Abstract
Protein Phosphatase-1 (PP1) constrains learning and memory formation in part through its effects on the induction threshold of long term potentiation (LTP) and depression (LTD). LTD induction requires both the enzymatic activity of PP1 and its proper anchoring to synaptic spines. We have shown previously that neurabin, a major synaptic scaffolding protein, targets PP1 to synapses for LTD induction. Here, we show that spinophilin, a close homologue of neurabin and another major synaptic PP1 anchoring protein, does not play a role in LTD induction, which suggests that neurabin plays a privileged role in nanodomain targeting of PP1 in LTD induction. We found that protein kinase A can significantly weaken the neurabin-PP1 interaction in neurons via phosphorylation of neurabin at serine 461, a phosphorylation site adjacent to the PP1 binding motif that is not conserved in spinophilin. Finally, we found that a neurabin mutation (S461E), which mimics phosphorylation, blocked AMPA receptor endocytosis and LTD induction. The results indicate the critical importance of nanodomain targeting of PP1 within synaptic spines and its regulation in LTD induction.
Keywords: protein phosphatase 1, neurabin and spinophilin, targeting, anchoring, phosphorylation regulation, AMPA receptor trafficking, long term depression, PKA
Introduction
Synaptic plasticity, i.e., activity dependent modification of synaptic strength, is critical for neural circuit formation during development and for experience-dependent brain functions, such as learning and memory. NMDA receptor-mediated long term potentiation (LTP) and long term depression (LTD) are two important forms of synaptic plasticity, with the underlying mechanism being AMPA receptor insertion and endocytosis, respectively (Malinow and Malenka 2002; Siddoway 2011). Kinases in general function to promote AMPA receptor insertion and LTP induction while phosphatases in general function to promote AMPA receptor endocytosis and LTD induction. However, recent studies suggest a more complex picture of kinases and phosphatase action in the induction of LTP and LTD (Lu et al. 2008; Sanderson et al. 2011; Coultrap et al. 2014; Woolfrey and Dell'Acqua 2015). It is known that phosphatases such as protein phosphatase-1 (PP1) negatively regulate LTP induction via attenuating CaMKII activity (Strack et al. 1997). However, how kinases can negatively regulate PP1 in synaptic plasticity is not clear.
Protein phosphatase-1 plays a critical role in AMPA receptor trafficking and LTD induction (Mulkey et al. 1993; Morishita et al. 2001; Hu et al. 2006; Jouvenceau et al. 2006; Hou et al. 2013), and plays a negative role in LTP induction (Blitzer et al. 1998; Jouvenceau et al. 2006). These synaptic functions of PP1 contribute to its role in constraining learning and memory formation (Genoux et al. 2002). PP1 function in synaptic plasticity requires both its enzymatic activity (Mulkey et al. 1993) as well as its anchoring proteins, which target PP1 to synapses (Morishita et al. 2001). Our work on neurabin, a major synaptic PP1-anchoring protein, indicated that Nrb is critical for LTD induction (Hu et al. 2006), and that Nrb targets PP1 to its substrates in synaptic spines (Hu et al. 2007). In this study, we sought to determine whether any PP1 anchoring proteins were involved in targeting PP1 to substrates in LTD. In this regard, we determined whether another synaptic PP1 anchoring protein, spinophilin (Sph), a close homologue of Nrb, can provide PP1 for LTD induction. We also sought to determine if targeting of PP1 to synapses for LTD induction is subjected to regulation by phosphorylation.
We found that Sph-targeted PP1 did not play a critical role in LTD induction. We also found that PKA kinase can negatively regulate PP1 function in LTD via attenuating its interaction with Nrb. Specifically, PKA attenuates the PP1-Nrb interaction by phosphorylating Nrb at serine 461 (S461), a site immediately adjacent to the PP1 binding motif. Significantly, the corresponding Ser461 site in spinophilin is not a conserved phosphorylation site. We also found that expression of a neurabin mutation at serine 461 (Nrb (S461E)), which mimics phosphorylation, attenuated both AMPA receptor endocytosis and LTD induction. Thus, our results show that nanodomain targeting specificity of PP1 as well as the modulation of PP1 targeting by phosphorylation regulate LTD induction.
Material and Methods
Plasmid constructions, HEK 293 Cells, and transfections
Plasmids encoding GFP-Nrb, GFP-Sph (F451A), GFP-Nrb (S461A), and GFP-Nrb (S461E) in pEGFP-C1 and pSinRep5 (nsP2S) (gift of Dr. Nicole Calakos, Duke University) plasmids were described previously (Hu et al. 2007). Growth and transfection of HEK293 cells (ATCC) by lipofectamine 2000 were as described previously (Gao et al. 2009). Both Nrb and Sph cDNA are from rat species.
Primary hippocampal cultures, packaging recombinant Sindbis virus, and infection
Hippocampal neuron cultures were prepared from mixed sex rat embryos as described previously (Hou et al. 2013; Siddoway et al. 2013). Briefly, hippocampi were dissected from embryonic day 18 (E18) rat embryos. The tissues were dissociated by trypsin digestion followed by trituration through glass pipettes. For immunocytochemistry, hippocampal neurons were plated at a density of 1.5-2.5×104 cells/cm2 on glass coverslips coated with poly-L-lysine in 24-well plates. For Western blotting, cultures of cortical neurons were prepared from E18 rats, and plated at a density of 1×105/cm2 in 6-well plates.
