Abstract
In vertebrates, the Eph/ephrin family of signaling molecules is a large group of membrane-bound proteins that signal through a myriad of mechanisms and effectors to play diverse roles in almost every tissue and organ system. Though Eph/ephrin signaling has functions in diverse biological processes, one core developmental function is in the regulation of cell position and tissue morphology by regulating cell migration and guidance, cell segregation, and boundary formation. Often, the role of Eph/ephrin signaling is to translate patterning information into physical movement of cells and changes in morphology that define tissue and organ systems. In this review, we focus on recent advances in the regulation of these processes, and our evolving understanding of the in vivo signaling mechanisms utilized in distinct developmental contexts.
1. Introduction
The Eph receptor tyrosine kinases were first identified based on their overexpression in human carcinoma (Hirai et al., 1987), and were later found to be expressed on initial outgrowths of neuronal axons, suggesting a role in axon guidance (Henkemeyer et al., 1994). Though their role in these contexts has been studied in great depth, Ephs and ephrins are also expressed in most tissues during embryonic development and are essential to a wide variety of developmental processes (Batlle and Wilkinson, 2012; Bush and Soriano, 2012; Egea and Klein, 2007; Kania and Klein, 2016; Klein and Kania, 2014; Kullander and Klein, 2002; Merlos-Suárez and Batlle, 2008; Pasquale, 2008; Wilkinson, 2001). This is perhaps unsurprising, as the Eph receptors are the largest family of receptor tyrosine kinases found in mammals (Gale et al., 1996; Henkemeyer et al., 1994; Kullander and Klein, 2002). In this review, we focus primarily on how Eph/ephrin signaling regulates cell position and tissue separation in development. Even so, it is not possible to comprehensively address all of the studies that have made important contributions in this area, and we have instead provided more extensive discussion of a subset of examples. In addition, roles for Eph/ephrin signaling in cell proliferation, apoptosis, axon guidance, and a myriad of other processes are documented, and are reviewed elsewhere (Bush and Soriano, 2012; Kania and Klein, 2016; Laussu et al., 2014; Merlos-Suárez and Batlle, 2008; Pasquale, 2008; Xu and Henkemeyer, 2012).
We will begin by reviewing the genetic support for our current understanding of in vivo signaling mechanisms. This area of research has been consistently active from the earliest studies of Eph/ephrin signaling, but our understanding of the broadly-used genetic tools, as well as the general principles derived from these studies, are continuing to evolve. From a cellular perspective, Eph/ephrin signaling has been widely implicated in regulating cell migration; the specific functions played in different developmental contexts differ somewhat, and we will compare some representative examples. Finally, there have been numerous recent advances in our understanding of the role of Eph/ephrin signaling in cell segregation; we will discuss proposed modes of action and how they relate to distinct conceptual models of this widely-occurring cellular process. In each of these areas, outcomes of recent studies challenge long-accepted roles for Eph/ephrin signaling, leading to interesting new questions concerning the complex ways in which these molecules impact morphogenesis.
2. Signaling mechanisms
The signaling partners of the Eph receptors are the ephrins, membrane-bound molecules separated into two classes: ephrin-As are membrane-bound through a GPI anchor, and ephrin-Bs are transmembrane molecules with a cytoplasmic domain (Gale et al., 1996). Eph receptors have also been separated into A and B classes based on sequence similarity and whether they bind to ephrin-A or ephrin-B signaling partners (Gale et al., 1996), although there is some overlap in binding affinity between the two classes (Himanen et al., 2004). Eph receptor oligomerization is necessary for propagation of a forward signal, with the size of the Eph receptor cluster determining the strength of the signal, such that trimers and tetramers signal maximally (Himanen et al., 2010; Schaupp et al., 2014; Seiradake et al., 2010). Biochemically, Eph/ephrin interactions have bidirectional signaling capacity (Brückner et al., 1997; Holland et al., 1996; Lin et al., 1999; Torres et al., 1998). Upon binding of an ephrin to an Eph receptor, signaling may be transduced into the receptor-expressing cell; this classical forward signaling is mediated by Eph tyrosine phosphorylation followed by binding of partners that mediate downstream signaling, though the in vivo utilization of these binding partners in distinct developmental contexts is largely unknown (Bush and Soriano, 2012). An Eph/ephrin binding event can also result in transduction of a signal into the ephrin-expressing cell, known as reverse signaling (Henkemeyer et al., 1996; Holland et al., 1996). Therefore, in addition to Eph and ephrin expression levels and degree of oligomerization, the extent to which forward, reverse, and bidirectional signaling modes are utilized represents another layer of regulation that contributes to modulating downstream signaling. The membrane-bound nature of Ephs and ephrins dictates that cell-cell contact is an important part of their signal transduction (Davis et al., 1994; Henkemeyer et al., 1994), and in many developmental contexts, Eph/ephrin signaling between adjacent cells is critical. However, Eph/ephrin signaling via cellular protrusions may be capable of mediating signaling between nonadjacent cells (Cayuso et al., 2016), and release of Ephs and ephrins by exosomes also allows for the possibility of signaling at greater distances (Gong et al., 2016). Whereas Eph and ephrin ectodomains can be proteolytically cleaved (Georgakopoulos et al., 2006; Hattori et al., 2000), the ectodomain alone is incapable of initiating oligomerization and is therefore unlikely to activate forward signaling; indeed, the unclustered ectodomain is often used as a competitive antagonist (Daar, 2012; Pegg et al., 2017), suggesting that antagonistic modulation of signaling at a distance by soluble Eph/ephrin ectodomains may be possible.
Adding an additional layer of complexity to the regulation of Eph/ephrin signaling, there is evidence that expression of Eph receptors and ephrins in the same cell (in cis) can negatively regulate Eph receptor signaling through ephrins in an adjacent cell (in trans) (Hornberger et al., 1999). Eph receptors have been proposed to interact with ephrins in cis using their canonical ligand-binding domain (Yin et al., 2004) as well as their membrane-proximal fibronectin type III domain (Carvalho et al., 2006). This cis-inhibition mechanism can fine-tune control of EphA receptor activation in a number of cellular contexts (Carvalho et al., 2006; Falivelli et al., 2013; Gatto et al., 2014; Hornberger et al., 1999; Kao and Kania, 2011; Marquardt et al., 2005). The breadth of functional relevance of cis-expression of Ephs and ephrins, and whether cis inhibition occurs for EphB receptors, however, remains unknown.
2.1 Genetic dissection of bidirectional signaling
Since the discovery that Eph receptor-ephrin interactions could result in signal transduction not only in the receptor-expressing cell (classical forward signaling), but also in the ephrin-expressing cell (reverse signaling) (Henkemeyer et al., 1996; Holland et al., 1996), many studies have delved into the possible mechanisms of ephrin reverse signaling. In biochemical studies, ephrin-Bs were found to use their cytoplasmic domains to mediate reverse signaling through phosphorylation of highly conserved tyrosines (Brückner et al., 1997) and interaction with PDZ-domain-containing proteins (Lin et al., 1999; Lu et al., 2001). Although ephrin-Bs themselves do not possess intrinsic kinase activity, therefore, they may mediate downstream signaling by recruitment of other signaling molecules. Ephrin-B reverse signaling may also occur by non-SH2/PDZ mechanisms, such as by interactions with scaffold proteins to regulate tight junctions or interactions with connexin-43 to regulate gap junctions (Daar, 2012; Davy et al., 2006). Although ephrin-As possess no intracellular domain, there is biochemical and genetic evidence for ephrin-A reverse signaling (Dudanova et al., 2012). The precise mechanisms of this remain unclear, but cell culture studies indicate that ephrin-As may mediate reverse signaling through interaction with Src family kinases in membrane microdomains (Davy et al., 1999) and interaction with integrins to promote adhesion (Davy and Robbins, 2000; Huai and Drescher, 2001). In addition, EphA3-dependent activation of the ADAM10 metalloprotease to cleave ephrin-As allows a switch from adhesion to repulsion, in essence modulating the cellular response of the ephrin-expressing cell (Hattori et al., 2000; Janes et al., 2005). Thus, upon the binding of an Eph to an ephrin on an adjacent cell and subsequent oligomerization, a signal can be transduced into the Eph-expressing cell, the ephrin-expressing cell, or into both cells simultaneously, generating a bidirectional signaling cascade. Which of these events is crucial to achieve the appropriate downstream signaling outcome seems to depend heavily on cellular context, and therefore has required direct evaluation in vivo in distinct cell and tissue types.
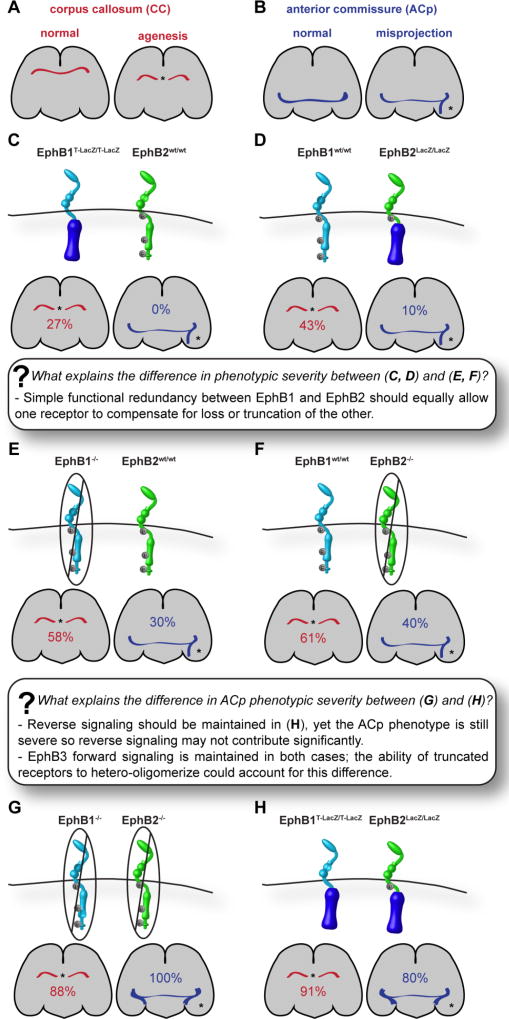
The prototypical in vivo roles for reverse signaling were first suggested by studying axon guidance phenotypes in mice lacking EphB receptor signaling capacity. Three receptors (EphB1, EphB2, EphB3), with similar affinity for B-type ephrins, and presumably similar intracellular signaling capabilities, are each involved in the patterning of two major interhemispheric commissural axon tracts, the anterior commissure (AC) and corpus callosum (CC) (Figure 1A, B) (Henkemeyer et al., 1996; Mendes, 2006; Orioli et al., 1996; Robichaux et al., 2016). Initial genetic evidence for the existence of reverse signaling was based on discrepant phenotypic outcomes upon complete loss of EphB2 compared with mutants in which most of the intracellular domain was replaced with β-galactosidase, while maintaining the extracellular, transmembrane, and juxtamembrane domains (Henkemeyer et al., 1996). Whereas all Ephb2−/− mutant embryos exhibited defects in the posterior projection of the AC (ACp) and 61% exhibited defects in the CC, Ephb2LacZ/LacZ mutant embryos did not exhibit either of these phenotypes, leading to the conclusion that they were attributable to loss of reverse and not forward signaling (Henkemeyer et al., 1996; Mendes, 2006). A recent study reexamining these mutant alleles in guidance of the AC confirmed that null loss of EphB2 led to a higher penetrance of ACp phenotypes than intracellular truncation of EphB2 in Ephb2LacZ/LacZ mutant embryos (Figure 1D, F). Further, while complete loss of EphB1 resulted in an abnormal ACp approximately 30% of the time (Figure 1E), EphB1 mutants in which the intracellular domain of EphB1 was replaced with LacZ (EphB1T-LacZ) did not exhibit defects in ACp development (Figure 1C) (Chenaux and Henkemeyer, 2011; Robichaux et al., 2016). Surprisingly, however, loss of forward signaling through EphB1 and EphB2 together resulted in ACp defects in 80% of Ephb1T-LacZ/T-LacZ; Ephb2LacZ/LacZ compound mutants, demonstrating that the intracellular domains of EphB1 and EphB2 have redundant function. An almost identical scenario was borne out for the CC; loss of forward signaling through EphB1 resulted in defects in the CC in 27% of Ephb1T-LacZ/T-LacZ mutants (Figure 1C) and 43% of Ephb2LacZ/LacZ mutants (Figure 1D), but loss of forward signaling through EphB1 and EphB2 resulted in CC defects in 91% of Ephb1T-LacZ/T-LacZ; Ephb2LacZ/LacZ compound mutants (Figure 1H). In both contexts, the penetrance upon loss of the intracellular domain was not as high as complete compound loss of EphB1 and EphB2 (100%) (Figure 1G), a discrepancy that was attributed to reverse signaling (Robichaux et al., 2016). However, this difference could instead be due to the retained presence of EphB3 forward signaling. Indeed, the penetrance of ACp or CC phenotypes in Ephb2−/−; Ephb3−/− compound mutants is 100%, and higher than in either homozygous mutant alone, demonstrating their redundancy (Mendes, 2006; Orioli et al., 1996). Based on these studies, we conclude that in the case of Ephb1T-LacZ/T-LacZ; Ephb2LacZ/LacZ mutants, reverse signaling activation should still be retained, but these mutants still exhibit high penetrance of AC and CC defects, arguing that it is not necessary to evoke the existence of a reverse signal to explain differences between EphB null and intracellular mutant phenotypic penetrance.
