Abstract
Metaiodobenzylguanidine (MIBG) is structurally similar to the neurotransmitter norepinephrine and specifically targets neuroendocrine cells including some neuroendocrine tumors. Iodine-131 (I-131)-labeled MIBG (I-131 MIBG) therapy for neuroendocrine tumors has been performed for more than a quarter-century. The indications of I-131 MIBG therapy include treatment-resistant neuroblastoma (NB), unresectable or metastatic pheochromocytoma (PC) and paraganglioma (PG), unresectable or metastatic carcinoid tumors, and unresectable or metastatic medullary thyroid cancer (MTC). I-131 MIBG therapy is one of the considerable effective treatments in patients with advanced NB, PC, and PG. On the other hand, I-131 MIBG therapy is an alternative method after more effective novel therapies are used such as radiolabeled somatostatin analogs and tyrosine kinase inhibitors in patients with advanced carcinoid tumors and MTC. No-carrier-aided (NCA) I-131 MIBG has more favorable potential compared to the conventional I-131 MIBG. Astatine-211-labeled meta-astatobenzylguanidine (At-211 MABG) has massive potential in patients with neuroendocrine tumors. Further studies about the therapeutic protocols of I-131 MIBG including NCA I-131 MIBG in the clinical setting and At-211 MABG in both the preclinical and clinical settings are needed.
Keywords: Iodine-131 metaiodobenzylguanidine, Neuroblastoma, Pheochromocytoma, Paraganglioma, Carcinoid tumors, Medullary thyroid cancer
Introduction
Metaiodobenzylguanidine (MIBG), which is derived from ganglionic blocking drug guanethidine, is an analog of norepinephrine (NE) and specifically targets neuroendocrine cells including some neuroendocrine tumors such as neuroblastoma (NB), pheochromocytoma (PC), and carcinoid tumors [1, 2]. MIBG, which can be labeled with iodine-131 (I-131) and iodine-123 (I-123), was first reported as adrenomedullary scintigraphic agents with use of their gamma-rays [1, 3]. Subsequently, I-131-labeled MIBG (I-131 MIBG) came into use as a therapeutic agent for patients with neuroendocrine tumors because of emitting a beta ray with cytocidal effect. Early I-131 MIBG therapies were reported in the 1980s. The first I-131 MIBG therapy was performed in patients with PC [4]. Additionally, I-131 MIBG therapies for patients with NB and carcinoid tumor were reported in 1986 [5, 6]. Since then, I-131 MIBG therapy has been widely used for some neuroendocrine tumors.
Mechanism of MIBG Uptake in Neuroendocrine Tumors
MIBG is structurally similar to NE and its uptake into neuroendocrine cells is similar to the uptake of NE. There are two pathways of the uptake of MIBG into neuroendocrine cells. One is a specific uptake system (uptake one) and the other is a nonspecific uptake system [7, 8]. Uptake one is an active pathway by a NE transporter (NET). It is sodium-dependent, temperature-dependent, energy-requiring, low-capacity, high-affinity, and saturable for MIBG and NE. The nonspecific uptake is a sodium-independent, temperature-dependent, energy-independent, unsaturable passive, and diffusional mechanism [7–9]. Under the clinical condition with I-131 MIBG therapy, the majority of MIBG uptake into neuroendocrine cells is via uptake-one by NET [9–11]. The uptake of MIBG into neuroendocrine cells is strongly linked to NET. Several neuroendocrine tumors originating from the neural crest such as NB, PC, carcinoid tumors, and medullary thyroid cancer (MTC) express NET with their cell membranes and transport MIBG into themselves.
After MIBG enters neuroendocrine tumor cells, most MIBG is stored in neurosecretory granules via the vesicular monoamine transporter (VMAT) within the tumor cells of PC and other well-differentiated tumors [12]. As with the expression of NET, the expression of VMAT is thought to be an important factor for the MIBG uptake in tumor cells [13]. By contrast, NB cells store MIBG mainly in the cytoplasm and/or mitochondria because of having fewer neurosecretory granules than other neuroendocrine tumors [14, 15].
Indications and Contraindications of I-131 MIBG Therapy
The current indications and contraindications of I-131 MIBG therapy for neuroendocrine tumors are stated in the European Association of Nuclear Medicine (EANM) procedure guidelines and the Japanese Society of Nuclear Medicine (JSNM) draft guidelines [16, 17].
In the EANM procedure guidelines, the indications of I-131 MIBG therapy include stage III or IV NB, inoperable PC and paraganglioma (PG), inoperable carcinoid tumors, and metastatic or recurrent MTC [16]. In the JSNM draft guidelines, the indications include treatment-resistant NB, unresectable or malignant PC and PG (PCPGs), unresectable or malignant carcinoid tumors, and unresectable MTC [17]. These tumors need MIBG uptake at the diagnostic scintigraphy with I-123 MIBG or I-131 MIBG before I-131 MIBG therapy. If tumors have no radiolabeled MIBG uptake at the pretherapy-diagnostic scintigraphy, I-131 MIBG therapy should not be performed. The most desirable aim of I-131 MIBG therapy is to achieve complete remission; however, it is rather difficult to obtain complete remission with I-131 MIBG therapy. The main aims of I-131 MIBG therapy are to constrict tumor progression, to relieve symptoms directly from primary or metastatic lesions, and to alleviate symptoms from hormonal abnormality.
Absolute contraindications include renal failure requiring short-term dialysis and life expectancy less than 3 months except in case of refractory bone pain, pregnancy, and patients who cannot stop breastfeeding. Relative contraindications include unmanageable medical risk and urinary contamination in the radiation-isolation room, decreased renal function with glomerular filtration (GFR) rate less than 30 mL/min, and myelosuppression with a white blood cell count less than 3000/μL or platelet count less than 100,000/μL. In the JSNM draft guidelines, life expectancy less than 1 month and decreased renal function with GFR less than 30 mL/min are included in the absolute contraindications [17].
Thyroid Blockage for I-131 MIBG Therapy
Free I-131, which is dissociated from I-131 MIBG, may accumulate in thyroid gland and cause hypothyroidism by irradiation. To inhibit the accumulation of free I-131 in thyroid gland, thyroid blockage with stable iodine is important [18]. In the JSNM draft guidelines, the oral administration of 300 mg/day potassium iodide or 1.5 mL/day Lugol’s solution from 1 to 3 days before to 7 to 14 days after I-131 MIBG administration is recommended [17]. Capsules of oral iodine are desirable for children because of their less bitter taste. More aggressive regimens of thyroid blockage may reduce the risk of hypothyroidism after I-131 MIBG therapy. The combination use of thyroxine, methimazole, and potassium iodide and the combination use of potassium iodide and potassium perchlorate resulted in better protection of thyroid function in patients with NB [19, 20].
Toxicities of I-131 MIBG Therapy
The most important toxicity of I-131 MIBG therapy is hematotoxicity which occurs dose-dependently at I-131 MIBG doses. The hematotoxicity usually appears a few weeks after I-131 MIBG administration and continues for a few months. The toxicity is more frequent in patients with bone marrow metastases and a higher whole-body radiation dose [21]. The maximum tolerated dose of I-131 MIBG therapy without hematopoietic cell transplantation (HCT) is considered as 444 MBq/kg (12 mCi/kg) in patients with NB [22, 23]. When considering I-131 MIBG therapy at a dose of more than 444 MBq/kg (12 mCi/kg), hematopoietic cell support should be prepared. In patients with NB, about one third of them treated with 666 MBq/kg (18 mCi/kg) required HCT [23]. In patients with PCPGs, severe hematotoxicities, which corresponded to grade 3 or 4 by the common terminology criteria for adverse events (CTCAE) version 4.0, were seen in more than 80% of 50 patients treated with the median 444 MB/kg (12 mCi/kg) of I-131 MIBG, and HCT was needed in 8% of them [24].
Common acute non-hematological toxicities include anorexia, nausea, and sialadenitis and usually occur within a few days after I-131 MIBG administration [25]. Most of them are mild and controllable. In a report of I-131 MIBG therapy at a dose of 157 to 804 MBq/kg (4.2 to 21.7 mCi/kg) for 66 patients with newly diagnosed NB, nausea and vomiting without exceeding grade II by the CTCAE version 4.0 were observed in 11 and 21% [26]. In a report from the USA, sialadenitis were observed in 5 of 10 patients with NB or PC within 24 h after 444 to 666 MBq/kg (12 to 18 mCi/kg) I-131 MIBG administrations [27]. However, all patients did not develop subsequent dry mouth and dysphagia. Severe non-hematological toxicities are relatively uncommon; however, they are potentially lethal, especially in patients treated with higher doses of I-131 MIBG. In a report of high-dose I-131 MIBG therapy for 50 patients with metastatic PCPGs, some severe non-hematological toxicities including two acute respiratory distress syndromes (ARDSs), two bronchiolitis obliterans-organizing pneumonias (BOOPs), and one pulmonary embolism were observed [24]. A patient with ARDS died after multiple organ failure. When considering high-dose I-131 MIBG therapy, physicians have to pay careful attention to not only hematological but also non-hematological toxicities.