Packaging and concentration of Sindbis virus has been described previously (Hu et al. 2006), except that the viral vector pSinRep5 nsp2s and DHBB helper were used to generate minimally toxic Sindbis viruses (Kim et al. 2004). Neurons (DIV21-DIV35) were infected with recombinant Sindbis viruses in conditioned medium for one hour, after which cells were washed and fresh conditioned medium added (>90% infection efficiency). Infected neurons were grown in conditioned medium for 24 hours before being used in experiments.
Co-immunoprecipitation (Co-IP) and Western blotting
Co-IP and subsequent Western blotting with GFP (Monoclonal antibody (Mab) from Roche), Nrb (Mab from BD bioscience), and/or PP1 (Mab from Santa Cruz biotech) antibodies were done as described previously (Gao et al. 2009).
AMPAR endocytosis assay, Laser confocal microscopy, and data analysis
AMPA receptor endocytosis was performed as described previously (Beattie et al. 2000). In brief, GluR1 antibody from Oncogene was used to label surface populations of GluR1 receptors in live neurons (15∼20 minutes followed by washing) before NMDA was added. Remaining cell surface-bound GluR1 antibody antigen complex was removed by acid wash, and endocytosed GluR1-GluR1 antibody complexes were visualized using a fluorescence-conjugated secondary antibody. Coverslips were mounted on glass slides with Fluoromount G (Electron Microscopy Sciences) for confocal imaging on a Zeiss LSM 510 (63× / 1.4 oil objective) as we have described previously (Siddoway et al. 2014b).
The same confocal microscope acquisition parameters were used for all scans for which fluorescence intensity was compared. All measurements were performed using MetaMorph software (Universal imaging, West Chester, PA). For quantitation, images were thresholded to subtract background fluorescence. Outlines of dendrites were carefully traced, and thresholded fluorescence intensity was then determined for the traced area. The fluorescence intensity was divided by the traced area, which was determined by setting a lower threshold level to outline the cell shape. For each experiment, internal fluorescence in cells was normalized by dividing the average fluorescence of untreated control cells. Each experiment was performed three times. Statistical analysis was performed with student's t test.
Organotypic slice culture and electrophysiological recordings
Organotypic slice cultures were prepared as we described previously (Hu et al. 2007). Electrophysiological recordings were also conducted exactly as described (Hu et al. 2007). All experimental protocols for live animals followed the ARRIVE guidelines (Kilkenny et al. 2010), and were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center, New Orleans.
Results
Sph-targeted PP1 does not play a critical role in LTD induction
We have shown that addition of a peptide that blocked the interaction of PP1 with its binding proteins resulted in defective LTD induction (Morishita et al. 2001). We have shown that Nrb, a major synaptic PP1 anchoring protein, is critical for LTD induction. Expression of a Nrb mutant unable to bind to PP1 (Nrb (F460A)) decreased synaptic PP1 protein levels (Hu et al. 2007) and inhibited LTD induction (Hu et al. 2006; Hu et al. 2007). These data suggest that Nrb targets PP1 to synaptic spines, the cellular domain where PP1 synaptic substrates (such as GluA1) localize, increasing substrate specificity of PP1. However, synaptic spines can accommodate thousands of protein molecules, and can be subdivided into sub-regions such as synaptic PSD nano-domain and perisynaptic regions; synaptic spines also contain Sph, another major synaptic PP1 anchoring protein, and a close homologue of Nrb (Grossman et al. 2002), which also targets PP1 to synaptic spines. Thus, we asked whether Sph-targeted PP1 supports induction of LTD.
Surprisingly, we found that LTD was normal in neurons expressing an Sph mutant, GFP-Sph (F451A), which corresponds to the GFP-Nrb (F460A) mutant, and is unable to bind PP1 (Fig. 1: at t=30 minutes post LTD stimulus: GFP-Nrb (F460A): 0.95+/- 0.1; GFP-Sph (F451A): 0.64+/-0.08; p<0.05, t-test) (Yan et al. 1999). The GFP tag on these constructs has been shown by us (Hu et al. 2006) and others (Zito et al. 2004; Terry-Lorenzo et al. 2005) not to interfere with Nrb function. Our data thus suggest that, although Nrb-targeted PP1 is essential, Sph-targeted PP1 does not play a critical role in LTD induction.
Figure 1. Spinophilin-targeted PP1 is not necessary for LTD induction.
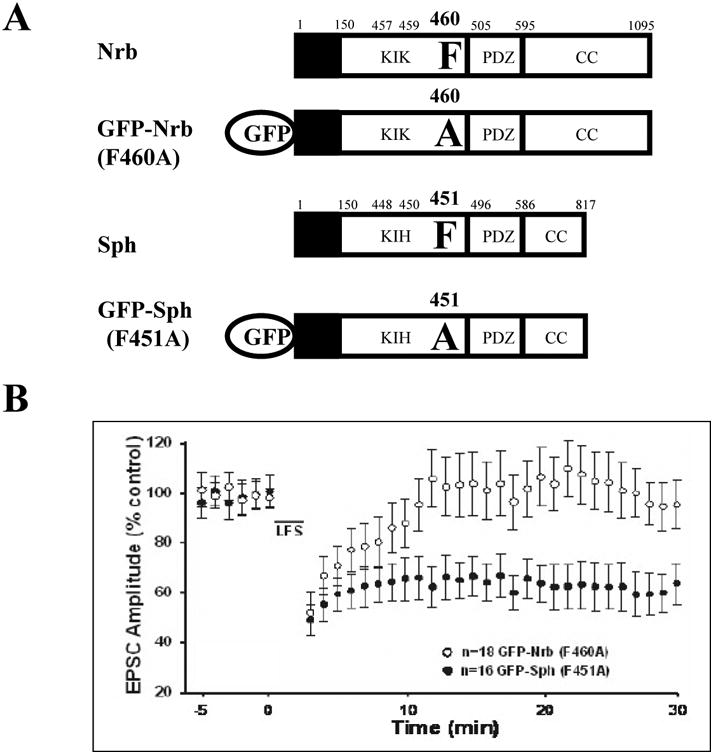
LTD was determined in CA1 neurons expressing a PP1-binding mutant of neurabin (GFP-Nrb (F460A)) or spinophilin (GFP-Sph (F451A)) in organotypic slice cultures. One-day expression of GFP fusion proteins was achieved by recombinant Sindbis viral infection in the CA1 cell body layer via injection. Error bars represent standard error of the means (SEM).