Figure 1. Differentiating Eph/ephrin forward and reverse signaling in the development of two axon tracts, the corpus callosum and the anterior commissure.
(A) Key to representations of a normal corpus callosum (CC) and agenesis of the corpus callosum (aCC). (B) Key to representations of a normal posterior branch of the anterior commissure (ACp) and of ACp misprojections. (C–F) Mice expressing a truncated version of EphB1 in which the intracellular domain is replaced with LacZ (EphB1T-LacZ) (C) have a 20% penetrance of aCC and no ACp defects. Similarly, mice expressing EphB2LacZ (D) have a 43% penetrance of aCC and a 10% penetrance of ACp defects. However, mice with total loss of EphB1 (E) or EphB2 (F) have a higher incidence of CC and ACp defects, raising the question of whether functional redundancy can truly explain the low penetrance of phenotypes in the truncated mutants. Functional reduncancy should allow one receptor to compensate for both loss and truncation of the other. (G–H) EphB1; EphB2 double knockout mice have 100% penetrance of severe ACp misprojection defects (G), while mice in which the intracellular domain of both receptors has been replaced with LacZ (H) have only 80% incidence of moderate-to-severe phenotypes. How can the difference between these cases be explained? It is possible that the maintenance of reverse signaling via receptor extracellular domains in (H) allows preservation of some function, though it is unlikely that reverse signaling plays a dominant role in CC or ACp formation, as the phenotype in (H) is still quite severe. Although EphB3 forward signaling may compensate somewhat, this should be the case in both mutants, and is unlikely to explain the difference. The most likely explanation is the possibility that truncated EphB1 and EphB2 receptors maintain the capacity to hetero-oligomerize with full-length Eph receptors, such as EphB3, to contribute to forward signaling and preserve a small amount of function.
If redundant receptor forward signaling function is responsible for the bulk of ACp and CC phenotypes, why is complete loss of EphB1 or EphB2 receptor more severe than loss of only the intracellular domain? In both cases, the C-terminally truncated fusion protein retains the extracellular and transmembrane domains; the EphB2LacZ mutant protein also retains the juxtamembrane region. Notably, Eph receptors can cocluster, or heterooligomerize, both within and beyond subclass, and Eph-Eph receptor interaction domains reside within the extracellular region that can mediate lateral propagation of signaling independent of ephrin ligation (Freywald et al., 2002; Janes et al., 2011; Lackmann et al., 1998; Marquardt et al., 2005; Wimmer-Kleikamp et al., 2004). This raises the question of whether these intracellular mutants may still retain some forward signaling function, possibly as a scaffold to the larger signaling complex when coexpressed with other active receptors possessing redundant intracellular signal transduction capabilities. If so, receptor heterooligomerization and retention of some forward signaling capacity is a plausible explanation for the decreased phenotypic severity of intracellular EphB receptor mutants compared to complete receptor knockouts. To distinguish between this possibility and that of a reverse signaling contribution, further in vivo exploration of both hypotheses will be required.
What, then, are the genetic data in support of reverse signaling in these contexts? Studies complementary to the above have created mouse mutants with changes to the intracellular domain of ephrin-Bs, either by replacing the entire domain with β-galactosidase (ephrin-B2LacZ) (Cowan et al., 2004; Dravis et al., 2004; Dravis and Henkemeyer, 2011; Yokoyama et al., 2001), or by making specific point mutations to the phosphorylatable tyrosines or the PDZ binding domain in the highly-conserved region of the cytoplasmic domain. The latter allows more specific assessment of phosphorylation or PDZ-dependent reverse signaling, but does not test other potential modes of reverse signaling (Bush and Soriano, 2009; Davy and Soriano, 2007; Dravis and Henkemeyer, 2011; Makinen, 2005; Xu et al., 2011; Xu and Henkemeyer, 2009). Null loss of function of ephrin-B1, or point mutations abrogating its ability to bind to PDZ-domain containing proteins, results in normal ACp development, but agenesis of the CC (aCC), in a 129S4 genetic background (Bush and Soriano, 2009). Though a recent study reported that Efnb1 null mutant embryos did not exhibit CC defects in a CD1 genetic background, human craniofrontonasal syndrome patients harboring mutations in EFNB1 also exhibit aCC, indicating that although the involvement of Efnb1 is genetic background-dependent in mice, its requirement is conserved across species (Twigg et al., 2004). Efnb2LacZ/LacZ mutant mice exhibited severe defects in the formation of the ACp axon tract, suggesting that ephrin-B2 reverse signaling is important in this context (Cowan et al., 2004). However, this allele has since been demonstrated to result in a gain of function of ephrin-B2 signaling in some contexts (described below), casting some uncertainty on the interpretation of phenotypes observed in these mutants (Zhang et al., 2015). Ephrin-B3 apparently regulates formation of the CC entirely via forward signaling, because complete loss of ephrin-B3 results in severe defects of the CC, whereas loss of the intracellular domain does not (Mendes, 2006). Null loss of ephrin-B3 does not disrupt formation of the AC, indicating it likely does not play a role in this context (Kullander et al., 2001). Together, these results support a major role for EphB forward signaling in formation of the AC and CC, with some reverse signaling contribution from the intracellular domain of B-type ephrins as well.
In some cases, examination of ephrin-B cytoplasmic signaling mutants has made a clear attribution of signaling directionality possible. For example, ephrin-B16FΔV mutant embryos do not have the craniofacial or skeletal defects seen in ephrin-B1null embryos, indicating that reverse signaling by PDZ- or phosphorylation-dependent mechanisms is not necessary for craniofacial and skeletal development (Bush and Soriano, 2009); instead, forward signaling has been implicated in these processes (Bush and Soriano, 2010; Risley et al., 2009). Mice expressing one copy of ephrin-B2F5, in which each of the five phosphorylatable tyrosines in the cytoplasmic tail of ephrin-B2 is mutated to phenylalanine, on a null background (Efnb2−/F5 mice) do not exhibit the early embryonic phenotypes exhibited by Efnb2−/− embryos, indicating that these defects are not caused by lack of ephrin-B2 tyrosine phosphorylation (Davy and Soriano, 2007). Efnb3ΔC/ΔC mice lacking the entire cytoplasmic domain of ephrin-B3 do not have the same corticospinal tract defects as Efnb3−/− mice, indicating that it is the forward signaling aspects of ephrin-B3 signaling that prevents EphA4-expressing axons from crossing the spinal cord midline (Yokoyama et al., 2001).
Recently, a study of Eph/ephrin-mediated cell segregation by our laboratory has implicated unidirectional signaling in tissue separation (O’Neill et al., 2016). Patients with CFNS are heterozygous for mutations in EFNB1, and after random X inactivation, they are mosaic for EPHRIN-B1 expression. This mosaicism leads to aberrant segregation of EPHRIN-B1 expressing and non-expressing cells, which can be demonstrated in Efnb1+/Δ mouse embryos as large patches in the limb bud (Compagni et al., 2003) and secondary palate (Bush and Soriano, 2010; Davy et al., 2006). We showed that ephrin-B1-mediated segregation first occurs in the neural plate neuroepithelium at E8.5, prior to neural tube closure. Through a series of genetic mouse mutants, we demonstrated that mosaic loss of reverse signaling (in Efnb1+/6FΔV embryos) does not result in cell segregation, indicating that mosaicism for ephrin-B1 reverse signaling alone is not sufficient to induce segregation. Mosaicism for loss of reverse signaling in an ephrin-B1 null embryo (in Efnb1Δ/6FΔV embryos) results in cell segregation indistinguishable from that seen in ephrin-B1 heterozygous embryos, indicating that the SH2/PDZ-dependent reverse signaling modes of ephrin-B1 are not required for segregation. Consistent with cell segregation being driven by forward signaling alone, ephrin-B1 heterozygous embryos lacking forward signaling through EphB2 (Efnb1+/Δ; Ephb2LacZ/LacZ) or through both EphB2 and EphB3 (Efnb1+/Δ; Ephb2LacZ/LacZ; Ephb3−/−) lose segregation. This forward signal requires EphB kinase activity, as demonstrated by a chemical genetic approach using Efnb1+/Δ mice also expressing mutant forms of EphB1, EphB2, and EphB3 engineered to be kinase-inhibited by the inhibitor 1-NA-PP1; these embryos exhibited robust segregation in the absence of 1-NA-PP1, but reduced segregation upon 1-NA-PP1 inhibition of kinase signaling. Therefore, reverse signaling through phosphorylation or PDZ mechanisms is neither necessary nor sufficient for cell segregation, whereas forward signaling is required.
Based on mutations to ephrin-B cytoplasmic domains in mice, reverse signaling by B-type ephrins has also been proposed in a variety of additional developmental contexts, including cardiac valve formation (Cowan et al., 2004), secondary palate development, foregut and urorectal morphogenesis (Dravis et al., 2004, p. 200; Dravis and Henkemeyer, 2011), lymphangiogenesis (Makinen, 2005), axon pruning (Xu and Henkemeyer, 2009), and postsynaptic neuron maturation (Xu et al., 2011). Studies using these mutant proteins to interrogate reverse signaling functionality, however, have sometimes resulted in confusing conclusions about the role of the ephrin-B cytoplasmic domain. Ephrin-B2LacZ/LacZ embryos have urorectal and hindgut defects (Dravis et al., 2004), defects in cardiac development (Cowan et al., 2004), tracheoesophageal fistula (TEF), and cleft palate (Dravis and Henkemeyer, 2011). Ephrin-B2ΔV/ΔV embryos (lacking PDZ interactions) and homozygous ephrin-B26YFΔV/6YFΔV embryos (lacking PDZ interactions and tyrosine phosphorylation), however, have no urorectal, tracheoesophageal, or palatal defects, whereas ephrin-B2LacZ/ΔV and ephrin-B2LacZ/6YFΔV embryos exhibit hypospadias (Dravis and Henkemeyer, 2011). This led to the conclusion that PDZ-dependent reverse signaling functions of ephrin-B2, in addition to a not-yet identified function of the intracellular domain, are important for midline closure of the embryo.
Further investigation of the effects of ephrin-B2 cytoplasmic mutants in the context of lymphatic valve development resulted in the surprising discovery that these mutants may affect not only reverse signaling, but also forward signaling. EphB4/ephrin-B2 signaling is critical for lymphatic valve development, which in turn is important for maintaining tissue fluid homeostasis by allowing drainage of interstitial fluid and preventing backflow of lymph (Zhang et al., 2015). Treating early neonatal mice (P1-P2) with antibodies that block ephrin-B2 or EphB4 function results in dilation of lymphatic vessels and defects in lymphatic valve structure soon thereafter, with complete absence of valves and death by postnatal day 8 (P8). However, Zhang and colleagues showed that co-administration of anti-ephrin-B2 with an antibody that is agonistic to EphB4 function, increasing its tyrosine phosphorylation, rescued these defects, suggesting that forward signaling may be sufficient in this context. These defects are similar to those seen in mice expressing a mutant ephrin-B2 lacking its PDZ domain interaction site (ephrin-B2ΔV) (Makinen, 2005) or in Efnb26YFΔV/6YFΔV mice that lack PDZ- and phosphorylation-dependent reverse signaling (Zhang et al., 2015), but EphB4 tyrosine phosphorylation is also significantly reduced, at least in Efnb26YFΔV/6YFΔV embryos, indicating that this ephrin-B2 mutant also affects forward signaling. Treatment with the EphB4 agonist antibody also rescues the lymphatic valve defects in EfnB26YFΔV/6YFΔV mutants, strongly suggesting that it is ephrin-B2-driven EphB4 forward signaling that is required for lymphatic valve development (Zhang et al., 2015). Although the mechanism by which mutating the cytoplasmic domain of ephrin-B2 could lead to defects in EphB4 forward signaling is not yet known, it has been shown that the intracellular region of ephrin-Bs can affect how a signal is processed in EphB expressing cells (Jorgensen et al., 2009). Further, Efnb2LacZ/LacZ mutant mice, with the entire cytoplasmic domain of ephrin-B2 replaced with LacZ, do not have lymphatic valve defects, and in fact have increased EphB4 tyrosine phosphorylation, probably due to tetramerization driven by the β-galactosidase (Zhang et al., 2015). This suggests that the ephrin-B2LacZ allele is hypermorphic for forward signaling rather than a reverse signaling mutant. Zhang and colleagues inhibited EphB4 kinase signaling using either a small molecule inhibitor or mice expressing an analog-sensitive EphB4 kinase mutant and showed that inhibiting EphB4 kinase signaling results in a severe lymphatic valve phenotype, without any manipulation of ephrin-B2 (Zhang et al., 2015). The results from the above experiments suggest that a variety of cytoplasmic mutations in ephrin-B2 disrupt forward signaling; in combination with the data that interruption of forward signaling alone is sufficient to recapitulate the lymphatic valve phenotypes under study, this work calls into question whether or not reverse signaling is involved in this developmental event.
