Abstract
Objective
To determine feasibility and preliminary effects of an occupational therapy treatment to improve upper extremity (UE) function in patients with early systemic sclerosis (SSc) who have UE contractures.
Methods
A one-arm pilot clinical rehabilitation trial was conducted at a university health system. Participants with SSc and > 1 UE contracture (n = 21) participated in a total of 8 weekly in-person occupational therapy sessions. The therapy consisted of thermal modalities, tissue mobilization, and UE mobility. Between sessions, participants were instructed to complete UE home exercises. Feasibility was measured by percent enrollment and session attendance and duration. The primary outcome measure was the QuickDASH, secondary and exploratory outcomes included PROMIS physical function, objective UE measures, and skin thickening. Linear mixed models were performed to determine treatment effects on primary and secondary outcomes.
Results
Fifty percent (24/48) of potentially eligible participants were interested. Of those, 88% (21/24) enrolled; and nineteen out of 21 (91%) completed all sessions. The mean (SD) age was 47.9 years (+ 16.1); 100% had diffuse SSc, and mean disease duration was 3.1 years. At 8 weeks, participants reported statistically significant improvement on QuickDASH and PROMIS physical function measures (p =.0012 and p = .004). Forty-seven and 53% percent of the sample achieved improvements that exceeded minimally important differences.
Conclusion
In-person treatment sessions were feasible for individuals with SSc and demonstrated statistically significant and clinically meaningful improvements on UE and physical function. Future studies need to examine effects against a control condition and examine durability of treatment effects.
Systemic sclerosis (SSc) is a rare, debilitating disease of the connective tissue that not only affects the skin, but also can cause severe damage to the internal organs. Despite gains in drug therapies to help control symptoms, patients with SSc face a significant challenge of managing a chronic disease that has a huge impact on daily life. Musculoskeletal complications of SSc can be severe, especially in early disease (1, 2). In particular, skin thickening and joint contractures in the upper extremities limit the ability to perform daily activities and are associated with disability (3, 4) and reduced quality of life (5-7).
Evidence-based, rehabilitation interventions for the upper extremity (UE) in SSc are limited.
Treatments that have some evidence supporting effects include thermal modalities such as paraffin wax baths (10-12); range of motion exercises (13, 14); and manual therapies including tissue mobilization and lymphatic drainage (15-17). Moreover, there are few high quality clinical trials (8, 9). Most studies are not randomized, have small samples, use many different outcome measures, and have various forms of treatments, treatment delivery, and dose which limits comparison (8, 9). To date, there has only been one large multi-site randomized controlled trial testing a rehabilitation intervention for SSc in which 220 participants were randomized either to an individualized 4-week physical and occupational therapy intervention or usual care (18). This study demonstrated that intensive rehabilitation treatment for SSc involving exercise not exclusive to the UE had, at minimum, short-term benefits on reported disability and some objective mobility measures. However, the intervention did not include evidence-based treatments such as thermal modalities or tissue mobilization, and a large portion of intervention was devoted to splinting, which has little evidence to support its use in SSc (9, 19, 20).
To address the shortcomings of the knowledge base on UE interventions in SSc, our study team was interested in testing an intervention that comprised evidence-based components and that could eventually be easily disseminated in practice. The majority of occupational therapists who come into contact with an SSc patient may have little to no experience treating this disease due to the rarity of the disease. Thus, part of this study was devoted to developing and testing a standardized treatment manual that included instruction for therapists regarding adaptations for patients with different UE problems that would facilitate translation into practice once the intervention is fully evaluated and support for effectiveness can be established.
The purpose of this pilot study was to test the feasibility and preliminary effects of a standard provision of an in-person 8-week occupational therapy treatment with prescribed home exercises to improve UE function in individuals with early SSC who had contractures. The intervention believed to be most effective for SSc patients by our team involved a minimum of 8 in-person visits with the occupational therapist. Because of the rarity of SSc and the fact that many SSc patients travel to the health system from long distances, it was necessary to examine feasibility of this intervention. In addition to feasibility, we examined the preliminary effects of treatment over time using the QuickDASH measure of UE function, the PROMIS measure of physical function; objective measures of UE mobility, strength, and coordination; and skin thickening. We hypothesized that the treatment would be feasible to deliver and that it would demonstrate preliminary effects on patient-reported functional measures.
Participants and Methods
Design
This pilot study used a one-group pretest posttest design with a target sample of 20 individuals with SSc. Outcome measures were collected at baseline, at 4 weeks (mid-treatment) and at 8 weeks (immediately following treatment).
