Abstract
Objectives
The associations between subclinical depressive symptoms, as well specific symptom subscales, on brain structure in aging are not completely elucidated. This study investigated the extent to which depressive symptoms were related to brain volumes in fronto-limbic structures in a sample of middle-aged to older adults.
Method
Eighty participants underwent structural neuroimaging and completed the Beck Depression Inventory, 2nd Edition (BDI-II), which comprises separate affective, cognitive, and somatic subscales. Gray matter volumes were extracted from the caudal and rostral anterior cingulate, posterior cingulate, hippocampus, and amygdala. Hierarchical regression models examined the relationship between brain volumes and (i) total depressive symptoms and (ii) BDI-II subscales were conducted.
Results
After adjusting for total intracranial volume, race, and age, higher total depressive symptoms were associated with smaller hippocampal volume (p = 0.005). For the symptom subscales, after controlling for the abovementioned covariates and the influence of the other symptom subscales, more somatic symptoms were related to smaller posterior cingulate (p = 0.025) and hippocampal (p < 0.001) volumes. In contrast, the affective and cognitive subscales were not associated with brain volumes in any regions of interest.
Conclusion
Our data showed that greater symptomatology was associated with smaller volume in limbic brain regions. These findings provide evidence for preclinical biological markers of major depression and specifically advance knowledge of the relationship between subclinical depressive symptoms and brain volume. Importantly, we observed variations by specific depressive symptom subscales, suggesting a symptom-differential relationship between subclinical depression and brain volume alterations in middle-aged and older individuals.
Keywords: aging, BDI-II, MRI, somatic symptoms, symptom dimensions
Introduction
Major depressive disorder (MDD) has been linked to alterations in brain structure and function. In frontal cortical regions, there are associations between MDD and decreased brain volume, metabolism, and perfusion have been reported (Bora, Fornito, Pantelis, & Yucel, 2012; Pandya, Altinay, Malone, & Anand, 2012). Abnormalities in limbic regions, such as the hippocampus and amygdala, have also been implicated, particularly with respect to alterations in brain volume (Lorenzetti, Allen, Fornito, & Yucel, 2009; Pandya et al., 2012). For late-life depression, depressive symptoms have been associated with gray matter structural abnormalities within frontal and limbic networks (Alexopoulos, 2002; Phillips, Drevets, Rauch, & Lane, 2003). Most often, volumetric decreases in these regions were reported (Andreescu et al., 2008; Du et al., 2014), though some studies suggest no significant volumetric differences between older adults with depression and healthy age-matched controls (Colloby et al., 2011; Koolschijn et al., 2010; Sexton et al., 2012).
There is evidence that aging is associated with decreased incidence of MDD (Beekman, Copeland, & Prince, 1999; Blazer & Hybels, 2005). Incidence of depressive symptoms that do not meet clinical criteria for major depression (i.e., subclinical, subthreshold, subsyndromal, or minor depression), however, increases with age (Polyakova et al., 2014). Subclinical depressive symptoms in older adults have been associated with similar structural brain alterations, health outcomes, and economic costs as MDD (Dotson, Davatzikos, Kraut, & Resnick, 2009; Meeks, Vahia, Lavretsky, Kulkarni, & Jeste, 2011; Szymkowicz, McLaren, O'Shea, et al., 2016; Taki et al., 2005; Zhou et al., 2016), and those individuals with subclinical depression are at increased risk for major depression and other psychiatric conditions (Laborde-Lahoz et al., 2015). However, subclinical depressive symptoms are often underdiagnosed and therefore undertreated (Cuijpers et al., 2013; Kumar, Jin, Bilker, Udupa, & Gottlieb, 1998), thus posing a significant public health concern.
Depression is a heterogeneous disorder that comprises many different symptom combinations (American Psychiatric Association, 2013). There is indication that age differences may exist with respect to depressive symptom presentation. Compared to younger adults, older adults are less likely to endorse affective symptoms of depression (e.g., dysphoria, loss of interest; Gallo, Anthony, & Muthen, 1994) and are more likely to endorse cognitive and somatic symptoms, such as concentration difficulties, sleep disturbance, and fatigue (Christensen et al., 1999; Fiske, Wetherell, & Gatz, 2009). This may relate to age-associated cognitive and physical changes and other medical comorbidities typical in older adults.
The Beck Depression Inventory, 2nd Edition (BDI-II; Beck, Steer, & Brown, 1996; Beck, Steer, Brown, & van der Does, 2002; Vanheule, Desmet, Groenvynck, Rosseel, & Fontaine, 2008) is a widely used depression scale that covers affective (e.g., sadness, loss of interest), cognitive (e.g., worthlessness, guilty feelings), and somatic (e.g., changes in sleep, tiredness or fatigue) symptoms that are common amongst depressed individuals. It is important to highlight that the BDI-II cognitive subscale is referring to cognitive symptoms of depression (e.g., dysfunctional beliefs, cognitive biases; Beck, Rush, Shaw, & Emery, 1987) and not cognitive dysfunction related to depression. To date, specific associations between these affective, cognitive, and somatic symptoms in healthy aging and brain volume alterations are not well understood, though a limited, but growing, body of evidence suggests differential relationships between symptom dimensions of depression and brain structure (Kirton, Resnick, Davatzikos, Kraut, & Dotson, 2014; Lener et al., 2016; McLaren et al., 2016, 2017).
Further advancing this research field, the present study integrated neuroimaging techniques with depressive symptomatology towards identification of preclinical biological markers of major depression. In particular, we systematically examined the extent to which self-reported total depressive symptomatology predicted brain volume in fronto-limbic regions (i.e., anterior and posterior cingulate [ACC and PCC, respectively], hippocampus, and amygdala) in a sample of healthy middle-aged to older adults. Secondary symptom-specific analyses examined the association of the three BDI-II subscales (i.e., affective, cognitive, and somatic symptoms) on these fronto-limbic brain volumes, as these relationships are also not well established yet.
Early conceptualization of the ACC dissociated it into dorsal-caudal “cognitive” and ventral-rostral “affective” subregions (Bush, Luu, & Posner, 2000). The dorsal portion interconnects with the dorsolateral prefrontal cortex, parietal cortex, and supplementary motor areas, and the more ventral subregion interconnects with limbic and paralimbic structures. Contrary to this early conceptualization, Etkin, Egner, and Kalisch (2011) concluded that both subregions make important contributions to emotion processing, with dorsal-caudal regions involved in the evaluation and expression of negative emotion and ventral-rostral regions involved in top-down regulation of limbic areas involved in the generation of emotional responses. Since our secondary analyses looked at depressive symptom subscales, we included both the caudal and rostral ACC into our analyses to determine whether there were any subregion-specific differential effects.
We also chose to break down the cingulate gyrus into both ACC and PCC subregions. This was based on recent work from our group in an independent study with older adults that had found distinct relationships between subregions of the cingulate and specific symptom dimensions of depression (McLaren et al., 2016). Specifically, higher depressed mood symptoms were associated with larger PCC and smaller isthmus volumes and higher somatic symptoms were related to smaller PCC volumes. A trend for higher scores on the lack of positive affect subscale was associated with greater ACC volume.
Consistent with previous literature, we expected that greater levels of self-reported depressive symptomatology would be associated with smaller volume in brain regions of interest (ROIs; ACC, PCC, hippocampus, and amygdala) in middle-aged to older adults (Hypothesis 1). With respect to specific symptom subscales, we expected greater levels of affective (Hypothesis 2) and somatic (Hypothesis 3) symptoms to be associated with smaller brain region volumes, as these subscales have shown significant relationships with brain alterations in our (Dotson, Davatzikos, et al., 2009; McLaren et al., 2016, 2017) and others (e.g., Pujol et al., 2000) work. Given the lack of findings in previous studies on the relationship between cognitive symptoms and brain structural alterations, however, we did not have a specific hypothesis for this subscale.