PKA activation regulates Nrb-PP1 binding through phosphorylation of Nrb at S461 in HEK 293 cells
Next we wanted to determine whether PP1 targeting of Nrb was regulated by phosphorylation. It has been reported that S461 immediately C terminal to the PP1 binding motif on Nrb (KIKFS), but not the homologous serine on Sph (KIHFS), can be phosphorylated (McAvoy et al. 1999; Oliver et al. 2002; Hu et al. 2006). We constructed mutants that mimicked or blocked phosphorylation of S461 in GFP-tagged Nrb constructs (Fig. 2A). After transfection into HEK 293 cells, expressed Nrb proteins in the soluble cell lysates were immunoprecipitated (IPed) by anti-GFP antibody, separated by SDS-PAGE, and Western blotted (Fig. 2B). Substantial PP1 was co-IPed with GFP-Nrb indicating that GFP-Nrb-PP1 complex formation (Fig. 2B). We found that significantly less PP1 was pulled down in immunoprecipitates from cells expressing the phosphorylation mimicking construct GFP-Nrb (S461E) than from cells expressing the phosphorylation blocking construct GFP-Nrb (S461A) (Fig. 2B), indicating that mimicking phosphorylation on S461 attenuated Nrb interaction with PP1. This result is consistent with the prediction that the introduction of a negative charge, via phosphorylation, into the hydrophobic PP1 binding motif, will interfere with PP1 binding. This is also consistent with the finding that phosphorylation (by PKA) of a purified Nrb fragment at S461, which is adjacent to the PP1 binding motif on Nrb (KIKF460), decreased its binding with PP1 in vitro (McAvoy et al. 1999).
Figure 2. Phosphorylation of Nrb at serine 461 weakens its binding to PP1.
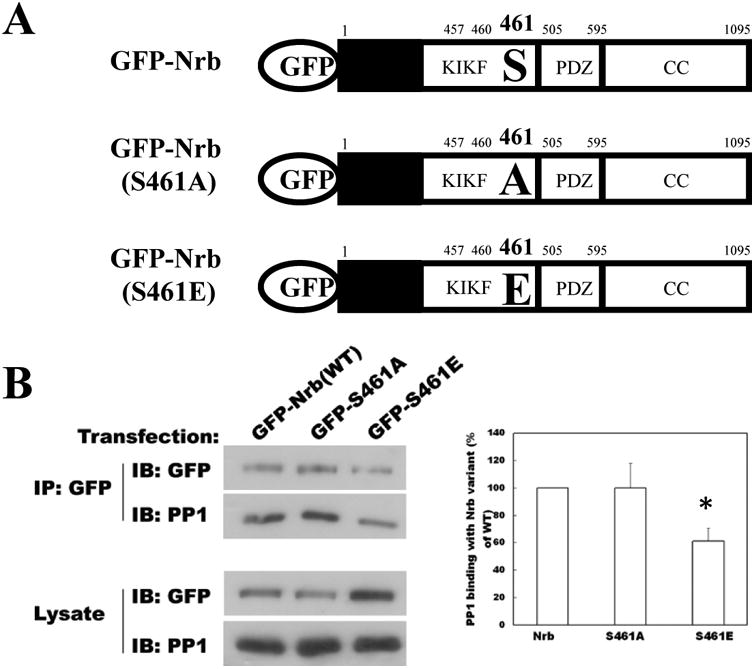
A Schematics of neurabin (Nrb) constructs used in the study. The key PP1 binding motif and mutated residues are explicitly noted. Other domains are also listed (PDZ and coiled coil (CC) domains) or are colored (black box: F-actin binding domain). B: Phosphorylation-mimicking mutant of Nrb at serine 461 exhibits decreased binding to PP1 compared to WT Nrb or Nrb phosphorylation-blocking mutant. Recombinant Nrb proteins were IPed with anti-GFP antibody and analyzed by Western blot for both GFP and PP1. Quantitation of co-IPed PP1 relative to input is shown to the right (N=3 for each group, p< 0.05, t-test).
We next determined whether phosphorylation attenuated Nrb-PP1 binding. Two days after transfection, HEK 293 cells were treated with forskolin to activate PKA. GFP-Nrb was IPed with anti-GFP antibody, and co-IPed PP1 was determined by Western blotting (Fig. 3). We found that following phosphorylation by PKA, the amount of PP1 that was co-IPed with the same amount of GFP-Nrb was significantly decreased (Fig. 3). We have observed similar phenomena using a different PKA activator, Sp-cAMP (Supplemental Fig. 1). These results indicate that the interaction of Nrb with PP1 is decreased by PKA activation.
Figure 3. PKA mediated-phosphorylation of Nrb at serine 461 weakens Nrb-PP1.