Sample
Participants were recruited from the Scleroderma Center at the University of Michigan Health System from September 2016 – May 2017. Potential participants were either contacted from an established research registry at the Scleroderma Center or were contacted at their clinic visit if they appeared to meet the inclusion criteria based on review of their electronic medical records. To be eligible for this study, participants needed to be 18 years of age or older; have SSc; have a contracture of the hand and another joint in at least one arm such as wrist or elbow with ability to demonstrate active range of motion in that arm; English speaking; have no active hand ulcers; no concurrent medical issues; and willingness to travel to the Scleroderma Center for treatments. We focused on patients with early SSc with diffuse cutaneous distribution as our hypothesis was that early UE contractures are related to active and progressive skin and joint disease and are amenable to treatment, whereas late disease reflected greater damage and does not improve with therapy. We considered early SSc to be less than 5 years after onset similar to a previous study (21).
Procedure
The research coordinator met with potential participants who were initially eligible based on a phone screening or review of their electronic medical records prior to a clinic visit. After eligibility was confirmed and informed consent was obtained, participants were scheduled for a baseline visit with the occupational therapist. The therapist administered questionnaires to evaluate UE function (QuickDASH) and overall physical function (PROMIS), and she conducted active and passive range of motion assessments, skin assessments, grip/pinch strength and administered tests of hand coordination. These outcome assessments occurred at baseline, at 4 weeks, and at 8 weeks. Treatment was conducted each week over 8 weeks at the outpatient rehabilitation clinic in the university health system. It involved preparatory thermal modalities, tissue mobilization, and UE mobility beginning with passive range of motion and ending with active range of motion (shown in Table 1). Tissue mobilization was done using the Physiotouch device, also called the Lymphatouch [Healthy Life Devices Ltd, Helsinki, Finland]. The Physiotouch is a negative pressure device that has been used primarily to decrease swelling in tissue (22), but is currently being used in our health system as a treatment for SSc patients because it delivers mild tissue mobilization (23) which may provide better mobilization than manual techniques. The therapist also instructed participants on a home range of motion exercise program that was tailored as needed to each participant based on the severity of their contractures and arm mobility. Participants were instructed to complete daily exercise sessions at home.
Table 1. Treatment Protocol.
| Focus Area | Technique |
|---|---|
| Preparation for Treatment | Thermal Modalities ■Hot packs – focused on areas with limitations ■Paraffin – focused on digital limitations |
| Tissue Mobilization | Physiotouch ■Applied proximal to distal in areas with pathological skin in sections |
| Arm Mobility | Passive Range of Motion ■Hold end position of joint for 3-10 seconds. (Dependent on skin and joint integrity) ■Repeat for each affected joint/digit Active Range of Motion Functional Activities (limited due to time) |
| Home Range of Motion Exercises | ■Tailored active and passive ROM based on limitations in upper extremity mobility |
Development of standardized treatment manual
The treating therapist, therapist consultant (a certified hand therapist with over 30 years of experience treating individuals with scleroderma), and principal investigator developed an initial guide for treatment reflected in Table 1. The treatment components were chosen based on support of their effects in the literature (thermal modalities, tissue mobilization, and range of motion) and reflected current practices within our clinic. During each treatment session, the therapist logged the duration of each treatment component and noted any adaptations made to treatment based on an individual's disease severity or specific impairments. After all participants completed treatment, the treatment manual was reviewed and detail was included to provide instruction on how to deliver the intervention. An excerpt of the treatment manual is provided in the Appendix.
Feasibility measures
We tested feasibility against a-priori criteria: 1) At least 50% of participants who were eligible for the study would enroll; 2) At least 80% of participants would attend all treatment sessions; and 3) The sessions that included both treatment and outcome assessments would not last on average more than 2 hours. We assessed how many people initially approached, either through call or in-person at their clinic visit, and were interested in participating in the treatment. We also assessed what percentage of participants completed all 8 sessions. We examined the feasibility of providing treatment that can potentially be provided via outpatient visits. Thus, we assessed the length of time needed to complete all procedures in the in-person sessions.
Primary outcome
The primary outcome was UE function as measured by the shortened Disability Arm Shoulder Hand questionnaire called the QuickDASH, a reliable and validated self-reported measure used in the SSc population (24, 25). This is an 11-item questionnaire in which difficulty in several tasks involving the upper extremity are rated as well as interference and severity of symptoms. Items are averaged and converted to a 0 – 100 scale; a higher score indicates worse function. This measure is responsive to change and the minimal clinically important difference in patients with shoulder and arm limitations is a 16 point decrease (26).
Secondary outcomes
The secondary outcomes included general reported physical function as assessed by the PROMIS physical function version 2.0 8 item short form; the US population mean score is 50 with SD of 10, and a higher score denotes better function (27). A 2-point improvement in T-score is considered a clinically meaningful improvement (28). The main measure of range of motion was total active hand motion for the right and left hands. This was calculated by summing the total active range of motion for each finger and thumb by goniometer (260 degrees in each finger and 135 in the thumb) (29); a total score of 1125 was possible for each hand. The therapist also took photos of participants demonstrating each range of motion in the UEs at each outcome assessments as another way to examine change over time. Coordination was measured by the 9 hole peg test, a commonly-used test of dexterity in which an individual needs to put 9 pegs in holes on a peg board while timed (30). Handgrip strength was measured in pounds of pressure by Jamar hand dynamometer [Lafayette Instruments, Lafayette IL] according to a standardized protocol in which the participant squeezes the dynamometer while seated with their elbow at a 90 degree angle (31). The value used was the maximum of 6 trials, 3 trials for each UE.