Methods
Participants
Data were obtained from the ACTIVE Brain Study (Nissim et al., 2016; O'Shea, Cohen, Porges, Nissim, & Woods, 2016; Porges et al., 2017; 1) at the University of Florida, an ongoing research project exploring behavioral and multi-modal neuroimaging predictors of age-related cognitive dysfunction. We recruited community-dwelling individuals in the Gainesville and North Central Florida region (N = 80; see Table 1). Exclusionary criteria were self-reported history of major neurological (e.g., stroke, multiple sclerosis), psychiatric (e.g., schizophrenia), or other medical (e.g., hepatitis C) illness, failure of a brief cognitive screener (Montreal Cognitive Assessment [MoCA] < 20; Nasreddine et al., 2005), or magnetic resonance imaging (MRI) contraindications. Individuals with self-reported MDD (i.e., reported as diagnosed by a physician) were not excluded in order to increase the range of depressive symptom severity in the sample. However, no participants were included that self-reported BDI-II scores ≥ 14, consistent with our focus on subclinical depressive symptoms. Twenty-two participants had a self-reported MDD diagnosis and seven participants reported antidepressant medication usage. All participants in the study underwent a battery of neuropsychological and cognitive testing (which will not be reported here) followed by an MRI session. The University of Florida Institutional Review board approved all procedures in this study. Prior to any study procedures, participants provided both verbal and written informed consent.
Table 1.
Sample characteristics (N = 80)
| Mean | SD | Observed Range | Possible Range | |
|---|---|---|---|---|
| Age (years) | 70.70 | 10.57 | 43 – 89 | -- |
| Sex (% female) | 60.00 | -- | -- | -- |
| Education (years) | 16.42 | 2.56 | 12 – 20 | -- |
| Race/Ethnicity | -- | -- | -- | -- |
| % Caucasian | 91.25 | -- | -- | -- |
| % Non-Hispanic | 97.50 | -- | -- | -- |
| Self-Report Depression (% yes) | 27.50 | -- | -- | -- |
| Self-Report Antidepressant Usage (% yes) | 8.75 | -- | -- | -- |
| CCI Total Score | 0.43 | 0.69 | 0 – 3 | 0 – 37 |
| MoCA Total Score | 26.00 | 2.61 | 20 – 30 | 0 – 30 |
| BDI-II Total Score | 4.10 | 3.76 | 0 – 13 | 0 – 63 |
| BDI-II Affective Subscale Score | 0.37 | 0.68 | 0 – 3 | 0 – 15 |
| BDI-II Cognitive Subscale Score | 0.82 | 1.49 | 0 – 7 | 0 – 21 |
| BDI-II Somatic Subscale Score | 2.77 | 2.32 | 0 – 8 | 0 – 27 |
| Brain Volume Measurements | -- | -- | -- | -- |
| Caudal ACC (mm3) | 1819.44 | 352.70 | 1157.00 – 2654.00 | -- |
| Rostral ACC (mm3) | 2203.79 | 408.48 | 1245.00 – 3453.50 | -- |
| PCC (mm3) | 2882.62 | 382.44 | 2023.50 – 3917.50 | -- |
| Hippocampus (mm3) | 3820.86 | 464.92 | 2463.90 – 4698.10 | -- |
| Amygdala (mm3) | 1523.01 | 239.33 | 1004.00 – 2065.50 | -- |
Note: CCI = Charlson Comorbidity Index; MoCA = Montreal Cognitive Assessment; BDI-II = Beck Depression Inventory, 2nd Edition; ACC = Anterior Cingulate Cortex; PCC = Posterior Cingulate Cortex; mm, millimeters
Measures
Depressive Symptoms
Depressive symptoms were measured using the BDI-II (Beck et al., 1996), a 21-item self-report questionnaire assessing the frequency and severity of depressive symptoms over the previous two weeks. The BDI-II has been used to screen for depression in various populations and has strong psychometric properties in community-dwelling middle-aged to older adults (Segal, Coolidge, Cahill, & O'Riley, 2008). The BDI-II can be broken down into separate affective, cognitive, and somatic subscales (Beck et al., 2002; Vanheule et al., 2008). Table 2 notes the items comprising each subscale that were used in our analyses.
Table 2.
Item content of the BDI-II subscales used in the current study
| Affective symptoms | Cognitive symptoms | Somatic symptoms |
|---|---|---|
| Sadness | Past Failure | Crying |
| Pessimism | Guilty Feelings | Agitation |
| Loss of Pleasure | Punishment Feelings | Loss of Energy |
| Suicidal Thoughts or Wishes | Self-Dislike | Changes in Sleeping Pattern |
| Loss of Interest | Self-Criticalness | Irritability |
| Indecisiveness | Changes in Appetite | |
| Worthlessness | Concentration Difficulty | |
| Tiredness or Fatigue | ||
| Loss of Interest in Sex |
Note: BDI-II = Beck Depression Inventory, 2nd Edition
Covariates
All analyses controlled for variables known to be associated with depressive symptoms and brain volume measurements. In particular, in line with previous studies on depression and structural neuroimaging, analyses were adjusted for demographic characteristics, including age, sex, education, and race (Akhtar-Danesh & Landeen, 2007; Kempton et al., 2011). There is literature to suggest differential relationships between both depression and structural brain measurements and general cognitive status (Chiao & Weng, 2016; O'Shea et al., 2016; Paul et al., 2011; Yaffe et al., 1999) and chronic medical conditions (Assari & Lankarani, 2016; Meurs et al., 2015; Srinivasa et al., 2016). Therefore, we controlled for general cognitive status via MoCA scores and assessed for chronic medical comorbidities via the total number of endorsed items on the Charlson Comorbidity Index (CCI; Charlson, Pompei, Ales, & MacKenzie, 1987). Moreover, given that we were interested in the effects of depressive symptoms, and not depression diagnosis, we controlled for self-reported depression diagnosis. As there are discrepancies in the literature regarding the associations between antidepressant use and brain volumes, with studies showing both negative (Geerlings et al., 2012) and positive (Tanis, Newton, & Duman, 2007) relationships in both human and non-human samples, we controlled for antidepressant use in this sample. Finally, we adjusted for total intracranial volume (ICV) in order to normalize brain segmental volumes and reduce bias from variations in head size (O'Brien et al., 2011).
MRI Data Acquisition
Participants were imaged in a Philips Achieva 3.0 Tesla scanner (Philips Electronics, Amsterdam, The Netherlands) in the McKnight Brain Institute at the University of Florida with a standard 32-channel receive-only head coil. A pillow was placed under the head and foam padding was used to limit motion during the scan. Participants were given headphones and earplugs to minimize noise while inside the scanner. A high-resolution 3D T1-weighted MPRAGE scan was performed to obtain anatomical images. Scanning parameters consisted of: voxel size = 1 mm isotropic, 1 mm slice thickness, TR = 7 ms, TE = 3.2 ms, FOV = 240 × 240, flip angle = 8 degrees, 170 slices acquired in a sagittal orientation.
