Abstract
Purpose
Bisphosphonate (BP) is the first-line therapy for the management of osteoporosis. BP-related osteonecrosis of the jaw (BRONJ) and atypical femoral fracture (AFF) are increasingly common comorbidities in patients with osteoporosis under long-term BP treatment. The aim of this study was to evaluate the incidence and risk factors for AFF features on bone scintigraphy in patients with BRONJ.
Methods
Among total of 373 BRONJ patients treated between September 2005 and July 2014, 237 (220 women, 17 men; median age 73 years) who underwent three-phase bone scintigraphy were enrolled for this retrospective study. AFF features on bone scintigraphy and the related clinical factors were assessed.
Results
Among 237 patients with BRONJ, 11 (4.6%) showed AFF features on bone scintigraphy. BP medication duration (p = 0.049) correlated significantly with AFF features on bone scintigraphy in patients with BRONJ. BP intake duration of 34 months was the cutoff value for predicting the presence of AFF features on bone scintigraphy. Among the patients with BRONJ, all those with AFF features on bone scintigraphy were female patients with osteoporosis who were on oral BP medication; however, these factors were not significantly different along with AFF features on bone scintigraphy.
Conclusion
The incidence of AFF features on bone scintigraphy was relatively high in patients with BRONJ. A careful observation of patients presenting with the AFF features on bone scintigraphy may be needed, particularly for female BRONJ patients with osteoporosis who have been on BP medication for over 34 months.
Keywords: Bone scintigraphy, Atypical femoral fracture, Bisphosphonate-related osteonecrosis of the jaw, Prevalence
Introduction
Bisphosphonate (BP) is widely used as a treatment for osteoporosis. Several studies have shown that BP lowers the prevalence of fracture risk in women with postmenopausal osteoporosis [1–3]. This drug has also been used for other diseases, including bone metastasis leading to hypercalcemia, Paget’s disease, and glucocorticoid-induced osteoporosis [4–6]. However, the long-term use of BP can cause adverse effects, including BP-related osteonecrosis of the jaw (BRONJ) [7–9] and atypical femoral fracture (AFF) [10–13]. The incidence of AFF and BRONJ are very low. The incidence rate for AFF has been reported to be 3 to 6 cases per 100,000 person-years, and is approximately 110 cases per 100,000 cases per year among patients who have been using BP medication for 8 years [14–16]. The incidence of BRONJ has been reported to be as low as 1 case per 10,000 patient-treatment years [7]. Although BP-related AFF is very rare, careful observation is required to detect any adverse effects in order to avoid the grave prognosis. Kharazmiet et al. reported that 39 (23%) out of 172 patients with AFF died during a mean follow-up period of 4 years [17]. Thus, the early identification of patients at high risk for AFF among those medicated with BP is clinically important. Although some cases of simultaneous AFF and BRONJ have been reported [18, 19], the incidence and risk factors of AFF features on bone scintigraphy in BRONJ patients have not been explored previously.
Three-phase bone scintigraphy may be useful for the evaluation of BRONJ [20–22]. In addition, whole body images usually obtained during the osseous phase may also provide additional information about the entire skeletal system. Since both BRONJ and AFF are adverse effects of BP medications, abnormal femoral cortical uptake, which may be related to future complete fractures [23, 24], can often be detected on whole-body bone scintigraphy images obtained from BRONJ patients.
Thus, the aim of this study was to evaluate the incidence and risk factors of AFF features on bone scintigraphy in patients with BRONJ.
Methods
Study Population and Design
Among the 373 BRONJ patients who were treated in the Department of Oral and Maxillofacial Surgery between September 2005 and July 2014 in Kyungpook National University hospital, 237 patients (220 women, 17 men; median age 73 years) who underwent three-phase bone scintigraphy were enrolled. Clinical factors were collected from medical records. Data collected included age, sex, type of disease, BRONJ stage, type of BP medicines, and the duration of BP use. Among these, the clinical factors predicting AFF features on bone scintigraphy were assessed.