Exploratory outcomes
Outcomes considered exploratory were measures considered by therapist team to be important in UE function but may not have been as directly impacted by the treatment. These outcomes included: 1) active range of motion of wrist flexion and elbow flexion for each UE measured by goniometer; 2) lateral pinch strength, measured by pinch gauge in which an average of 3 trials was used (31); and 3) skin thickness assessed by the modified Rodnan skin score (32). The modified Rodnan score was assessed at baseline and at 8 weeks by a clinic rheumatologist who was not part of the study team.
Sample size determination
Twenty participants was the target sample size, which was thought to be sufficient to establish feasibility over the one year period of the study. With 20 participants, we determined that at 80% power we could detect an effect of .67 standard deviation units, which is about a 16 point change on the QuickDASH measure, a cut-off reported for the minimally clinically important difference in patients with shoulder limitations (25).
Statistical Analysis
We used descriptive statistics to examine feasibility of study processes and treatment protocol and compared them to our a-priori criteria for success. To examine the change over time from baseline, 4- and 8-week assessments in our primary and secondary outcome measures, we used linear mixed models using all available data which served as an intent-to-treat analysis. For exploratory outcomes, we performed a per-protocol analysis in which completer data was examined for change over time using one way repeated measures analyses of variance or paired t-tests.
Results
Participant flow and characteristics
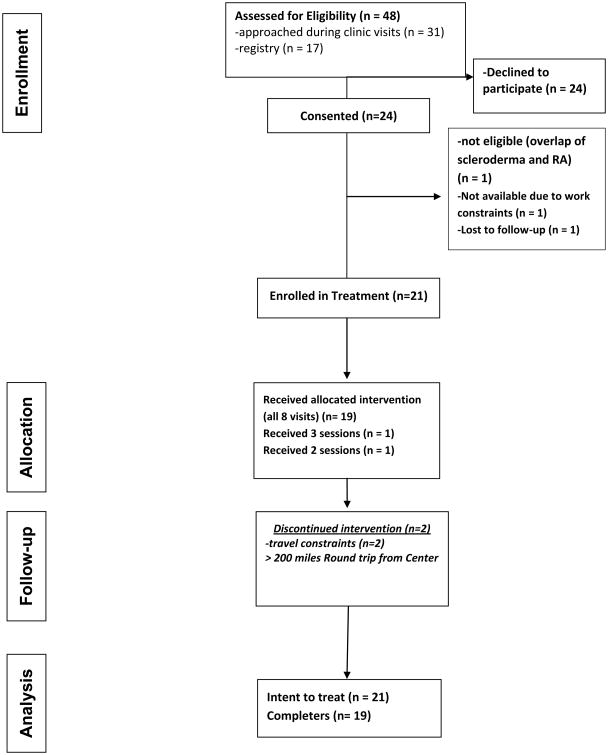
Figure 1 shows the participant flow through the study. There were 48 potentially eligible participants from the chart review and early scleroderma research registry who were either approached at a clinic visit or by phone (if on the registry) and of these, 24 (50%) were interested and consented. A main reason for not participating was travel burden. Individuals who were interested were screened in person and completed the informed consent process. One person did not meet the study eligibility criteria, and two people did not choose to participate due to not being able to get time off of work or being unable to schedule for visits. Twenty one participants were enrolled in the treatment and two were lost to follow-up due to travel constraints; despite their interest in participating, round trip mileage to the Center for treatment ranged between 200 – 550 miles per session.
Figure 1. Participant Flow Diagram.
Baseline characteristics of the sample (N = 21) are shown in Table 2. Participants were predominantly female and 38% identified as a racial minority, with almost one quarter of the sample identifying as African American. Participants ranged in age from 20 – 75 years, with a mean of 47.9 years. All participants had diffuse cutaneous SSc. The mean duration of SSc was 3.1 + 2.3 years; with a mean (SD) Rodnan score of 17.6 (9.7) indicating moderate skin disease. Majority of patients were being treated with immunosuppressive therapy or were in ongoing clinical trials for their aggressive skin disease.