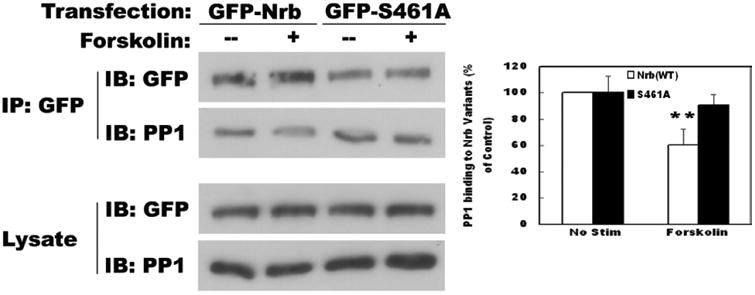
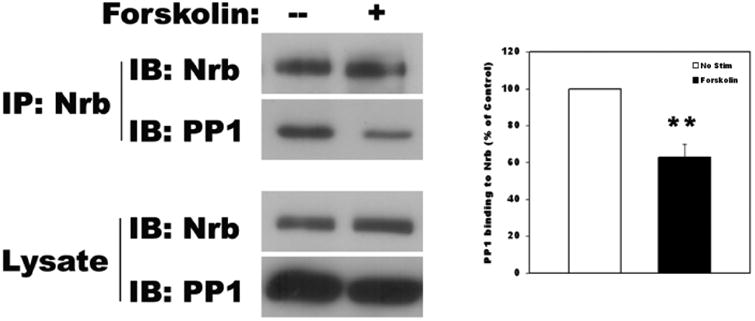
interaction. Phosphorylation-blocking mutant of Nrb at serine 461 blocked the decrease in binding between Nrb WT and PP1 induced by forskolin application. Quantitation is shown in the right panel (n=3 for each group, p< 0.05, t-test).
We then determined whether the decreased binding between Nrb and PP1, in response to forskolin application, was mediated by phosphorylation at S461. In contrast to the reduction of co-IPed PP1 in WT GFP-Nrb IP experiment, the amount of PP1 co-IPed with GFP-Nrb (S461A) was not affected by forskolin treatment (Fig. 3). This result indicates that decreased binding between Nrb and PP1 caused by PKA activation in vivo is mostly mediated by phosphorylation of S461 of Nrb.
Regulation of endogenous Nrb-PP1 interactions in neurons
To determine whether endogenous Nrb-PP1 complex in neurons can be modulated by PKA activation, primary cortical neuron cultures were prepared for biochemical analysis. When the neurons were stimulated with forskolin, the amount of PP1 co-IPed with Nrb was decreased to 62.8±7.0% of no forskolin control (Fig. 4A). This suggests that endogenous Nrb-PP1 complex in neurons is subject to PKA regulation.
Figure 4. Regulation of Nrb-PP1 interactions in neurons affects AMPA receptor endocytosis.
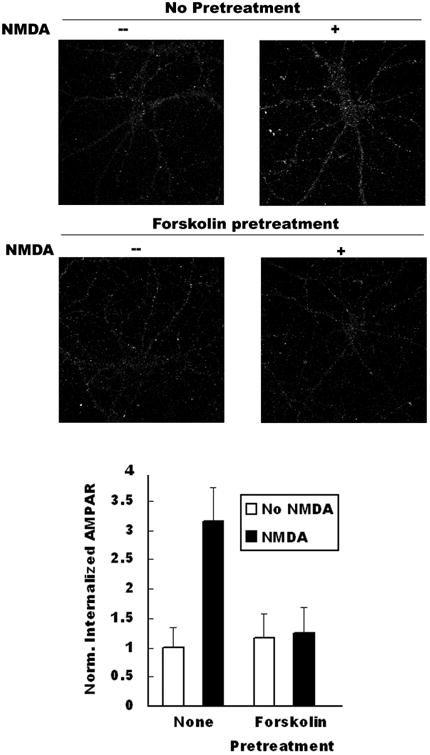
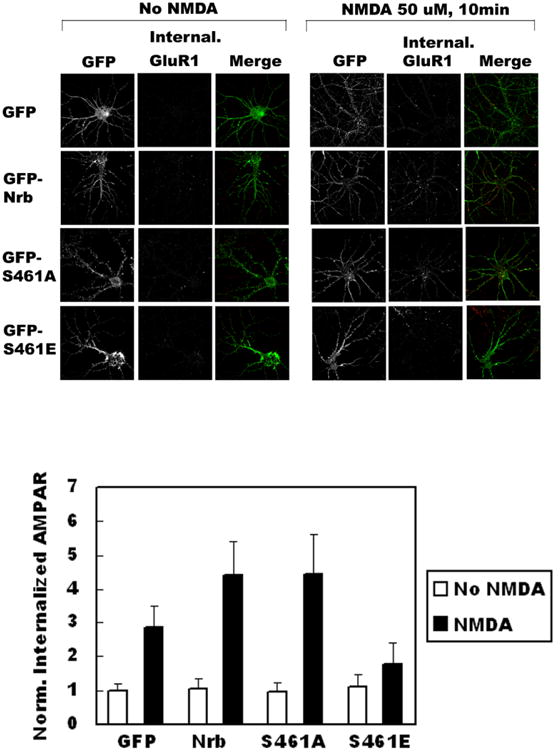
A Co-IP of PP1 by Nrb antibody from neurons following PKA activation through forskolin application. Quantitation is shown in bottom panel (N=3, p< 0.05, t-test). B: Effect of PKA activation on AMPA receptor endocytosis in neurons. Quantitation is shown in bottom panel (no pretreat and no NMDA: n=28, no pretreat and NMDA: n=32, p< 0.05, t-test; forskolin pretreat and no NMDA treat: n=31, forskolin/NMDA treat: n=38, p>0.05, t-test). C. Effect of expression of phosphorylation-mimicking mutant of Nrb on NMDA-induced AMPA receptor endocytosis. Quantitation is shown at right (GFP: no stim (n=20), NMDA stim (n=30), p<0.05, t-test; GFP-Nrb: no stim (n=26), NMDA stim (n=29), p<0.05, t-test; GFP-Nrb (S461A): no stim (n=26), NMDA stim (n=30), p<0.05; GFP-Nrb (S461E): no stim (n=28), NMDA stim (n=32), p>0.05, t-test).
