Abstract
The sporophytic system of self-incompatibility is a widespread genetic phenomenon in plant species, promoting out-breeding and maintaining genetic diversity. This phenomenon is of commercial importance in hybrid breeding of Brassicaceae crops and is controlled by single S locus with multiple S haplotypes. The molecular genetic studies of Brassica ‘S’ locus has revealed the presence of three tightly linked loci viz. S-receptor kinase (SRK), S-locus cysteine-rich protein/S-locus protein 11 (SCR/SP11), and S-locus glycoprotein (SLG). On self-pollination, the allele-specific ligand–receptor interaction activates signal transduction in stigma papilla cells and leads to rejection of pollen tube on stigmatic surface. In addition, arm-repeat-containing protein 1 (ARC1), M-locus protein kinase (MLPK), kinase-associated protein phosphatase (KAPP), exocyst complex subunit (Exo70A1) etc. has been identified in Brassica crops and plays a key role in self-incompatibility signaling pathway. Furthermore, the cytoplasmic calcium (Ca2+) influx in papilla cells also mediates self-incompatibility response in Brassicaceae, but how this cytoplasmic Ca2+ influx triggers signal transduction to inhibit pollen hydration is still obscure. There are many other signaling components which are not well characterized yet. Much progress has been made in elucidating the downstream multiple pathways of Brassica self-incompatibility response. Hence, in this review, we have made an effort to describe the recent advances made on understanding the molecular aspects of genetic mechanism of self-incompatibility in Brassicaceae.
Keywords: Self-incompatibility, Brassicaceae, S haplotypes, Molecular mechanism, Ca2+ influx
Introduction
The genetic phenomenon of self-incompatibility (SI) has emerged as an indispensable widespread mechanism in the flowering plants causing physiological hindrance to self-pollination, self-fruitfulness, and enforcing out-crossing (Takayama and Isogai 2005). Hence, it allows gene flow through pollen, thus maintaining and enhancing the genetic diversity in plant species. The term self-incompatibility is defined as the inability of plants, producing functional gametes, to set viable seed on self-pollination or when crossed with some of their genetic relatives (Brewbaker 1957). This phenomenon was first described in plants by Koelreuter in 1764 when he observed no seed set after self-pollination in the flowers of purple mullen (Verbascum phoeniceum), but produced abundant seeds when crossed with other nearby plants. However, the term ‘self-incompatibility’ was first coined by Stout (1917) and it is the genetic mechanism used to avoid self-fertilization (Suzuki 2009). Self-incompatibility can be divided into heteromorphic and homomorphic types. In the heteromorphic system, self-incompatibility is associated with differences in floral morphology and pollination is compatible only between the flowers of different morphological types, whereas in the homomorphic type, flowers of the same species have same morphological types (de Nattencourt 2001). The homomorphic system can further be classified into gametophytic self-incompatibility (GSI) and sporophytic self-incompatibility (SSI), based on the genetic control of pollen incompatibility reactions. In the sporophytic system (SSI), the pollen behavior in the SI interaction is determined by the genotype of plant on which it is produced (sporophytic control), whereas in the gametophytic system (GSI), it is determined by the genotype of pollen grain or tube itself (Nasrallah 2002; Hiscock and Tabah 2003). The system of self-incompatibility is widely distributed in more than 100 angiosperm families (Igic et al. 2008) and is controlled by multiple alleles, up to 200 SI haplotypes (Lawrence 2000). The gametophytic system is thought to be more common as compared to sporophytic system, but sporophytic type is more characterized, and both systems evidently evolved independently (Kao and McCubbin 1996). Both the genetic systems are controlled by single locus, with multiple S alleles (S haplotypes) (S1, S2, S3, …Sn) in most of the plant species.
