D, Cabrera L, Loli S, et al. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis. 2014;58(4):483–91. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheesbrough JS, Barkess-Jones L, Brown DW. Possible prolonged environmental survival of small round structured viruses. J Hosp Infect. 1997;35(4):325–6. doi: 10.1016/s0195-6701(97)90230-9. [DOI] [PubMed] [Google Scholar]
- 9.Jones EL, Kramer A, Gaither M, Gerba CP. Role of fomite contamination during an outbreak of norovirus on houseboats. Int J Environ Health Res. 2007;17(2):123–31. doi: 10.1080/09603120701219394. [DOI] [PubMed] [Google Scholar]
- 10.Wikswo ME, Cortes J, Hall AJ, Vaughan G, Howard C, Gregoricus N, et al. Disease transmission and passenger behaviors during a high morbidity Norovirus outbreak on a cruise ship, January 2009. Clin Infect Dis. 2011;52(9):1116–22. doi: 10.1093/cid/cir144. [DOI] [PubMed] [Google Scholar]
- 11.Gotz H, Ekdahl K, Lindback J, de Jong B, Hedlund KO, Giesecke J. Clinical spectrum and transmission characteristics of infection with Norwalk-like virus: findings from a large community outbreak in Sweden. Clin Infect Dis. 2001;33(5):622–8. doi: 10.1086/322608. [DOI] [PubMed] [Google Scholar]
- 12.Alfano-Sobsey E, Sweat D, Hall A, Breedlove F, Rodriguez R, Greene S, et al. Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiol Infect. 2012;140(2):276–82. doi: 10.1017/S0950268811000665. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan JE, Goodman RA, Schonberger LB, Lippy EC, Gary GW. Gastroenteritis due to Norwalk virus: an outbreak associated with a municipal water system. J Infect Dis. 1982;146(2):190–7. doi: 10.1093/infdis/146.2.190. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Gu H, Leung RK, Liu R, Chen Q, Wu Y, et al. Evidence-Based interventions of Norovirus outbreaks in China. BMC Public Health. 2016;16(1):1072. doi: 10.1186/s12889-016-3716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornley CN, Emslie NA, Sprott TW, Greening GE, Rapana JP. Recurring norovirus transmission on an airplane. Clin Infect Dis. 2011;53(6):515–20. doi: 10.1093/cid/cir465. [DOI] [PubMed] [Google Scholar]
- 16.Baron RC, Murphy FD, Greenberg HB, Davis CE, Bregman DJ, Gary GW, et al. Norwalk gastrointestinal illness: an outbreak associated with swimming in a recreational lake and secondary person-to-person transmission. Am J Epidemiol. 1982;115(2):163–72. doi: 10.1093/oxfordjournals.aje.a113287. [DOI] [PubMed] [Google Scholar]
- 17.ter Waarbeek HL, Dukers-Muijrers NH, Vennema H, Hoebe CJ. Waterborne gastroenteritis outbreak at a scouting camp caused by two norovirus genogroups: GI and GII. J Clin Virol. 2010;47(3):268–72. doi: 10.1016/j.jcv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Arvelo W, Sosa SM, Juliao P, Lopez MR, Estevez A, Lopez B, et al. Norovirus outbreak of probable waterborne transmission with high attack rate in a Guatemalan resort. J Clin Virol. 2012;55(1):8–11. doi: 10.1016/j.jcv.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Hoebe CJ, Vennema H, de Roda Husman AM, van Duynhoven YT. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J Infect Dis. 2004;189(4):699–705. doi: 10.1086/381534. [DOI] [PubMed] [Google Scholar]
- 20.Ng TL, Chan PP, Phua TH, Loh JP, Yip R, Wong C, et al. Oyster-associated outbreaks of Norovirus gastroenteritis in Singapore. J Infect. 2005;51(5):413–8. doi: 10.1016/j.jinf.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14(10):1553–7. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eddelbuettel D. In: Seamless R and C++ Integration with Rcpp. Springer, editor. New York: 2013. [Google Scholar]
- 23.Yao D, Chen J, Ye W, Ou X. Etiology and genotype features analysis of an acute gastroenteritis outbreak associated with Norovirus GII.17. Chinese Journal of Zoonoses. 2016;32(7):641–50. [Google Scholar]
- 24.Zelner JL, Lopman BA, Hall AJ, Ballesteros S, Grenfell BT. Linking time-varying symptomatology and intensity of infectiousness to patterns of norovirus transmission. PLoS One. 2013;8(7):e68413. doi: 10.1371/journal.pone.0068413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potter GE, Handcock MS, Longini IM, Jr, Halloran ME. Estimating within-Household Contact Networks from Egocentric Data. Ann Appl Stat. 2011;5(3):1816–38. doi: 10.1214/11-aoas474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauchemez S, Bhattarai A, Marchbanks TL, Fagan RP, Ostroff S, Ferguson NM, et al. Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci U S A. 2011;108(7):2825–30. doi: 10.1073/pnas.1008895108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heijne JC, Teunis P, Morroy G, Wijkmans C, Oostveen S, Duizer E, et al. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg Infect Dis. 2009;15(1):24–30. doi: 10.3201/eid1501.080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franck KT, Nielsen RT, Holzknecht BJ, Ersboll AK, Fischer TK, Bottiger B. Norovirus Genotypes in Hospital Settings: Differences Between Nosocomial and Community-Acquired Infections. J Infect Dis. 2015;212(6):881–8. doi: 10.1093/infdis/jiv105. [DOI] [PubMed] [Google Scholar]
- 29.Klinkenberg D, Nishiura H. The correlation between infectivity and incubation period of measles, estimated from households with two cases. J Theor Biol. 2011;284(1):52–60. doi: 10.1016/j.jtbi.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Tsang TK, Lau LL, Cauchemez S, Cowling BJ. Household Transmission of Influenza Virus. Trends Microbiol. 2016;24(2):123–33. doi: 10.1016/j.tim.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costantini VP, Cooper EM, Hardaker HL, Lee LE, Bierhoff M, Biggs C, et al. Epidemiologic, Virologic, and Host Genetic Factors of Norovirus Outbreaks in Long-term Care Facilities. Clin Infect Dis. 2016;62(1):1–10. doi: 10.1093/cid/civ747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teunis PF, Sukhrie FH, Vennema H, Bogerman J, Beersma MF, Koopmans MP. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect. 2015;143(8):1710–7. doi: 10.1017/S095026881400274X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Graaf M, Villabruna N, Koopmans MP. Capturing norovirus transmission. Curr Opin Virol. 2017;22:64–70. doi: 10.1016/j.coviro.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Kirby AE, Streby A, Moe CL. Vomiting as a Symptom and Transmission Risk in Norovirus Illness: Evidence from Human Challenge Studies. PLoS One. 2016;11(4):e0143759. doi: 10.1371/journal.pone.0143759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sukhrie FH, Teunis P, Vennema H, Copra C, Thijs Beersma MF, Bogerman J, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012;54(7):931–7. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 36.Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Center for Disease Control and Prevention. Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60(RR03):1–15. [PubMed] [Google Scholar]
- 37.Cho M. The Specter of “the People”: Urban Poverty in Northeast China. Ithaca and London: Cornell University Press; 2013. [Google Scholar]
- 38.Bain R, Cronk R, Hossain R, Bonjour S, Onda K, Wright J, Yang H, Slaymaker T, Hunter P, Pruss-Ustun A, Bartram J. Global assessment of exposure to gaecal contamination through drinking water based on a systematic review. Trop Med Int Health. 2014;19(8):917–27. doi: 10.1111/tmi.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