Hypothyroidism is a typical late toxicity, despite thyroid blockage with potassium iodine. In a report from the Netherlands, 22 of 42 patients with NB presented thyrotropin elevation and 8 patients required thyroxine replacement after a mean of 1.4 years of I-131 MIBG therapy [28]. The same institution reported that 8 of 16 survivors with NB developed hypothyroidism required with thyroxine after a median of 15.5 years of I-131 MIBG therapy [29].
The cumulative incidences of secondary malignancies after the first I-131 MIBG therapy were 7.6% at 5 years and 14.3% at 10 years in patients with advanced NB [30]. These results were similar to those of myeloablative therapy. Leukemia, angiomatoid fibrous histiocytoma, schwannoma, and rhabdomyosarcoma were reported after I-131 MIBG therapy in NB patients [31, 32]. Because most patients who receive I-131 MIBG therapy have been treated with several other intensive therapies before and after I-131 MIBG therapy, it is difficult to evaluate the effect of I-131 MIBG therapy on secondary malignancies.
I-131 MIBG Therapy for NB
NB derives from embryonic neural crest cells that potentially differentiate into sympathetic nerve and arises from the tissue of the sympathetic nervous system typically from adrenal medulla and extra-adrenal paraganglia [33]. The tumor is the most frequent extracranial malignant solid tumor in childhood. It accounts for 8 to 10% of all childhood malignant tumors and annually occurs in 1.02 cases per 100,000 children under 15 years of age [34]. More than one third and more than 90% of the patients are diagnosed before 1 and 5 years of age, and the median age at diagnosis is 18.8 months [35, 36]. About half of the patients have hematogenous metastases such as bone, bone marrow, liver, and lymph nodes [37]. Patients with an age greater than 12 or 18 months at diagnosis, metastatic lesions, and MYCN amplification have more unfavorable outcomes [35, 37, 38]. Despite the current intensive treatment with induction, consolidation and post-consolidation therapies, the 5-year overall survival (OS) rate in patients with high-risk NB is less than 50% [39, 40]. In patients with refractory or relapsed NB, the 5-year OS rate is even worse at less than 20% [41].
As NB originates in sympathetic nerve-related neural crest cells, the tumor has high expression of the NE transporter and approximately 90% of them have MIBG uptake in their cells [42, 43]. Therefore, radiolabeled MIBG is reasonable for the therapy of NB. Since the first I-131 therapy for NB was performed [5], many studies of monotherapy with I-131 MIBG for refractory or relapsed NB have shown that the objective response rates were 0 to 57% (Table 1) [22, 23, 44–47]. Compared to lower doses of I-131 MIBG, higher doses tend to achieve more favorable responses. A Dutch phase II study with 3.7 to 7.4 GBq (100 to 200 mCi) of I-131 MIBG for 53 patients with refractory or relapsed NB reported that the objective response rate was 57%, and 7 of 53 patients could achieve complete responses (CR) [44]. In a French phase II study, 26 patients with refractory or relapsed NB received 1.1 to 4.0 GBq (30 to 108 mCi) of I-131 MIBG per each therapy [45]. Despite obtaining pain reduction in 50% of the patients, the objective response rate was 0%. In an Israeli study with a dose of 185 MBq/kg (5 mCi/kg) per each therapy for 10 symptomatic patients with refractory NB, pain reductions were obtained in 90 and 75% of patients at the first and the second therapies without anticipated toxicities [48]. Though lower doses of I-131 MIBG therapy obtain fewer objective responses, the method is safe and useful for the aim of disease palliation for symptomatic patients with refractory and relapsed NB. In a dose escalation phase I study with each dose of 111 to 666 MBq/kg (3 to 18 mCi/kg) for 30 patients with refractory or relapsed NB, the most important toxicity was myelosuppression, and the maximum tolerated dose without hematopoietic cell support was 444 MBq/kg (12 mCi/kg) [22]. Objective responses were achieved in 37% of all the 30 patients. The doses at 444 MBq/kg (12 mCi/kg) or higher had improved responses compared to the lower doses. In a phase II study in the USA for patients with refractory or relapsed NB, 16 patients without HCT and 148 patients with HCT were treated with 444 MBq/kg (12 mCi/kg) and 666 MBq/kg (18 mCi/kg) I-131 MIBG [23]. The objective responses were 25 and 37% in patients treated with a dose of 444 MBq/kg (12 mCi/kg) and 666 MBq/kg (18 mCi/kg). The 1-year event-free survival (EFS) rate and OS rate were 18 and 49%, respectively. The EFS rate was significantly longer in patients with objective responses at I-131 MIBG therapy, older age, and fewer prior treatments. As a single therapy, I-131 MIBG therapy is one of the highest effective procedures in patients with refractory or relapsed NB.
Table 1.
Results of I-131 MIBG therapy for neuroblastoma
| Ref. No. | No. of pts | Single dose of I-131 MIBG therapy | Combination therapy | Response (%) | Survival | ||
|---|---|---|---|---|---|---|---|
| CR + PR | CR | SR | |||||
| Monotherapy of I-131 MIBG therapy | |||||||
| 38 | 53 | 3.7–7.4 GBq | – | 57 | 13 | – | – |
| 39 | 24 | 1.1–4.0 GBq | – | 0 | – | 50 | – |
| 41 | 43 | 2.5–5.5 GBq | – | 30 | 2 | – | Median OS: 59 m |
| 42 | 10 | 185 MBq/kg | – | – | – | 90 | – |
| 19 | 30 | 111 to 666 MBq/kg (dose escalation) | – | 37 | 3 | – | – |
| 20 | 16 | 444 MBq/kg without HCT | – | 25 | 6 | – | 1-year OS = 49%, 2-year OS = 29% |
| 148 | 666 MBq/kg with HCT | – | 37 | 8 | – | ||
| Combination with I-131 MIBG therapy and other therapy | |||||||
| 43 | 16 | 7.4 GBq | Cisplatin and cyclophosphamide with/without etoposide and vincristine | 75 | – | – | – |
| 45 | 32 | 555 or 666 MBq/kg | Vincristine and irinotecan | 28 | 16 | – | – |
| 46 | 27 | 296 to 666 MBq/kg (dose escalation) | Vorinostat | 12 | – | – | – |
| 47 | 19 | 444 or 666 MBq/kg | Arsenic trioxide | 0 | 0 | – | 5-year OS = 37% |
| 53 | 24 | 444 to 666 MBq/kg (dose escalation) | Carboplatin, etoposide, melphalan | 27 | 5 | – | 3-year OS = 58% |
| 55 | 8 | 666 MBq/kg | Busulfan, melphalan | 63 | 38 | – | – |
CR complete response, PR partial response, SR symptomatic response, OS overall survival, HCT hematopoietic cell transplantation
To improve the therapeutic effect, several groups attempted the combination use of I-131 MIBG and other therapies such as radiation sensitizer, myeloablative chemotherapy, and HCT (Table 1). An Italian group treated refractory or relapsed NBs with cisplatin and cyclophosphamide with or without etoposide and vincristine in addition to 7.4 GBq (200 mCi) I-131 MIBG [49]. Objective responses were obtained in 75% of 16 patients. The main toxicity was myelosuppression associated with the chemotherapy. Regardless of the relatively low dose of the study, the result was superior to the results of I-131 MIBG monotherapies. The New Approaches to Neuroblastoma Therapy (NANT) consortium tried to combine some chemotherapy agents with I-131 MIBG. A phase I and I/II study with vincristine, irinotecan, and I-131 MIBG and a phase I study with vorinostat and I-131 MIBG were reported [50–52]. The combined uses with 666 MBq/kg (18 mCi/kg) I-131 MIBG and these agents were well tolerable with controllable toxicities. Now, a phase II randomized study of I-131 MIBG alone, I-131 MIBG with vincristine and irinotecan, and I-131 MIBG with vorinostat is in progress (Clinical Trials. Gov identifier: NCT02035137). In a phase II study from the USA [53], 19 patients with refractory or relapsed NB were treated with 444 MBq/kg (12 mCi/kg) or 666 MBq/kg (18 mCi/kg) I-131 MIBG and arsenic trioxide, which was preclinically proven to act as a radiation sensitizer of several tumors such as glioblastoma and fibrosarcoma [54, 55]. The combination therapy was well tolerated without unanticipated toxicity. However, there was no radiation-sensitizing effect on I-131 MIBG therapy in patients with NB. After several pilot and feasibility studies of I-131 MIBG therapy with myeloablative chemotherapy supported by autologous HCT (auto-HCT) [56–58], a phase I dose escalation study from the NANT consortium evaluated the feasibility and effectivity of I-131 MIBG therapy with myeloablative chemotherapy in patients with refractory NB [59]. Twenty-four patients with refractory NB were treated with an escalation dose of I-131 MIBG on day − 21 along with carboplatin, etoposide, and melphalan (CEM) on days − 7 to − 4 supported by auto-HCT on day 0. The maximum tolerated doses in patients with normal renal function were 444 MBq/kg (12 mCi/kg) of I-131 MIBG, 1500 mg/m2 of carboplatin, 1200 mg/m2 of etoposide, and 210 mg/m2 of melphalan. The estimated 3-year EFS rate and OS rate were 31 and 58%, respectively. In a phase II study from the same group, 50 patients with high-risk NB were divided into efficacious and inefficacious cohorts after induction therapy [60]. They were treated with I-131 MIBG therapy on day − 21 along with CEM on days − 7 to − 4 and auto-HCT on day 0. Objective responses were seen in 38% of all 8 patients in the efficacious group and in 10% of the evaluable 41 patients in the inefficacious group. The 3-year EFS rate and OS rate were 38 and 75% in the efficacious group and 20 and 62% in the inefficacious group, respectively. There was no statistical difference of EFS and OS between the two groups. A USA group reported the combination therapy with I-131 MIBG, busulfan, melphalan, and auto-HCT [61]. Eight patients with refractory NB were treated with 666 MBq/kg (18 mCi/kg) I-131 MIBG on day − 13 and auto-HCT on day 0. After 6 to 8 weeks, they received busulfan on days − 6 to − 2, melphalan on day − 1, and auto-HCT on day 0. After the combination therapy, three CRs and two partial responses (PRs) were obtained in the evaluable seven patients. The combined use with I-131 MIBG and other therapies may provide additional benefit for refractory and relapsed NB. Recently, a USA group reported that I-131 MIBG therapy may wield more favorable effect for refractory NB than relapsed NB [62].