The extent to which studies using mutations of the ephrin-B cytoplasmic domain could lead to incorrect conclusions about the respective roles of forward and reverse signaling in contexts outside of lymphatic development is unclear. It is unlikely that gain-of-function activity of the ephrin-B2LacZ allele is responsible for all of the exhibited phenotypes, because Efnb2LacZ/6FΔV and Efnb2LacZ/ΔV mutants still exhibit urorectal malformations with a penetrance significantly higher than Efnb2LacZ/+ embryos (Dravis and Henkemeyer, 2011). Further, the Efnb2CR conditional loss of function model also exhibits urorectal malformations and TEF, indicating that these phenotypes are loss of function phenotypes that may be caused by loss of reverse signaling (Lewis et al., 2015). Cleft palate, on the other hand, does not occur in these Efnb2CR mice, suggesting that this phenotype in Efnb2LacZ/LacZ mice could be caused by hyperactivation of forward signaling.
The data that have emerged regarding the effects of ephrin-B reverse-signaling mutants on Eph receptor forward signaling suggests that the interplay between forward and reverse signaling will prove to be an even more complicated subject than was previously thought. In determining the contributions of forward and reverse signaling to in vivo phenotypes, it will be essential to examine forward signaling activation in reverse signaling mutants to rule out contributions of cytoplasmic ephrin-B mutants to hyper- or hypo-activation of forward signaling. It should also be noted that very similar mutants to those described in the above section are frequently re-expressed, over-expressed, or mis-expressed to evaluate signaling function in other model systems where knock-ins are not technically straightforward. Nearly all of the potential caveats and complications considered above are also possible in these systems, which additionally must account for the consequences of exogenous expression. Thus, these studies highlight that our understanding of in vivo Eph/ephrin signaling mechanisms is still evolving and will benefit from additional approaches. For example, mutations that selectively block Eph receptor kinase signaling, such as those that use kinase-dead or analog-sensitive kinase mutant Eph receptors, have been recently used to study kinase signaling mechanisms in a number of contexts (O’Neill et al., 2016; Robichaux et al., 2014, 2016; Soskis et al., 2012; Zhang et al., 2015). In the future, it will be valuable to identify effectors downstream of forward and reverse signaling activation relevant to specific contexts and demonstrate that loss of function of these downstream pathways contributes to the observed phenotype.
3. Cell migration
Eph/ephrin signaling is essential to axon guidance, with repulsive interactions between Ephs and ephrins expressed in axons and in their target zone cells mediating correct formation of synapses in many different areas of the developing nervous system, as reviewed elsewhere (Egea and Klein, 2007). Analogous to this role in axonal pathfinding, Ephs and ephrins have also been described as guidance cues that mediate migration of cells over long distances by repeated short-range interactions. Recent studies have reexamined the role of Eph/ephrin signaling in neural crest migration, have demonstrated new interactions between Eph/ephrin signaling and canonical neuronal migration guidance pathways in the brain, and have implicated Eph/ephrin signaling in the migration of cells to establish left-right asymmetry.
3.1 Neural crest migration
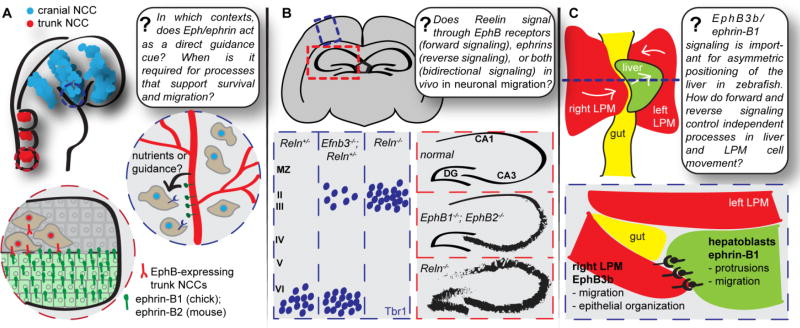
Eph/ephrin signaling has repeatedly been shown to be critical for development of neural crest cells (NCCs). From the earliest stages, Eph/ephrin signaling regulates NCC development in Xenopus. Forward signaling from ephrin-B1 and ephrin-B2 in the dorsal mesoderm inhibits Wnt signaling required to induce NCCs; the Adam13 metalloprotease cleaves ephrin-B1 and ephrin-B2 to relieve this inhibition and allow NCC induction (Wei et al., 2010). Studies in chick and Xenopus have proposed a repulsive mode of neural crest guidance, wherein disruption of Eph/ephrin signaling results in invasion of NCCs into ectopic territories. Using in vitro stripe assays in which soluble ephrin ligand is immobilized in narrow stripes on cover slips, cultured chick or rat NCCs, which express EphB receptors, avoid stripes of ephrin-B protein, instead preferring to migrate alongside (Krull et al., 1997; Wang and Anderson, 1997). In rat and mouse embryos, ephrin-B2 expression is restricted to the caudal half of the somite, whereas in chick embryos ephrin-B1 exhibits this pattern of expression, leading to the idea that caudally-expressed ephrin-B guides EphB-expressing trunk NCC migration to the rostral half of the somite (Figure 2A). Eph/ephrin signaling has been implicated in cranial neural crest guidance as well. In Xenopus, rhombomeric boundaries set up by expression of EphA4 and ephrin-B2 expression in r5 and r4, respectively, extend to migratory NCC populations derived from those rhombomeres, whereas EphB1 is expressed in NCCs destined for branchial arches (BA) 3 and 4. Disruption of EphA4/EphB1 function by overexpression of truncated receptors, or by blinding the NCCs to the position of ephrin-B2 signal by overexpression of ephrin-B2, results in NCC intermingling during migration (Smith et al., 1997). In addition to a role in segmental guidance, Eph/ephrin signaling in Xenopus has also been demonstrated to regulate migration of NCCs along the ventral or dorsolateral pathways. Expression of multiple ephrins along the dorsolateral pathway repels and guides early-migrating NCCs expressing EphB receptors along the ventral pathway. Interestingly, migration of melanoblast NCCs, which also express EphB receptors, is promoted along the dorsoventral pathway by increasing adhesion (Santiago and Erickson, 2002).
Figure 2. Eph/ephrin signaling provides support and guidance for migrating cells during development.
(A) Ephrin-Bs have been implicated in both cranial and trunk neural crest cell (NCC) migration. Recent genetic evidence in mice suggests that the role of ephrin-B2 in cranial NCC migration is secondary to its role in the vasculature, and therefore that ephrin-B2 signaling in this context acts not as a guidance cue, but rather as a supplier of support and nutrients critical to NCC survival. During trunk NCC migration, ephrin-B1 (in chick) or ephrin-B2 (in mouse) is thought to act as a guidance cue for migrating cells, with their expression in the posterior half of the somite restricting migration of EphB-expressing NCCs to the anterior half of the somite. (B) The secreted neuronal guidance cue Reelin interacts biochemically with Eph receptors, and compound EphB receptor mutant mice as well as compound ephrin-B mutant mice have reeler (Reln−/−)-like neuronal migration phenotypes in both the cortex and hippocampus. Although many Eph or ephrin compound mutant phenotypes are not as severe as Reln−/− phenotypes, this raises the intriguing possibility that Eph/ephrin signaling and Reelin signaling may synergize to promote neuronal migration in the cortex and hippocampus. (C) EphB/ephrinB signaling, with EphB3b expressed in the lateral plate mesoderm (LPM) and ephrin-B1 expressed in the hepatoblasts, is essential for correct asymmetric positioning of the liver during zebrafish development. NCC, neural crest cell; LPM, lateral plate mesoderm.
In mouse, genetic disruption of ephrin-B2 also perturbs normal NCC development, with Efnb2null mouse embryos exhibiting both angiogenic remodeling defects and NCC defects that result in abnormal BA development and trunk NCC segmentation (Davy and Soriano, 2007). It has been difficult to assess the exact role of ephrin-B2 in NCC development in vivo, as angiogenic defects lead to early embryonic lethality of Efnb2null embryos. An attempt to separate the role of ephrin-B2 in the vasculature from its contributions to NCC migration resulted in the surprising discovery that rescuing expression of ephrin-B2 only in the vascular endothelium (VE) is sufficient to obtain normal NCC migration and BA development (Lewis et al., 2015). In mice with a conditional rescue of ephrin-B2 in the vasculature in an otherwise ephrin-B2 null embryo (Tie2-Cre; Efnb2CR), not only angiogenesis defects, but also BA morphogenesis and cranial and trunk NCC defects, are rescued. Further, loss of ephrin-B2 specifically in the vasculature, again mediated by Tie2-Cre, resulted in angiogenic and neural crest phenotypes, as well as increased cell death in migrating cranial and trunk NCCs, suggesting that without proper vascular development, NCC survival is compromised. From these studies, it is apparent that in mouse, proper ephrin-B2 expression, or angiogenesis regulated by ephrin-B2, is a requirement for NCC development, and that ephrin-B2 may not have a role in repulsive NCC guidance as previously proposed. The role of the VE in regulating NCC migration, however, remains unclear. EphB4 plays an important role in ephrin-B2 signaling in the context of angiogenesis: Ephb4−/− mice have similar angiogenic remodeling defects to Efnb2null mice, as well as similar NCC and BA morphogenesis phenotypes. However, because EphB4 is not expressed in NCCs, it seems unlikely that signaling to NCCs from ephrin-B2 in the VE is required (Lewis et al., 2015). Even without pinpointing the exact mechanism by which angiogenic remodeling contributes to NCC migration, it seems likely that the role of ephrin-B2 in the process is a secondary one in allowing the vasculature to develop and function normally, which in turn provides an as yet unknown, but essential, support to NCCs. It is possible that ephrin-B2 signaling from the VE may provide a guidance role for NCCs; further studies will be needed to determine whether this is the case, or whether angiogenesis plays a more permissive role in NCC survival. For example, normal angiogenesis may be required for delivery of oxygen, nutrients, or other signaling molecules may be required for NCC survival (Figure 2A) (Lewis et al., 2015). Interestingly, Eph/ephrin signaling may also have a more direct role in NCC survival. Ectopic expression of ephrin-A5-Fc in mouse dorsal neuroectoderm and NCCs, mediated by Wnt1-Cre, led to decreased NCC survival and diminished NCCs of the frontonasal process and BA1 and BA2 (Noh et al., 2014).
Like the trunk NCCs from which they are derived, the sympathetic ganglia (SG) are also segregated to the rostral half of each somite. This organization, however, is not the consequence of early migratory guidance of NCCs, which lose their segregated pattern and intermix along the anteroposterior axis upon arrival at the site of SG formation, but of later re-sorting of NCCs into discrete ganglia following their arrival at sympathetic ganglia target sites (Kasemeier-Kulesa et al., 2005). Live imaging studies in chick have demonstrated that the segregation of NCCs into SG involves repulsive Eph/ephrin signaling wherein expression of ephrin-B1 in the rostral half of the somite expands to the mesoderm, signaling to NCCs expressing EphB2 to drive their condensation into discrete SG (Kasemeier-Kulesa et al., 2006). Disruption of this late repulsive cue disrupts SG formation even when early segmental NCC guidance is maintained due to loss of directionality in their repulsion.
3.2 Neuronal migration
Eph/ephrin signaling plays important roles in development of the mammalian neocortex and hippocampus by regulating radial and tangential neuronal migration. In the cortex, tightly controlled migration of neurons born at the ventricular zone leads to formation of a highly organized, layered structure. As the location of different excitatory neurons with different functions in each layer is essential to the future function of the cortex, newly born neurons at the proliferative ventricular zone of the cortex must migrate radially to a distinct position, with each successive generation of neurons migrating past older-born neurons. In the hippocampus, migration of neuronal precursors is also essential for the differentiation of these neurons to form mature structures. Interestingly, several studies have indicated that Eph/ephrin signaling functions together with Reelin signaling in neuronal guidance in the cortex and hippocampus (Bouché et al., 2013; Catchpole and Henkemeyer, 2011; Sentürk et al., 2011). Reelin is a secreted glycoprotein that regulates neuronal migration and cortical layering, and reeler (Reln−/−) mice have cortical phenotypes including “inside-out” layering, with late-born neurons aberrantly located in deeper layers of the cortex and earlier-born neurons in upper layers. Efnb1/b2/b3 compound knockout mice mimic reeler cortical phenotypes, and loss of one copy of Reelin on an Efnb2−/− or Efnb3−/− background leads to alterations in cortical layering and hippocampal structures similar to those seen in reeler mice, though Reln+/− mice alone are unaffected (Sentürk et al., 2011). Although this suggests that Reelin and ephrin-Bs interact to facilitate correct radial migration of neurons to form the laminated cortex (Figure 2B), these phenotypes seem to depend on the Efnb3 knockout strategy used, as well as the genetic background of the mouse strain (Pohlkamp et al., 2016).
In the hippocampus, loss of forward signaling from ephrin-B1 to EphB2 leads to a reduction in granule cell neurons in the lateral suprapyramidal blade (LSB) of the dentate gyrus, resulting from decreased migration of precursor cells into this area (Catchpole and Henkemeyer, 2011). Notably, EphB2 and ephrin-B1 mutant mice demonstrate a decrease in Reelin expression adjacent to the LSB, suggesting that forward signaling through EphB2 is necessary for Reelin expression to promote migration of precursor cells into the LSB (Catchpole and Henkemeyer, 2011). However, the direct mechanisms by which EphB2/ephrin-B1 influences the expression and secretion of Reelin in the hippocampus are unknown.