MRI Data Extraction
Using FreeSurfer software (version 5.3.0, http://surfer.nmr.mgh.harvard.edu; Fischl et al., 2002), T1 volumes were segmented into gray, white, cerebrospinal fluid, and non-brain tissues. Gray and white matter volumes were calculated through the fully automated FreeSurfer recon-all processing stream that was completed on all participants. Following preprocessing, all results underwent quality control to confirm correct detection of gray and white matter. Any errors in cortical segmentation were corrected manually (via control points, fixing white matter defects and tissue incorrectly identified as white matter, and pial editing) and volumes were re-processed through FreeSurfer via recon-all-make, producing results that are validated against manual segmentation (Morey et al., 2009). Subcortical segmentations were not manually edited. Participants with visually bad raw structural data were excluded from the dataset. For more technical details, see the following: Dale and Sereno (1993); Fischl and Dale (2000); Fischl, Liu, and Dale (2001); Fischl et al. (2002); Fischl et al. (2004); Segonne et al. (2004); and Sled, Zijdenbos, and Evans (1998). For each hemisphere, gray matter volumes were extracted for the caudal and rostral ACC, PCC, hippocampus, and amygdala, and total ICV was estimated. Right and left volumes were averaged to obtain a total volumetric value for each ROI, as our hypotheses were not hemisphere-specific.
Statistical Analyses
Analyses were conducted using SPSS 24.0 software (IBM Corp., 2016). Prior to statistical analysis, we screened data for outliers by computing standardized z-scores for all dependent and independent variables. Outliers were identified as z > ±3 standard deviations and removed from analyses (n = 2; one individual was removed from the analysis pertaining to the BDI-II depression total score, one individual from the analysis pertaining to cognitive symptoms).
We computed correlational analyses to determine the relationship between mood symptoms and brain volume measures. Two sets of analyses were then conducted, each involving separate hierarchical regressions for each of the five ROIs. Estimated total ICV, race (Caucasian/non-Caucasian), and age (years) were entered into the first block as covariates. Total depressive symptoms were entered into the second block of the first set of regression models. For the other set of regression models, the BDI-II subscales (i.e., affective, cognitive, and somatic) were entered simultaneously into the second block to examine the association of each subscale with brain volumes while controlling for the other subscale scores. Sex (male/female), education (years), general cognitive status (MoCA scores), self-reported depression (no/yes), self-reported antidepressant usage (absent/present), and medical comorbidities (CCI scores) were initially entered as covariates, but were removed from final analyses due to lack of statistical significance. All variables were entered into the models as continuous variables, with the exception of sex, race, self-reported depression, and self-reported antidepressant usage.
In order to reduce the number of analyses and risk for type I error, analyses were restricted to fronto-limbic brain regions based on previous studies showing their sensitivity of these regions to depression (Drevets, Price, & Furey, 2008). We conducted exploratory analyses investigating age by depressive symptom interactions (both for total symptoms and symptom subscales). The abovementioned hierarchical regressions were run for each of the five ROIs, with estimated total ICV and race entered as covariates into the first block, age and either (i) total depressive symptoms or (ii) the BDI-II subscales entered into the second block, and their interaction(s) entered into the third block. Additional exploratory analyses investigating relationships between additional frontal ROIs and total depressive symptoms and subscale scores were also conducted, as previous studies had found specific relationships between depressive symptoms and frontal regions in older adults (Dotson, Davatzikos, et al., 2009; Taki et al., 2005). In particular, the abovementioned hierarchical regressions from the primary analyses were run separately for the following regions: precentral gyrus, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, orbitofrontal cortex (OFC), and the frontal pole. The following regions were summed together intrahemispherically to create total right and left regional volumes and then averaged to obtain a total volumetric value: middle frontal gyrus = caudal + rostral middle frontal gyri; inferior frontal gyrus = pars opercularis + pars orbitalis + pars triangularis; OFC = lateral + medial OFC.
We applied a statistical significance threshold of α ≤ 0.05, uncorrected, and indicated when results met significance after Bonferroni correction for multiple comparisons (total and subscale primary (p < (0.05/5 = 0.01)) and exploratory [interactions (p < 0.01) and additional frontal ROIs (p < (0.05/6 = 0.008)] analyses). Effect sizes were reported as partial eta-squared (ηp2), for which 0.01 indicates a small effect, 0.06 a medium effect, and 0.14 a large effect (Cohen, 1969).
Results
Sample Characteristics
As shown in Table 1, the sample had a mean age of 70.7 years, mean education of 16.4 years, and was primarily female (60.0%) and Caucasian (91.3%). With respect to depression, 27.5% of the sample self-reported a history of depression and 8.8% self-reported current antidepressant use. The average MoCA total score was 26.0, which suggested that the sample was non-demented. The average BDI-II total score was 4.10, suggesting minimal total depressive symptoms.
Correlations
Table 3 depicts the relationship between age, mood symptoms, and brain volumes. Age was not significantly correlated with depressive symptoms; however, there were significant negative correlations with posterior cingulate (r = −0.27, p = 0.015), hippocampal (r = −0.57, p < 0.001, and amygdala (r = −0.44, p < 0.001) volumes. BDI-II total score and somatic symptoms were negatively correlated with hippocampal volume (r = −0.26, p = 0.020 and r = −0.41, p < 0.001, respectively). Somatic symptoms were also negatively correlated with posterior cingulate volume (r = −0.25, p = 0.024). All brain volume measures were positively intercorrelated (r’s > 0.43, p’s < 0.001).
Table 3.
Correlations between age, mood measures, and brain volumes
| Age | BDI-II total | Affective | Cognitive | Somatic | Caudal ACC | Rostral ACC | PCC | Amygdala | |
|---|---|---|---|---|---|---|---|---|---|
| Age | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| BDI-II total | 0.01 | -- | -- | -- | -- | -- | -- | -- | -- |
| Affective | −0.02 | 0.56* | -- | -- | -- | -- | -- | -- | -- |
| Cognitive | 0.02 | 0.77* | 0.34* | -- | -- | -- | -- | -- | -- |
| Somatic | 0.15 | 0.87* | 0.37* | 0.47* | -- | -- | -- | -- | -- |
| Caudal ACC | −0.17 | −0.08 | 0.06 | 0.08 | −0.16 | -- | -- | -- | -- |
| Rostral ACC | −0.20 | −0.11 | 0.01 | 0.03 | −0.22 | 0.63* | -- | -- | -- |
| PCC | −0.27* | −0.17 | 0.04 | −0.01 | −0.25* | 0.64* | 0.62* | -- | -- |
| Amygdala | −0.44* | −0.10 | −0.09 | 0.02 | −0.20 | 0.44* | 0.49* | 0.48* | -- |
| Hippocampus | −0.57* | −0.26* | −0.14 | −0.03 | −0.41* | 0.33* | 0.49* | 0.43* | 0.65* |
Note. BDI-II = Beck Depression Inventory, 2nd Edition; ACC = Anterior Cingulate Cortex; PCC = Posterior Cingulate Cortex.
p < 0.05.