Some groups tried to use I-131 MIBG therapy for newly diagnosed NB. In a pilot study from Italy, 13 patients with newly diagnosed advanced NB received the combined therapy with chemotherapy and I-131 MIBG therapy [63]. Two CRs, six very good PRs, four PRs, and one mixed response were observed without non-hematologic toxicity. European groups evaluated the efficacy of upfront I-131 MIBG therapy and chemotherapy in patients with newly diagnosed high-risk NB. Patients were treated with two cycles of I-131 MIBG and topotecan before the standard induction therapy, surgery, and myeloablative chemotherapy supported by auto-HCT [64]. The objective response rate after auto-HCT was 57% of 16 patients, and the 10-year OS rate was only 6%. A similar European group evaluated the efficacy of two upfront cycles of I-131 MIBG therapy followed by German Pediatric Oncology Group 2004 Neuroblastoma protocol, myeloablative therapy, and auto-HCT in patients with newly diagnosed high-risk NB [65]. The objective response rates after I-131 MIBG therapy and auto-HCT were 38 and 71%, respectively. They concluded that the induction therapy with upfront I-131 MIBG was effective and tolerable in patients with newly diagnosed high-risk NB. A Korean group incorporated MIBG therapy at a dose of 444 MBq/kg (12 mCi/kg) or 666 MBq/kg (18 mCi/kg) into tandem myeloablative chemotherapy and auto-HCT in patients with newly diagnosed high-risk NB [66]. The study replaced only I-131 MIBG therapy with total body irradiation (TBI) in the previous SMC NB-2004 study from the same group in order to reduce toxicities [67]. The 5-year EFS rate and OS rate were 58 and 72%, respectively. Compared with TBI, I-131 MIBG therapy incorporated into tandem myeloablative chemotherapy and auto-HCT could achieve an equivalent survival rate with lower toxicities. To clarify the efficacy of I-131 MIBG therapy incorporated in the treatment regimen of newly diagnosed NB, further studies are needed.
Recently, peptide receptor radionuclide therapy (PRRT) with radioactive somatostatin analogs such as Lutetium-177 (Lu-177) DOTA-TATE has been developed for neuroendocrine tumors. Some studies demonstrated their favorable efficacy in patients with refractory or relapsed NB [68, 69]. Compared to PRRT, I-131 MIBG therapy has the advantage in more evidence and longer experience for patients with NB.
I-131 MIBG Therapy for PCPGs
PCPGs are tumors of chromaffin tissues derived from neural crest. PC arises from the adrenal medulla and PG from the extra-adrenal tissue mainly along with the sympathetic and parasympathetic nervous system [70]. Most PCPGs without parasympathetic PG secrete catecholamines which cause various symptoms such as paroxysmal or sustained hypertension, headaches, palpitation, and obstipation. PCPGs are very rare and the annual incidence of both tumors is approximately 0.2 to 1.0 per 100,000 [71–73]. The tumors are mostly diagnosed in the third to fifth decade and are equally common in men and women [74]. About 10% of patients with PC have metastases at their diagnosis, and patients with PG have it more frequently [75, 76]. The 5-year OS rate of metastatic PCPGs is approximately 50% and the 10-year OS rate is 25% [77, 78]. In the 2017 World Health Organization classification of endocrine tumors, benign PC and malignant PC were combined into a single section “PC,” because both benign and malignant PCs have the same histological findings and could have the same metastatic potential [79]. Thus, the term “malignant” is replaced with “metastatic” in patients with metastatic lesions. The change in terminology applies to PG.
PCPGs take MIBG in their cells through NET and store MIBG mainly in their neuroendocrine granules, and thus approximately 90% of PC and 70% of PG have MIBG uptake in their cells [12, 80]. I-131 MIBG therapy has been widely used for metastatic or unresectable MIBG-avid PCPGs. Several groups have tried single or repeated therapy with a variable dose of I-131 MIBG and reported that objective, hormonal, and symptomatic response rates ranged from 0 to 63%, 10 to 71%, and 23 to 90%, respectively (Table 2) [24, 81–90].
Table 2.
Results of I-131 MIBG therapy for pheochromocytoma and paraganglioma
| Ref. no. | No. of pts | I-131 MIBG therapy | Response (%) | Survival | ||||
|---|---|---|---|---|---|---|---|---|
| Single dose (GBq) | No. of doses | OR | HR | SR | OCR | |||
| Low dose of I-131 MIBG therapy | ||||||||
| 73 | 28 | 3.6–11.1 | 1–6 | 29 | 43 | – | – | – |
| 74 | 116 | 3.6–11.1 (Mean 5.8) | 1–11 (Mean 3.3) | 30 | 45 | 76 | 4 | – |
| 75 | 10 | – (Mean 5.4) | 1–4 (Mean 2.1) | 30 | 50 | 50 | 0 | – |
| 76 | 26 | 3.7 or 7.4 (Median 7.4) | 1–6 | 0 | 36 | 52 | 0 | 5-year OS = 50% |
| 78 | 22 | 5.0–11.3 (Mean 10.0) | 1–5 (Median 2) | 19 | 10 | 23 | – | – |
| Mixed dose of I-131 MIBG therapy | ||||||||
| 79 | 33 | – (Mean 14.5) | 1–6 (Median 1) | 38 | 60 | 86 | – | 5-year OS = 50% |
| 80 | 19 | 6.8–25.9 (Median 7.4) | 1–10 (Median 3) | 47 | 67 | 89 | 0 | Median OS = 42 m |
| High dose of I-131 MIBG therapy | ||||||||
| 81 | 12 | 14.3–32.0 (Median 29.6) | 1–3 (Median 1.5) | 36 | 71 | 90 | 17 | – |
| 82 | 30 | 20.6–43.8 (Median 30.8) | 1 | 63* | – | 13 | 5-year OS = 75% | |
| 21 | 50 | 18.2–42.9 (Median 30.3) | 1–3 (Median 1) | 27 | 35 | – | 9 | 5-year OS = 64% |
OR objective response which means objective complete response plus objective partial response, HR hormonal response, SR symptomatic response, OCR overall complete response, OS overall survival
*Rate with both OR and HR
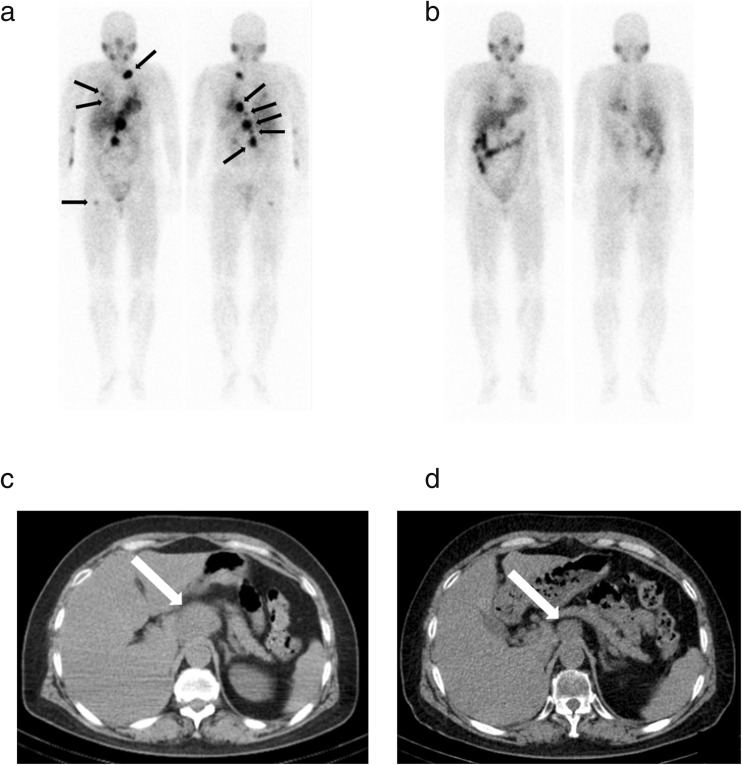
Though low-dose I-131 MIBG therapy, which was approximately less than 11.1 GBq (300 mCi), could not achieve a satisfactory objective response ranged from 0 to 30% and seldom achieved CR, the method could achieve a comparatively high hormonal response rate ranging from 10 to 50% and a symptomatic response rate ranging from 23 to 76% without severe hematological and non-hematological toxicities (Table 2) [81–84, 86]. In a report from the USA, 116 patients with metastatic PCPGs were treated with a single dose of I-131 MIBG ranging from 3.6 to 11.1 GBq (96 to 300 mCi) [82]. Objective, hormonal, and symptomatic response rates were 30, 45, and 76%, respectively, and five patients obtained complete objective and hormonal responses. Patients with soft tissue metastases had better therapeutic effects than those with bone metastases. Our group treated 26 metastatic PCPGs with a single dose of 3.7 GBq (100 mCi) or 7.4 GBq (200 mCi) of I-131 MIBG and obtained hormonal responses in 36% and symptomatic responses in 52% [84]. Though the objective response rate after the first therapy was zero, 85% of patients could be maintained in a stable condition, and the 5-year OS rate after I-131 MIBG therapy of the study was 50%. We demonstrated that no hormonal progressive disease (PD), no objective PD, no symptoms from bone metastases, and multiple times of I-131 MIBG therapy were good prognostic factors of low-dose I-131 MIBG therapy for patients with metastatic PCPGs. Because of low frequency of severe hematological and non-hematological toxicities, patients with metastatic PCPGs can receive repeated low-dose of I-131 MIBG therapy in safety and obtain long-term disease control [91]. Figure 1 shows the representative case with good therapeutic effect of repeated low-dose I-131 MIBG therapies.