Canonical Reelin signaling involves Reelin binding to lipoprotein receptors VLDLR and ApoER2, leading to tyrosine phosphorylation of Dab1, but VLDLR and ApoER2 have no intrinsic kinase activity, which is instead fulfilled by the recruitment of Src-family kinases (D’Arcangelo et al., 1999; Howell et al., 1999). Physical interactions between Reelin, EphB, and ephrin-B proteins have been demonstrated in both the cortex and the hippocampus, suggesting that EphB/ephrin-B signaling may synergize with Reelin signaling to affect neuronal migration. Specifically, Reelin interacts with ephrin-B2 and ephrin-B3 proteins, and stimulation of cultured cortical neurons with Reelin results in clustering of ephrin-Bs (Sentürk et al., 2011). Sentürk and colleagues point to reverse signaling through ephrin-Bs as a “missing link” that recruits Src family kinases to phosphorylate Dab1. Stimulation of cortical neurons with EphB3-Fc results in tyrosine phosphorylation of Dab1, and Efnb1/b2/b3 knockout mice have decreased Dab1 phosphorylation (Sentürk et al., 2011), though genetic evidence supporting the involvement of the ephrin-B cytoplasmic domain in Dab1 phosphorylation has not yet been shown. Reelin also coimmunoprecipitates with recombinant EphB1, EphB2, and EphB3 in cultured neurons and induces EphB tyrosine phosphorylation, proteolytic processing, and cytoskeletal responses in Cos1 cells (Bouché et al., 2013). These effects are independent of the canonical Reelin receptors (Bouché et al., 2013). However, the in vivo relevance of these biochemical interactions to cortical lamination, and the mechanisms of synergy between Eph/ephrin signaling and Reelin signaling, remain uncertain. Loss of EphB2 kinase signaling results in aberrant cell dispersal in the CA3 region of the hippocampus (Figure 2B), demonstrating that EphB forward signaling is required for this in vivo event. However, these studies face a challenge in distinguishing Reelin-to-Eph signaling from ephrin-to-Eph signaling. Bouché and colleagues argue that the hippocampal CA3 defects in compound Ephb1; Ephb2 mutant mice are a result of Reelin-mediated forward signaling, not ephrin-B1-mediated forward signaling, because ephrin-B1 knockout mice have a much milder CA3 phenotype than the Ephb1; Ephb2 mutants, whereas the reeler mouse phenotype is similar to the Ephb1; Ephb2 mutant phenotype (Bouché et al., 2013). However, although neither ephrin-B2 nor ephrin-B3 were found to be expressed in the CA3 region during hippocampal neuronal migration, it is impossible to rule out the contribution of ephrin-B1, or as yet unknown contributions from other ephrins that may bind to and activate EphB1/EphB2. In the future, studies that examine compound EphB receptor/Reelin mutant mice, probe EphB receptor signaling changes in reeler mice, or differentiate the downstream signaling pathways of EphB receptors activated by ephrins vs. activated by Reelin, may shed further light on the in vivo relevance of these interactions. Although the mechanism of Reelin interactions with EphBs or ephrinBs to facilitate neuronal migration remains unclear, it is apparent that Reelin can interact genetically and biochemically with several members of this pathway, demonstrating that Eph/ephrin signaling molecules are able to integrate with Reelin signaling to assist in mediating signaling outcomes that lead to correct positioning of neurons.
Ephrin-B1 also plays an early role in maintenance of the structural integrity of the apical surface of the developing cortex, which is necessary for correct cortical lamination. Maintenance of apical attachment of neural progenitors at the ventricular zone and attachment of neighboring apical progenitors to each other via adherens junctions are regulated by ephrin-B1 signaling, and Efnb1+/− and Efnb1−/− embryos demonstrate abnormal folding of the apical surface of the neuroepithelium (Arvanitis et al., 2013). Efnb1 mutant embryos demonstrate changes in cell-ECM adhesion at the apical surface, with decreases in apical localization, but not mRNA or protein expression of integrin-β1. Notably, cell-cell adhesion, examined via N-cadherin expression, and cell polarity, marked by the apical distribution of β-catenin, are unaffected in these mutants. In ex vivo cortical slices, acute loss of ephrin-B1 results in loss of elongated cell morphology and detachment of cells from the apical surface of the ventricular zone. Ephrin-B1 tyrosine phosphorylation and the PDZ interaction domain of the ephrin-B1 intracellular domain are not necessary for maintenance of apical adhesion, as expression of ephrin-B1 lacking its cytoplasmic domain rescues the cell-ECM adhesion defects (Arvanitis et al., 2013). By synergizing with integrin-β1 signaling to maintain the integrity of the apical surface of the ventricular zone of the cortex, therefore, ephrin-B1 signaling plays a critical role in coordinating the development of the cortex. Here, ephrin-B1 does not serve directly as a guidance cue, but rather plays an indirect role, serving as a structural support system for neural progenitors to allow maintenance of apical adhesion during the early development of the cortex. Future studies defining the mechanisms of Eph/ephrin support of neuronal migration, through Reelin signaling or other pathways, will provide more insight into the roles of this pathway in defining correct cell positioning in the developing brain.
3.3 Guidance of cell migration to establish left-right asymmetry
Eph/ephrin signaling has recently been implicated in establishing left-right asymmetry in the developing zebrafish embryo. The dorsal forerunner cells (DFCs), precursor cells to the Kupffer’s vesicle (KV), the left-right organizer in zebrafish, express EphB4b, while the surrounding mesendodermal cells express ephrin-B2b, and signaling between them maintains DFC cluster integrity as the cells migrate past each other (Zhang et al., 2016). These interactions are mediated by extension of protrusions from DFCs that are then repulsed by the neighboring cells, and loss of EphB4b leads to loss of RhoA and pMLC expression at the DFC-mesendoderm boundary, slowing of this repulsive response, and subsequent dispersal of DFCs, ultimately resulting in a smaller or absent KV and defects in later asymmetric positioning of organs (Zhang et al., 2016). Eph/ephrin signaling is also involved in epithelial-mesenchymal interactions that control asymmetrical positioning of the liver (Cayuso et al., 2016). Cayuso and colleagues demonstrate that EphB3b/ephrin-B1 signaling between the lateral plate mesoderm (LPM) and the hepatoblasts of the forming liver results in hepatoblast repulsion that is critical for correct asymmetric positioning of the liver (Figure 2C), challenging the existing model that the LPM actively pushes hepatoblasts, which are passively corralled into place. Hepatoblasts form long cellular protrusions that are lost in ephrin-B1 morpholino-treated embryos, but increased with loss of direction in ephb3b morphants (Cayuso et al., 2016). Expressing reverse signaling mutant ephrin-B1 proteins in an ephrin-B1-deficient background has varying effects on protrusion formation. Adding ephrin-B16F rescues hepatoblast protrusion formation, whereas adding ephrin-B1ΔV does not. The authors conclude that PDZ-dependent reverse signaling mediates protrusion formation, independent of tyrosine phosphorylation of the cytoplasmic domain of ephrin-B1, to permit hepatoblast movement away from the midline and facilitate asymmetric positioning of the liver. Though, no examination of possible changes to forward signaling in LPM cells was made, ectopic expression of EphB3bΔICD (lacking the intracellular domain) in the left LPM where there is little endogenous EphB3b, resulted in movement of the hepatoblasts to the right side, consistent with a role of reverse signaling in repulsion of hepatoblasts from the LPM and establishing the asymmetric positioning of the liver. Further studies of the role of Eph/ephrin signaling in liver positioning, as well as in the establishment of asymmetry in other organ systems, will elucidate the signaling mechanisms involved in these processes.
4. Tissue separation
Establishing and maintaining separation between tissues during development is a multifaceted process requiring patterning to establish cell fate, segregation of different cell types to form boundaries, and prevention of cell migration and intermingling across these boundaries. A classical view of Eph/ephrin-mediated tissue separation involves reciprocal expression of Ephs and ephrins in separate compartments, with bidirectional signaling across the Eph/ephrin expression boundary preventing intermingling (Kania and Klein, 2016). This model stems from studies of Eph receptors and ephrins in the organization of the developing embryo: in the brain, spinal cord, branchial arches, limb buds, and somites, Eph receptor and ephrin expression appeared mutually exclusive and restricted to defined domains, leading to the proposal that Ephs may encounter ephrins only at the boundaries between these domains (Gale et al., 1996). Further study suggested that reciprocal expression can contribute to tissue separation and maintenance of boundaries between morphologically defined compartments, such as the rhombomeres of the zebrafish hindbrain (Xu et al., 1999). More recent evidence demonstrates the existence of more complex patterns of Eph/ephrin expression and signaling during development (Barrios et al., 2003; O’Neill et al., 2016; Rohani et al., 2014). Here, we review boundary formation in early neural development, gastrulation and somitogenesis, where Eph/ephrin signaling has long been studied as a mediator of boundary formation and maintenance. Recent studies explore more complex Eph/ephrin expression patterns and signaling, providing evidence for a more nuanced role of Eph/ephrin signaling at tissue boundaries that depends both on context and on the combinatorial expression of Eph receptors and ephrins in the tissue in question. In each of these situations, Eph/ephrin signaling is a downstream effector of boundary formation and maintenance, translating patterning information to physical separation. The mechanisms by which tissue separation is achieved are still under study, but include changes to adhesion, cytoskeletal dynamics, cellular repulsion, and cell migration.
4.1 Tissue separation during early neurodevelopment
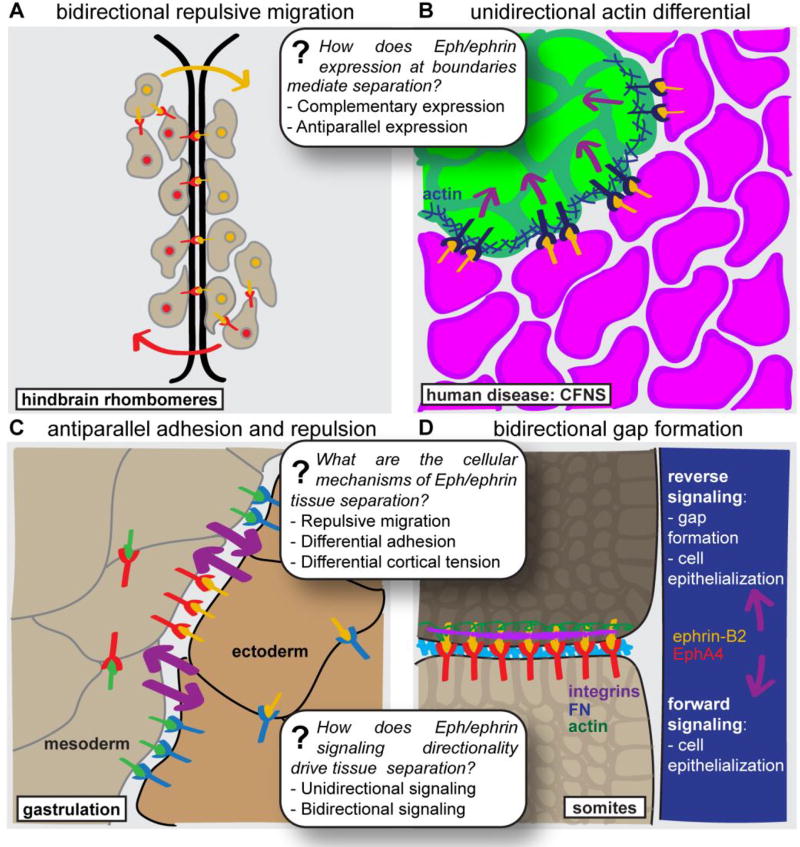
The first discovery of Eph/ephrin signaling in cell segregation and boundary formation was in the developing nervous system. Segmentation of the vertebrate hindbrain during development leads to the formation of compartments termed rhombomeres, which demarcate boundaries between different areas of the hindbrain that will eventually develop into different adult structures. Importantly, the organization of the rhombomere separates precursor cells of different neuronal subtypes and contributes to the later organization of hindbrain neurons (Moens and Prince, 2002); inappropriate cell mixing must therefore be prevented to preserve normal adult hindbrain function. Eph receptors are expressed in odd-numbered rhombomeres, and ephrins are expressed in even-numbered rhombomeres, leading to the proposal that signaling mediates repulsive interactions at rhombomere boundaries (Figure 3A) (Xu et al., 1999) or differential adhesion between cells of different rhombomeres (Cooke et al., 2005; Kemp et al., 2009).
Figure 3. Eph/ephrin signaling mechanisms in developmental tissue separation.
(A) A classical view of Eph/ephrin signaling mechanisms in the rhombomeres of the zebrafish hindbrain involves complementary expression of Eph receptors and ephrins in adjacent rhombomere compartments, with bidirectional signaling mediating repulsive migration of Eph-expressing cells away from the ephrin-expressing compartment and vice versa. (B) Mosaicism for ephrin-B1 expression in the congenital craniofacial disease craniofrontonasal syndrome (CFNS) leads to aberrant cell segregation, which is characterized by unidirectional signaling from ephrin-B1 expressing cells (magenta) to ephrin-B1 non-expressing (EphB-expressing) cells (green), leading to upregulation of actin and formation of an actin cable in the EphB-expressing cells. (C) In the Xenopus gastrula, the mesoderm migrates along the ectodermal blastocoel roof without integrating into the ectoderm. This simultaneous migration and separation is mediated by antiparallel Eph/ephrin forward signaling across the ectoderm-mesoderm boundary, leading to repeated attachment and detachment of the migrating mesoderm cells. (D) Bidirectional signaling between ephrin-B2 in the posterior region of a newly formed somite and EphA4 in the anterior region of a forming somite contributes to somite gap formation and subsequent cell epithelialization at the somite border. While gap formation requires reverse signaling but not forward signaling, cell epithelialization requires both reverse signaling and forward signaling through direct or cell-non-autonomous mechanisms. CFNS, craniofrontonasal syndrome; FN, fibronectin.