BRONJ
According to the American Association of Oral and Maxillofacial Surgeons (AAOMS), patients may be diagnosed with BRONJ if they have been under treatment with BP for at least 12 months, have a maxillofacial bone exposure that has persisted for more than 8 weeks after identification, and have no history of treatment with local radiotherapy [8]. All patients were staged using the AAOMS BRONJ staging system. Stage 1 involves an exposed necrotic bone in asymptomatic patients with no evidence of infection. Stage 2 involves an infected exposed/necrotic bone, as evidenced by pain and erythema in the region of the exposed bone. Stage 3 includes an infected exposed/necrotic bone causing pain, and one or more of the following features: a pathologic fracture and an extra-oral fistula extending into the inferior border of the sinus floor.
Bone Scintigraphy
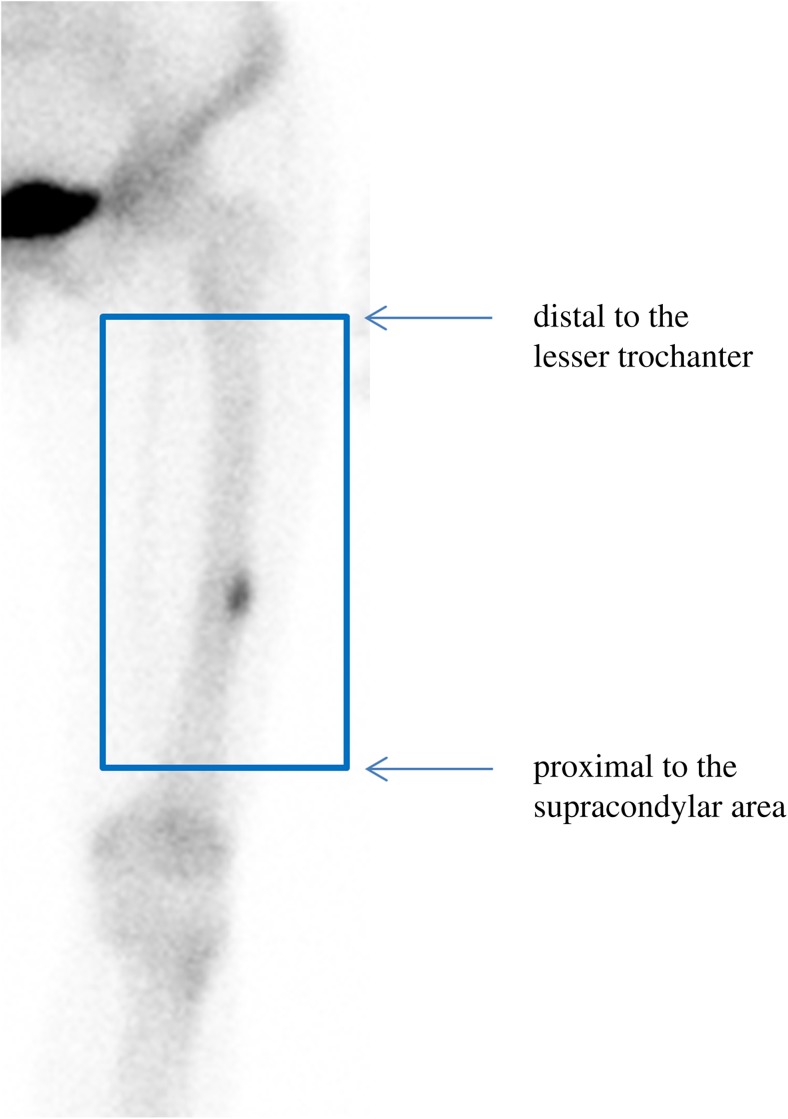
Bone scintigraphy was performed immediately after a bolus injection of 740 MBq (20 mCi) of Tc-99m hydroxymethylene diphosphonate (HDP). A dual-headed gamma camera equipped with a high-resolution collimator (Infinia, GE, Milwaukee, WI, USA) was used. Focal or diffusely increased tracer accumulation over the surrounding background or contralateral bone was considered an indicator of BRONJ. According to the criteria put forth by the American Society for Bone and Mineral Research (ASBMR), AFF is defined as a fracture located in the femur, from just distal to the lesser trochanter to just proximal to the supracondylar area; complete fractures extend through both cortices and may be associated with a medial spike, whereas incomplete fractures involve only the lateral cortex [10, 25]. In the current study, linear tracer uptake (suggesting the presence of a complete fracture) and focal tracer uptake along the cortex of the femur (suggesting the presence of an incomplete fracture), located within the femur, from just distal to the lesser trochanter to just proximal to the supracondylar area, were considered AFF features on bone scintigraphy (Fig. 1) [24]. An additional bone scintigraphy image of a particular region of the femur, such as an anterior, posterior, or lateral image, was obtained for all patients with suspected AFF features to determine whether the focal tracer uptake indeed represented cortical uptake.