AMPAR endocytosis is attenuated in neurons expressing Nrb phosphorylation mimicking-mutant at S461
In light of our previous finding that the Nrb-PP1 interaction is critical for LTD induction (Hu et al. 2006), we assessed the consequences of reducing the interaction between Nrb and PP1 in AMPA receptor endocytosis, a cellular model for LTD. To this end, we determined the effect on AMPA receptor endocytosis of blocking Nrb phosphorylation. Consistent with prior reports, we found that treatment of cultures with NMDA caused significant internalization of AMPARs (Fig. 4B, upper panel, untreated: 1.00±0.35, n=14; NMDA treated: 3.15±0.59, n=16; internalized AMAPR immunoreactivity normalized to untreated control). Treatment of neurons with forskolin before (20 minutes) and during NMDA application (10 minutes) largely abolished NMDA-induced AMPAR internalization (Fig. 4B, bottom panel, untreated: 1.17±0.40, n=14; NMDA treated: 1.25±0.44, n=19). This again is consistent with literature reports that PKA activation blocks AMPA receptor endocytosis (Ehlers 2000) and LTD induction (Mulkey et al. 1994). After establishing the endocytosis assay, we expressed recombinant Nrb proteins in hippocampal neurons through infection with recombinant Sindbis virus encoding GFP, GFP-Nrb (S461A), or GFP-Nrb (S461E). We observed strong attenuation of AMPA receptor endocytosis in neurons expressing the Nrb phosphorylation-mimicking mutant, GFP-Nrb (S461E) (No NMDA: 1.11±0.39, n=14; NMDA-treated: 1.77±0.64, n=16), while neurons expressing WT GFP-Nrb (No NMDA: 1.03±0.32, n=13; NMDA-treated: 4.42±0.98, n=15) or phosphorylation-blocking GFP-Nrb (S461A) (No NMDA: 0.96±0.30, n=13; NMDA-treated: 4.42±1.19, n=15) exhibited increased amounts of endocytosed AMPA receptor compared to the GFP control (No NMDA: 1.00±0.22, n=10; NMDA treated: 2.87±0.62, n=15; Fig. 4C). These data indicate that modulating the Nrb-PP1 interaction via Nrb phosphorylation on S461 has significant effects on AMPA receptor endocytosis.
LTD is defective in neurons expressing Nrb mutant whose interaction with PP1 is weakened
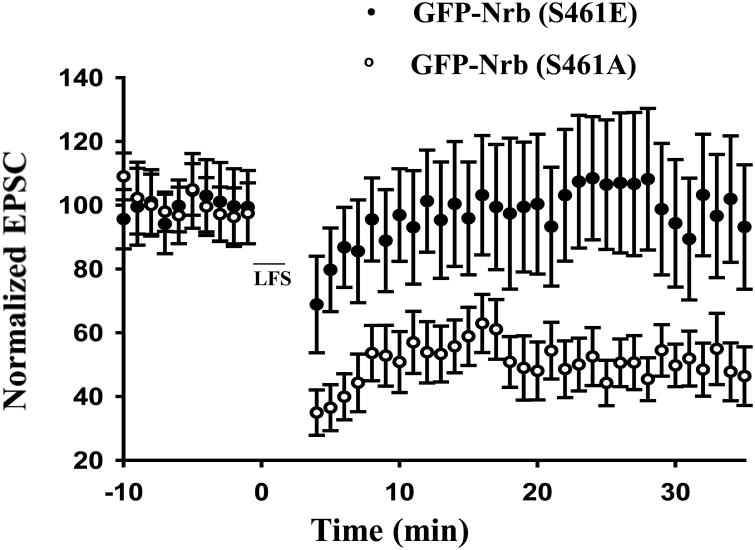
We next analyzed LTD directly in organotypic hippocampal CA1 neurons infected with Sindbis virus expressing GFP-Nrb mutants. Robust LTD was observed in GFP-Nrb (S461A)-expressing CA1 pyramidal neurons (41 ± 8%; n=7; Fig. 5) using the 5Hz/3 minute LTD stimulation protocol (Morishita et al. 2001; Hu et al. 2006). In contrast, the same stimulus elicited no synaptic depression in neurons expressing GFP-Nrb (S461E) (98 ± 8%, n=5; Fig. 4). These data are consistent with defective AMPA receptor endocytosis in neurons expressing phosphorylation-mimicking GFP-Nrb (S461E), indicating that the phosphorylation of Nrb at S461 interferes with LTD induction.
Figure 5. Expression of phosphorylation-mimicking mutant of Nrb attenuates LTD induction in organotypic hippocampal slice cultures.
Effect of expression of Nrb phosphorylation-mimicking mutant, GFP-Nrb (S461E), on LTD induction in CA1 neurons in hippocampal organotypic slice cultures. Control expression is Nrb phosphorylation-blocking mutant, Nrb (S461A) (N=5 for each group. P< 0.05, t-test).