In the developing zebrafish hindbrain, gene expression boundaries become morphological boundaries, which manifest as shallow indentations between rhombomeres by 15 hours post fertilization. Morpholino knockdown of EphA4a, which is usually expressed in rhombomeres r3 and r5, resulted in jagged edges to rhombomere boundaries, with krx20-expressing cells from r3 and r5 invading into the adjacent even-numbered rhombomeres (Calzolari et al., 2014). Invading cells do not immediately change their fate, suggesting that Eph/ephrin signaling acts downstream of patterning cues, and indeed, Eph/ephrin expression patterns in rhombomeres are established in part through the transcription factors Krox20 and HoxB4. In zebrafish, Krox20 binds and drives expression from an r3/r5 enhancer element harbored by EphA4 and deletion of this binding site leads to loss of r3/r5 enhancer activity, suggesting that EphA4 is directly regulated by Krox20 (Theil et al., 1998). Krox20 also directly regulates the expression of multiple Hox genes, suggesting a means of coupling downstream A-P cell fate specification with physical separation of rhombomeres (Theil et al., 1998). More recently, it has been suggested that coupling cell fate with tissue separation in the hindbrain may entail a more complicated hierarchy that also depends on A-P specification; indeed, loss of Hox genes disrupts not only A-P identity, but also rhombomere boundaries (Prin et al., 2014). In mouse and chick, Hoxb4 and Hoxd4 share their anterior expression border at the r6/r7 boundary, and loss of both resulted in loss of the r6/r7 boundary. In addition, widespread misexpression of Hoxb4 also disrupted rhombomere boundaries, and mosaic misexpression of Hoxb4 or any of several other Hox genes caused aberrant segregation within a rhombomere, indicating that a differential in Hoxb4 (and its targets) drives rhombomere formation. One such target may be EphA7: upon loss of Hoxb4 and Hoxd4, EphA7 was upregulated caudal to the site of the r6/r7 border, while ectopic expression of Hoxb4 resulted in EphA7 repression rostral to this border. Functional evidence for a requirement of EphA7 in r6/r7 boundary formation is so far lacking, however, and complementary ephrin expression at this boundary has not been identified. Mosaic expression of several different Hox proteins induced cell segregation, but the segregating effect of Krox20 expression predominated, suggesting that a hierarchy of combinatorial regulation of Eph/ephrin genes by multiple transcription factors couples A-P cell fate specification to tissue separation.
As the morphological changes that define rhombomere boundaries take place, actin and myosin II accumulate to form a cable between rhombomeres (Calzolari et al., 2014), suggesting a mechanism by which Eph/ephrin signaling may direct both cell segregation and the formation of a physical barrier to cell intermingling. Work in Drosophila, Xenopus, and mouse, as well as in human cell culture, similarly support the broad idea that regulation of actomyosin contractility contribute to Eph/ephrin-mediated segregation (Aliee et al., 2012; Monier et al., 2010; O’Neill et al., 2016; Rohani et al., 2011; Umetsu et al., 2014). In the rhombomere, cells can move past a boundary via cell division but are rapidly pushed back into their proper position, perhaps by the elastic boundary formed by the actomyosin cable (Calzolari et al., 2014). The actomyosin cables appear to colocalize with EphA4a in r3/r5, and disrupting actin or myosin II using ROCK inhibitors or Blebbistatin results in dismantling of the cables and invasion of r3/r5 cells into even-numbered rhombomeres, creating jagged rhombomere boundaries. Conversely, stabilizing phosphorylated myosin by treatment with calyculin A results in even more pronounced rhombomere boundaries. Importantly, morpholino knockdown of EphA4a disrupts actomyosin cable formation in a similar manner to ROCK inhibition or Blebbistatin treatment, and the jagged boundaries that form can be rescued by treatment with calyculin A (Calzolari et al., 2014), indicating that EphA4a acts upstream of myosin to modulate formation of this physical boundary. Further, ectopic activation of EphA4a in even-numbered rhombomeres results in accumulation of actomyosin in EphA4a-expressing cells only if they are surrounded by ephrin-expressing cells, suggesting that Eph/ephrin signaling acts to upregulate actomyosin. Boundary sharpening appeared to begin before actomyosin enrichment could be observed, however, (Calzolari et al., 2014), suggesting that additional mechanisms may be at play in initial rhombomere boundary sharpening.
Eph/ephrin signaling also acts downstream of patterning cues during forebrain development in zebrafish. During neurulation, the cells of the prospective eye field evaginate to form the optic vesicles, which must remain separated from the cells of the prospective telencephalon and diencephalon for proper eye development. Eph receptor expression in the telencephalon and ephrin expression in the eye field are essential to this process, as morpholino knockdown or misexpression of these molecules results in delayed optic vesicle evagination and aberrant localization of eye field cells within the telencephalon (Cavodeassi et al., 2013). In addition, misexpression of the normally telencephalon-restricted EphB4a in eye field cells causes them to segregate into the telencephalon while retaining eye field markers, indicating that Eph/ephrin expression determines their movement regardless of fate (Cavodeassi et al., 2013). In zebrafish mutants that have lost expression of the eye field transcription factor Rx3, Eph receptor expression expands into the eye field while ephrin expression remains normal, placing Eph/ephrin signaling downstream of patterning (Cavodeassi et al., 2013). Although the boundary between the eye field and the telencephalon is enriched in both F-actin and pMLCII, this study does not investigate whether Eph/ephrin signaling directly regulates the cytoskeleton in this context.
In addition to maintaining normal tissue boundaries during development, Eph/ephrin signaling can also mediate pathogenic cell segregation in cells of the early neural plate neuroepithelium when the normal expression of pathway members is disrupted, as was recently demonstrated in the congenital disease craniofrontonasal syndrome (Niethamer et al., 2017; O’Neill et al., 2016). Craniofrontonasal syndrome (CFNS) is caused by mutations in EFNB1, encoding EPHRIN-B1, which is expressed from the X chromosome. As a result, female CFNS patients are heterozygous for EFNB1 mutations and demonstrate mosaic expression of EPHRIN-B1. Mosaicism for ephrin-B1 expression leads to aberrant segregation of cells in the early neural plate neuroepithelium in Efnb1+/− mice to generate large patches of ephrin-B1 expression and non-expression (O’Neill et al., 2016). These patches are also apparent in later tissue structures, such as the palate (Bush and Soriano, 2010) and limb mesenchyme (Compagni et al., 2003) and may contribute to craniofacial and limb phenotypes in CFNS patients, as segregation also occurs in human iPSC-derived neuroepithelial cells (Niethamer et al., 2017). Importantly, this process also requires Eph/ephrin modulation of the cytoskeleton (Figure 3B); genetic disruption or pharmacological inhibition of ROCK dramatically decreased pathogenic cell segregation. Interestingly, genetic interaction studies also indicated that loss of Cdc42 or Rac1 function did not reduce segregation, consistent with the possibility that actomyosin contractility per se, and not cell migratory capacity more generally, may drive segregation.
4.2 Germ layer separation during gastrulation
Gastrulation represents one of the earliest tissue separation events in the embryo. The propensity for separation between cells of the germ layers is so strong that in the classic experiments of Townes and Holtfreter, even when cells of the amphibian germ layers were dissociated into single cells and forcibly mixed, they segregated from one another in culture to re-aggregate in groups of like cells (Townes and Holtfreter, 1955). Eph/ephrin signaling is required to maintain the boundary between the ectoderm and mesoderm during Xenopus gastrulation. Recent studies of the formation and maintenance of these boundaries have added a layer of complexity to our understanding of Eph/ephrin regulation of these early tissue separation processes.
In the Xenopus gastrula, the mesoderm translocates across the ectodermal blastocoel roof (BCR), producing a paradox whereby mesoderm cells must be prevented from integrating into the ectoderm as they adhere to it while migrating. This process can be reconstituted in vitro using explant culture experiments, and live imaging of the mesoderm-ectoderm explant boundary reveals fast cell detachment and reattachment events, which may enable the adhesive contacts that mediate the collective migration of the mesoderm along the BCR, while preventing intermingling (Rohani et al., 2011). Multiple EphB and ephrin-B proteins are expressed in both the mesoderm and the ectoderm, and morpholino knockdown of ephrin-B1, ephrin-B2, or EphB4 in either the mesoderm or the ectoderm leads to increased integration of mesoderm cells into the BCR, suggesting a more complex situation than in the classical complementary expression model. Competitive inhibition of forward signaling by overexpression of truncated EphB4 resulted in diminished separation, and while combinatorial morpholino knockdown of ephrin-B expression in one explant and EphB expression in the opposing explant did not further decrease segregation, loss of both ephrin-B and EphB expression in the same explant does. These data have led to the conclusion that ectoderm-mesoderm separation is likely governed by multiple antiparallel forward signaling pathways across the boundary (Figure 3C) (Rohani et al., 2011, 2014). Addition of preclustered ephrin-B-Fc fusion proteins to activate forward signaling can rescue attachment-detachment cell behaviors and separation from ephrin-B knockdown mesoderm or endoderm, further indicating that activation of Eph receptors in both tissues mediates segregation. This result is surprising in that it suggests that positional information conferred by cellular ephrin signaling within each cell population may not be important; activation of Eph receptor signaling in all cells in the explant results in tissue separation only at the explant boundaries. It seems, therefore, that activation of Eph receptor signaling in either population renders the populations somehow less miscible. The outcome of Eph/ephrin signaling across the ectoderm-mesoderm boundary is at least partly mediated by activation of RhoA and Rac and results in enrichment of F-actin at the boundary (Rohani et al., 2011). In contrast to reciprocal Eph/ephrin expression, these data demonstrate that more complex mechanisms involving multiple coexpressed Ephs and ephrins may be required to localize cytoskeletal and cell behavioral changes to a boundary. These results also indicate that unidirectional signaling is sufficient to produce separation, although in this case it is required in both tissues (Rohani et al., 2011).
If Ephs and ephrins are expressed throughout both the ectoderm and mesoderm, how is tissue separation restricted only to their interface? Whereas complete specificity of Eph/ephrin pair binding, with signaling only at the boundary, is unlikely based on known receptor-ligand affinities, completely promiscuous binding would result in repulsion within both the ectoderm and mesoderm, leading to complete dissolution of the tissue. Instead, work by Rohani and colleagues suggests that an intermediate situation exists, in which antiparallel signaling between complementary pairs of Ephs and ephrins creates the strongest signaling interface at the heterotypic mesoderm-ectoderm boundary (Rohani et al., 2014). Importantly, although complete specificity is not necessary for separation in this model (both ephrin-B2 and ephrin-B3 can signal through EphA4, for example), neither are the Ephs and ephrins functionally redundant. Only ephrin-B2 signals through EphB4, and the phenotypes of morpholino knockdown of one Eph/ephrin family member are not rescued by expression of another. Further, chimeric Eph receptors composed of the extracellular domain of one receptor and the intracellular domain of another can only rescue depletion of the Eph whose extracellular domain they possess, indicating that specificity depends on differential Eph/ephrin binding occurring at the boundary, which may activate different downstream signaling cascades. A baseline level of adhesion between homotypic cell pairs of the ectoderm and mesoderm, provided by cadherin expression, is required to maintain the homotypic tissue cohesivity required for border sharpening (Rohani et al., 2014; Taylor et al., 2017). Whether homotypic repulsion is simply overcome by differential adhesive forces provided by cadherins, or whether other mechanisms, such as cis-inhibition of signaling, also contribute to blunting homotypic signaling outcomes, is not yet known.
An important remaining question involves the mechanisms that establish and maintain the differential expression of Eph and ephrin proteins in different germ layers. It is possible that transcription factor patterning alone maintains Eph/ephrin expression domains, but transcription factors that affect Eph/ephrin expression in the Xenopus ectoderm and mesoderm are not yet known. However, during gastrulation, Eph/ephrin protein stability is regulated through degradation of ephrin-B1 by ubiquitination, which is mediated by Smad ubiquitin regulatory factors (Smurfs). Interactions between ephrin-B1 and Smurf2 lead to ephrin-B1 ubiquitination and degradation, whereas interactions between ephrin-B1 and Smurf1 prevent its interaction with Smurf2, forestalling degradation (Hwang et al., 2013). Smurf2-mediated degradation of ephrin-B1 prevented separation between the endoderm and mesoderm, but Smurf1 binding to ephrin-B1 inhibits this interaction to allow separation. Loss of separation upon loss of Smurf1 can be rescued by subsequent knockdown of Smurf2 (Hwang et al., 2013). This reveals that modulating protein stability is one mechanism by which ephrin-B1 can be regulated to eventuate tissue separation during gastrulation.