Fig. 1.
An atypical femur fracture feature on bone scintigraphy was identified as focal tracer uptake along the cortex of the femur, located within the femur, from just distal to the lesser trochanter to just proximal to the supracondylar area
Statistical Analysis
Continuous data are presented as medians and ranges. Chi-squared tests were performed for categorical comparisons, and continuous variables were compared using the Student’s t test and the Mann-Whitney U test. Receiver operating characteristic (ROC) area under the curve analysis was used to identify an optimal cutoff of clinical variables for predicting the presence of AFF features on bone scintigraphy. MedCalc Statistical Software version 13.2 (MedCalc Software, Ostend, Belgium) was used for all statistical calculations, and p values < 0.05 were considered statistically significant.
Results
Patient Characteristics
Among the 237 patients with clinically confirmed BRONJ, 204 were diagnosed with osteoporosis, 15 with multiple myeloma, and 18 with malignancies. As for the type of BP medication, 116 patients were taking alendronate, 11 were taking ibandronate, 15 were on pamidronate, 40 were on risedronate, 23 were on zoledronate, and 32 patients took more than one type of BP medicine. The route of the BP medicine administration was oral for 191 patients and intravenous (IV) for 46.
Bone Scintigraphy and Clinical Characteristics of BRONJ Patients
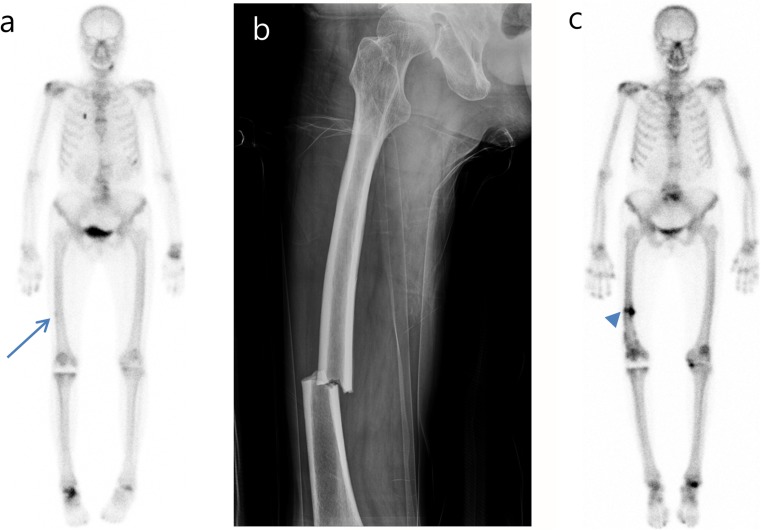
Among the 237 BRONJ patients, 11 (4.6%) exhibited AFF features on bone scintigraphy. Table 1 shows bone scintigraphic, radiological, and clinical characteristics of BRONJ patients with AFF features on bone scintigraphy. Among these 11 patients with abnormal cortical femur findings, 9 had newly diagnosed cortical uptake on bone scintigraphy, and the remaining 2 patients with previously diagnosed AFF had undergone surgery. All patients with no AFF history showed focal tracer uptake in the lateral cortex of the femur on bone scintigraphy, suggesting the presence of an incomplete fracture (Fig. 2a). Two patients had rheumatoid arthritis, while another had no previous disease history.