Total Depressive Symptoms
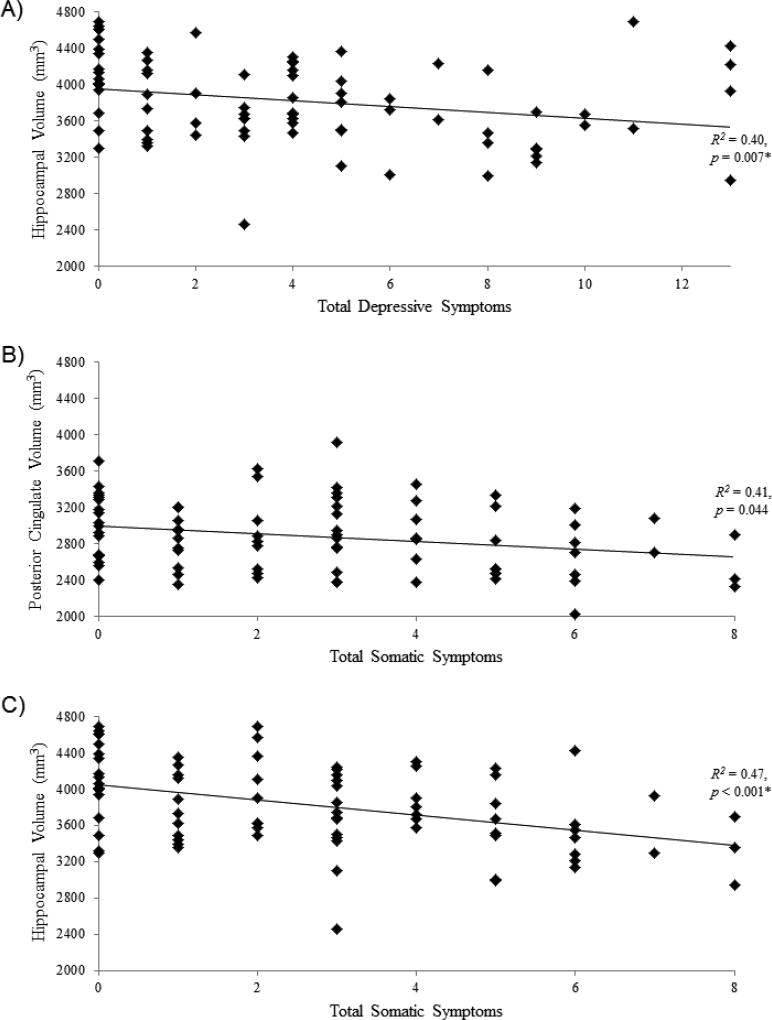
As summarized in Table 4, after controlling for total ICV, race, and age, more self-reported total depressive symptoms were associated with smaller hippocampal volume (p = 0.007, ηp2 = 0.094; significant after Bonferroni correction; Figure 1A). This partially supported Hypothesis 1.
Table 4.
Regression coefficients for total depressive symptoms and covariates on total brain volumes
| Caudal ACC | Rostral ACC | PCC | Hippocampus | Amygdala | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | |
| Total ICV | 0.23 | 0.04 | 0.06 | 0.40 | <0.01* | 0.16 | 0.51 | <0.01* | 0.28 | 0.12 | 0.21 | 0.02 | 0.34 | <0.01* | 0.14 |
| Race | −0.23 | 0.04 | 0.06 | −0.03 | 0.78 | 0.00 | −0.18 | 0.06 | 0.05 | −0.04 | 0.68 | 0.00 | −0.07 | 0.51 | 0.01 |
| Age | −0.27 | 0.02 | 0.08 | −0.27 | 0.01 | 0.08 | −0.36 | <0.01* | 0.16 | −0.59 | <0.01* | 0.36 | −0.49 | <0.01* | 0.25 |
| BDI-II total | −0.05 | 0.64 | 0.00 | −0.11 | 0.31 | 0.01 | −0.15 | 0.13 | 0.03 | −0.25 | <0.01* | 0.09 | −0.09 | 0.38 | 0.01 |
| F | 3.09 | 4.91 | 10.44 | 12.52 | 8.22 | ||||||||||
| R2 | 0.14 | 0.21 | 0.36 | 0.40 | 0.31 | ||||||||||
| Model p | 0.02 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||
Note. ICV = Intracranial Volume; BDI-II = Beck Depression Inventory, 2nd Edition. ACC = Anterior Cingulate Cortex; PCC = Posterior Cingulate Cortex.
Bold data indicates significance at p < 0.05, uncorrected;
p < 0.01, Bonferroni-corrected.
Figure 1.
Significant results for relationships between a) total depressive symptoms and hippocampal volume (p = 0.007), b) total somatic symptoms and posterior cingulate volume (p = 0.044), and c) total somatic symptoms and hippocampal volume (p < 0.001). Greater symptoms were associated with less volume across all measures. mm = millimeters, * = results significant after Bonferroni correction.
Depressive Symptom Subscales
Affective symptoms
As summarized in Table 5, after controlling for total ICV, race, age, and the influence of the other subscales, there were no significant effects of the affective symptoms subscale on any of the ROI brain volumes. These results did not support Hypothesis 2.
Table 5.
Regression coefficients for depressive symptom subscales and covariates on total brain volumes
| Caudal ACC | Rostral ACC | PCC | Hippocampus | Amygdala | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | |
| Total ICV | 0.22 | 0.05 | 0.05 | 0.38 | <0.01* | 0.15 | 0.50 | <0.01* | 0.29 | 0.09 | 0.34 | 0.01 | 0.33 | <0.01* | 0.13 |
| Race | −0.23 | 0.04 | 0.06 | −0.03 | 0.80 | 0.00 | −0.19 | 0.05 | 0.06 | −0.03 | 0.71 | 0.00 | −0.07 | 0.52 | 0.01 |
| Age | −0.26 | 0.03 | 0.07 | −0.25 | 0.03 | 0.07 | −0.36 | <0.01* | 0.17 | −0.53 | <0.01* | 0.32 | −0.46 | <0.01* | 0.22 |
| Affective | 0.11 | 0.35 | 0.01 | 0.06 | 0.58 | 0.00 | 0.13 | 0.21 | 0.02 | −0.06 | 0.51 | 0.01 | −0.09 | 0.41 | 0.01 |
| Cognitive | 0.05 | 0.67 | 0.00 | 0.10 | 0.43 | 0.01 | 0.04 | 0.70 | 0.00 | 0.18 | 0.08 | 0.04 | 0.10 | 0.37 | 0.01 |
| Somatic | −0.15 | 0.24 | 0.02 | −0.23 | 0.07 | 0.05 | −0.23 | 0.04 | 0.06 | −0.39 | <0.01* | 0.16 | −0.12 | 0.30 | 0.02 |
| F | 2.37 | 3.73 | 8.19 | 10.36 | 5.40 | ||||||||||
| R2 | 0.17 | 0.24 | 0.41 | 0.47 | 0.31 | ||||||||||
| Model p | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||
Note. ICV = Intracranial Volume; ACC = Anterior Cingulate Cortex; PCC = Posterior Cingulate Cortex.
Bold data indicates significance at p < 0.05, uncorrected;
p < 0.01, Bonferroni-corrected.
Cognitive symptoms
There also were no significant effects of the cognitive symptoms subscale on any of the ROI brain volumes (Table 5).
Somatic symptoms
In partial support of Hypothesis 3, there were significant relationships between somatic symptoms and PCC (Figure 1B) and hippocampal volumes (Figure 1C), such that greater somatic symptoms were associated with smaller volume in these two regions (p = 0.044, ηp2 = 0.056 and p < 0.001, ηp2 = 0.162, respectively; latter significant after Bonferroni correction; Table 5).
Exploratory Analyses
Interactions with age
There were no significant (i) age by total depressive symptom or (ii) age by depressive symptom subscale effects on any of the ROI brain volumes (all p’s > 0.18).
Additional frontal ROIs
Total depressive symptoms
Table 6 shows that greater self-reported total depressive symptoms were associated with larger frontal pole volume (p = 0.007, ηp2 = 0.095; significant after Bonferroni correction). There were no significant effects of total depressive symptoms on any of the other frontal ROI brain volumes.
Table 6.