Fig. 1.
A 57-year-old female with metastatic paraganglioma receives three cycles of I-131 MIBG therapy. Each dose is 7.4 GBq (200 mCi). A left supraclavicular lymph node metastasis, multiple para-aortic lymph node metastases, bilateral lung metastases, and a right femur metastasis are detected with I-123 MIBG scintigram before the first therapy (a black arrows). I-123 MIBG scintigram after the third therapy shows decreasing uptakes in all lesions (b). Plain CT images before the first therapy (c) and after the third therapy (d) show decrease in size of a para-aortic lymph node swelling (white arrows)
Compared to low-dose I-131 MIBG therapy, high-dose I-131 MIBG therapy may achieve better therapeutic responses (Table 2) [24, 89, 90]. A USA group demonstrated that more than 18.5 GBq (500 mCi) doses of I-131 MIBG at their first therapies improved patient survival [87]. They also reported that hormonal and symptomatic responses were good prognostic factors of I-131 MIBG therapy. In a phase II study of high-dose I-131 MIBG therapy for 30 patients with metastatic PCPGs, a single dose of I-131 MIBG which ranged from 20.6 to 43.8 GBq (557 to 1185 mCi) (median, 444 MBq/kg (12 mCi/kg)) achieved therapeutic responses in 63% of subjects including CR in 13% [90]. The 5-year OS rate after I-131 MIBG therapy was 75%. CR rates of high-dose I-131 MIBG therapy were higher than that of low-dose I-131 MIBG therapy. However, severe hematological toxicities were seen in over 70% of patients, and some severe non-hematological toxicities such as ARDS and BOOP were observed [24, 89, 90].
A USA group tried to combine I-131 MIBG therapy and chemotherapy with cyclophosphamide, dacarbazine, and vincristine for six metastatic PCs [92]. Although the combination therapy provided additional therapeutic effect, the therapy increased toxicity and there was difficulty maintaining the planned chemotherapy protocol. Further evaluation of the availability of the combination therapy with I-131 MIBG and other therapies is needed.
There is no consensus about the dosage and the frequency of I-131 MIBG therapy for patients with PCPGs. There is little evidence on the combination use of I-131 MIBG and other therapies. However, I-131 MIBG therapy is effective in preventing disease progression and reducing symptoms derived from hormonal abnormality and metastatic lesions in patients with metastatic PCPGs.
As with PRRT for NB, several groups reported the favorable efficacy of PRRT for patients with PCPGs [93–95]. A UK group reported that PRRT with Lu-177 DOTA-TATE or Yttrium-90 (Y-90) DOTA-TATE achieved increased OS and EFS compared to I-131 MIBG therapy in patients with progressive or metastatic PG [95]. In the near future, PRRT may replace I-131 MIBG therapy as the first-line radionuclide therapy in patients with somatostatin receptor-positive PCPGs.
I-131 MIBG Therapy for Carcinoid Tumors
Carcinoid tumors derive from enterochromaffin or Kulchitsky’s cells of the aerodigestive tract and are mainly located in the gastrointestinal tract and bronchopulmonary system [96]. Now, carcinoid tumors generically correspond to well-differentiated neuroendocrine tumors and mainly arise from the aerodigestive tract. The incidence of the tumors was reported as 4.7 and 4.4 per 100,000 from the USA and Sweden [97, 98]. Some carcinoid tumors typically derive from embryonic midgut secrete vasoactive substances such as serotonin, histamine, bradykinin, and prostaglandins [99]. These products cause carcinoid syndrome such as cutaneous flushing, venous telangiectasia, diarrhea, and bronchospasm. In a report from the USA, 19% of 9512 patients with neuroendocrine tumors had carcinoid syndrome [100]. Carcinoid tumors have the strong potential to metastasize [101]. The most metastatic site is liver and other sites are mesentery, retroperitoneum, bone, etc. The 5-year OS rate of metastatic carcinoid tumors was reported as 39% [102].
Carcinoid tumors theoretically take I-131 MIBG into their cells through the NET system [12]; however, the uptake rates of I-131 MIBG in carcinoid tumors are not so high. The sensitivity of I-123 MIBG scintigraphy was reported as 36 to 61% [103–105]. On the other hand, somatostatin analogs such as Indium-111 pentetreotide and Gallium-68 DOTA-TATE are more sensitive in the detection of carcinoid tumors [104–106]. As with diagnostic agents, therapeutic radioactive somatostatin analogs such as Lu-177 DOTA-TATE and Y-90 DOTA-TOC are more favorable to the therapy in patients with carcinoid tumors. Several studies have demonstrated the remarkable efficacy of newer somatostatin analogs in patients with neuroendocrine tumors including carcinoid tumors [107–109]. Thus, the interest in I-131 MIBG therapy for carcinoid tumors has gradually subsided. Nonetheless, I-131 MIBG therapy remains a good alternative therapeutic choice, for example, in patients with absolute or relative contraindications for the therapy with radioactive somatostatin analogs because of impaired renal function or other reasons [110].
Since the first I-131 MIBG therapy for carcinoid tumors was reported in 1986 [6], several groups have tried I-131 MIBG therapy for the tumors and reported objective, biochemical, and symptomatic response rates which ranged from 0 to 28%, 29 to 37%, and 49 to 82%, respectively, and 5-year OS rates which ranged from 22 to 63% [111–114]. A USA group treated 98 patients with metastatic carcinoid tumors with I-131 MIBG [112]. The median initial I-131 MIBG dose was 11.3 GBq (306 mCi) and the median total dose was 14.8 GBq (401 mCi). Objective, biochemical, and symptomatic response rates were 15, 37, and 49, respectively, and the 5-year OS rate was 22%. Patients with more than 14.8 GBq (400 mCi) of I-131 MIBG at their initial therapies and symptomatic responses after the therapy had better survival rates. In a German study, 20 patients with metastatic carcinoid tumors received 11.1 GBq (300 mCi) I-131 MIBG administrations and achieved 82% of symptomatic responses [114]. Though the objective response rate was zero, 80% of patients could be maintained at stable disease (SD) level. In carcinoid tumors, the main effect of I-131 MIBG therapy is symptomatic reduction and prevention of disease progression.
I-131 MIBG Therapy for MTC
MTC is a tumor of parafollicular C-cell derived from embryonic neural crest and accounts for 1 to 2% of all thyroid cancers [115, 116]. Approximately one third of the tumors are hereditary diseases, which occur in the form of the multiple neuroendocrine neoplasia type 2 [116, 117]. The tumor secretes calcitonin and other substances, which cause diarrhea, facial flushing, and Cushing’s syndrome, especially in advanced patients. Distant metastases are seen in 5 to 10% of the patients and mainly occur in the liver, lung, and bone [116, 118]. Disease stage is one of the important prognostic factors. In patients with MTC, 10-year OS rates with stage I–IV were 100, 93, 71, and 21%, respectively [119].
Though the indication of I-131 MIBG therapy for MTC is described as unresectable or a metastatic state in the guidelines [16, 17], there have been few reports of I-131 MIBG therapy for MTC because of its rare frequency and its relatively low MIBG uptake rate in patients with MTC [120, 121]. An Italian group treated 13 MTC patients with I-131 MIBG at the total dose of 16.6 to 50.0 GBq (448 to 1350 mCi) and obtained PR in 31% and SD in 31% [121]. A Chinese group treated three MTC patients with one to three administrations of 11.1 GBq (300 mCi) I-131 MIBG and obtained one PR and to SDs without severe hematological and non-hematological toxicities [122]. In a case report, a MTC patient with two liver metastases was treated with 5.6 GBq (150 mCi) of I-131 MIBG and achieved PR for 10 years [123]. Though there have been no prospective and randomized trials of I-131 MIBG therapy in patients with MTC, these reports indicate the beneficial potential of I-131 MIBG therapy. Recently, some tyrosine kinase inhibitors (TKIs) such as cabozantinib and vandetanib have been widely approved as effective and are recommended in patients with advanced or symptomatic MTC. I-131 MIBG therapy could be an alternative method in patients with resistance or contraindication to TKIs.