Table 1.
Bone scintigraphic, radiological, and clinical characteristics of BRONJ patients
| Number | Age (year) | Disease | Duration of BP (months) | BP type | AFF history | X-ray feature | Bone scan feature | Bilateral fracture | Previous disease related to fracture |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | Osteoporosis | 48 | Risedronate, alendronate, pamidronate, ibandronate | Yes | Left cortical thickening, right fracture | Left lateral focal uptake, right linear uptake | Yes | None |
| 2 | 79 | Osteoporosis | 42 | Alendronate | No | No X-ray data | Right lateral cortex focal uptake | No | Rheumatoid arthritis |
| 3 | 58 | Osteoporosis | 36 | Risedronate | No | No X-ray data | Right lateral cortex focal uptake | No | Rheumatoid arthritis |
| 4 | 73 | Osteoporosis | 60 | Alendronate | No | Cortical thickening | Left lateral cortex focal uptake | No | None |
| 5 | 74 | Osteoporosis | 60 | Alendronate | No | No X-ray data | Right lateral cortex focal uptake | No | None |
| 6 | 86 | Osteoporosis | 60 | Alendronate | Yes | Right cortical thickening, left fracture | Right lateral focal uptake, left linear uptake | Yes | None |
| 7 | 82 | Osteoporosis | 120 | Risedronate | No | No X-ray data | Both lateral cortex focal uptake | Yes | None |
| 8 | 83 | Osteoporosis | 36 | Ibandronate | No | No X-ray data | Right lateral cortex focal uptake | No | None |
| 9 | 71 | Osteoporosis | 120 | Alendronate, risedronate | No | No X-ray data | Right lateral cortex focal uptake | No | None |
| 10 | 78 | Osteoporosis | 24 | Alendronate | No | No X-ray data | Right lateral cortex focal uptake | No | None |
| 11 | 75 | Osteoporosis | 72 | Risedronate | No | No X-ray data | Left lateral cortex focal uptake | No | None |
BRONJ bisphosphonate-related osteonecrosis of the jaw, AFF atypical femur fracture, BP bisphosphonate, X-ray plain radiography
Fig. 2.
A 58-year-old female patient with bisphosphonate-related osteonecrosis of the jaw after risedronate medication use for 3 years exhibited subtle cortical uptake at the mid-shaft of the right femur (arrow), suggesting the presence of an atypical femur fracture feature on bone scintigraphy (a). Two years later, a complete fracture of the right femur occurred at the same level where the atypical femur fracture feature was noted on the bone scintigraphy (b). Post-operative bone scintigraphy showed intense linear tracer uptake at the fracture site (arrowhead) (c)
Risk Factors for AFF Features on Bone Scintigraphy
Table 2 shows the clinical characteristics of patients with and without AFF features on bone scintigraphy. Only the duration of BP medication use was significantly higher in patients with than in those without AFF features on bone scintigraphy (p = 0.049). Median duration of BP medication treatment in patients with AFF features on bone scintigraphy was 60 months (range 24 to 120). A BP intake duration of 34 months was found to be the cutoff value (sensitivity 90.9%, specificity 41.8%, AUC 0.674) for predicting the presence of AFF features on bone scintigraphy using ROC analysis. When evaluating only the nine patients with newly diagnosed AFF features on bone scintigraphy and no AFF history, the association between BP medication duration and risk of AFF features approached statistical significance (p = 0.083), and a BP duration of 34 months was found to be the cutoff value based on ROC analysis (i.e., only in the patients newly diagnosed with AFF features on bone scintigraphy). Only one patient with AFF features on bone scintigraphy had been on BP medication for less than 34 months (Table 1).
Table 2.