Regression coefficients from exploratory analyses on total depressive symptoms and covariates on total brain volumes in frontal regions
| Precentral | Superior Frontal | Middle Frontal | Inferior Frontal | OFC | Frontal Pole | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | |
| Total ICV | 0.62 | <0.01* | 0.41 | 0.64 | <0.01* | 0.44 | 0.62 | <0.01* | 0.40 | 0.50 | <0.01* | 0.26 | 0.71 | <0.01* | 0.51 | 0.44 | <0.01* | 0.21 |
| Race | 0.01 | 0.94 | 0.00 | 0.02 | 0.81 | 0.00 | −0.05 | 0.55 | 0.01 | −0.17 | 0.09 | 0.04 | −0.10 | 0.22 | 0.02 | −0.23 | 0.02 | 0.07 |
| Age | −0.31 | <0.01* | 0.14 | −0.37 | <0.01* | 0.20 | −0.33 | <0.01* | 0.16 | −0.31 | <0.01* | 0.12 | −0.29 | <0.01* | 0.14 | −0.03 | 0.80 | 0.00 |
| BDI-II total | −0.17 | 0.06 | 0.05 | −0.05 | 0.56 | 0.01 | −0.11 | 0.22 | 0.02 | −0.05 | 0.61 | 0.00 | −0.04 | 0.59 | 0.00 | 0.27 | <0.01* | 0.10 |
| F | 14.96 | 17.44 | 14.32 | 8.42 | 20.20 | 7.70 | ||||||||||||
| R2 | 0.45 | 0.49 | 0.44 | 0.31 | 0.52 | 0.29 | ||||||||||||
| Model p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||
Note. ICV = Intracranial Volume; BDI-II = Beck Depression Inventory, 2nd Edition. OFC = Orbitofrontal Cortex.
Bold data indicates significance at p < 0.05, uncorrected;
p < 0.01, Bonferroni-corrected.
Depressive symptom subscales
There was a significant negative relationship between somatic symptoms and superior frontal volume (p = 0.047, ηp2 = 0.054; Table 7). No other associations between subscale scores and frontal ROI volumes were found.
Table 7.
Regression coefficients from exploratory analyses on depressive symptom subscales and covariates on total brain volumes in frontal regions
| Precentral | Superior Frontal | Middle Frontal | Inferior Frontal | OFC | Frontal Pole | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | β | p | η2p | |
| Total ICV | 0.63 | <0.01* | 0.41 | 0.63 | <0.01* | 0.44 | 0.61 | <0.01* | 0.39 | 0.49 | 0.01* | 0.26 | 0.70 | <0.01* | 0.51 | 0.43 | <0.01* | 0.21 |
| Race | 0.00 | 0.97 | 0.00 | 0.02 | 0.82 | 0.00 | −0.05 | 0.59 | 0.01 | −0.17 | 0.08 | 0.04 | −0.11 | 0.19 | 0.02 | −0.23 | 0.03 | 0.07 |
| Age | −0.30 | <0.01* | 0.13 | −0.34 | <0.01* | 0.18 | −0.30 | <0.01* | 0.13 | −0.29 | <0.01* | 0.11 | −0.29 | <0.01* | 0.14 | −0.03 | 0.81 | 0.00 |
| Affective | 0.02 | 0.84 | 0.00 | 0.06 | 0.49 | 0.01 | −0.01 | 0.94 | 0.00 | 0.17 | 0.11 | 0.04 | 0.00 | 0.98 | 0.00 | 0.11 | 0.33 | 0.01 |
| Cognitive | −0.15 | 0.15 | 0.03 | 0.15 | 0.13 | 0.03 | 0.02 | 0.84 | 0.00 | 0.41 | 0.71 | 0.00 | 0.14 | 0.13 | 0.03 | 0.13 | 0.26 | 0.02 |
| Somatic | −0.06 | 0.58 | 0.00 | −0.20 | 0.05 | 0.05 | −0.14 | 0.19 | 0.02 | −0.18 | 0.13 | 0.03 | −0.14 | 0.14 | 0.03 | 0.12 | 0.34 | 0.01 |
| F | 9.75 | 12.83 | 9.16 | 6.41 | 14.53 | 4.91 | ||||||||||||
| R2 | 0.46 | 0.52 | 0.44 | 0.35 | 0.55 | 0.29 | ||||||||||||
| Model p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | ||||||||||||
Note. ICV = Intracranial Volume; OFC = Orbitofrontal Cortex.
Bold data indicates significance at p < 0.05, uncorrected;
p < 0.01, Bonferroni-corrected.
Discussion
Integrating structural neuroimaging with self-reported symptomatology, the present study investigated relationships between subclinical depressive symptoms, both total symptoms and symptom subscales, and fronto-limbic brain volumes in an age-heterogeneous sample of middle-aged to older adults. We expected greater depressive symptoms to predict smaller volume in fronto-limbic brain regions known to be affected by major depression. Further, we expected this relationship to be associated with affective and somatic symptoms, while we had no specific predictions for cognitive symptoms given lack of previous findings for this subscale.
We provide partial support for our hypothesis that greater total depressive symptoms were associated with volume of select brain regions. Our results did not support relationships between affective and cognitive symptoms and brain volumes in individuals with subclinical depression. We did find support, however, for a relationship between greater somatic symptoms of depression and smaller regional brain volumes. These results will be discussed in more detail below.
We observed significant effects for total depressive symptoms and hippocampal volume, such that higher self-reported subclinical depressive symptoms were associated with smaller hippocampal volume. This is consistent with a recent cross-sectional study suggesting that older adults with subclinical depression, compared to healthy controls, had smaller right parahippocampal volumes (Zhou et al., 2016), which was associated with depressive symptoms. Not all previous studies investigating subclinical depression in older adults, however, have found this relationship. Dotson, Davatzikos, et al. (2009) observed cross-sectional associations between higher depressive symptoms and temporal gray matter volumes, but did not find a specific relationship for the hippocampus, nor were depressive symptoms associated with longitudinal decline in temporal lobe volume. In addition, Taki et al. (2005) did not find a relationship between hippocampal volume and subthreshold depression in older adults. Methodological differences between studies may help explain these conflicting results, as different age ranges, depression rating scales (e.g., BDI-II; Center for Epidemiologic Studies Depression Scale [CES-D; Radloff, 1977]; Geriatric Depression Scale [Yesavage et al., 1982]), and analytical approaches (e.g., whole brain versus ROI; continuous depression ratings versus group differences) were used across studies. More research is needed, with larger sample sizes, a wider range of depressive symptoms, and more consistent analytical approaches, as well as longitudinal designs, to better understand the significance of the depressive symptom–hippocampal relationship in aging.
While we observed significant associations between total depressive symptoms and hippocampal volume, we did not find effects in the other predicted regions (i.e., ACC, PCC, and amygdala). This is particularly interesting, as other studies in subclinical depression in older adults have found smaller volumes in frontal regions (Dotson, Davatzikos, et al., 2009; Taki et al., 2005). Our exploratory analyses did not support relationships with the frontal regions identified in the aforementioned studies; however, we did find a positive association between total depressive symptoms and frontal pole volume. This is inconsistent with previously published findings that indicate smaller frontal pole volumes in depression (Bludau et al., 2016; Grieve, Korgaonkar, Koslow, Gordon, & Williams, 2013). This finding may be due to the fact that our sample was generally healthy with low levels of depressive symptoms compared to other studies that investigate structural alterations in MDD. Other studies investigating brain volume in subclinical depression have found both similar and differential effects in regions that are typically associated with MDD, with reports of reduced volumes (Hayakawa et al., 2013; Li et al., 2015; Webb, Weber, Mundy, & Killgore, 2014), enlarged volumes (Romanczuk-Seiferth et al., 2014; Szymkowicz, McLaren, O'Shea, et al., 2016), and null effects (Allan et al., 2016). Again, methodological differences may account for differential findings across studies. For example, it is possible that the low levels of depressive symptoms obtained in this study, and in other studies investigating subclinical depression, may not have allowed for sufficient sensitivity to detect effects in specific brain regions.