No-Carrier-Aided I-131 MIBG
I-131 MIBG, which is synthesized by conventional method, includes non-radiolabeled MIBG molecules in high proportions. Non-radiolabeled MIBG competes against I-131 MIBG in NET and could reduce the therapeutic effect of I-131 MIBG therapy [124]. Some groups produced NCA I-131 MIBG and proved its favorable accumulations in tumor cells compared to conventional I-131 MIBG in preclinical settings [125, 126]. Based on a phase I study from the USA in patients with PCPGs and carcinoid tumors [127], 15 patients with refractory or relapsed NB were treated with NCA I-131 MIBG at an escalation dose of 444 to 666 MBq/kg (12 to 18 mCi/kg) supported by HCT in a phase II study [128]. All patients could achieve the therapy without dose-limiting toxicity, and objective responses were obtained in 27% of the patients. A phase II study of I-131 Iobenguane in patients with metastatic PCPGs is now ongoing (Clinical Trials. Gov identifier: NCT02961491).
Astatine-211 (At-211)-Labeled Meta-astatobenzylguanidine
At-211 emits alpha particles with high linear energy transfer within a very short range of only a few cell diameters in tissues [129]. Compared to beta particles, alpha particles are more suitable for the radionuclide therapy theoretically for small tumors because of their shorter range, higher linear energy transfer, and stronger cytotoxicity. A clinical pilot study with At-211 labeled antitenascin monoclonal antibodies in patients with brain tumors demonstrated their feasibility, safety, and efficacy without severe toxicities [130]. At-211-labeled meta-astatobenzylguanidine (At-211 MABG) was developed and proven to have stronger cytotoxicity compared to I-131 MIBG in human NB cells with NETs expressions [131, 132]. For more convenient use of At-211 MABG, a kit method of the synthesis of At-211 MABG also has been developed [133]. However, there have been no clinical studies of At-211 MABG for therapeutic use. Further studies both in clinical and preclinical settings related to At-211 MABG are needed.
Conclusion
A number of studies about I-131 MIBG therapy indicate their efficacy, especially in patients with advanced NB and PCPGs. Though other newer therapies such as radiolabeled somatostatin analogs for advanced carcinoid tumors and TKIs for advanced MTC have become common, I-131 MIBG therapy remains a good alternative therapeutic option in patients with advanced carcinoid tumors and MTC. Further studies about therapeutic protocols of I-131 MIBG including NCA I-131 MIBG in the clinical setting and At-211 MABG in both the preclinical and clinical settings are needed.
Compliance with Ethical Standards
Conflict of Interest
Daiki Kayano and Seigo Kinuya declare no conflict of interest.
Ethical Statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Not applicable.
References
- 1.Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergi neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med. 1980;21:349–353. [PubMed] [Google Scholar]
- 2.McEwan AJ, Shapiro B, Sisson JC, Beierwaltes WH, Ackery DM. Radio-iodobenzylguanidine for the scintigraphic location and therapy of adrenergic tumors. Semin Nucl Med. 1985;15:132–153. doi: 10.1016/s0001-2998(85)80022-2. [DOI] [PubMed] [Google Scholar]
- 3.Nakajo M, Shapiro B, Copp J, Kalff V, Gross MD, Sisson JC, et al. The normal and abnormal distribution of the adrenomedullary imaging agent m-[I-131]iodobenzylguanidine (I-131 MIBG) in man: evaluation by scintigraphy. J Nucl Med. 1983;24:672–682. [PubMed] [Google Scholar]
- 4.Sisson J, Shapiro B, Beierwaltes WH, Nakajo M, Glowniak J, Mangner T, et al. Treatment of malignant pheochromocytoma with a new radiopharmaceutical. Trans Assoc Am Phys. 1983;96:209–217. [PubMed] [Google Scholar]
- 5.Treuner J, Klingebiel T, Feine U, Buck J, Bruchelt G, Dopfer R, et al. Clinical experiences in the treatment of neuroblastoma with 131I-metaiodobenzylguanidine. Pediatr Hematol Oncol. 1986;3:205–216. doi: 10.3109/08880018609031220. [DOI] [PubMed] [Google Scholar]
- 6.Hoefnagel CA, Den Hartog Jager FC, Van Gennip AH, Marcuse HR, Taal BG. Diagnosis and treatment of a carcinoid tumor using iodine-131 meta-iodobenzylguanidine. Clin Nucl Med. 1986;11:150–152. doi: 10.1097/00003072-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Jaques S, Jr, Tobes MC, Sisson JC, Baker JA, Wieland DM. Comparison of the sodium dependency of uptake of meta-lodobenzylguanidine and norepinephrine into cultured bovine adrenomedullary cells. Mol Pharmacol. 1984;26:539–546. [PubMed] [Google Scholar]
- 8.Tobes MC, Jaques S, Jr, Wieland DM, Sisson JC. Effect of uptake-one inhibitors on the uptake of norepinephrine and metaiodobenzylguanidine. J Nucl Med. 1985;26:897–907. [PubMed] [Google Scholar]
- 9.Jaques S, Jr, Tobes MC, Sisson JC. Sodium dependency of uptake of norepinephrine and m-iodobenzylguanidine into cultured human pheochromocytoma cells: evidence for uptake-one. Cancer Res. 1987;47:3920–3928. [PubMed] [Google Scholar]
- 10.Buck J, Bruchelt G, Girgert R, Treuner J, Niethammer D. Specific uptake of m-[125I]iodobenzylguanidine in the human neuroblastoma cell line SK-N-SH. Cancer Res. 1985;45:6366–6370. [PubMed] [Google Scholar]
- 11.Smets LA, Loesberg C, Janssen M, Metwally EA, Huiskamp R. Active uptake and extravesicular storage of m-iodobenzylguanidine in human neuroblastoma SK-N-SH cells. Cancer Res. 1989;49:2941–2944. [PubMed] [Google Scholar]
- 12.Kolby L, Bernhardt P, Levin-Jakobsen AM, Johanson V, Wangberg B, Ahlman H, et al. Uptake of meta-iodobenzylguanidine in neuroendocrine tumours is mediated by vesicular monoamine transporters. Br J Cancer. 2003;89:1383–1388. doi: 10.1038/sj.bjc.6601276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson O, Jakobsen AM, Kolby L, Bernhardt P, Forssell-Aronsson E, Ahlman H. Importance of vesicle proteins in the diagnosis and treatment of neuroendocrine tumors. Ann N Y Acad Sci. 2004;1014:280–283. doi: 10.1196/annals.1294.032. [DOI] [PubMed] [Google Scholar]
- 14.Lashford LS, Hancock JP, Kemshead JT. Meta-iodobenzylguanidine (mIBG) uptake and storage in the human neuroblastoma cell line SK-N-BE(2C) Int J Cancer. 1991;47:105–109. doi: 10.1002/ijc.2910470119. [DOI] [PubMed] [Google Scholar]
- 15.Gaze MN, Huxham IM, Mairs RJ, Barrett A. Intracellular localization of metaiodobenzyl guanidine in human neuroblastoma cells by electron spectroscopic imaging. Int J Cancer. 1991;47:875–880. doi: 10.1002/ijc.2910470615. [DOI] [PubMed] [Google Scholar]
- 16.Giammarile F, Chiti A, Lassmann M, Brans B, Flux G. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur J Nucl Med Mol Imaging. 2008;35:1039–1047. doi: 10.1007/s00259-008-0715-3. [DOI] [PubMed] [Google Scholar]
- 17.Kinuya S, Yoshinaga K, Higuchi T, Jinguji M, Kurihara H, Kawamoto H. Draft guidelines regarding appropriate use of (131)I-MIBG radiotherapy for neuroendocrine tumors: guideline drafting Committee for Radiotherapy with (131)I-MIBG, Committee for Nuclear Oncology and Immunology, the Japanese Society of Nuclear Medicine. Ann Nucl Med. 2015;29:543–552. doi: 10.1007/s12149-015-0960-z. [DOI] [PubMed] [Google Scholar]
- 18.Verger P, Aurengo A, Geoffroy B, Le Guen B. Iodine kinetics and effectiveness of stable iodine prophylaxis after intake of radioactive iodine: a review. Thyroid. 2001;11:353–360. doi: 10.1089/10507250152039082. [DOI] [PubMed] [Google Scholar]
- 19.van Santen HM, de Kraker J, van Eck BL, de Vijlder JJ, Vulsma T. Improved radiation protection of the thyroid gland with thyroxine, methimazole, and potassium iodide during diagnostic and therapeutic use of radiolabeled metaiodobenzylguanidine in children with neuroblastoma. Cancer. 2003;98:389–396. doi: 10.1002/cncr.11523. [DOI] [PubMed] [Google Scholar]