Table 2. Baseline Characteristics of Sample N = 21.
| Characteristics | |
|---|---|
| Age, (mean, SD) | 47.9 (16.1) range 20 - 75 |
| Female (%, n) | 86%, 18 |
| Race (%, n) | 38.1%, 8 |
| Black/African American | 23.8% 5 |
| White | 61.9% 13 |
| Other | 14.3% 7 |
| Ethnicity (%, n) | 4.8% 1 |
| Hispanic or Latino | 61.9% 13 |
| Other | 33.3% 7 |
| Married (%, n) | 47.6%, 10 |
| High school education or less (%, n) | 38.1%, 8 |
| Modified Rodnan Skin Score, (mean, SD)* | 17.6 (9.7) range 1 – 37 |
| Disease Duration, (mean, SD) | 3.1 (2.3) years |
| Diffuse cutaneous SSc | 100% |
| Interstitial Lung Disease (%, n) | 42.9% |
| Gastrointestinal Involvement (%, n) | 81.0% |
| Raynaud's Phenomenon (%, n) | 90.5% (19) |
| Use of Immunosuppressive Agents (%, n) | 48% (10) Mycophenolate Mofetil (MMF) |
| 23.8% (5) Methotrexate | |
| 9.5% (2) MMF and Methotrexate | |
| 19.1% (4) Investigational agents | |
| 4.8% (1) Abatacept* | |
| 19.1% (4) None |
n = 20
Feasibility outcomes
Nineteen participants (91% of the enrolled sample) completed the protocol as intended, attending all 8 in-person sessions. These participants traveled a mean of 103.4 + 82.5 miles round trip for each session with 37% traveling between 100 and approximately 340 miles each session. There were a few protocol deviations due to timing of sessions. One participant stopped and restarted treatment two months later due to travel issues, but then was able to attend all 8 sessions. Fifteen of the 19 participants who attended all sessions attended them weekly; whereas the remaining four had at least one cancellation and rescheduled for the next available slot (usually the following week). There was also a protocol deviation due to a participant being treated with an active hand ulcer and one person having a fingernail fall off during the course of treatment, which are relatively common phenomenon in SSc. Both participants received modified treatment modalities (such as no paraffin treatment to affected hands) in these cases. We also evaluated the time it took to administer sessions in which evaluation plus treatment sessions were combined (at baseline, 4 weeks, and 8 weeks) for feasibility of administration. Ten percent of evaluation plus treatment sessions (6/59 total sessions) lasted longer than 2 hours, which exceeded our feasibility target; however, five of those sessions occurred at baseline and the therapist was able to improve process efficiency at almost all the subsequent sessions.
Adverse events and unanticipated problems
With regard to adverse events and unanticipated problems, there were no related adverse events or unanticipated problems of treatment. One participant experienced a fingernail falling off prior to returning for the last session of treatment and was considered unrelated to the treatment provided. For the last session of treatment, any treatment or outcome measures concerning that digit were not performed.
Effects of treatment
Table 3 shows the results of the each outcome using linear mixed models. Participants had a mean of 6.6 point improvement on the QuickDASH at 4 weeks which was not significant; however, participants continued to improve from 4 to 8 weeks with a 14 point mean improvement from baseline [t (2,36) = 3.53; p =.0012]. Using a previously cited clinically meaningful cut-point of 16 point improvement on the QuickDASH (25); 47% of participants who completed the intervention (9/19) met this threshold.
Table 3. Least Square Means (SE) of Change over Time from Linear Mixed Models.
| Baseline | Mid-treatment (4 weeks) | Post-treatment (8 weeks) | P value | |
|---|---|---|---|---|
| Primary Outcome | ||||
| QuickDASH† | 49.3 (4.6) | 42.7 (4.8) | 35.2 (4.8) | 0.0012 |
| Secondary Outcomes | ||||
| PROMIS Physical Function‡ | 38.0 (1.3) | 38.5 (1.4) | 41.1 (1.4) | 0.004 |
| Left Total Active Motion§ | 736.5 (41.0) | 797.3 (41.3) | 778.0 (41.3) | 0.013 |
| Right Total Active Motion | 745.2 (43.1) | 775.5 (43.4) | 758.0 (43.4) | 0.49 |
| Left 9 Hole Peg Test (sec) | 25.4 (1.6) | 21.5 (1.6) | 22.9 (1.6) | 0.03 |
| Right 9 Hole Peg Test (sec) | 23.6 (1.6) | 21.9 (1.6) | 21.8 (1.6) | 0.15 |
| Handgrip Strength¶ | 45.8 (4.1) | 45.4 (4.1) | 43.3 (4.1) | 0.06 |
A higher score denotes worse function.
A higher score denotes better function.
total active motion is 1175 degrees of movement total-260 degrees for each finger and 135 for thumb; right hand total active motion n = 20.
Handgrip strength is the maximum value from either hand.
On the PROMIS physical function measure, participants had a significant improvement over the 8 week period from baseline. Similar to the QuickDASH trends, change from baseline to 4 weeks was not significant; however, improvements continued from 4 to 8 weeks and was a significant effect [t(2,36) = -3.08; p = .004]. The mean improvement over time was 3.1 points on the PROMIS which demonstrates a third of a standard deviation change; larger than the minimally important difference of 2 points on the PROMIS 20-item physical function scale in a rheumatoid arthritis sample (28). Fifty three percent achieved a 2-point increase on the PROMIS after 8 weeks.