4.3 Somitogenesis
The formation of somites is a key step in the axis elongation and segmentation of the vertebrate embryo. The cells of the somites are precursors to vertebral and rib structures, as well as to skeletal muscle and dermis of the skin. Segmentation must be tightly regulated to ensure that segment number is strictly maintained, which is essential to further normal development; although the number of somites may vary between species, it is generally fixed within a species (Bénazéraf and Pourquié, 2013). Somites form with a defined period, the length of which is dependent on the species (Bénazéraf and Pourquié, 2013). To achieve this, Wnt, FGF, and Notch signaling pathways are activated in pulses in the presomitic mesoderm (PSM), leading to a “traveling wave” of mRNA expression of their transcriptional targets. Many of these targets encode negative-feedback inhibitors of Wnt, FGF, and Notch signaling, which may explain the periodic control of signaling through these pathways (Bénazéraf and Pourquié, 2013). Downstream of this feedback loop, expression of segmentation genes such as Mesp2 is seen in the PSM of the future somite, preceding the morphological changes that mediate somite boundary formation.
After the future somite is defined by patterning of segmentation genes, intercellular signaling is required to translate these cues into the morphogenetic processes that result in boundary formation and separation. Several Eph receptors and ephrins are expressed in the somites in developing mouse embryos (Gale et al., 1996), and expression of ephrin-A1, ephrin-B2, and EphA4 in segmented patterns in the zebrafish PSM suggested that these signaling molecules may play a role in somite formation (Durbin et al., 1998). Indeed, overexpression of truncated Eph or ephrin proteins, which serve a dominant-negative function by binding their cognate ephrin or Eph and blocking downstream signaling, disrupted somite boundary formation, resulting in absent or abnormally-shaped somites (Durbin et al., 1998). Perturbation of Eph/ephrin signaling did not affect segmental prepatterning, but downstream markers of somite differentiation reflected the organization of the ectopic somite, indicating that Eph/ephrin signaling mediates the translation of segmental prepattern into physical somite separation. Activation of Eph/ephrin signaling is sufficient for boundary formation, because exogenous expression of EphA4 in zebrafish fss− mutants, which lack segmental expression of several Eph/ephrins and fail to form somites, was sufficient to induce somite boundary formation at the interface between clusters of EphA4-expressing and –nonexpressing cells (Barrios et al., 2003; Durbin et al., 2000). Separation was also induced when cells expressed a truncated EphA4 that lacks the intracellular tyrosine kinase domain, indicating that tissue separation can be induced in the absence of kinase signaling in this context (Barrios et al., 2003). These data suggest that Eph/ephrin signaling may be directly involved in the morphogenetic processes that occur at the somite boundary, which include the formation of an indentation or furrow between adjacent somites; mesenchymal-to-epithelial transition of cells at either side of the boundary, which assume a columnar shape; accumulation of adhesion complex components, such as beta-catenin, at the apical pole of the cell; basal movement of cell nuclei towards the somite boundary; and apical movement of centrosomes. Whereas initial gap formation requires reverse signaling, several of the subsequent epithelialization changes require forward signaling through either direct or non-cell-autonomous means (Barrios et al., 2003).
Later work in chicken embryos suggests that both forward and reverse Eph/ephrin signaling may act in initial formation of the somite gap (Watanabe et al., 2009). In a gap-inducing assay, transplantation of a cMeso-1-expressing explant into the PSM of a host chick embryo results in upregulation of EphA4 and formation of a gap at the cMeso-1 expression boundary (Watanabe et al., 2009). Likewise, overexpression of EphA4 or ephrin-B2 is sufficient to induce an ectopic gap. Overexpression of EphA4 lacking its intracellular domain also creates an ectopic gap, indicating that the intracellular domain of EphA4 is dispensable for somitic gap formation. Consistent with this, overexpression of ephrin-B2 lacking its intracellular domain or ephrin-B2YF (lacking three phosphorylatable tyrosines) does not induce an ectopic gap, indicating that phosphorylation-dependent ephrin-B2 reverse signaling is necessary for gap formation (Watanabe et al., 2009). Overexpression of constitutively active Cdc42 with ephrin-B2 negates the ability of ectopic ephrin-B2 expression to induce an ectopic somite gap, but suppression of Cdc42 alone is not sufficient to induce the gap, though it is sufficient to rescue cell epithelialization. Inhibition of Cdc42 in conjunction with overexpression of ephrin-B2YF rescues both gap formation and cell epithelialization (Watanabe et al., 2009). The authors conclude that tyrosine phosphorylation of ephrin-B2 likely regulates Cdc42 to enable somite gap formation. Although it is clear that some portion of the cytoplasmic domains of both Ephs and ephrins are required for various aspects of the somite gap formation and cell epithelialization process, the consequences of these mutations must be considered in light of new knowledge of the effects of ephrin cytoplasmic mutants on forward signaling and much remains to be learned about the underlying mechanism. In particular, it remains to be determined whether somites, like the ectoderm and mesoderm in Xenopus gastrulation, require antiparallel forward signaling in addition to bidirectional signaling across a boundary.
Changes in adhesion molecules and extracellular matrix interactions are key outcomes of Eph/ephrin signaling to promote somite separation. As somites form and boundary cells undergo MET, a fibronectin (FN) matrix is assembled along the somite boundary. Both FN and its receptor, the heterodimer Integrinα5β1, are required for somite formation (Koshida et al., 2005). Live imaging and genetic studies in transgenic zebrafish expressing the Itgα5-GFP fusion protein show that Itgα5 clustering occurs along the basal side of cells during formation of the somite border followed by the emergence of a FN matrix (Jülich et al., 2009). “Inside-out” signaling (that is, cellular signaling to integrins resulting in clustering and crosslinking of FN to form a matrix), rather than “outside-in” signaling (signaling from accumulated FN to the integrins inducing integrin clustering) is therefore likely required for somite separation (Jülich et al., 2009). Reverse signaling through EphA4/ephrin-B2a induced Itgα5 clustering, FN matrix assembly, and the formation of “actin belts” in mosaic experiments, implicating Eph/ephrin signaling as a candidate regulator for inside-out activation of Itgα5 in somite separation (Jülich et al., 2009). However, the signaling mechanisms by which ephrin-B2 reverse signaling might drive Itgα5 clustering remain unknown.
4.4 Cellular mechanisms of segregation
In each of these systems, a common underlying question exists regarding the cellular mechanisms of segregation mediated by Eph/ephrin signaling. Generally, cell segregation was originally hypothesized to result from differences in affinities between two cell types that could be driven by quantitative or qualitative differences in adhesiveness conferred by distinct constellations or levels of adhesion molecule expression (Nose et al., 1988; Steinberg, 1970; Townes and Holtfreter, 1955). The differential adhesion hypothesis (DAH) proposes the specific case in which a quantitiative difference in cell adhesion between two cell populations drives the segregation of randomly-migrating cells (Steinberg, 1970). Indeed, multiple studies have implicated direct regulation of cell adhesion by Eph/ephrin signaling as a driver of cell segregation. In the intestinal epithelium, EphB activation by ephrin-B1 results in the recruitment of the ADAM10 metalloproteinase, which cleaves E-cadherin and therefore presumably results in differential adhesion at the Eph/ephrin signaling interface (Cortina et al., 2007; Solanas et al., 2011).
Eph/ephrin-mediated segregation is more widely attributed to a “repulsive” mechanism, though the cell biological definition and biophysical consequences of “repulsive” Eph/ephrin cellular interactions are varied. In many contexts, Eph/ephrin signaling guides cell migration by a repulsion mechanism analogous to axon guidance in which cellular collapse and disengagement of Eph- and ephrin-expressing cells is followed by directional migration, as in contact inhibition of locomotion (Astin et al., 2010; Pasquale, 2005; Poliakov et al., 2004). In cell segregation, this guidance activity would result in a repeated redirection of cells away from their heterotypic partners, resulting in segregation of the heterotypic populations by a trial and error process that would be predicted to result in a greater distance travelled over the course of segregation compared to other models of segregation. Indeed, in cell segregation assays in low density HEK293 cell culture, EphB2-expressing cells exhibit collapse and directional migration away with an increased migration speed and travel a greater total distance than cells not undergoing segregation (O’Neill et al., 2016; Poliakov et al., 2008; Taylor et al., 2017). However, at high density, EphB2 cells undergoing segregation do not exhibit an increased distance travelled, probably due to constraints imposed by the confluent culture conditions (O’Neill et al., 2016). Nevertheless, cells segregate robustly at high density, consistent with the fact that cell segregation in the embryo is a behavior that occurs at high cell densities, which leads to the question of whether directional migratory guidance is required for Eph/ephrin cell segregation in the embryo (O’Neill et al., 2016; Taylor et al., 2017).
A separate model, the differential interfacial tension hypothesis (DITH), has proposed that differences in cortical tension, driven primarily by actomyosin contractility, oppose cell adhesion and drive differences in the ability of cells to establish and maintain stable contacts (Brodland, 2002; Krieg et al., 2008). Based on elevated Lifeact-RFP signals in EphB2 cells during live imaging of cell segregation, and on the apparently highly conserved involvement of actomyosin contractility in cell segregation, we previously proposed that cellular collapse upon ephrin-B activation of Eph receptor signaling may result in a change in cortical tension, leading to minimization of heterotypic contacts in favor of homotypic contacts (O’Neill et al., 2016). In cases where Eph and ephrin populations begin fully intermixed, as in cell mixing assays or in CFNS, the “signaling interface” initially includes all EphB2 cells. In other situations, such as Xenopus gastrulation, however, the signaling interface is limited to a patterned interface where Eph/ephrin signaling sharpens and maintains the existing boundary. A recently proposed heterotypic interfacial tension (HIT) model that relates to this specific case observes that there need not be differences in cortical tension or homotypic adhesion between the entirety of two populations if local differences in these properties occur specifically at heterotypic cell contacts (Canty et al., 2017). Such a local difference would, of course be the case in boundaries generated by Eph/ephrin signaling, which relies on cell contact for signaling. Interestingly, atomic force microscopy to infer cortical tension showed that in fact ectoderm cells were stiffer than mesoderm cells, but levelling these differences by myosin depletion in the ectoderm or overexpression of constitutively active Rho in the mesoderm had no effect on separation. Similarly, a dissociation assay indicated that ectoderm cells were more adherent than mesoderm cells, and in this case, levelling these differences decreased separation only modestly. These data led to the conclusion that tissue separation in Xenopus gastrulation is governed by local Eph/ephrin-mediated repulsion leading to reduced contact at the heterotypic interface, though whether cortical tension is changed specifically at that interface remains untested (Canty et al., 2017).
In contrast, at the Xenopus notochord-PSM boundary, Eph/ephrin signaling may regulate both adhesion and cortical tension (Fagotto et al., 2013). Cadherin clustering at interfaces between heterotypic notochord-PSM cell pairs at the boundary is less pronounced than that at interfaces between homotypic cell pairs, which possess larger cadherin clusters. Smaller or absent cadherin clusters at the heterotypic interface allow for cell protrusions and blebbing at the boundary. This cadherin pattern is dependent on myosin, which in turn depends on Eph/ephrin signaling between notochord and PSM cells at the boundary (Fagotto et al., 2013). This suggests that separation at this boundary does not depend on differing levels of expression of cadherin complexes between cells of the different tissue types, but rather on differences in recruitment at different cell interfaces. Differences in heterotypic and homotypic Eph/ephrin signaling therefore result in differential cell contractility at the notochord-PSM boundary, with protrusions and blebbing of the boundary cells, which then inhibits cadherin clustering between heterotypic cell pairs.
Importantly, these studies reinforce the idea that a boundary cannot be defined simply by examining the adhesive or contractile properties of each individual tissue. Instead, the interaction between heterotypic cell pairs at the boundary must somehow differ from that of homotypic cell pairs within each individual tissue. This requires an intricately regulated interface with many small interactions between cells of each tissue, resulting in dynamic changes to the cytoskeleton and to adhesion complexes that mediate boundary maintenance. The Eph/ephrin signaling family, with its large variety of molecules and complex regulatory abilities, is a perfect fit for this role. Further investigation of this complex interplay at other embryonic boundaries will reveal the mechanisms by which Eph/ephrin signaling mediates tissue separation in different contexts.
5. Conclusion
It is readily apparent that Eph/ephrin signaling acts to regulate cell movement and positioning to effect tissue morphogenesis in a wide variety of developmental processes (Table 1), perhaps unsurprising considering the expression of pathway members in almost every tissue during development. Eph/ephrin signaling is unique among major signaling pathways that direct developmental processes on a large scale, in that it generally appears to have relatively little influence on establishing transcription of downstream target genes in the contexts studied so far. Instead, the members of this pathway act more directly to define tissue architecture by acting as a “middleman” between upstream transcription factor patterning and the cell physical and behavioral changes required to direct cells to their correct positions. Specifically, a major role for this pathway has been identified in regulation of cytoskeletal changes. This mediation of cellular changes downstream of transcription factor expression can be aided by the ability of Ephs and ephrins to signal in both the receptor and ephrin-expressing cells, and is essential for Eph/ephrin signaling to mediate cell migration and tissue separation. In this review, we have summarized a number of challenges addressed by recent developments in the field: these include analysis of the complexity of signaling directionality and signaling mechanisms in vivo, identification of new contexts for Eph/ephrin regulation of cell migration, the role of combinatorial expression of Ephs and ephrins in tissue separation, and the role for differing cellular mechanisms of segregation to effect tissue separation in a variety of contexts. The results of these recent studies have deepened our understanding of how Eph/ephrin signaling regulates cell position and have made clear the need for continuing studies in these areas to fully understand how this complex pathway regulates developmental morphogenesis.