Examining the BDI-II subscales, the present study also found that greater self-reported somatic symptoms were associated with smaller volume in the PCC and the hippocampus. Findings of this relationship in the PCC are consistent with a recent study from our group using an independent, but comparable, aging population (McLaren et al., 2016), which found that higher somatic symptoms were associated with smaller PCC volumes. The PCC is part of the default mode network (Buckner, Andrews-Hanna, & Schacter, 2008) and is involved in self-referential processing (Gusnard, Akbudak, Shulman, & Raichle, 2001). A recent meta-analysis found that regions associated with personal introspection, such as the PCC, were important in somatoform disorders (Boeckle, Schrimpf, Liegl, & Pieh, 2016). Other studies have specifically indicated PCC dysfunction in somatization and somatoform disorders (Fayed et al., 2012; Lemche et al., 2013). Our finding with respect to the hippocampus was also intriguing, as a recent investigation found that dysfunction in a brain network that included the hippocampus was related to the manifestation of somatic symptoms (Gondo et al., 2012). While dysfunction does not necessarily speak to the structural integrity of a region, these results, in conjunction with findings from the current study, suggest that the hippocampus may play a role in the manifestation of somatic symptoms.
Further, our exploratory analyses suggested a negative relationship between somatic symptoms and superior frontal volume. The superior frontal gyrus has been implicated in emotion regulation (D'Argembeau et al., 2007) and pain catastrophizing in somatic disorders (Gracely et al., 2004). Further, structural changes to medial frontal regions, including the superior frontal gyrus, may lead to increased attention towards and emotional reactions to somatic symptoms (Lutz et al., 2008). More research is needed to understand the relationship between somatic depressive symptoms and these brain regions in aging, with the current study suggesting potential regions for future investigations.
The current study did not find significant relationships between affective or cognitive symptoms and fronto-limbic regions. We used the BDI-II to investigate depressive symptom dimensions. Other studies in middle-aged to older adults have used the CES-D and found differential relationships between the depressed mood subscale and volumes of the cingulate (Dotson, Davatzikos, et al., 2009), with higher symptoms associated with smaller volumes in younger, but not older, adults. The exploratory findings of the current study did not find differential age effects. When controlling for age and other demographic variables, positive relationships between the depressed mood subscale and PCC (McLaren et al., 2016) and temporal brain (McLaren et al., 2017) volumes, and a negative relationship between the somatic symptoms subscale and PCC volume (McLaren et al., 2016), have been reported. This pattern of findings across studies highlights the complexity of the relationship specific symptoms of depression and brain volumes. While age may be an important moderator of the link between depressive symptoms and brain structure for some studies, other factors (i.e., other demographic or health variables) and their interactions may also play a role and should be explored in future research.
To the best of our knowledge, there are no previous studies reporting relationships between cognitive symptoms of depression and structural brain alterations. However, there is evidence that motivational symptoms (which correspond most closely with the BDI-II cognitive subscale), but not mood symptoms, are predictive of future diagnosis of Alzheimer’s disease (Berger, Fratiglioni, Forsell, Winblad, & Backman, 1999) and are related to memory dysfunction in non-depressed older adults (Backman, Hill, & Forsell, 1996). Given these findings, one could have expected that cognitive symptoms of depression were related to brain volumes, which was not supported in the current study. It may be that cognitive symptoms of depression are related to functional brain alterations that are associated with mood-congruent biases in the processing of emotional and neutral stimuli that may eventually lead to structural brain alterations (Disner, Beevers, Haigh, & Beck, 2011), rather than underlying structural brain alterations that cause dysfunctional beliefs and negative biases. This hypothesis warrants investigation in future studies. Of note, we were unable to conduct our own factor analyses of the BDI-II due to our sample size (>100 participants; Mundfrom, Shaw, & Ke, 2005). It is possible that the BDI-II has a different factor structure when used in individuals with subclinical depressive symptoms compared to those with clinical depression, which could affect its relationship with brain volumes.
The underlying pathophysiology of depression-related hippocampal alterations is outside the scope of the current study. Smaller hippocampal volumes, however, are commonly reported in the literature (McKinnon, Yucel, Nazarov, & MacQueen, 2009; Videbech & Ravnkilde, 2004). While the exact mechanisms for this effect are unclear, it is postulated that neuronal remodeling, neuronal death, and suppressed neurogenesis, resulting from increased glucocorticoid levels, are potential causative factors for reduced hippocampal volume (Czeh & Lucassen, 2007; Sapolsky, 2000). Depressed individuals often exhibit hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis, which results in increased levels of glucocorticoids (Pariante, 2009). The hippocampus is involved in modulation of the HPA-axis and is highly concentrated with glucocorticoid receptors (McEwen & Olie, 2005), the latter of which makes it particularly vulnerable to the effects of stress (McEwen, Nasca, & Gray, 2016). In this study, we were able to show a relationship between subclinical depressive symptoms (i.e., nonclinical levels of psychological stress) and hippocampal volumes, with medium-to-large effect sizes. To date, it is unclear whether smaller hippocampal volumes in depression is the result of the disease process, or whether they are a pre-existing trait that predisposes individuals to pathological stress reactions. Nevertheless, preclinical models suggest that acute stress induces hippocampal alterations (Kirby et al., 2013; Rocher, Spedding, Munoz, & Jay, 2004). Future longitudinal research investigating depressive symptoms across the lifespan will help further elucidate the nature and direction of this relationship.
Our findings should be interpreted in the context of limitations of the current study. Our sample of middle-aged to older adults was highly educated, fairly healthy, and predominantly Caucasian, which may limit the generalizability of these results to other community samples. It is possible that a small minority of our participants met criteria for mild cognitive impairment and/or dementia, as our sample had a wide range of MoCA scores (20–30). Thus, findings may be related to an underlying neurodegenerative process rather than depressive symptoms. However, MoCA scores were not significantly related to brain volumes in any of our analyses and a recent study suggests that the optimal cut-off score for cognitive impairment on the MoCA may be lower than previously reported (≤ 20 rather than < 26), as the latter score may overpathologize those who perform poorly on the measure and do not actually have true cognitive impairment (Waldron-Perrine & Axelrod, 2012). Replication of these findings in a larger, independent sample where cognitive status will be assessed via comprehensive neuropsychological testing is needed.
We were unable to determine the influence of anxiety on the present results, as depressive and anxious symptoms are often comorbid (Gorman, 1996; Hek et al., 2011; Wu & Fang, 2014) and previous research has shown that anxious symptoms can interact with depressive symptoms to differentially impact brain functioning (Dotson et al., 2014). Moreover, this research is cross-sectional in nature, which does not allow specification of the direction of the observed relationship. It is possible that having greater depressive symptoms leads to decreases in brain volume or that the inverse is true ─ decreases in brain volume may cause worsening of depressive symptoms. Thus, longitudinal research across the adult lifespan investigating the direction of these relationships is warranted.
Finally, our sample had low levels of depressive symptoms overall; thus, our conclusions are limited to community-dwelling middle-aged to older adults. Despite our subclinical sample, we did find large effect sizes in the hippocampus for both total depressive and somatic symptoms. Future research needs to set out to determine the extent to which our findings can be replicated in both subclinical and clinically depressed samples.