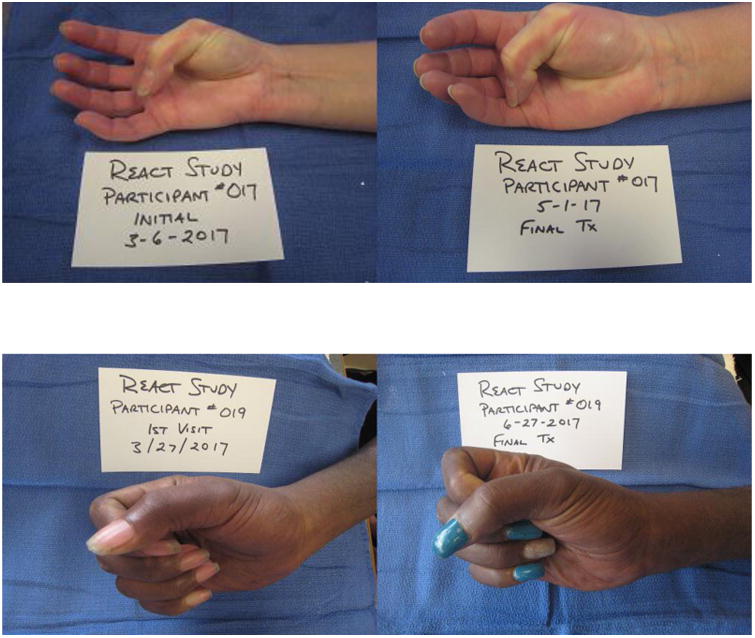
Of the objective UE measures, left total active hand function and left 9 hole peg test scores were significantly improved after the intervention. On average, participants gained 41.5 degrees of active hand motion at 8 weeks and were 2.5 seconds faster at performing the 9 hole peg test with their left hands. There are no established clinically important differences in either of these measures. Figure 2 shows examples of improvement from baseline to 8 weeks in active range of motion. No significant improvement was shown on active hand motion or coordination for the right hand after 8 weeks. Handgrip strength did not improve, and participants had a slightly weaker handgrip at 8 weeks, although findings were not statistically significant (p = .06). For the exploratory outcomes, there were no statistically significant changes in wrist or elbow flexion or lateral pinch measures. Skin thickness, evaluated by modified Rodnan score was captured on 15 participants at post-test, and a paired t-test on completer data showed no significant change and slight worsening from baseline to 8 weeks [17.9 (+7.9) baseline, 20.1 (+9.5) 8 weeks; t (1,14) = -1.4; p = .18].
Figure 2. Participant Photos Depicting Change in Upper Extremity Mobility from Baseline to 8 Weeks.
Discussion
In this study, we examined the feasibility and preliminary effects of an 8 session in-person occupational therapy treatment to improve UE outcomes in individuals with SSc. In general, the study and the treatment were feasible supported by our ability to enroll and retain participants despite the travel burden to the center. Of 47 eligible participants, 48 potentially eligible individuals minus one who did not meet the inclusion criteria for contracture type, 51% were enrolled in the treatment, which was slightly above our target of 50%. Attendance at all 8 sessions for those enrolled exceeded our expectations in that 91% of the sample met this metric despite the burden of traveling a mean distance of over 100 miles roundtrip for each session. In an attempt to reduce participant travel burden, we consolidated treatment and outcome visits at baseline, 4 weeks, and 8 weeks. We examined feasibility of conducting these combined sessions in a 2 hour period and 90% of sessions met this criterion. The sessions that exceeded the time limit occurred early in the study and timing improved as the therapist was able to streamline processes. The treatment provided showed the strongest effects on reported improvement in UE function and general physical function and improvements were considered clinically important for approximately half of the sample on the QuickDASH and PROMIS physical function measures (47% and 53% respectively) per established cut-offs in other populations (26, 28). Without studies in SSc evaluating these clinically important differences, it is not clear whether this is an accurate reflection of who benefited as a result of this treatment. For instance, depending upon the study, different values of minimal clinically important differences for QuickDASH have been reported; one study reported a cut-off score of 8 and another reported 14 (24, 33). Thus, our chosen cut-off of 16 points is likely conservative, and more individuals in our sample may have benefitted.
Improvements were shown in some but not all objective measures, and most improvement occurred in hand mobility and coordination. Significant effects were shown only in the left hand, although both hands had similar trends of improvement. Interestingly, more gains occurred in the first 4 weeks of treatment. However, gains in these measures continued from 4 – 8 weeks showing that the additional sessions were valuable. It remains unclear how many sessions are optimal for sustaining gains made during treatment. Most studies investigating UE rehabilitation interventions in SSc were designed to measure short-term efficacy with end-points spanning from 4 weeks to 3 months (11, 12, 14-17) and most clinic-based interventions lasted from 3 – 8 weeks, however intensity of these interventions was variable. The highest quality randomized controlled trial in this evidence base from Rannou and colleagues showed a 3-week intensive intervention, consisting of 36 treatment hours and a prescribed daily home exercise program, had short term effects on disability on the Health Assessment Questionnaire that diminished over time at 6 months and 12 months follow-up (18). Although long-term adherence to the home exercise program was poor, participants who adhered to the daily home exercises had better effects than those that did not over time, supporting the inclusion of home exercise in future interventions. Home exercise programs likely need to be more engaging to participants to improve adherence in future studies.