Table 1.
Eph/ephrin relationships and roles in cell positioning in the developing embryo.
| Signaling partner(s) |
Role in cell positioning | Signaling directionality |
References | |
|---|---|---|---|---|
| ephrin-B1 | EphB1, EphB2, EphB3 | Axon guidance of the corpus callosum | Forward Reverse - ? | (Henkemeyer et al., 1996; Mendes, 2006; Orioli et al., 1996; Robichaux et al., 2016) |
| EphB2, EphB3 | Craniofacial and skeletal development | Forward | (Bush and Soriano, 2010; Niethamer et al., 2017; O’Neill et al., 2016) | |
| EphB2, EphB3 | Cell segregation | Forward | (O’Neill et al., 2016) | |
| EphB2 | Sorting of neural crest cells into sympathetic ganglia | Forward | (Kasemeier-Kulesa et al., 2005) | |
| EphB2 | Apical attachment of neural progenitor cells | Forward | (Arvanitis et al., 2013) | |
| EphB1, EphB2 | Migration of precursor cells in the hippocampus | Forward* | (Bouché et al., 2013; Catchpole and Henkemeyer, 2011) | |
| EphB3 | Cortical neuronal migration | Reverse | (Sentürk et al., 2011) | |
| EphB3 | Asymmetric liver positioning | Bidirectional | (Cayuso et al., 2016) | |
| EphA4, EphB2 | Xenopus ectoderm-mesoderm separation during gastrulation | Forward | (Rohani et al., 2014) | |
| ephrin-B2 | EphB1, EphB2, EphB3 | Axon guidance of the anterior commissure | Reverse - ? | (Cowan et al., 2004; Robichaux et al., 2016) |
| EphB2, EphB3, EphB4 | Midline closure of the embryo | Reverse - ? | (Cowan et al., 2004; Dravis et al., 2004; Dravis and Henkemeyer, 2011) | |
| EphB3 | Cortical neuronal migration | Reverse | (Sentürk et al., 2011) | |
| EphB4 | Lymphatic valve development | Forward Reverse - ? | (Makinen, 2005; Zhang et al., 2015) | |
| EphB4 | Neural crest migration (indirect); angiogenic remodeling | Forward | (Davy and Soriano, 2007; Lewis et al., 2015) | |
| EphB4 | Dorsal forerunner cell migration to form the Kupffer’s vesicle | Bidirectional - ? | (Zhang et al., 2016) | |
| EphB2, EphB4 | Xenopus ectoderm-mesoderm separation during gastrulation | Forward | (Rohani et al., 2011, 2014) | |
| EphA4 | Somite formation | Reverse | (Jülich et al., 2009; Watanabe et al., 2009) | |
| ephrin-B3 | EphB1, EphB2, EphB3 | Axon guidance of the corpus callosum | Forward | (Kullander et al., 2001; Mendes, 2006) |
| EphB3 | Cortical neuronal migration | Reverse | (Sentürk et al., 2011) | |
| EphA4 | Axon guidance in the spinal cord | Forward | (Yokoyama et al., 2001) | |
| EphA4 | Xenopus ectoderm-mesoderm separation duringgastrulation | Forward | (Rohani et al., 2014) |
Bouché et al. also propose a role for Reelin-to-Eph forward signaling in hippocampal progenitor migration in the CA3 region.
The complexity of Eph/ephrin signaling has led to longstanding challenges in understanding signaling mechanisms in vivo; these challenges may also impact our consideration of cellular mechanisms. Whereas genetic evidence for bidirectional signaling in development remains, refinement of this understanding continues, causing us to reevaluate the relevance of this paradigm in specific situations. While Eph/ephrin signaling mutants are tremendously valuable for determining signaling mechanisms involved in the formation and maintenance of boundaries, they must also be interpreted with care, especially when overexpression is employed. It has become clear that a simple complementary expression model, with bidirectional signaling across the expression divide, will no longer be sufficient in most developmental scenarios. First and foremost, in many developing tissues, both Eph receptors and ephrins are co-expressed. For example, both EphB receptors and ephrin-Bs are expressed in the early mouse neural plate neuroepithelium prior to induction of the neural crest (O’Neill et al., 2016), as well as in mouse palatal shelves later in development (Bush and Soriano, 2010). It is also clear that co-expression of EphAs and ephrinAs, such as in the developing chick retina (Hornberger et al., 1999), can play critical roles in axon guidance. In addition, recent evidence indicates that unidirectional Eph/ephrin signaling is sufficient to drive the formation of boundaries during development, calling into question the idea that bidirectional signaling is necessary for Eph/ephrin signaling-mediated processes. Rather, parallel or anti-parallel combinations of Eph- and ephrin- expression may work together to drive separation of tissues.
Studies of tissue separation in disparate contexts often seem to converge on Eph/ephrin regulation of the cytoskeleton, firmly establishing the paradigm of this signaling pathway as a “middleman” between transcription factor patterning and the cellular changes necessary for morphogenetic processes to occur. What then are the upstream mechanisms by which differential Eph/ephrin expression is determined? Often, it seems that expression patterns of these signaling molecules are determined in parallel to the definition of cell fate by upstream transcription factors. For example, tbx24 (fss) in the zebrafish PSM, cMeso-1 in forming chick somites, Krox20 in rhombomeres 3 and 5 in the mouse (Theil et al., 1998), val in rhombomeres 5/6 in zebrafish (Cooke et al., 2001), and rx3 in the zebrafish eye field (Cavodeassi et al., 2013) can all affect the proper expression of Ephs and ephrins that is necessary for later boundary formation. Loss of Eph/ephrin expression does not seem to affect expression of these transcription factors, suggesting that gene expression patterning is not generally an important outcome of Eph/ephrin signaling. In considering complementary expression domains, we must also consider the effect of post-transcriptional mechanisms. In some cases, what appears as reciprocal expression of Eph and ephrin proteins could be caused by signaling leading to cleavage or endocytosis of receptor-ligand complexes, this high signaling turnover appearing as a reduction in Eph receptor expression in populations of cells with high ephrin expression, or vice versa.
How Eph/ephrin signaling leads to cell segregation and boundary formation has long been under debate, with differential adhesion, repulsive migration, and differential cortical tension all accumulating evidence to support these various hypotheses. Whether the outcome of Eph/ephrin signaling is tissue-specific, or whether one of these hypotheses will dominate in all contexts in which Eph/ephrin-mediated tissue separation occurs, remains to be determined by future careful studies of these processes.
Highlights.
The Ephs and ephrins compose a large signaling family that is critical for a wide array of morphogenetic functions in vertebrates.
Recent discoveries cast new light on the in vivo relevance of bidirectional signaling.
Eph/ephrin signaling often acts at the interface of tissue patterning and physical morphogenesis.
Cell sorting is a fundamental function of Eph/ephrin signaling and ongoing discoveries continue to clarify the self-organizing properties of this family.
Acknowledgments
We apologize to many colleagues whose work we could not discuss due to space limitations. J.O.B was supported by grant R01DE0233375 and R21DE025923 from the NIH/NIDCR; T.K.N was supported by grant F31DE026059 from the NIH/NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliee M, Röper J-C, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr. Biol. CB. 2012;22:967–976. doi: 10.1016/j.cub.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Arvanitis DN, Béhar A, Tryoen-Tóth P, Bush JO, Jungas T, Vitale N, Davy A. Ephrin B1 maintains apical adhesion of neural progenitors. Dev. Camb. Engl. 2013;140:2082–2092. doi: 10.1242/dev.088203. [DOI] [PubMed] [Google Scholar]
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 2010;12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr. Biol. CB. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Batlle E, Wilkinson DG. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008227. doi: 10.1101/cshperspect.a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénazéraf B, Pourquié O. Formation and segmentation of the vertebrate body axis. Annu. Rev. Cell Dev. Biol. 2013;29:1–26. doi: 10.1146/annurev-cellbio-101011-155703. [DOI] [PubMed] [Google Scholar]
- Bouché E, Romero-Ortega MI, Henkemeyer M, Catchpole T, Leemhuis J, Frotscher M, May P, Herz J, Bock HH. Reelin induces EphB activation. Cell Res. 2013;23:473–490. doi: 10.1038/cr.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J. Biomech. Eng. 2002;124:188–197. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- Brückner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Eph/ephrin signaling: genetic, phosphoproteomic, and transcriptomic approaches. Semin. Cell Dev. Biol. 2012;23:26–34. doi: 10.1016/j.semcdb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes Dev. 2010;24:2068–2080. doi: 10.1101/gad.1963210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush JO, Soriano P. Ephrin-B1 regulates axon guidance by reverse signaling through a PDZ-dependent mechanism. Genes Dev. 2009;23:1586–1599. doi: 10.1101/gad.1807209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari S, Terriente J, Pujades C. Cell segregation in the vertebrate hindbrain relies on actomyosin cables located at the interhombomeric boundaries. EMBO J. 2014;33:686–701. doi: 10.1002/embj.201386003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty L, Zarour E, Kashkooli L, François P, Fagotto F. Sorting at embryonic boundaries requires high heterotypic interfacial tension. Nat. Commun. 2017;8:157. doi: 10.1038/s41467-017-00146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RF, Beutler M, Marler KJM, Knöll B, Becker-Barroso E, Heintzmann R, Ng T, Drescher U. Silencing of EphA3 through a cis interaction with ephrinA5. Nat. Neurosci. 2006;9:322–330. doi: 10.1038/nn1655. [DOI] [PubMed] [Google Scholar]
- Catchpole T, Henkemeyer M. EphB2 tyrosine kinase-dependent forward signaling in migration of neuronal progenitors that populate and form a distinct region of the dentate niche. J. Neurosci. Off. J. Soc. Neurosci. 2011;31:11472–11483. doi: 10.1523/JNEUROSCI.6349-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F, Ivanovitch K, Wilson SW. Eph/Ephrin signalling maintains eye field segregation from adjacent neural plate territories during forebrain morphogenesis. Dev. Camb. Engl. 2013;140:4193–4202. doi: 10.1242/dev.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayuso J, Dzementsei A, Fischer JC, Karemore G, Caviglia S, Bartholdson J, Wright GJ, Ober EA. EphrinB1/EphB3b Coordinate Bidirectional Epithelial-Mesenchymal Interactions Controlling Liver Morphogenesis and Laterality. Dev. Cell. 2016;39:316–328. doi: 10.1016/j.devcel.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenaux G, Henkemeyer M. Forward signaling by EphB1/EphB2 interacting with ephrin-B ligands at the optic chiasm is required to form the ipsilateral projection. Eur. J. Neurosci. 2011;34:1620–1633. doi: 10.1111/j.1460-9568.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev. Cell. 2003;5:217–230. doi: 10.1016/s1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Cooke J, Moens C, Roth L, Durbin L, Shiomi K, Brennan C, Kimmel C, Wilson S, Holder N. Eph signalling functions downstream of Val to regulate cell sorting and boundary formation in the caudal hindbrain. Dev. Camb. Engl. 2001;128:571–580. doi: 10.1242/dev.128.4.571. [DOI] [PubMed] [Google Scholar]
- Cooke JE, Kemp HA, Moens CB. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. CB. 2005;15:536–542. doi: 10.1016/j.cub.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Cortina C, Palomo-Ponce S, Iglesias M, Fernández-Masip JL, Vivancos A, Whissell G, Humà M, Peiró N, Gallego L, Jonkheer S, Davy A, Lloreta J, Sancho E, Batlle E. EphB-ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev. Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Daar IO. Non-SH2/PDZ reverse signaling by ephrins. Semin. Cell Dev. Biol. 2012;23:65–74. doi: 10.1016/j.semcdb.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Gale NW, Murray EW, Klinghoffer RA, Soriano P, Feuerstein C, Robbins SM. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy A, Soriano P. Ephrin-B2 forward signaling regulates somite patterning and neural crest cell development. Dev. Biol. 2007;304:182–193. doi: 10.1016/j.ydbio.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev. Biol. 2011;355:138–151. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravis C, Yokoyama N, Chumley MJ, Cowan CA, Silvany RE, Shay J, Baker LA, Henkemeyer M. Bidirectional signaling mediated by ephrin-B2 and EphB2 controls urorectal development. Dev. Biol. 2004;271:272–290. doi: 10.1016/j.ydbio.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Dudanova I, Kao T-J, Herrmann JE, Zheng B, Kania A, Klein R. Genetic evidence for a contribution of EphA:ephrinA reverse signaling to motor axon guidance. J. Neurosci. Off. J. Soc. Neurosci. 2012;32:5209–5215. doi: 10.1523/JNEUROSCI.5707-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, Brennan C, Shiomi K, Cooke J, Barrios A, Shanmugalingam S, Guthrie B, Lindberg R, Holder N. Eph signaling is required for segmentation and differentiation of the somites. Genes Dev. 1998;12:3096–3109. doi: 10.1101/gad.12.19.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin L, Sordino P, Barrios A, Gering M, Thisse C, Thisse B, Brennan C, Green A, Wilson S, Holder N. Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Dev. Camb. Engl. 2000;127:1703–1713. doi: 10.1242/dev.127.8.1703. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Rohani N, Touret A-S, Li R. A Molecular Base for Cell Sorting at Embryonic Boundaries: Contact Inhibition of Cadherin Adhesion by Ephrin/Eph-Dependent Contractility. Dev. Cell. 2013;27:72–87. doi: 10.1016/j.devcel.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Falivelli G, Lisabeth EM, Rubio de la Torre E, Perez-Tenorio G, Tosato G, Salvucci O, Pasquale EB. Attenuation of eph receptor kinase activation in cancer cells by coexpressed ephrin ligands. PloS One. 2013;8:e81445. doi: 10.1371/journal.pone.0081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freywald A, Sharfe N, Roifman CM. The Kinase-null EphB6 Receptor Undergoes Transphosphorylation in a Complex with EphB1. J. Biol. Chem. 2002;277:3823–3828. doi: 10.1074/jbc.M108011200. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Gatto G, Morales D, Kania A, Klein R. EphA4 receptor shedding regulates spinal motor axon guidance. Curr. Biol. CB. 2014;24:2355–2365. doi: 10.1016/j.cub.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–52. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Körner R, Gaitanos L, Klein R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 2016;214:35–44. doi: 10.1083/jcb.201601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Marengere LE, McGlade J, Olivier JP, Conlon RA, Holmyard DP, Letwin K, Pawson T. Immunolocalization of the Nuk receptor tyrosine kinase suggests roles in segmental patterning of the brain and axonogenesis. Oncogene. 1994;9:1001–1014. [PubMed] [Google Scholar]