Comparison of clinical parameters between patients with and without femoral abnormality on bone scintigraphy (n = 237)
| No femoral abnormality on bone scintigraphy (n = 226) | Femoral abnormality on bone scintigraphy (n = 11) | p value | |
|---|---|---|---|
| Age (years) | 0.229 | ||
| 73 (40 to 90) | 75 (58 to 86) | ||
| Sex | 0.346 | ||
| Female | 209 | 11 | |
| Male | 17 | 0 | |
| Medication route | 0.096 | ||
| Oral | 180 | 11 | |
| IV | 46 | 0 | |
| Disease | 0.393 | ||
| Osteoporosis | 193 | 11 | |
| Multiple myeloma | 15 | 0 | |
| Cancer | 18 | 0 | |
| BRONJ stage | 0.781 | ||
| 0 | 12 | 0 | |
| 1 | 8 | 0 | |
| 2 | 79 | 4 | |
| 3 | 127 | 7 | |
| Drug | 0.650 | ||
| Alendronate | 111 | 5 | |
| Ibandronate | 10 | 1 | |
| Pamidronate | 15 | 0 | |
| Risedronate | 37 | 3 | |
| Zoledronate | 23 | 0 | |
| Mixed | 30 | 2 | |
| BP duration (months) | 0.049 | ||
| 36 (3 to 156) | 60 (24 to 120) | ||
Data are presented as number of patients or median (range)
BRONJ bisphosphonate-related osteonecrosis of the jaw, IV intravenous, BP bisphosphonate
Among the BRONJ patients, all those presenting with AFF features on bone scintigraphy were women with osteoporosis who were under treatment with oral BP. However, these factors were not statistically significant predictors of AFF features on bone scintigraphy. Other factors, such as age, BRONJ stage, and type of BP medication, were also not significantly different between patients with and without AFF features on bone scintigraphy. Alendronate or risedronate was prescribed to all but one patient with AFF features on bone scintigraphy (Table 1). Three (7.5%) out of the 40 patients taking only risedronate and 5 (4.5%) out of the 111 patients taking only alendronate exhibited AFF features on bone scintigraphy.
Discussion
In this study, we evaluated the incidence and risk factors of AFF features on bone scintigraphy in 237 patients with BRONJ, which is a major side effect of BP medication. To our knowledge, this is the first study evaluating the incidence rate of AFF features on bone scintigraphy in BRONJ patients, which was observed to be relatively high. Eleven (4.6%) of the 237 patients with BRONJ exhibited AFF features on bone scintigraphy. Nine (81.8%) of these 11 patients presented with focal femoral uptake, suggesting the presence of an incomplete fracture on bone scintigraphy, which might be associated with future complete AFF [24]. However, incidence rates for AFF and BRONJ are reported to be very low: the incidence of AFF in patients under BP medication is reported to be 3 to 6 cases per 100,000 person-years [14–16] and that of BRONJ is reported to be 1 per 10,000 patient-treatment years [7, 26]. Due to the relatively high prevalence of AFF features on bone scintigraphy in BRONJ patients, a careful follow-up in cases of BRONJ is warranted.
It is well known that long-term use of BP medications can cause adverse effects such as BRONJ and AFF [7–11]. In some studies, it has been observed that treatment with BP medicines for more than 5 years increased the risk of subtrochanteric fracture or fracture of the femoral shaft [15]. Moreover, the incidence of BRONJ among these patients was found to increase over time, from nearly 0% at baseline to 0.2% after 4 or more years of BP treatment [8]. The long-term use of BP medications may be a common risk factor of BRONJ and AFF. Consistent with previous studies, BP duration was significantly different between patients with and without AFF features on bone scintigraphy (p = 0.049, cutoff 34 months). The median value of BP medication treatment duration in 11 BRONJ patients with AFF features was 60 months. When evaluating only the nine patients who were newly diagnosed with cortical uptake on bone scintigraphy and had no AFF history, the association of BP treatment duration with the risk of AFF features still approached significance; the same ROC cutoff value was observed (p = 0.083, cutoff 34 months). Only one patient showed AFF features on bone scintigraphy after less than 34 months of BP medication intake (Table 1). A careful observation of femoral lesions on bone scintigraphy may be necessary for BRONJ patients who have been on BP medication for over 34 months.