Our study adds to a growing body of literature that has investigated preclinical biological markers of major depression by demonstrating relationships between subclinical depressive symptoms and structural brain measures (Dotson, Davatzikos, et al., 2009; Dotson, Zonderman, Davatzikos, Kraut, & Resnick, 2009; Kumar et al., 1998; Li et al., 2015; McLaren et al., 2016, 2017; Szymkowicz, McLaren, Kirton, et al., 2016; Taki et al., 2005). Our sample size was similar, if not larger, than previous cross-sectional studies in the field, and we considered interindividual differences in our approach. We also examined a larger age range than previous studies and solely focused on subclinical depressive symptoms (BDI-II scores < 14). We found that greater self-reported total depressive symptomatology, and more specifically somatic symptoms, predicted smaller regional brain volumes in a sample of middle-aged to older adults. These findings are important in their own right given evidence that low levels of depressive symptoms are associated with negative consequences in older adults (Laborde-Lahoz et al., 2015; Naismith, Norrie, Mowszowski, & Hickie, 2012). Increasing our understanding of the relationship between symptoms of depression and brain structure across the lifespan is important, as it could inform future development of personalized treatments for depression based on an individual’s specific symptom presentation.
Acknowledgments
This work was supported by the McKnight Brain Research Foundation; the Center for Cognitive Aging and Memory at the University of Florida, and the National Institute on Aging, under Grants T32AG020499-11, R01AG054077-01, and K01AG050707-A101; the National Center for Advancing Translational Science under Grants UL1TR000064 and KL2TR000065; the National Institute of Mental Health, under Grant R03MH109336-02; the National Institute on Alcohol Abuse and Alcoholism, under Grants P01AA19072-07 and U01AA020797-06; and the National Institute of Diabetes and Digestive and Kidney Diseases, under Grant R01DK099334-03. Neuroimaging was performed at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.
References
- Akhtar-Danesh N, Landeen J. Relation between depression and sociodemographic factors. Int J Ment Health Syst. 2007;1(1):4. doi: 10.1186/1752-4458-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10(6):687–695. [PubMed] [Google Scholar]
- Allan CL, Sexton CE, Filippini N, Topiwala A, Mahmood A, Zsoldos E, Ebmeier KP. Sub-threshold depressive symptoms and brain structure: A magnetic resonance imaging study within the Whitehall II cohort. J Affect Disord. 2016;204:219–225. doi: 10.1016/j.jad.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, Aizenstein H. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology. 2008;33(11):2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S, Lankarani MM. Chronic Medical Conditions and Negative Affect; Racial Variation in Reciprocal Associations Over Time. Front Psychiatry. 2016;7:140. doi: 10.3389/fpsyt.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Hill RD, Forsell Y. The influence of depressive symptomatology on episodic memory functioning among clinically nondepressed older adults. J Abnorm Psychol. 1996;105(1):97–105. doi: 10.1037//0021-843x.105.1.97. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford Press; 1987. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beck AT, Steer RA, Brown GK, van der Does AJW. BDI-II-NL Handleiding [BDI-II-Dutch Manual] Lisee, The Netherlands: Psychological Corporation; 2002. [Google Scholar]
- Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- Berger AK, Fratiglioni L, Forsell Y, Winblad B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology. 1999;53(9):1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- Blazer DG, 2nd, Hybels CF. Origins of depression in later life. Psychol Med. 2005;35(9):1241–1252. doi: 10.1017/S0033291705004411. [DOI] [PubMed] [Google Scholar]
- Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, Eickhoff SB. Medial Prefrontal Aberrations in Major Depressive Disorder Revealed by Cytoarchitectonically Informed Voxel-Based Morphometry. Am J Psychiatry. 2016;173(3):291–298. doi: 10.1176/appi.ajp.2015.15030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckle M, Schrimpf M, Liegl G, Pieh C. Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. Neuroimage Clin. 2016;11:606–613. doi: 10.1016/j.nicl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138(1–2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chiao C, Weng LJ. Mid-life socioeconomic status, depressive symptomatology and general cognitive status among older adults: inter-relationships and temporal effects. BMC Geriatr. 2016;16:88. doi: 10.1186/s12877-016-0257-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Jorm AF, Mackinnon AJ, Korten AE, Jacomb PA, Henderson AS, Rodgers B. Age differences in depression and anxiety symptoms: a structural equation modelling analysis of data from a general population sample. Psychol Med. 1999;29(2):325–339. doi: 10.1017/s0033291798008150. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. New York, NY: Academic Press; 1969. [Google Scholar]
- Colloby SJ, Firbank MJ, Vasudev A, Parry SW, Thomas AJ, O'Brien JT. Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord. 2011;133(1–2):158–164. doi: 10.1016/j.jad.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202(1):22–27. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257(5):250–260. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Salmon E. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved Localization of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Szymkowicz SM, Kirton JW, McLaren ME, Green ML, Rohani JY. Unique and interactive effect of anxiety and depressive symptoms on cognitive and brain function in young and older adults. J Depress Anxiety. 2014;(Suppl 1) doi: 10.4172/2167-1044.S1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Zonderman AB, Davatzikos C, Kraut MA, Resnick SM. Frontal Atrophy and Attention Deficits in Older Adults with a History of Elevated Depressive Symptoms. Brain Imaging Behav. 2009;3(4):358. doi: 10.1007/s11682-009-9078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, Gong Q. Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci. 2014;39(6):397–406. doi: 10.1503/jpn.130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayed N, Andres E, Rojas G, Moreno S, Serrano-Blanco A, Roca M, Garcia-Campayo J. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand. 2012;126(2):115–125. doi: 10.1111/j.1600-0447.2011.01820.x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. doi: 10.1146/annurev.clinpsy.032408.153621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo JJ, Anthony JC, Muthen BO. Age differences in the symptoms of depression: a latent trait analysis. J Gerontol. 1994;49(6):P251–264. doi: 10.1093/geronj/49.6.p251. [DOI] [PubMed] [Google Scholar]
- Geerlings MI, Brickman AM, Schupf N, Devanand DP, Luchsinger JA, Mayeux R, Small SA. Depressive symptoms, antidepressant use, and brain volumes on MRI in a population-based cohort of old persons without dementia. J Alzheimers Dis. 2012;30(1):75–82. doi: 10.3233/JAD-2012-112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo M, Moriguchi Y, Kodama N, Sato N, Sudo N, Kubo C, Komaki G. Daily physical complaints and hippocampal function: an fMRI study of pain modulation by anxiety. Neuroimage. 2012;63(3):1011–1019. doi: 10.1016/j.neuroimage.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4(4):160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(Pt 4):835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–339. doi: 10.1016/j.nicl.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa YK, Sasaki H, Takao H, Mori H, Hayashi N, Kunimatsu A, Ohtomo K. Structural brain abnormalities in women with subclinical depression, as revealed by voxel-based morphometry and diffusion tensor imaging. J Affect Disord. 2013;144(3):263–268. doi: 10.1016/j.jad.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Hek K, Tiemeier H, Newson RS, Luijendijk HJ, Hofman A, Mulder CL. Anxiety disorders and comorbid depression in community dwelling older adults. Int J Methods Psychiatr Res. 2011;20(3):157–168. doi: 10.1002/mpr.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kirby ED, Muroy SE, Sun WG, Covarrubias D, Leong MJ, Barchas LA, Kaufer D. Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. Elife. 2013;2:e00362. doi: 10.7554/eLife.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirton JW, Resnick SM, Davatzikos C, Kraut MA, Dotson VM. Depressive symptoms, symptom dimensions, and white matter lesion volume in older adults: a longitudinal study. Am J Geriatr Psychiatry. 2014;22(12):1469–1477. doi: 10.1016/j.jagp.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Schnack HG, Janssen J, Hulshoff Pol HE, Kahn RS. Cortical thickness and voxel-based morphometry in depressed elderly. Eur Neuropsychopharmacol. 2010;20(6):398–404. doi: 10.1016/j.euroneuro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Kumar A, Jin Z, Bilker W, Udupa J, Gottlieb G. Late-onset minor and major depression: early evidence for common neuroanatomical substrates detected by using MRI. Proc Natl Acad Sci U S A. 1998;95(13):7654–7658. doi: 10.1073/pnas.95.13.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde-Lahoz P, El-Gabalawy R, Kinley J, Kirwin PD, Sareen J, Pietrzak RH. Subsyndromal depression among older adults in the USA: prevalence, comorbidity, and risk for new-onset psychiatric disorders in late life. Int J Geriatr Psychiatry. 2015;30(7):677–685. doi: 10.1002/gps.4204. [DOI] [PubMed] [Google Scholar]