- 20.Quach A, Ji L, Mishra V, Sznewajs A, Veatch J, Huberty J, et al. Thyroid and hepatic function after high-dose 131 I-metaiodobenzylguanidine (131 I-MIBG) therapy for neuroblastoma. Pediatr Blood Cancer. 2011;56:191–201. doi: 10.1002/pbc.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuBois SG, Messina J, Maris JM, Huberty J, Glidden DV, Veatch J, et al. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol. 2004;22:2452–2460. doi: 10.1200/JCO.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 23.Matthay KK, Yanik G, Messina J, Quach A, Huberty J, Cheng SC, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 24.Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27:4162–4168. doi: 10.1200/JCO.2008.21.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kayano D, Kinuya S. Iodine-131 metaiodobenzylguanidine therapy for neuroblastoma: reports so far and future perspective. ScientificWorldJournal. 2015;2015:189135. doi: 10.1155/2015/189135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleeker G, Schoot RA, Caron HN, de Kraker J, Hoefnagel CA, van Eck BL, et al. Toxicity of upfront (1)(3)(1)I-metaiodobenzylguanidine ((1)(3)(1)I-MIBG) therapy in newly diagnosed neuroblastoma patients: a retrospective analysis. Eur J Nucl Med Mol Imaging. 2013;40:1711–1717. doi: 10.1007/s00259-013-2510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modak S, Pandit-Taskar N, Kushner BH, Kramer K, Smith-Jones P, Larson S, et al. Transient sialoadenitis: a complication of 131I-metaiodobenzylguanidine therapy. Pediatr Blood Cancer. 2008;50:1271–1273. doi: 10.1002/pbc.21391. [DOI] [PubMed] [Google Scholar]
- 28.van Santen HM, de Kraker J, van Eck BL, de Vijlder JJ, Vulsma T. High incidence of thyroid dysfunction despite prophylaxis with potassium iodide during (131)I-meta-iodobenzylguanidine treatment in children with neuroblastoma. Cancer. 2002;94:2081–2089. doi: 10.1002/cncr.10447. [DOI] [PubMed] [Google Scholar]
- 29.Clement SC, van Eck-Smit BL, van Trotsenburg AS, Kremer LC, Tytgat GA, van Santen HM. Long-term follow-up of the thyroid gland after treatment with 131I-Metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatr Blood Cancer. 2013;60:1833–1838. doi: 10.1002/pbc.24681. [DOI] [PubMed] [Google Scholar]
- 30.Huibregtse KE, Vo KT, DuBois SG, Fetzko S, Neuhaus J, Batra V, et al. Incidence and risk factors for secondary malignancy in patients with neuroblastoma after treatment with (131)I-metaiodobenzylguanidine. Eur J Cancer. 2016;66:144–152. doi: 10.1016/j.ejca.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garaventa A, Gambini C, Villavecchia G, Di Cataldo A, Bertolazzi L, Pizzitola MR, et al. Second malignancies in children with neuroblastoma after combined treatment with 131I-metaiodobenzylguanidine. Cancer. 2003;97:1332–1338. doi: 10.1002/cncr.11167. [DOI] [PubMed] [Google Scholar]
- 32.Weiss B, Vora A, Huberty J, Hawkins RA, Matthay KK. Secondary myelodysplastic syndrome and leukemia following 131I-metaiodobenzylguanidine therapy for relapsed neuroblastoma. J Pediatr Hematol Oncol. 2003;25(7):543. doi: 10.1097/00043426-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Bonisch H, Bruss M. The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol. 2006;175:485–524. doi: 10.1007/3-540-29784-7_20. [DOI] [PubMed] [Google Scholar]
- 34.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s oncology group. J Clin Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 36.Voo S, Bucerius J, Mottaghy FM. I-131-MIBG therapies. Methods. 2011;55:238–245. doi: 10.1016/j.ymeth.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 38.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 39.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 40.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 41.Mueller S, Matthay KK. Neuroblastoma: biology and staging. Curr Oncol Rep. 2009;11:431–438. doi: 10.1007/s11912-009-0059-6. [DOI] [PubMed] [Google Scholar]
- 42.Treuner J, Feine U, Niethammer D, Muller-Schaumburg W, Meinke J, Eibach E, et al. Scintigraphic imaging of neuroblastoma with [131-I]iodobenzylguanidine. Lancet. 1984;1:333–334. doi: 10.1016/s0140-6736(84)90375-1. [DOI] [PubMed] [Google Scholar]
- 43.Carlin S, Mairs RJ, McCluskey AG, Tweddle DA, Sprigg A, Estlin C, et al. Development of a real-time polymerase chain reaction assay for prediction of the uptake of meta-[(131)I]iodobenzylguanidine by neuroblastoma tumors. Clin Cancer Res. 2003;9:3338–3344. [PubMed] [Google Scholar]
- 44.Hoefnagel CA, Voute PA, De Kraker J, Valdes Olmos RA. [131I]metaiodobenzylguanidine therapy after conventional therapy for neuroblastoma. J Nucl Biol Med. 1991;35:202–206. [PubMed] [Google Scholar]
- 45.Lumbroso J, Hartmann O, Schlumberger M. Therapeutic use of [131I]metaiodobenzylguanidine in neuroblastoma: a phase II study in 26 patients. “Societe Francaise d'Oncologie Pediatrique” and nuclear medicine co-investigators. J Nucl Biol Med. 1991;35:220–223. [PubMed] [Google Scholar]
- 46.Matthay KK, Huberty JP, Hattner RS, Ablin AR, Engelstad BL, Zoger S, et al. Efficacy and safety of [131I]metaiodobenzylguanidine therapy for patients with refractory neuroblastoma. J Nucl Biol Med. 1991;35:244–247. [PubMed] [Google Scholar]
- 47.Garaventa A, Bellagamba O, Lo Piccolo MS, Milanaccio C, Lanino E, Bertolazzi L, et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: a mono-institutional experience with 43 patients. Br J Cancer. 1999;81:1378–1384. doi: 10.1038/sj.bjc.6694223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weyl Ben-Arush M, Ben Barak A, Bar-Deroma R, Ash S, Goldstein G, Golan H, et al. Targeted therapy with low doses of 131I-MIBG is effective for disease palliation in highly refractory neuroblastoma. Isr Med Assoc J. 2013;15:31–34. [PubMed] [Google Scholar]
- 49.Mastrangelo S, Tornesello A, Diociaiuti L, Pession A, Prete A, Rufini V, et al. Treatment of advanced neuroblastoma: feasibility and therapeutic potential of a novel approach combining 131-I-MIBG and multiple drug chemotherapy. Br J Cancer. 2001;84(4):460. doi: 10.1054/bjoc.2000.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DuBois SG, Chesler L, Groshen S, Hawkins R, Goodarzian F, Shimada H, et al. Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res. 2012;18:2679–2686. doi: 10.1158/1078-0432.CCR-11-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuBois SG, Allen S, Bent M, Hilton JF, Hollinger F, Hawkins R, et al. Phase I/II study of (131)I-MIBG with vincristine and 5 days of irinotecan for advanced neuroblastoma. Br J Cancer. 2015;112:644–649. doi: 10.1038/bjc.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DuBois SG, Groshen S, Park JR, Haas-Kogan DA, Yang X, Geier E, et al. Phase I study of Vorinostat as a radiation sensitizer with 131I-Metaiodobenzylguanidine (131I-MIBG) for patients with relapsed or refractory neuroblastoma. Clin Cancer Res. 2015;21:2715–2721. doi: 10.1158/1078-0432.CCR-14-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Modak S, Zanzonico P, Carrasquillo JA, Kushner BH, Kramer K, Cheung NK, et al. Arsenic trioxide as a radiation sensitizer for 131I-metaiodobenzylguanidine therapy: results of a phase II study. J Nucl Med. 2016;57:231–237. doi: 10.2967/jnumed.115.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ning S, Knox SJ. Increased cure rate of glioblastoma using concurrent therapy with radiotherapy and arsenic trioxide. Int J Radiat Oncol Biol Phys. 2004;60:197–203. doi: 10.1016/j.ijrobp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Chiu HW, Lin JH, Chen YA, Ho SY, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances cell-killing effects in human fibrosarcoma cells in vitro and in vivo through induction of both autophagy and apoptosis. Autophagy. 2010;6:353–365. doi: 10.4161/auto.6.3.11229. [DOI] [PubMed] [Google Scholar]
- 56.Gaze MN, Wheldon TE, O'Donoghue JA, Hilditch TE, McNee SG, Simpson E, et al. Multi-modality megatherapy with [131I]meta-iodobenzylguanidine, high dose melphalan and total body irradiation with bone marrow rescue: feasibility study of a new strategy for advanced neuroblastoma. Eur J Cancer. 1995;31a:252–256. doi: 10.1016/0959-8049(94)e0036-4. [DOI] [PubMed] [Google Scholar]
- 57.Klingebiel T, Bader P, Bares R, Beck J, Hero B, Jurgens H, et al. Treatment of neuroblastoma stage 4 with 131I-meta-iodo-benzylguanidine, high-dose chemotherapy and immunotherapy. A pilot study. Eur J Cancer. 1998;34:1398–1402. doi: 10.1016/s0959-8049(98)00130-0. [DOI] [PubMed] [Google Scholar]
- 58.Miano M, Garaventa A, Pizzitola MR, Piccolo MS, Dallorso S, Villavecchia GP, et al. Megatherapy combining I(131) metaiodobenzylguanidine and high-dose chemotherapy with haematopoietic progenitor cell rescue for neuroblastoma. Bone Marrow Transplant. 2001;27:571–574. doi: 10.1038/sj.bmt.1702846. [DOI] [PubMed] [Google Scholar]