In the current study, all 11 patients with AFF features on bone scintigraphy were female patients on osteoporotic medication. This might be related to the high incidence of the prescription of BP medications in postmenopausal osteoporotic women [1–3]. However, factors such as sex and type of disease did not show statistically significant associations with AFF features on bone scintigraphy. In the current study, even though we observed that all patients with AFF features on bone scintigraphy were female and on osteoporotic medication, it must be noted that most of the included patients themselves were also females on osteoporosis medication. Thus, these factors did not show statistically significant associations with the risk of AFF features on bone scintigraphy.
In the current study, all but one patient with AFF features on bone scintigraphy was under treatment with either alendronate or risedronate (Table 1). Among the patients with BRONJ, 3 (7.5%) of the 40 patients taking risedronate and 5 (4.5%) of the 111 patients taking alendronate exhibited AFF features on bone scintigraphy. However, the use of two medications did not show significant associations with the presence of AFF features on bone scintigraphy, and whether the type of BP medication influences AFF could not be elucidated. However, in previous published case reports, most AFF patients on BP medication were under treatment with oral alendronate monotherapy or oral risedronate therapy [10]. Moreover, 160 of 189 AFF patients presented with side effects of BP medication that occurred after oral alendronate monotherapy, 12 patients were treated with oral risedronate, and the rest with other types of BP medication. Consistent with this report, the results of the current study revealed that 12 of the 13 patients with AFF features took alendronate or risedronate for the treatment of osteoporosis. However, it must be noted that most of the patients who were included in the study also took alendronate or risedronate. Thus, these factors were not significantly associated with the risk of AFF features on bone scintigraphy.
BRONJ is characterized by the presence of bone exposure in the maxillofacial region, in response to BP medication, in the absence of radiation therapy to the jaw [8], and risk factors for BRONJ include age, concomitant malignant disease, diabetes, and preexisting dental disease. Some studies have reported the incidence of the risk for osteonecrosis of the jaw from high doses of IV BP medicines to be 1–12%, over 36 months of administration, while oral BP medication in osteoporotic patients resulted in a risk of less than 1 per 100,000 person-years for BRONJ [9]. The current study showed that none of the patients on IV BP medication exhibited AFF features on bone scintigraphy. The risk factors for BRONJ, such as age and malignant disease, were not significantly different between patients with and without AFF features on bone scintigraphy. In addition, the BRONJ stage, reflecting the severity of BRONJ, was not significantly different between patients with AFF features on bone scintigraphy and patients without such features. This suggests that the pathophysiology of BRONJ and AFF may be different.
The pathophysiology of BRONJ and AFF was not fully elucidated, but has been reviewed separately [7, 10]. Recently, some reports have suggested a model for the development of BRONJ and AFF as a common origin with divergent outcomes. This model suggests that attenuated bone remodeling provides a common mechanism for BRONJ and AFF [27]. The incidence of BRONJ was high with IV BP and correlated with BP dosage [8, 9]. BRONJ pathogenesis is combined with bone defects followed by suppression of bone remodeling, mucosal dehiscence, as BP medications inhibit angiogenesis, and the resultant secondary infections [8, 28, 29]. In contrast, the incidence of AFF correlates with the duration of BP and may be the result of long-term, low-dose BP therapy. Bone remodeling may be sufficient to prevent necrosis, however, over time long-term changes including reduced heterogeneity of the microstructure, and microdamage or high strain may lead to mechanical failure [30]. According to this model, the current study revealed that 43 (19.4%) BRONJ patients took bisphosphonate medications via IV administration, although no patients with IV BP administration revealed AFF features on bone scintigraphy. Duration of BP medication use was significantly longer in patients with AFF features on bone scintigraphy than in patients without such features.