A main strength of this study includes testing a treatment informed by available evidence supporting specific treatment components in SSc that capitalized on the extensive experience of our therapist team who commonly provide UE treatment for individuals with SSc. In addition, the creation of a standardized manual as done in this study (see Appendix for an excerpt) will be important for further testing of this intervention and has the potential to provide an evidence-based guide for therapists who treat patients with SSc on a broad scale.
This study is limited by conclusions that can be drawn due to its one group design. Thus, the assessment of outcomes in this study does not provide definitive evidence of efficacy of treatment. Further, due to the size and scope of this study, the therapist also served as the outcome assessor and therefore was not blinded. Tracking of home exercise needs to be strengthened in future studies as we did not formally assess adherence. Thus, it was not possible to disentangle the effects of home exercise from that of in-person sessions. Understanding the effects of in-person intervention versus home exercise will be important in future research studies since participation in the intervention was mainly precluded by travel to the center. Given that our sample included all participants with diffuse SSc who were in the early stages of the disease (within 5 years of diagnosis), our findings can only be generalized to this population. In addition, we are uncertain if these improvements are maintained after the in-person sessions were completed in the trial.
In conclusion, this pilot one group trial supported the feasibility of an 8 session occupational therapy intervention to address UE function in individuals with SSc. Preliminary effects were found at 8 weeks with reported improvements in UE disability, physical function, and objective measures of hand mobility and coordination. Although definitive treatment effects cannot be drawn from this study, therapists unfamiliar with treating SSc may benefit from reviewing information on the treatment provided to gain knowledge of progression of treatment components and recommended adaptations based on individual differences. Further larger studies are needed that include a control group or comparator arm and examine durability of treatment effects.
Significance and Innovations.
In a pilot test of occupational therapy treatment consisting of thermal modalities, tissue mobilization, range of motion exercises, functional activities and home exercises, improvements in reported UE and physical function and some objective performance measures were found in a small cohort of individuals with early systemic sclerosis.
Eight weekly occupational therapy sessions plus home exercises was highly feasible for participants despite burden of travel to the clinic.
The standardized therapy manual created in this study has the potential to be disseminated to the occupational therapy community after further testing in larger studies which could increase clinical uptake of an evidence-based intervention for early systemic sclerosis.
Acknowledgments
This research study was supported by the Rehabilitation Research Resource to Enhance Clinical Trials (REACT) Center at the University of Alabama (P2CHD086851). Dr Khanna was also supported by the NIH/NIAMS Grant # K24 AR063120.
Appendix. Excerpt from the Treatment Protocol
This manual was developed by Susan Murphy, Mary Barber and Carole Dodge with input from the entire study team
Description of Treatment
The occupational therapy practitioner will work individually with patients following the general pattern below.
Treatment will be tailored based on specific characteristics of the participant (severity of contractures and inflammation) and will be progressed as participants improve.
Each session will end with a description and reminder to complete the home exercise program.
- At the beginning of each session, the interventionist will discuss the following with the participant:
- Any symptoms or functional issues that have been problematic during the last week as these may be important to focus on during treatment.
- The participant's ability to engage in the home exercise program and will go over any questions or concerns.
- How often the participant completed the home exercises in the last week and will emphasize the importance of completing the exercises if adherence is low.
Table 2. Overview of treatment.
Additional explanation for focus areas follow
| Focus Area | Treatment | Instructions | Time |
|---|---|---|---|
| Preparation for Treatment | Thermal Modalities ■Hot packs ■Paraffin |
|
15 minutes |
| Tissue Mobilization | Physiotouch (also called Lymphatouch)*
|
|
10-13 minutes per extremity |
| Arm Mobility\Range of Motion | Passive Range of Motion Active Range of Motion\Functional Activities |
■PROM Hold end position of joint for 3-10 seconds; 3-5 reps (Dependent on skin and joint integrity) see Appendix ■Repeat for each affected joint/digit AROM – 10 reps - see Appendix Functional Activities utilizing available ROM, grip/pinch |
10 minutes per extremity 3-5 minutes per extremity (or remaining time in session) |
| Home Program | ■Tailored active and passive ROM based on limitations in upper extremity mobility | *Adaptions may be needed if skin ulcers are present by teaching alternative methods for holding/stretching joints, or if patient experiences lasting increase of musculoskeletal pain by decreasing repetitions. |
Preparation for Treatment
Thermal modalities are recommended to prepare the upper extremity for movement.
The thermal modalities used in this treatment are hot packs and paraffin.