Discussion
It is known that PP1 plays a critical role in many synaptic plasticity processes including Hebbian synaptic plasticity, homeostatic synaptic plasticity, and metaplasticity (Mulkey et al. 1993; Blitzer et al. 1998; Genoux et al. 2011; Siddoway et al. 2013; Siddoway et al. 2014a). Because of the indiscriminate nature of the actions of PP1 on its substrates, both the activity and proper targeting/anchoring of PP1 are presumed to be important for the regulation of PP1 in these forms of synaptic plasticity. In this report, we have made two important findings. First, PP1-targeting required for LTD induction exhibits exquisite nanodomain specificity. PP1 targeted by Nrb supports LTD induction, while PP1 targeted by Sph, a close Nrb homologue, does not. Secondly, we found that PKA kinase can negatively regulate PP1 function via attenuating PP1 targeting by Nrb. PKA negatively regulates PP1 targeting by weakening the Nrb-PP1 interaction via phosphorylation of Nrb at S461, a site immediately adjacent to the PP1 binding motif.
Our finding that Sph-targeted PP1 does not play a role in LTD induction is unexpected because (1) Sph and Nrb are close homologues of each other and in general bind to same set of proteins through their many conserved domains (Hu et al. 2006; Baucum et al. 2010; Ragusa et al. 2010; Baucum et al. 2012; Baucum et al. 2013), (2) specifically, Sph and Nrb are two major synaptic PP1-anchoring proteins (Colbran et al. 1997; Terry-Lorenzo et al. 2002) and (3) a role for Sph in LTD induction is suggested from studies performed in Sph KO mice (Feng et al. 2000). Both Nrb and Sph are primarily targeted to synaptic spines via their N terminal F-acting binding domains (ABD) (Grossman et al. 2002; Zito et al. 2004). However, there exist differences in their sequences that could potentially affect their nanodomain localization within the spines, either directly or indirectly. For example, there are many differences in phosphorylation sites between the core ABDs (aa 1-144) in Nrb and Sph (Hsieh-Wilson et al. 2003; Grossman et al. 2004). Moreover, Sph, but not Nrb, can interact with certain G-protein coupled receptors (GPCR) through sites adjacent to the ABD domain (aa 145-285) (Smith et al. 1999; Richman et al. 2001). These differential interactions underlie the opposing functions of Nrb and Sph in GPCR signaling (Wang et al. 2005; Wang et al. 2007). It was recently reported that Nrb, but not Sph, interacts with A1R through aa 146-453 on Nrb (Chen et al. 2012), further illustrating that the difference of this divergent domain on Nrb and Sph contributes to the functional difference of Nrb and Sph function. It will be important to determine whether this structural difference results in subtle differential anchoring of Nrb and Sph within the synaptic spines, thus contributing to the distinct effects of PP1 targeted by Nrb and Sph in LTD. Future super-resolution studies utilizing specific Nrb and Sph antibodies will be needed to test this prediction. Other regions beyond ABD that are not conserved between Nrb and Sph, such as the domain between ABD and the PP1 binding motif on Nrb and Sph (∼120 amino acids), may also contribute to the distinct functions of Nrb and Sph on LTD. This domain on Nrb and Sph may interact with different proteins and/or help Nrb/Sph localize to different nanodomains in the spine as well.
Our data on Sph-targeted PP1 in LTD is in apparent contradiction with the published null LTD phenotype in Sph KO mice (Feng et al. 2000), which indicates an essential role of Sph in LTD. However, all Sph binding proteins including PP1 are perturbed in Sph KO mice. For example, CaMKII can also bind Sph (Baucum et al. 2012). Moreover, binding is age-dependent (Baucum et al. 2012), and also presumably participates in different complex formation in different brain regions (Baucum et al. 2013). Thus, the null LTD phenotype in Sph KO mice does not necessarily indicate a contribution of PP1 bound to Sph in LTD. Moreover, Sph KO mice exhibited a deformed hippocampal structure, CA3 layer-formation defects, spine density changes, and other abnormalities (Feng et al. 2000; Bielas et al. 2007). Therefore, the null LTD phenotype in Sph KO mice may be an indirect result of developmental compensation rather than a direct effect of Sph, via its binding proteins, on synaptic depression.
Our work presented here illustrates a novel example of how kinases can inhibit phosphatase signaling, a mechanism that may play a critical role in the induction of synaptic plasticity. We found that PKA can phosphorylate Nrb at S461, thereby weakening the interaction between Nrb and PP1. This, thus, reduces the amount of PP1 that can be targeted to the distinct nanodomain within spines defined by Nrb localization. We further showed that mimicking Nrb phosphorylation at S461 blocks both AMPA receptor endocytosis and LTD induction, suggesting functional importance of PKA action on synaptic plasticity via Nrb-PP1 signaling. Serine 845 on GluA1 is also a PKA site important for AMPA receptor trafficking and LTD induction (Ehlers 2000; Tavalin et al. 2002; Lee et al. 2010). On the other hand, a deficit in LTD induction was observed in a knock-in mouse in which the binding site for PKA on AKAP79/150 was deleted (Lu et al. 2008). Thus, it will be important to determine the exact sources of the PKA critical for phosphorylation of Nrb in synaptic plasticity.