It is well known that bone scintigraphy is more sensitive than plain radiography to detect bone lesions because it images the activity of osteoblasts. Simple radiography can detect bone lesions with 30–50% changes in bone mineral mass, whereas bone scintigraphy can detect bone lesions with 3–5% changes in bone mineral mass. Therefore, early skeletal pathologic lesions can be detected by the bone scintigraphy [31]. Even though bone scintigraphy demonstrated more sensitive results to identify bone lesions including fracture, the definition of AFF by ASBMR task force [14] is based on simple radiography. In the current study, 3 of 11 patients with AFF features on bone scintigraphy had plain radiography, which were consistent with bone scintigraphy result. When plain radiography showed complete fracture, bone scintigraphy revealed linear tracer uptake, and when plain radiography showed cortical thickening suggesting incomplete fracture, bone scintigraphy revealed focal tracer uptake (Table 1). Further studies on the role of bone scintigraphy in AFF are warranted.
This study has some limitations that may affect the results. First, the retrospective nature of the study precludes the full investigation of known risk factors of AFF, including the use of drugs such as glucocorticoids to inhibit bone remodeling and diseases such as rheumatoid arthritis. Further investigation is needed in future studies to understand whether these factors could influence the presence of AFF features on bone scintigraphy. Second, it was not assessed whether the AFF features that appeared as focal tracer uptake on bone scintigraphy, suggesting the presence of an incomplete fracture in the femur, progressed to complete fractures in the future.
Conclusion
In the present study, 4.6% of BRONJ patients exhibited AFF features on bone scintigraphy. The duration of BP medication use was a risk factor for AFF features on bone scintigraphy. All patients exhibiting AFF features on bone scintigraphy were female and osteoporosis medication. Therefore, careful examination of bone scintigraphy images for AFF features may be warranted for female BRONJ patients with osteoporosis who have been using BP for more than 34 months.
Compliance with Ethical Standards
Conflict of Interest
Seung Hyun Son, Chae Moon Hong, Ju Hye Jeong, Shin Young Jeong, Sang-Woo Lee, Jaetae Lee, Tae-Geon Kwon, and Byeong-Cheol Ahn declare that they have no conflict of interest.
Ethical Approval
The study protocol had been approved by the Ethics Committee of the Kyungpook National University Hospital (KNUH 2018–02-029). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. No informed consent was needed, because of the retrospective design of our study.
Informed Consent
The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Contributor Information
Chang-Hee Lee, Phone: +82-53-420-5577, Email: kune2151@naver.com.
Byeong-Cheol Ahn, Phone: 82-53-420-5583, Email: abc2000@knu.ac.kr.
References
- 1.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the fracture intervention trial long-term extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 2.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 3.Chesnut CH, 3rd, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 4.Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- 5.Devogelaer JP. Modern therapy for Paget's disease of bone: focus on bisphosphonates. Treat Endocrinol. 2002;1:241–257. doi: 10.2165/00024677-200201040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Polascik TJ. Bisphosphonates in oncology: evidence for the prevention of skeletal events in patients with bone metastases. Drug Des Devel Ther. 2009;3:27–40. [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 8.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Khan AA, Sandor GK, Dore E, Morrison AD, Alsahli M, Amin F, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36:478–490. doi: 10.3899/jrheum.080759. [DOI] [PubMed] [Google Scholar]
- 10.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294. doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 11.Ott SM. What is the optimal duration of bisphosphonate therapy? Cleve Clin J Med. 2011;78:619–630. doi: 10.3949/ccjm.78a.11022. [DOI] [PubMed] [Google Scholar]