- Lemche E, Giampietro VP, Brammer MJ, Surguladze SA, Williams SC, Phillips ML. Somatization severity associated with postero-medial complex structures. Sci Rep. 2013;3:1032. doi: 10.1038/srep01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lener MS, Kundu P, Wong E, Dewilde KE, Tang CY, Balchandani P, Murrough JW. Cortical abnormalities and association with symptom dimensions across the depressive spectrum. J Affect Disord. 2016;190:529–536. doi: 10.1016/j.jad.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wei D, Sun J, Chen Q, Zhang Q, Qiu J. Brain structural alterations associated with young women with subthreshold depression. Sci Rep. 2015;5:9707. doi: 10.1038/srep09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117(1–2):1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58(12):3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Olie JP. Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry. 2005;10(6):525–537. doi: 10.1038/sj.mp.4001648. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(1):41–54. [PMC free article] [PubMed] [Google Scholar]
- McLaren ME, Szymkowicz SM, O'Shea A, Woods AJ, Anton SD, Dotson VM. Dimensions of depressive symptoms and cingulate volumes in older adults. Transl Psychiatry. 2016;6:e788. doi: 10.1038/tp.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren ME, Szymkowicz SM, O'Shea A, Woods AJ, Anton SD, Dotson VM. Vertex-wise examination of depressive symptom dimensions and brain volumes in older adults. Psychiatry Res. 2017;260:70–75. doi: 10.1016/j.pscychresns.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in "a minor" can "b major": a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129(1–3):126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurs M, Groenewold NA, Roest AM, van der Wee NJ, Veltman DJ, van Tol MJ, de Jonge P. The associations of depression and hypertension with brain volumes: Independent or interactive? Neuroimage Clin. 2015;8:79–86. doi: 10.1016/j.nicl.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, 2nd, Lewis DV, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundfrom DJ, Shaw DJ, Ke TL. Minimal sample size recommendations for conducting factor analyses. Int J Testing. 2005;5(2):159–168. doi: 10.1207/s15327574ijt0502_4. [DOI] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98(1):99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nissim NR, O'Shea AM, Bryant V, Porges EC, Cohen R, Woods AJ. Frontal Structural Neural Correlates of Working Memory Performance in Older Adults. Front Aging Neurosci. 2016;8:328. doi: 10.3389/fnagi.2016.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien LM, Ziegler DA, Deutsch CK, Frazier JA, Herbert MR, Locascio JJ. Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Res. 2011;193(2):113–122. doi: 10.1016/j.pscychresns.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ. Cognitive Aging and the Hippocampus in Older Adults. Front Aging Neurosci. 2016;8:298. doi: 10.3389/fnagi.2016.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA, Jr, Anand A. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634–642. doi: 10.1007/s11920-012-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci. 2009;1179:144–152. doi: 10.1111/j.1749-6632.2009.04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Lane EM, Tate DF, Heaps J, Romo DM, Akbudak E, Conturo TE. Neuroimaging signatures and cognitive correlates of the montreal cognitive assessment screen in a nonclinical elderly sample. Arch Clin Neuropsychol. 2011;26(5):454–460. doi: 10.1093/arclin/acr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Polyakova M, Sonnabend N, Sander C, Mergl R, Schroeter ML, Schroeder J, Schonknecht P. Prevalence of minor depression in elderly persons with and without mild cognitive impairment: a systematic review. J Affect Disord. 2014;152–154:28–38. doi: 10.1016/j.jad.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Porges EC, Woods AJ, Edden RA, Puts NA, Harris AD, Chen H, Cohen RA. Frontal Gamma-Aminobutyric Acid Concentrations Are Associated With Cognitive Performance in Older Adults. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):38–44. doi: 10.1016/j.bpsc.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Bello J, Deus J, Cardoner N, Marti-Vilalta JL, Capdevila A. Beck Depression Inventory factors related to demyelinating lesions of the left arcuate fasciculus region. Psychiatry Res. 2000;99(3):151–159. doi: 10.1016/s0925-4927(00)00061-5. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex. 2004;14(2):224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Romanczuk-Seiferth N, Pohland L, Mohnke S, Garbusow M, Erk S, Haddad L, Heinz A. Larger amygdala volume in first-degree relatives of patients with major depression. Neuroimage Clin. 2014;5:62–68. doi: 10.1016/j.nicl.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Segal DL, Coolidge FL, Cahill BS, O'Riley AA. Psychometric properties of the Beck Depression Inventory II (BDI-II) among community-dwelling older adults. Behav Modif. 2008;32(1):3–20. doi: 10.1177/0145445507303833. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Seider TR, Fieo RA, O'Shea A, Porges EC, Woods AJ, Cohen RA. Cognitively Engaging Activity Is Associated with Greater Cortical and Subcortical Volumes. Front Aging Neurosci. 2016;8:94. doi: 10.3389/fnagi.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, Ebmeier KP. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69(7):680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Srinivasa RN, Rossetti HC, Gupta MK, Rosenberg RN, Weiner MF, Peshock RM, King KS. Cardiovascular Risk Factors Associated with Smaller Brain Volumes in Regions Identified as Early Predictors of Cognitive Decline. Radiology. 2016;278(1):198–204. doi: 10.1148/radiol.2015142488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowicz SM, McLaren ME, Kirton JW, O'Shea A, Woods AJ, Manini TM, Dotson VM. Depressive symptom severity is associated with increased cortical thickness in older adults. Int J Geriatr Psychiatry. 2016;31(4):325–333. doi: 10.1002/gps.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowicz SM, McLaren ME, O'Shea A, Woods AJ, Anton SD, Dotson VM. Depressive symptoms modify age effects on hippocampal subfields in older adults. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, Fukuda H. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. 2005;88(3):313–320. doi: 10.1016/j.jad.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Newton SS, Duman RS. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6(2):151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]
- Vanheule S, Desmet M, Groenvynck H, Rosseel Y, Fontaine J. The factor structure of the Beck Depression Inventory-II: an evaluation. Assessment. 2008;15(2):177–187. doi: 10.1177/1073191107311261. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Waldron-Perrine B, Axelrod BN. Determining an appropriate cutting score for indication of impairment on the Montreal Cognitive Assessment. Int J Geriatr Psychiatry. 2012;27(11):1189–1194. doi: 10.1002/gps.3768. [DOI] [PubMed] [Google Scholar]
- Webb CA, Weber M, Mundy EA, Killgore WD. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol Med. 2014;44(13):2833–2843. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Fang Y. Comorbidity of depressive and anxiety disorders: challenges in diagnosis and assessment. Shanghai Arch Psychiatry. 2014;26(4):227–231. doi: 10.3969/j.issn.1002-0829.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–430. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhou H, Li R, Ma Z, Rossi S, Zhu X, Li J. Smaller gray matter volume of hippocampus/parahippocampus in elderly people with subthreshold depression: a cross-sectional study. BMC Psychiatry. 2016;16:219. doi: 10.1186/s12888-016-0928-0. [DOI] [PMC free article] [PubMed] [Google Scholar]