- 59.Matthay KK, Tan JC, Villablanca JG, Yanik GA, Veatch J, Franc B, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to neuroblastoma therapy consortium study. J Clin Oncol. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 60.Yanik GA, Villablanca JG, Maris JM, Weiss B, Groshen S, Marachelian A, et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant. 2015;21:673–681. doi: 10.1016/j.bbmt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 61.French S, DuBois SG, Horn B, Granger M, Hawkins R, Pass A, et al. 131I-MIBG followed by consolidation with busulfan, melphalan and autologous stem cell transplantation for refractory neuroblastoma. Pediatr Blood Cancer. 2013;60:879–884. doi: 10.1002/pbc.24351. [DOI] [PubMed] [Google Scholar]
- 62.Zhou MJ, Doral MY, DuBois SG, Villablanca JG, Yanik GA, Matthay KK. Different outcomes for relapsed versus refractory neuroblastoma after therapy with (131)I-metaiodobenzylguanidine ((131)I-MIBG) Eur J Cancer. 2015;51:2465–2472. doi: 10.1016/j.ejca.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mastrangelo S, Rufini V, Ruggiero A, Di Giannatale A, Riccardi R. Treatment of advanced neuroblastoma in children over 1 year of age: the critical role of (1)(3)(1)I-metaiodobenzylguanidine combined with chemotherapy in a rapid induction regimen. Pediatr Blood Cancer. 2011;56:1032–1040. doi: 10.1002/pbc.22986. [DOI] [PubMed] [Google Scholar]
- 64.Kraal KC, Tytgat GA, van Eck-Smit BL, Kam B, Caron HN, van Noesel M. Upfront treatment of high-risk neuroblastoma with a combination of 131I-MIBG and topotecan. Pediatr Blood Cancer. 2015;62:1886–1891. doi: 10.1002/pbc.25580. [DOI] [PubMed] [Google Scholar]
- 65.Kraal KC, Bleeker GM, van Eck-Smit BL, van Eijkelenburg NK, Berthold F, van Noesel MM, et al. Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy followed by German pediatric oncology group neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer. 2017;76:188–196. doi: 10.1016/j.ejca.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Lee JW, Lee S, Cho HW, Ma Y, Yoo KH, Sung KW, et al. Incorporation of high-dose 131I-metaiodobenzylguanidine treatment into tandem high-dose chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: results of the SMC NB-2009 study. J Hematol Oncol. 2017;10:108. doi: 10.1186/s13045-017-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung KW, Son MH, Lee SH, Yoo KH, Koo HH, Kim JY, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk neuroblastoma: results of SMC NB-2004 study. Bone Marrow Transplant. 2013;48:68–73. doi: 10.1038/bmt.2012.86. [DOI] [PubMed] [Google Scholar]
- 68.Gains JE, Bomanji JB, Fersht NL, Sullivan T, D'Souza D, Sullivan KP, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. 2011;52(7):1041. doi: 10.2967/jnumed.110.085100. [DOI] [PubMed] [Google Scholar]
- 69.Kong G, Hofman MS, Murray WK, Wilson S, Wood P, Downie P, et al. Initial experience with gallium-68 DOTA-Octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J Pediatr Hematol Oncol. 2016;38:87–96. doi: 10.1097/MPH.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 70.Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28:213–227. doi: 10.1007/s12022-017-9484-5. [DOI] [PubMed] [Google Scholar]
- 71.Beard CM, Sheps SG, Kurland LT, Carney JA, Lie JT. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin Proc. 1983;58:802–804. [PubMed] [Google Scholar]
- 72.Stenstrom G, Svardsudd K. Pheochromocytoma in Sweden 1958-1981. An analysis of the National Cancer Registry data. Acta Med Scand. 1986;220:225–232. [PubMed] [Google Scholar]
- 73.Andersen GS, Toftdahl DB, Lund JO, Strandgaard S, Nielsen PE. The incidence rate of phaeochromocytoma and Conn’s syndrome in Denmark, 1977-1981. J Hum Hypertens. 1988;2:187–189. [PubMed] [Google Scholar]
- 74.Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009;209:727–732. doi: 10.1016/j.jamcollsurg.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 75.Sutton MG, Sheps SG, Lie JT. Prevalence of clinically unsuspected pheochromocytoma. Review of a 50-year autopsy series. Mayo Clin Proc. 1981;56:354–360. [PubMed] [Google Scholar]
- 76.Ezzat Abdel-Aziz T, Prete F, Conway G, Gaze M, Bomanji J, Bouloux P, et al. Phaeochromocytomas and paragangliomas: a difference in disease behaviour and clinical outcomes. J Surg Oncol. 2015;112:486–491. doi: 10.1002/jso.24030. [DOI] [PubMed] [Google Scholar]
- 77.Amar L, Baudin E, Burnichon N, Peyrard S, Silvera S, Bertherat J, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–3828. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 78.Szalat A, Fraenkel M, Doviner V, Salmon A, Gross DJ. Malignant pheochromocytoma: predictive factors of malignancy and clinical course in 16 patients at a single tertiary medical center. Endocrine. 2011;39:160–166. doi: 10.1007/s12020-010-9422-5. [DOI] [PubMed] [Google Scholar]
- 79.Lloyd RV, Osamura RY, Klöppel G, Rosai J. WHO classification of tumours of endocrine organs. 4. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 80.Wiseman GA, Pacak K, O'Dorisio MS, Neumann DR, Waxman AD, Mankoff DA, et al. Usefulness of 123I-MIBG scintigraphy in the evaluation of patients with known or suspected primary or metastatic pheochromocytoma or paraganglioma: results from a prospective multicenter trial. J Nucl Med. 2009;50:1448–1454. doi: 10.2967/jnumed.108.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shapiro B, Sisson JC, Wieland DM, Mangner TJ, Zempel SM, Mudgett E, et al. Radiopharmaceutical therapy of malignant pheochromocytoma with [131I]metaiodobenzylguanidine: results from ten years of experience. J Nucl Biol Med. 1991;35:269–276. [PubMed] [Google Scholar]
- 82.Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Investig. 1997;20:648–658. doi: 10.1007/BF03348026. [DOI] [PubMed] [Google Scholar]
- 83.Shilkrut M, Bar-Deroma R, Bar-Sela G, Berniger A, Kuten A. Low-dose iodine-131 metaiodobenzylguanidine therapy for patients with malignant pheochromocytoma and paraganglioma: single center experience. Am J Clin Oncol. 2010;33:79–82. doi: 10.1097/COC.0b013e31819e2c28. [DOI] [PubMed] [Google Scholar]
- 84.Wakabayashi H, Taki J, Inaki A, Nakamura A, Kayano D, Fukuoka M, et al. Prognostic values of initial responses to low-dose (131)I-MIBG therapy in patients with malignant pheochromocytoma and paraganglioma. Ann Nucl Med. 2013;27:839–846. doi: 10.1007/s12149-013-0755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshinaga K, Oriuchi N, Wakabayashi H, Tomiyama Y, Jinguji M, Higuchi T, et al. Effects and safety of (1)(3)(1)I-metaiodobenzylguanidine (MIBG) radiotherapy in malignant neuroendocrine tumors: results from a multicenter observational registry. Endocr J. 2014;61:1171–1180. doi: 10.1507/endocrj.EJ14-0211. [DOI] [PubMed] [Google Scholar]
- 86.Rutherford MA, Rankin AJ, Yates TM, Mark PB, Perry CG, Reed NS, et al. Management of metastatic phaeochromocytoma and paraganglioma: use of iodine-131-meta-iodobenzylguanidine therapy in a tertiary referral Centre. QJM. 2015;108:361–368. doi: 10.1093/qjmed/hcu208. [DOI] [PubMed] [Google Scholar]
- 87.Safford SD, Coleman RE, Gockerman JP, Moore J, Feldman JM, Leight GS, Jr, et al. Iodine -131 metaiodobenzylguanidine is an effective treatment for malignant pheochromocytoma and paraganglioma. Surgery. 2003;134:956–962. doi: 10.1016/s0039-6060(03)00426-4. [DOI] [PubMed] [Google Scholar]
- 88.Gedik GK, Hoefnagel CA, Bais E, Olmos RA. 131I-MIBG therapy in metastatic phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2008;35:725–733. doi: 10.1007/s00259-007-0652-6. [DOI] [PubMed] [Google Scholar]
- 89.Rose B, Matthay KK, Price D, Huberty J, Klencke B, Norton JA, et al. High-dose 131I-metaiodobenzylguanidine therapy for 12 patients with malignant pheochromocytoma. Cancer. 2003;98:239–248. doi: 10.1002/cncr.11518. [DOI] [PubMed] [Google Scholar]
- 90.Fitzgerald PA, Goldsby RE, Huberty JP, Price DC, Hawkins RA, Veatch JJ, et al. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG) Ann N Y Acad Sci. 2006;1073:465–490. doi: 10.1196/annals.1353.050. [DOI] [PubMed] [Google Scholar]
- 91.Lam MG, Lips CJ, Jager PL, Dullaart RP, Lentjes EG, van Rijk PP, et al. Repeated [131I]metaiodobenzylguanidine therapy in two patients with malignant pheochromocytoma. J Clin Endocrinol Metab. 2005;90:5888–5895. doi: 10.1210/jc.2004-2290. [DOI] [PubMed] [Google Scholar]