When Nrb was phosphorylated at S461, the amount of PP1 binding to Nrb was only reduced by half. However, this reduced amount of PP1 bound to Nrb did not support AMPA receptor endocytosis and LTD induction, suggesting that PP1 is a rate limiting factor. This is consistent with observations from our previous work showing that increasing the amount of PP1 available in CA1 neurons, via including purified PP1 in the recording pipette, reduced the threshold of LTD induction (Morishita et al. 2001).
In summary, our findings here indicate that PP1 bound on Sph does not play a critical role in LTD induction, but PP1 bound on Nrb does, and Nrb dephosphorylation at serine 461, a site not conserved in Sph, leads to maximal PP1 targeting by Nrb to the correct nanodomain within spines for synaptic depression.
Supplementary Material
Supplemental Figure 1: PKA activator, Sp-cAMP, weakens the interaction between Nrb and PP1. Recombinant GFP-Nrb proteins were IPed with anti-GFP antibody while co-IPed PP1 is blotted. Cells are pre-treated with PKA activator, Sp-cAMP.
Highlights.
Spinophilin-targeted PP1 is not necessary for LTD induction.
Neurabin-PP1 interaction is weakened in neurons via phosphorylation by PKA at serine 461, which is immediately adjacent to the PP1 binding motif.
Neurabin phosphorylation-mimicking mutant at serine 461 blocks AMPA receptor endocytosis and LTD induction.
Acknowledgments
This work was supported by funding from NSF (IOS-1457336) and NIH (R01MH109719) to HX. The confocal images were taken with the Zeiss LSM510 imaging system, a core facility supported by NIH/NIGMS Grant P30-GM103340.
Footnotes
There are no conflicts of interest noted by any of the authors.
References
- Baucum AJ, 2nd, Strack S, Colbran RJ. Age-dependent targeting of protein phosphatase 1 to Ca2+/calmodulin-dependent protein kinase II by spinophilin in mouse striatum. PloS one. 2012;7:e31554. doi: 10.1371/journal.pone.0031554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Brown AM, Colbran RJ. Differential association of postsynaptic signaling protein complexes in striatum and hippocampus. Journal of neurochemistry. 2013;124:490–501. doi: 10.1111/jnc.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ, Colbran RJ. Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Mol Cell Proteomics. 2010;9:1243–1259. doi: 10.1074/mcp.M900387-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature neuroscience. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Bielas SL, Serneo FF, Chechlacz M, Deerinck TJ, Perkins GA, Allen PB, Ellisman MH, Gleeson JG. Spinophilin facilitates dephosphorylation of doublecortin by PP1 to mediate microtubule bundling at the axonal wrist. Cell. 2007;129:579–591. doi: 10.1016/j.cell.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Connor JH, Brown GP, Wong T, Shenolikar S, Iyengar R, Landau EM. Gating of CaMKII by cAMP-regulated protein phosphatase activity during LTP. Science (New York, NY. 1998;280:1940–1942. doi: 10.1126/science.280.5371.1940. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu Y, Cottingham C, McMahon L, Jiao K, Greengard P, Wang Q. Neurabin scaffolding of adenosine receptor and RGS4 regulates anti-seizure effect of endogenous adenosine. J Neurosci. 2012;32:2683–2695. doi: 10.1523/JNEUROSCI.4125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran RJ, Bass MA, McNeill RB, Bollen M, Zhao S, Wadzinski BE, Strack S. Association of brain protein phosphatase 1 with cytoskeletal targeting/regulatory subunits. Journal of neurochemistry. 1997;69:920–929. doi: 10.1046/j.1471-4159.1997.69030920.x. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Freund RK, O'Leary H, Sanderson JL, Roche KW, Dell'Acqua ML, Bayer KU. Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell reports. 2014;6:431–437. doi: 10.1016/j.celrep.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97:9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Siddoway B, Huang Q, Xia H. Inactivation of CREB mediated gene transcription by HDAC8 bound protein phosphatase. Biochemical and biophysical research communications. 2009;379:1–5. doi: 10.1016/j.bbrc.2008.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoux D, Bezerra P, Montgomery JM. Intra-spaced stimulation and protein phosphatase 1 dictate the direction of synaptic plasticity. The European journal of neuroscience. 2011;33:1761–1770. doi: 10.1111/j.1460-9568.2011.07669.x. [DOI] [PubMed] [Google Scholar]
- Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Hsieh-Wilson LC, Allen PB, Nairn AC, Greengard P. The actin-binding domain of spinophilin is necessary and sufficient for targeting to dendritic spines. Neuromolecular medicine. 2002;2:61–69. doi: 10.1385/NMM:2:1:61. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Futter M, Snyder GL, Allen PB, Nairn AC, Greengard P, Hsieh-Wilson LC. Spinophilin is phosphorylated by Ca2+/calmodulin-dependent protein kinase II resulting in regulation of its binding to F-actin. Journal of neurochemistry. 2004;90:317–324. doi: 10.1111/j.1471-4159.2004.02491.x. [DOI] [PubMed] [Google Scholar]
- Hou H, Sun L, Siddoway BA, Petralia RS, Yang H, Gu H, Nairn AC, Xia H. Synaptic NMDA receptor stimulation activates PP1 by inhibiting its phosphorylation by Cdk5. The Journal of cell biology. 2013;203:521–535. doi: 10.1083/jcb.201303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh-Wilson LC, Benfenati F, Snyder GL, Allen PB, Nairn AC, Greengard P. Phosphorylation of spinophilin modulates its interaction with actin filaments. The Journal of biological chemistry. 2003;278:1186–1194. doi: 10.1074/jbc.M205754200. [DOI] [PubMed] [Google Scholar]