Hot packs
Materials needed
2 medium-size hot packs
2 bath-size towels (should be 8 layers between hot pack and skin)
Instructions
Hot packs to elbows and forearms can be applied immediately prior to the treatment session (such as while participant is in reception area) or concurrently with the paraffin wax dips to the hands.
Hot packs should be encased in hot pack cover and towels and applied to elbows and forearms.
Important Points
NEVER put a hot pack over the paraffin as this may cause harm to the participant.
ALWAYS listen to the participant and adjust heat and application of hot packs according to participant tolerance. For instance, more towels can be added around heat packs to reduce the heat.
Paraffin Wax
Paraffin is a thermal modality which is used to reduce pain, reduce joint stiffness, improve blood flow, moisturize skin and improve scar elasticity.
Materials needed
Paraffin unit with adjustable heat control
Paraffin wax (approximately 6 lbs. of prepared wax)
Candy or meat thermometer
Exam gloves – latex or non-latex (if needed)
Medical tape (if needed)
Additional small bottle of mineral oil (if needed to lower temperature)
Instructions
Heat wax to at least 120 degrees Fahrenheit but no more than 130 degrees - test with a thermometer.
Have participant prepare for treatment by removing any rings, washing hands with soapy water, and rinsing and drying hands.
Dip one hand with a straight wrist and fingers as relaxed and separated as possible into the wax (being careful not to crack the wax).
Immediately pull hand out of wax after dipping.
After no more wax drips off of the hand, dip again for a recommended 8 – 12 times in the same manner (See Important Points section below).
Insert hand into a plastic bag and then wrap in a towel or mitt to retain heat.
Repeat process of dipping with the other hand.
Important Points
Listen to the participant regarding their tolerance. Ask participant after a few dips how it feels, and if the temperature is getting too warm, do not continue to dip.
If patient has an ulcer on one of their digits or thumb, paraffin can still be used if the area is adequately protected. For instance, an exam glove can be used over the hand with tape applied around the top of glove to keep wax from getting in the wound.
Footnotes
The authors have no financial conflicts of interest with Healthy Life Devices that manufacture the Physiotouch device used in this study.
References
- 1.Denton C, Khanna D. Systemic sclerosis. The Lancet. 2017;390(10103):1685–99. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 2.Bálint Z, Farkas H, Farkas N, Minier T, Kumánovics G, Horváth K, et al. A three-year follow-up study of the development of joint contractures in 131 patients with systemic sclerosis. Clin Exp Rheumatol. 2014;32:S68–74. [PubMed] [Google Scholar]
- 3.Sandqvist G, Eklund M, Åkesson A, Nordenskiöld U. Daily activities and hand function in women with scleroderma. Scand J Rheumatol. 2004;33(2):102–7. doi: 10.1080/03009740410006060. [DOI] [PubMed] [Google Scholar]
- 4.Sandqvist G, Scheja A, Hesselstrand R. Pain, fatigue and hand function closely correlated to work ability and employment status in systemic sclerosis. Rheumatology. 2010;49:1739–46. doi: 10.1093/rheumatology/keq145. [DOI] [PubMed] [Google Scholar]
- 5.Frantz C, Avouac J, Distler O, Amrouche F, Godard D, Kennedy AT, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Semin Arthritis Rheum. 2016;46:115–23. doi: 10.1016/j.semarthrit.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Khanna D, Yan X, Tashkin D, et al. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheumatol. 2007;56:1676–84. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 7.Maddali-Bongi S, Rosso AD, Mikhaylova S, Francini B, Branchi A, Baccini M, et al. Impact of hand and face disabilities on global disability and quality of life in systemic sclerosis patients. Clin Exp Rheumatol. 2014;32:S15–20. [PubMed] [Google Scholar]
- 8.Willems LM, Vriezekolk JE, Schouffoer AA, Poole JL, Stamm TA, Boström C, et al. Effectiveness of nonpharmacologic interventions in systemic sclerosis: A systematic review. Arthritis Care Res. 2015;67:1426–39. doi: 10.1002/acr.22595. [DOI] [PubMed] [Google Scholar]
- 9.Poole JL. Musculoskeletal rehabilitation in the person with scleroderma. Curr Opin Rheumatol. 2010;22:205–12. doi: 10.1097/BOR.0b013e328335a7d2. [DOI] [PubMed] [Google Scholar]
- 10.Horvath J, Balint Z, Szep E, Deiszinger A, Minier T, Farkas N, et al. Efficacy of intensive hand physical therapy in patients with systemic sclerosis. Clin Exp Rheumatol. 2017 in press. [PubMed] [Google Scholar]