- 92.Sisson JC, Shapiro B, Shulkin BL, Urba S, Zempel S, Spaulding S. Treatment of malignant pheochromocytomas with 131-I metaiodobenzylguanidine and chemotherapy. Am J Clin Oncol. 1999;22:364–370. doi: 10.1097/00000421-199908000-00008. [DOI] [PubMed] [Google Scholar]
- 93.Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52:334–340. [PubMed] [Google Scholar]
- 94.Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017;102:3278–3287. doi: 10.1210/jc.2017-00816. [DOI] [PubMed] [Google Scholar]
- 95.Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M, et al. Peptide receptor radionuclide treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol. 2017;115:425–434. doi: 10.1002/jso.24553. [DOI] [PubMed] [Google Scholar]
- 96.Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–122. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 98.Hemminki K, Li X. Incidence trends and risk factors of carcinoid tumors: a nationwide epidemiologic study from Sweden. Cancer. 2001;92:2204–2210. doi: 10.1002/1097-0142(20011015)92:8<2204::aid-cncr1564>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 99.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 100.Halperin DM, Shen C, Dasari A, Xu Y, Chu Y, Zhou S, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol. 2017;18:525–534. doi: 10.1016/S1470-2045(17)30110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology. 2009;89:471–476. doi: 10.1159/000197899. [DOI] [PubMed] [Google Scholar]
- 102.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 103.Feldman JM, Blinder RA, Lucas KJ, Coleman RE. Iodine-131 metaiodobenzylguanidine scintigraphy of carcinoid tumors. J Nucl Med. 1986;27:1691–1696. [PubMed] [Google Scholar]
- 104.Kaltsas G, Korbonits M, Heintz E, Mukherjee JJ, Jenkins PJ, Chew SL, et al. Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:895–902. doi: 10.1210/jcem.86.2.7194. [DOI] [PubMed] [Google Scholar]
- 105.Le Rest C, Bomanji JB, Costa DC, Townsend CE, Visvikis D, Ell PJ. Functional imaging of malignant paragangliomas and carcinoid tumours. Eur J Nucl Med. 2001;28:478–482. doi: 10.1007/s002590100475. [DOI] [PubMed] [Google Scholar]
- 106.Herrmann K, Czernin J, Wolin EM, Gupta P, Barrio M, Gutierrez A, et al. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: the referring physician’s perspective. J Nucl Med. 2015;56:70–75. doi: 10.2967/jnumed.114.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 108.Brabander T, van der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, de Herder WW, et al. Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23:4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 109.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of (177)Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grunwald F, Ezziddin S. 131I-metaiodobenzylguanidine therapy of neuroblastoma and other neuroendocrine tumors. Semin Nucl Med. 2010;40:153–163. doi: 10.1053/j.semnuclmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 111.Sywak MS, Pasieka JL, McEwan A, Kline G, Rorstad O. 131I-meta-iodobenzylguanidine in the management of metastatic midgut carcinoid tumors. World J Surg. 2004;28:1157–1162. doi: 10.1007/s00268-004-7603-1. [DOI] [PubMed] [Google Scholar]
- 112.Safford SD, Coleman RE, Gockerman JP, Moore J, Feldman J, Onaitis MW, et al. Iodine-131 metaiodobenzylguanidine treatment for metastatic carcinoid. Results in 98 patients. Cancer. 2004;101:1987–1993. doi: 10.1002/cncr.20592. [DOI] [PubMed] [Google Scholar]
- 113.Nwosu AC, Jones L, Vora J, Poston GJ, Vinjamuri S, Pritchard DM. Assessment of the efficacy and toxicity of (131)I-metaiodobenzylguanidine therapy for metastatic neuroendocrine tumours. Br J Cancer. 2008;98:1053–1058. doi: 10.1038/sj.bjc.6604273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ezziddin S, Sabet A, Logvinski T, Alkawaldeh K, Yong-Hing CJ, Ahmadzadehfar H, et al. Long-term outcome and toxicity after dose-intensified treatment with 131I-MIBG for advanced metastatic carcinoid tumors. J Nucl Med. 2013;54:2032–2038. doi: 10.2967/jnumed.112.119313. [DOI] [PubMed] [Google Scholar]
- 115.Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114–118. doi: 10.1136/jcp.19.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kameyama K, Takami H. Medullary thyroid carcinoma: nationwide Japanese survey of 634 cases in 1996 and 271 cases in 2002. Endocr J. 2004;51:453–456. doi: 10.1507/endocrj.51.453. [DOI] [PubMed] [Google Scholar]
- 118.Pacini F, Castagna MG, Cipri C, Schlumberger M. Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22:475–485. doi: 10.1016/j.clon.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 119.Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC study group. Groupe d'etude des tumeurs a calcitonine. Clin Endocrinol. 1998;48:265–273. doi: 10.1046/j.1365-2265.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- 120.Ilias I, Divgi C, Pacak K. Current role of metaiodobenzylguanidine in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin Nucl Med. 2011;41:364–368. doi: 10.1053/j.semnuclmed.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Castellani MR, Seregni E, Maccauro M, Chiesa C, Aliberti G, Orunesu E, et al. MIBG for diagnosis and therapy of medullary thyroid carcinoma: is there still a role? Q J Nucl Med Mol Imaging. 2008;52:430–440. [PubMed] [Google Scholar]
- 122.Gao Z, Biersack HJ, Ezziddin S, Logvinski T, An R. The role of combined imaging in metastatic medullary thyroid carcinoma: 111In-DTPA-octreotide and 131I/123I-MIBG as predictors for radionuclide therapy. J Cancer Res Clin Oncol. 2004;130:649–656. doi: 10.1007/s00432-004-0588-1. [DOI] [PubMed] [Google Scholar]
- 123.Maiza JC, Grunenwald S, Otal P, Vezzosi D, Bennet A, Caron P. Use of 131 I-MIBG therapy in MIBG-positive metastatic medullary thyroid carcinoma. Thyroid. 2012;22:654–655. doi: 10.1089/thy.2011.0174. [DOI] [PubMed] [Google Scholar]
- 124.Mairs RJ, Cunningham SH, Russell J, Armour A, Owens J, McKellar K, et al. No-carrier-added iodine-131-MIBG: evaluation of a therapeutic preparation. J Nucl Med. 1995;36:1088–1095. [PubMed] [Google Scholar]
- 125.Vaidyanathan G, Zalutsky MR. No-carrier-added synthesis of meta-[131I]iodobenzylguanidine. Appl Radiat Isot. 1993;44:621–628. doi: 10.1016/0969-8043(93)90179-e. [DOI] [PubMed] [Google Scholar]
- 126.Barrett JA, Joyal JL, Hillier SM, Maresca KP, Femia FJ, Kronauge JF, et al. Comparison of high-specific-activity ultratrace 123/131I-MIBG and carrier-added 123/131I-MIBG on efficacy, pharmacokinetics, and tissue distribution. Cancer Biother Radiopharm. 2010;25:299–308. doi: 10.1089/cbr.2009.0695. [DOI] [PubMed] [Google Scholar]
- 127.Coleman RE, Stubbs JB, Barrett JA, de la Guardia M, Lafrance N, Babich JW. Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid. Cancer Biother Radiopharm. 2009;24:469–475. doi: 10.1089/cbr.2008.0584. [DOI] [PubMed] [Google Scholar]
- 128.Matthay KK, Weiss B, Villablanca JG, Maris JM, Yanik GA, Dubois SG, et al. Dose escalation study of no-carrier-added 131I-metaiodobenzylguanidine for relapsed or refractory neuroblastoma: new approaches to neuroblastoma therapy consortium trial. J Nucl Med. 2012;53:1155–1163. doi: 10.2967/jnumed.111.098624. [DOI] [PubMed] [Google Scholar]
- 129.Zalutsky MR, Vaidyanathan G. Astatine-211-labeled radiotherapeutics: an emerging approach to targeted alpha-particle radiotherapy. Curr Pharm Des. 2000;6:1433–1455. doi: 10.2174/1381612003399275. [DOI] [PubMed] [Google Scholar]
- 130.Zalutsky MR, Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, et al. Clinical experience with alpha-particle emitting 211At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008;49:30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vaidyanathan G, Zalutsky MR. 1-(m-[211At]astatobenzyl)guanidine: synthesis via astato demetalation and preliminary in vitro and in vivo evaluation. Bioconjug Chem. 1992;3:499–503. doi: 10.1021/bc00018a006. [DOI] [PubMed] [Google Scholar]
- 132.Strickland DK, Vaidyanathan G, Zalutsky MR. Cytotoxicity of alpha-particle-emitting m-[211At]astatobenzylguanidine on human neuroblastoma cells. Cancer Res. 1994;54:5414–5419. [PubMed] [Google Scholar]
- 133.Vaidyanathan G, Affleck DJ, Alston KL, Zhao XG, Hens M, Hunter DH, et al. A kit method for the high level synthesis of [211At]MABG. Bioorg Med Chem. 2007;15:3430–3436. doi: 10.1016/j.bmc.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]