- Hu XD, Huang Q, Yang X, Xia H. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J Neurosci. 2007;27:4674–4686. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XD, Huang Q, Roadcap DW, Shenolikar SS, Xia H. Actin-associated neurabin-protein phosphatase-1 complex regulates hippocampal plasticity. Journal of neurochemistry. 2006;98:1841–1851. doi: 10.1111/j.1471-4159.2006.04070.x. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Hedou G, Potier B, Kollen M, Dutar P, Mansuy IM. Partial inhibition of PP1 alters bidirectional synaptic plasticity in the hippocampus. The European journal of neuroscience. 2006;24:564–572. doi: 10.1111/j.1460-9568.2006.04938.x. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Dittgen T, Nimmerjahn A, Waters J, Pawlak V, Helmchen F, Schlesinger S, Seeburg PH, Osten P. Sindbis vector SINrep(nsP2S726): a tool for rapid heterologous expression with attenuated cytotoxicity in neurons. Journal of neuroscience methods. 2004;133:81–90. doi: 10.1016/j.jneumeth.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. Journal of neurophysiology. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhang M, Lim IA, Hall DD, Allen M, Medvedeva Y, McKnight GS, Usachev YM, Hell JW. AKAP150-anchored PKA activity is important for LTD during its induction phase. The Journal of physiology. 2008;586:4155–4164. doi: 10.1113/jphysiol.2008.151662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annual review of neuroscience. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McAvoy T, Allen PB, Obaishi H, Nakanishi H, Takai Y, Greengard P, Nairn AC, Hemmings HC., Jr Regulation of neurabin I interaction with protein phosphatase 1 by phosphorylation. Biochemistry. 1999;38:12943–12949. doi: 10.1021/bi991227d. [DOI] [PubMed] [Google Scholar]
- Morishita W, Connor JH, Xia H, Quinlan EM, Shenolikar S, Malenka RC. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science (New York, NY. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- Oliver CJ, Terry-Lorenzo RT, Elliott E, Bloomer WA, Li S, Brautigan DL, Colbran RJ, Shenolikar S. Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Mol Cell Biol. 2002;22:4690–4701. doi: 10.1128/MCB.22.13.4690-4701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragusa MJ, Dancheck B, Critton DA, Nairn AC, Page R, Peti W. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nature structural & molecular biology. 2010;17:459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. The Journal of biological chemistry. 2001;276:15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, Dell'Acqua ML. NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2)(+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron. 2011;89:1000–1015. doi: 10.1016/j.neuron.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway B, Hou H, Xia H. Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology. 2014a;78:38–44. doi: 10.1016/j.neuropharm.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway B, Hou H, Yang H, Petralia R, Xia H. Synaptic activity bidirectionally regulates a novel sequence-specific S-Q phosphoproteome in neurons. Journal of neurochemistry. 2014b;128:841–851. doi: 10.1111/jnc.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway BA, Altimimi HF, Hou H, Petralia RS, Xu B, Stellwagen D, Xia H. An essential role for inhibitor-2 regulation of protein phosphatase-1 in synaptic scaling. J Neurosci. 2013;33:11206–11211. doi: 10.1523/JNEUROSCI.5241-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway BHH, Xia H. Glutamatergic Synapses: Molecular Organization. Encyclopedia of Life Sciences. 2011 doi: 10.1002/9780470015902.a0000235.pub2. [DOI] [Google Scholar]
- Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. The Journal of biological chemistry. 1999;274:19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- Strack S, Barban MA, Wadzinski BE, Colbran RJ. Differential inactivation of postsynaptic density-associated and soluble Ca2+/calmodulin-dependent protein kinase II by protein phosphatases 1 and 2A. Journal of neurochemistry. 1997;68:2119–2128. doi: 10.1046/j.1471-4159.1997.68052119.x. [DOI] [PubMed] [Google Scholar]
- Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Roadcap DW, Otsuka T, Blanpied TA, Zamorano PL, Garner CC, Shenolikar S, Ehlers MD. Neurabin/protein phosphatase-1 complex regulates dendritic spine morphogenesis and maturation. Mol Biol Cell. 2005;16:2349–2362. doi: 10.1091/mbc.E04-12-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-Lorenzo RT, Carmody LC, Voltz JW, Connor JH, Li S, Smith FD, Milgram SL, Colbran RJ, Shenolikar S. The neuronal actin-binding proteins, neurabin I and neurabin II, recruit specific isoforms of protein phosphatase-1 catalytic subunits. The Journal of biological chemistry. 2002;277:27716–27724. doi: 10.1074/jbc.M203365200. [DOI] [PubMed] [Google Scholar]
- Wang X, Zeng W, Kim MS, Allen PB, Greengard P, Muallem S. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. The EMBO journal. 2007;26:2768–2776. doi: 10.1038/sj.emboj.7601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nature cell biology. 2005;7:405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- Woolfrey KM, Dell'Acqua ML. Coordination of Protein Phosphorylation and Dephosphorylation in Synaptic Plasticity. The Journal of biological chemistry. 2015;290:28604–28612. doi: 10.1074/jbc.R115.657262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nature neuroscience. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- Zito K, Knott G, Shepherd GM, Shenolikar S, Svoboda K. Induction of spine growth and synapse formation by regulation of the spine actin cytoskeleton. Neuron. 2004;44:321–334. doi: 10.1016/j.neuron.2004.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: PKA activator, Sp-cAMP, weakens the interaction between Nrb and PP1. Recombinant GFP-Nrb proteins were IPed with anti-GFP antibody while co-IPed PP1 is blotted. Cells are pre-treated with PKA activator, Sp-cAMP.