- 12.Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. 2003;5:65–74. doi: 10.1007/s11926-003-0085-6. [DOI] [PubMed] [Google Scholar]
- 13.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 14.Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2014;29:1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 15.Park-Wyllie LY, Mamdani MM, Juurlink DN, Hawker GA, Gunraj N, Austin PC, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA. 2011;305:783–789. doi: 10.1001/jama.2011.190. [DOI] [PubMed] [Google Scholar]
- 16.Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, et al. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012;27:2544–2550. doi: 10.1002/jbmr.1719. [DOI] [PubMed] [Google Scholar]
- 17.Kharazmi M, Hallberg P, Schilcher J, Aspenberg P, Michaelsson K. Mortality after atypical femoral fractures: a cohort study. J Bone Miner Res. 2016;31:491–497. doi: 10.1002/jbmr.2767. [DOI] [PubMed] [Google Scholar]
- 18.Chiu WY, Lee JJ, Tsai KS. Atypical femoral fractures shortly after osteonecrosis of the jaw in a postmenopausal woman taking alendronate for osteoporosis. J Clin Endocrinol Metab. 2013;98:E723–E726. doi: 10.1210/jc.2012-4144. [DOI] [PubMed] [Google Scholar]
- 19.Won Y, Lim JR, Kim YH, Song HK, Yang KH. Atypical femoral fracture combined with osteonecrosis of jaw during osteoporosis treatment with bisphosphonate. J Bone Metab. 2014;21:155–159. doi: 10.11005/jbm.2014.21.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenport MS, Brown RK, Frey KA. Utility of delayed whole-body bone scintigraphy after directed three-phase scintigraphy. AJR Am J Roentgenol. 2009;193:338–342. doi: 10.2214/AJR.08.2142. [DOI] [PubMed] [Google Scholar]
- 21.O'Ryan FS, Khoury S, Liao W, Han MM, Hui RL, Baer D, et al. Intravenous bisphosphonate-related osteonecrosis of the jaw: bone scintigraphy as an early indicator. J Oral Maxillofac Surg. 2009;67:1363–1372. doi: 10.1016/j.joms.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Hong CM, Ahn BC, Choi SY, Kim DH, Lee SW, Kwon TG, et al. Implications of three-phase bone scintigraphy for the diagnosis of bisphosphonate-related osteonecrosis of the jaw. Nucl Med Mol Imaging. 2012;46:162–168. doi: 10.1007/s13139-012-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards MH, McCrae FC, Young-Min SA. Alendronate-related femoral diaphysis fracture--what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int. 2010;21:701–703. doi: 10.1007/s00198-009-0986-y. [DOI] [PubMed] [Google Scholar]
- 24.Papandrianos N, Alexiou S, Xouria X, Apostolopoulos DJ. Atypical bilateral stress fractures of the femoral shaft diagnosed by bone scintigraphy in a woman with osteoporosis. Clin Nucl Med. 2013;38:910–912. doi: 10.1097/RLU.0b013e3182a75940. [DOI] [PubMed] [Google Scholar]
- 25.Schilcher J, Koeppen V, Ranstam J, Skripitz R, Michaelsson K, Aspenberg P. Atypical femoral fractures are a separate entity, characterized by highly specific radiographic features. A comparison of 59 cases and 218 controls. Bone. 2013;52:389–392. doi: 10.1016/j.bone.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/S0278-2391(09)01877-1. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian G, Fritton JC, Quek SY. Osteonecrosis and atypical fractures-common origins? Osteoporos Int. 2013;24:745–746. doi: 10.1007/s00198-012-1953-6. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian G, Cohen HV. Quek SY. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide's potential role in its resolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:744–753. doi: 10.1016/j.tripleo.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Yin G, Bai Y, Luo E. Angiogenic suppression of osteoclasts may play a role in developing bisphosphonate-related osteonecrosis of the jaw. Med Hypotheses. 2011;76:347–349. doi: 10.1016/j.mehy.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Unnanuntana A, Saleh A, Mensah KA, Kleimeyer JP, Lane JM. Atypical femoral fractures: what do we know about them?: AAOS exhibit selection. J Bone Joint Surg Am 2013;95:e8 1–13. [DOI] [PubMed]
- 31.Yang DC, Ratani RS, Mittal PK, Chua RS, Pate SM. Radionuclide three-phase whole-body bone imaging. Clin Nucl Med. 2002;27:419–426. doi: 10.1097/00003072-200206000-00007. [DOI] [PubMed] [Google Scholar]