- 11.Mancuso T, Poole JL. The effect of paraffin and exercise on hand function in persons with scleroderma: a series of single case studies. J Hand Ther. 2009;22:71–8. doi: 10.1016/j.jht.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Sandqvist G, Åkesson A, Eklund M. Evaluation of paraffin bath treatment in patients with systemic sclerosis. Disabil Rehabil. 2004;26:981–7. doi: 10.1080/09638280410001702405. [DOI] [PubMed] [Google Scholar]
- 13.Mugii N, Hasegawa M, Matsushita T, Kondo M, Orito H, Yanaba K, et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol. 2006;33:1586–92. [PubMed] [Google Scholar]
- 14.Stefanantoni K, Sciarra I, Iannace N, Vasile M, Caucci M, Sili Scavalli A, et al. Occupational therapy integrated with a self-administered stretching programme on systemic sclerosis patients with hand involvement. Clin Exp Rheumatol. 2016;34:157–61. [PubMed] [Google Scholar]
- 15.Maddali Bongi S, Del Rosso A, Galluccio F, Tai G, Sigismondi F, Passalacqua M, et al. Efficacy of a tailored rehabilitation program for systemic sclerosis. Clin Exp Rheumatol. 2009;27:S44–50. [PubMed] [Google Scholar]
- 16.Maddali Bongi S, Del Rosso A, Passalacqua M, Miccio S, Cerinic MM. Manual lymph drainage improving UE edema and hand function in patients with systemic sclerosis in edematous phase. Arthritis Care Res. 2011;63:1134–41. doi: 10.1002/acr.20487. [DOI] [PubMed] [Google Scholar]
- 17.Maddali Bongi S, Rosso A, Galluccio F, Sigismondi F, Miniati I, Conforti ML, et al. Efficacy of connective tissue massage and Mc Mennell joint manipulation in the rehabilitative treatment of the hands in systemic sclerosis. Clin Rheumatol. 2009;28:1167–73. doi: 10.1007/s10067-009-1216-x. [DOI] [PubMed] [Google Scholar]
- 18.Rannou F, Boutron I, Mouthon L, Sanchez K, Tiffreau V, Hachulla E, et al. Personalized physical therapy versus usual care for patients with systemic sclerosis: a randomized controlled trial. Arthritis Care Res. 2017;69:1050–9. doi: 10.1002/acr.23098. [DOI] [PubMed] [Google Scholar]
- 19.Harvey LA, Katalinic OM, Herbert RD, Moseley AM, Lannin NA, Schurr K. Stretch for the treatment and prevention of contractures. Cochrane Database Syst Rev. 2017:CD007455. doi: 10.1002/14651858.CD007455.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeger MW, Furst DE. Effects of splinting in the treatment of hand contractures in progressive systemic sclerosis. Am J Occup Ther. 1987;41:118–21. doi: 10.5014/ajot.41.2.118. [DOI] [PubMed] [Google Scholar]
- 21.Khanna D, Hayes B, Furst D. Functional disability and other health-related quality-of-life domains: points to consider for clinical trials in systemic sclerosis. Rheumatology. 2017;56:v17–22. doi: 10.1093/rheumatology/kex193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iivarinen JT, Korhonen RK, Jurvelin JS. Modeling of interstitial fluid movement in soft tissue under negative pressure – relevance to treatment of tissue swelling. Comput Methods Biomech Biomed Engin. 2016;19:1089–98. doi: 10.1080/10255842.2015.1101073. [DOI] [PubMed] [Google Scholar]
- 23.Adams A. Technical Note: Physiotouch. Rehabilitation Oncology. 2016;34:49–50. [Google Scholar]
- 24.Kennedy CA. Measurement properties of the QuickDASH (Disabilities of the Arm, Shoulder and Hand) outcome measure and cross-cultural adaptations of the QuickDASH: a systematic review. Qual Life Res. 2013;22:2509–47. doi: 10.1007/s11136-013-0362-4. [DOI] [PubMed] [Google Scholar]
- 25.Varju C, Balint Z, Solym A, et al. Cross-cultural adaptation of the disability of the arm, shoulder, and hand (DASH) questionnaire into Hungarian and investigation of its validity in patients with systemic sclerosis. Clin Exp Rheumatol. 2008;26:776–84. [PubMed] [Google Scholar]
- 26.Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (quickDASH) J Orthop Sports Phys Ther. 2014;44:30–9. doi: 10.2519/jospt.2014.4893. [DOI] [PubMed] [Google Scholar]
- 27.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epid. 2010;63:1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the Patient-Reported Outcomes Measurement Information System (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis. 2014;74:104–7. doi: 10.1136/annrheumdis-2013-204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams L, Greene L, Topoozian E. Range of Motion. In: American Society of Hand Therapists (ASHT), editor. Clinical Assessment Recommendations. Chicago: ASHT; 1992. pp. 55–70. [Google Scholar]
- 30.Grice KO, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available nine hole peg test for finger dexterity. Am J Occup Ther. 2003;57:570–3. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–6. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 32.Clements P, Lachenbruch P, Seibold J, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–6. [PubMed] [Google Scholar]
- 33.Sorensen AA, Howard D, Tan WH, Ketchersid J, Calfee RP. Minimal clinically important differences of 3 patient-rated outcomes instruments. J Hand Surg Am. 2013;38(4):641–9. doi: 10.1016/j.jhsa.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]