- Henkemeyer M, Orioli D, Henderson JT, Saxton TM, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- Himanen J-P, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, Dhe-Paganon S. Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Hornberger MR, Dütting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22:731–742. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine [corrected] phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- Hwang Y-S, Lee H-S, Kamata T, Mood K, Cho HJ, Winterbottom E, Ji YJ, Singh A, Daar IO. The Smurf ubiquitin ligases regulate tissue separation via antagonistic interactions with ephrinB1. Genes Dev. 2013;27:491–503. doi: 10.1101/gad.208355.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Griesshaber B, Atapattu L, Nievergall E, Hii LL, Mensinga A, Chheang C, Day BW, Boyd AW, Bastiaens PI, Jørgensen C, Pawson T, Lackmann M. Eph receptor function is modulated by heterooligomerization of A and B type Eph receptors. J. Cell Biol. 2011;195:1033–1045. doi: 10.1083/jcb.201104037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen J-P, Lackmann M, Nikolov DB. Adam Meets Eph: An ADAM Substrate Recognition Module Acts as a Molecular Switch for Ephrin Cleavage In trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jorgensen C, Sherman A, Chen GI, Pasculescu A, Poliakov A, Hsiung M, Larsen B, Wilkinson DG, Linding R, Pawson T. Cell-Specific Information Processing in Segregating Populations of Eph Receptor Ephrin-Expressing Cells. Science. 2009;326:1502–1509. doi: 10.1126/science.1176615. [DOI] [PubMed] [Google Scholar]
- Jülich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Dev. Camb. Engl. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- Kao T-J, Kania A. Ephrin-mediated cis-attenuation of Eph receptor signaling is essential for spinal motor axon guidance. Neuron. 2011;71:76–91. doi: 10.1016/j.neuron.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005;132:235–245. doi: 10.1242/dev.01553. [DOI] [PubMed] [Google Scholar]
- Kemp HA, Cooke JE, Moens CB. EphA4 and EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev. Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Kania A. Ephrin signalling in the developing nervous system. Curr. Opin. Neurobiol. 2014;27:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev. Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech P-H, Käfer J, Graner F, Müller DJ, Heisenberg C-P. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- Krull CE, Lansford R, Gale NW, Collazo A, Marcelle C, Yancopoulos GD, Fraser SE, Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. CB. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Lackmann M, Oates AC, Dottori M, Smith FM, Do C, Power M, Kravets L, Boyd AW. Distinct subdomains of the EphA3 receptor mediate ligand binding and receptor dimerization. J. Biol. Chem. 1998;273:20228–20237. doi: 10.1074/jbc.273.32.20228. [DOI] [PubMed] [Google Scholar]
- Laussu J, Khuong A, Gautrais J, Davy A. Beyond boundaries--Eph:ephrin signaling in neurogenesis. Cell Adhes. Migr. 2014;8:349–359. doi: 10.4161/19336918.2014.969990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AE, Hwa J, Wang R, Soriano P, Bush JO. Neural crest defects in ephrin-B2 mutant mice are non-autonomous and originate from defects in the vasculature. Dev. Biol. 2015;406:186–195. doi: 10.1016/j.ydbio.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J. Biol. Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZRGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Makinen T. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Shirasaki R, Ghosh S, Andrews SE, Carter N, Hunter T, Pfaff SL. Coexpressed EphA receptors and ephrin-A ligands mediate opposing actions on growth cone navigation from distinct membrane domains. Cell. 2005;121:127–139. doi: 10.1016/j.cell.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Mendes SW. Multiple Eph Receptors and B-Class Ephrins Regulate Midline Crossing of Corpus Callosum Fibers in the Developing Mouse Forebrain. J. Neurosci. 2006;26:882–892. doi: 10.1523/JNEUROSCI.3162-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos-Suárez A, Batlle E. Eph-ephrin signalling in adult tissues and cancer. Curr. Opin. Cell Biol. 2008;20:194–200. doi: 10.1016/j.ceb.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Moens CB, Prince VE. Constructing the hindbrain: insights from the zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2002;224:1–17. doi: 10.1002/dvdy.10086. [DOI] [PubMed] [Google Scholar]
- Monier B, Pélissier-Monier A, Brand AH, Sanson B. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol. 2010;12:60–69. doi: 10.1038/ncb2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethamer TK, Larson AR, O’Neill AK, Bershteyn M, Hsiao EC, Klein OD, Pomerantz JH, Bush JO. EPHRIN-B1 Mosaicism Drives Cell Segregation in Craniofrontonasal Syndrome hiPSC-Derived Neuroepithelial Cells. Stem Cell Rep. 2017;8:529–537. doi: 10.1016/j.stemcr.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Park E, Park S. In vivo expression of ephrinA5-Fc in mice results in cephalic neural crest agenesis and craniofacial abnormalities. Mol. Cells. 2014;37:59–65. doi: 10.14348/molcells.2014.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- O’Neill AK, Kindberg AA, Niethamer TK, Larson AR, Ho H-YH, Greenberg ME, Bush JO. Unidirectional Eph/ephrin signaling creates a cortical actomyosin differential to drive cell segregation. J. Cell Biol. 2016;215:217–229. doi: 10.1083/jcb.201604097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. Sek4 and Nuk receptors cooperate in guidance of commissural axons and in palate formation. EMBO J. 1996;15:6035. [PMC free article] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pegg CL, Cooper LT, Zhao J, Gerometta M, Smith FM, Yeh M, Bartlett PF, Gorman JJ, Boyd AW. Glycoengineering of EphA4 Fc leads to a unique, long-acting and broad spectrum, Eph receptor therapeutic antagonist. Sci. Rep. 2017;7:6519. doi: 10.1038/s41598-017-06685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlkamp T, Xiao L, Sultana R, Bepari A, Bock HH, Henkemeyer M, Herz J. Ephrin Bs and canonical Reelin signalling. Nature. 2016;539:E4–E6. doi: 10.1038/nature20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Poliakov A, Cotrina ML, Pasini A, Wilkinson DG. Regulation of EphB2 activation and cell repulsion by feedback control of the MAPK pathway. J. Cell Biol. 2008;183:933–947. doi: 10.1083/jcb.200807151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prin F, Serpente P, Itasaki N, Gould AP. Hox proteins drive cell segregation and non-autonomous apical remodelling during hindbrain segmentation. Dev. Camb. Engl. 2014;141:1492–1502. doi: 10.1242/dev.098954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risley M, Garrod D, Henkemeyer M, McLean W. EphB2 and EphB3 forward signalling are required for palate development. Mech. Dev. 2009;126:230–239. doi: 10.1016/j.mod.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Robichaux MA, Chenaux G, Ho H-YH, Soskis MJ, Dravis C, Kwan KY, Šestan N, Greenberg ME, Henkemeyer M, Cowan CW. EphB receptor forward signaling regulates area-specific reciprocal thalamic and cortical axon pathfinding. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2188–2193. doi: 10.1073/pnas.1324215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaux MA, Chenaux G, Ho H-YH, Soskis MJ, Greenberg ME, Henkemeyer M, Cowan CW. EphB1 and EphB2 intracellular domains regulate the formation of the corpus callosum and anterior commissure. Dev. Neurobiol. 2016;76:405–420. doi: 10.1002/dneu.22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N, Canty L, Luu O, Fagotto F, Winklbauer R. EphrinB/EphB Signaling Controls Embryonic Germ Layer Separation by Contact-Induced Cell Detachment. PLoS Biol. 2011;9:e1000597. doi: 10.1371/journal.pbio.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani N, Parmeggiani A, Winklbauer R, Fagotto F. Variable combinations of specific ephrin ligand/Eph receptor pairs control embryonic tissue separation. PLoS Biol. 2014;12:e1001955. doi: 10.1371/journal.pbio.1001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago A, Erickson CA. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Dev. Camb. Engl. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Schaupp A, Sabet O, Dudanova I, Ponserre M, Bastiaens P, Klein R. The composition of EphB2 clusters determines the strength in the cellular repulsion response. J. Cell Biol. 2014;204:409–422. doi: 10.1083/jcb.201305037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat. Struct. Mol. Biol. 2010;17:398–402. doi: 10.1038/nsmb.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentürk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472:356–360. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. CB. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- Solanas G, Cortina C, Sevillano M, Batlle E. Cleavage of E-cadherin by ADAM10 mediates epithelial cell sorting downstream of EphB signalling. Nat. Cell Biol. 2011;13:1100–1107. doi: 10.1038/ncb2298. [DOI] [PubMed] [Google Scholar]
- Soskis MJ, Ho H-YH, Bloodgood BL, Robichaux MA, Malik AN, Ataman B, Rubin AA, Zieg J, Zhang C, Shokat KM, Sharma N, Cowan CW, Greenberg ME. A Chemical Genetic Approach Reveals Distinct Mechanisms of EphB Signaling During Brain Development. Nat. Neurosci. 2012;15:1645–1654. doi: 10.1038/nn.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 1970;173:395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- Taylor HB, Khuong A, Wu Z, Xu Q, Morley R, Gregory L, Poliakov A, Taylor WR, Wilkinson DG. Cell segregation and border sharpening by Eph receptor–ephrin-mediated heterotypic repulsion. J. R. Soc. Interface. 2017;14:20170338. doi: 10.1098/rsif.2017.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil T, Frain M, Gilardi-Hebenstreit P, Flenniken A, Charnay P, Wilkinson DG. Segmental expression of the EphA4 (Sek-1) receptor tyrosine kinase in the hindbrain is under direct transcriptional control of Krox-20. Dev. Camb. Engl. 1998;125:443–452. doi: 10.1242/dev.125.3.443. [DOI] [PubMed] [Google Scholar]
- Townes PL, Holtfreter J. Directed movements and selective adhesion of embryonic amphibian cells. J. Exp. Zool. 1955;128:53–120. doi: 10.1002/jez.1401280105. [DOI] [PubMed] [Google Scholar]
- Twigg SR, Kan R, Babbs C, Bochukova EG, Robertson SP, Wall SA, Morriss-Kay GM, Wilkie AO. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D, Dunst S, Dahmann C. An RNA interference screen for genes required to shape the anteroposterior compartment boundary in Drosophila identifies the Eph receptor. PloS One. 2014;9:e114340. doi: 10.1371/journal.pone.0114340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite segmentation. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Xu G, Bridges LC, Williams P, White JM, DeSimone DW. ADAM13 induces cranial neural crest by cleaving class B Ephrins and regulating Wnt signaling. Dev. Cell. 2010;19:345–352. doi: 10.1016/j.devcel.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of eph receptors and ephrins in neural development. Nat. Rev. Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PIH, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J. Cell Biol. 2004;164:661–666. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N-J, Henkemeyer M. Ephrin reverse signaling in axon guidance and synaptogenesis. Semin. Cell Dev. Biol. 2012;23:58–64. doi: 10.1016/j.semcdb.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N-J, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat. Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N-J, Sun S, Gibson JR, Henkemeyer M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat. Neurosci. 2011;14:1421–1429. doi: 10.1038/nn.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Mellitzer G, Robinson V, Wilkinson DG. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yamashita Y, Noda H, Okafuji T, Go MJ, Tanaka H. EphA receptor tyrosine kinases interact with co-expressed ephrin-A ligands in cis. Neurosci. Res. 2004;48:285–296. doi: 10.1016/j.neures.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29:85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Zhang G, Brady J, Liang W-C, Wu Y, Henkemeyer M, Yan M. EphB4 forward signalling regulates lymphatic valve development. Nat. Commun. 2015;6(6625) doi: 10.1038/ncomms7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jiang Z, Liu X, Meng A. Eph/ephrin signaling maintains the boundary of dorsal forerunner cell cluster during morphogenesis of the zebrafish embryonic left-right organizer. Dev. Camb. Engl. 2016;143:2603–2615. doi: 10.1242/dev.132969. [DOI] [PMC free article] [PubMed] [Google Scholar]