Homomorphic sporophytic self-incompatibility system has been reported to be known from ten families of angiosperms (Igic et al. 2008) and has been best characterized and exploited commercially in the family Brassicaceae, especially in cole vegetables. Self-incompatibility (SI) system has evolved as models for investigating cell-to-cell signaling, as it involves cell-to-cell interaction between pollen and pistil, and signal transduction in stigma/style (Watanabe et al. 2012). The SSI system has been proved effective for commercial hybrid seed production in Brassica crops (Shen et al. 2008). Thus, an understanding of fundamental biology and molecular mechanisms of SSI in Brassicaceae vegetables is an important research target. During last two decades, intense molecular studies of self-incompatibility (SI) have focused on identifying and characterizing male and female determinants of SI in different plant families. Currently, both the determinants have been identified in angiosperms along with other players of SI response. Here, in this review, we have discussed the progress made towards characterization and identification of SI-signaling components in Brassica crops.
Self-incompatibility system in Brassica vegetables
In all the members of Brassicaceae family including cole crops, homomorphic sporophytic self-incompatibility (SSI) system exists, associated with trinucleate pollen and inhibition of self-pollen germination on ‘dry’ stigmatic surface (Bateman 1952, 1955; Kroh 1964; Ockendon 1972). The SSI system has been confirmed in kale (Thompson 1957), sprouting broccoli (Sampson 1957a), radish (Sampson 1957b), cabbage (Adamson 1965), and in cauliflower (Hoser-Krauze 1979). The exploitation of sporophytic system of self-incompatibility (SSI) for hybrid breeding in Brassica was first suggested by Pearson (1932); however, development of hybrids on commercial scale was achieved in Japan in 1950, and where first hybrid in cabbage based on SI system was developed (Odland and Noll 1950). Subsequently, in the United States, commercial hybrid seed production exploiting SSI was achieved in 1954 and in the Europe in 1966 (Haruta 1962; Wallace and Nasrallah 1968; Johnson 1972; Wallace 1979). Thompson and Taylor (1966) reported that ancestral Brassica oleracea var. sylvestris is highly self-incompatible. On this basis, it is natural that cole vegetables would be self-incompatible. However, there is wide variation present in the level of self-incompatibility (SSI) in Brassica vegetables. Among the cole group, Kale (Thompson and Taylor 1965) and round-headed cabbage (Adamson 1965) have high level of self-incompatibility. On the other hand, broccoli has relatively low level of self-incompatibility as compared to kale and cabbage, and very low level of self-incompatibility is present in early summer cauliflower owing to weak S-alleles (Watts 1965). Systematic studies on self-incompatibility in cauliflower (Watts 1963, 1965) suggested that among the European types, the biennial winter- and autumn-type cauliflower has higher level of self-incompatibility, and in European, summer-type cauliflower (snowball, alpha, and erfurt) has low level of SSI (Singh et al. 2013). Investigations on the level of self-incompatibility in Indian cauliflower revealed that varieties/lines under maturity group I have strongest self-incompatibility followed by maturity group II and III and maturity group IV has weak self-incompatibility level (Vidyasagar 1981; Chatterjee and Swarup 1984; Singh et al. 2013).
In the self-incompatible plants of Brassicaceae family, following the self-pollination, pollen grains either do not germinate on the stigma or they germinate, but pollen tube fails to penetrate the stigma papillar wall which is having the same S haplotypes as the pollen parent. The self-pollen is recognized during the interaction of pollen grain with stigma papillar cell wall and inhibition occurs at stigmatic surface. The inhibition of pollen tube at stigmatic surface in SSI is due to response of papillae to incompatible pollinations by the deposition of the β-1, 3-glucan callose (Kanno and Hinnata 1969; Roberts et al. 1980, 1983; Sulaman et al. 1997). On the occasions of both germination and penetration of pollen tube, its development halts at the callose barrier synthesized by the cytoplasm of the stigmatic papillar cells (Dickinson and Lewis 1973a; Elleman and Dickinson 1990; Edlund et al. 2004). Thus, failure of pollen grain adhesion, hydration, germination, pollen tube development, and penetration to stigma papillar wall leads to the expression of self-incompatibility (Chapman and Goring 2010; Singh et al. 2013).
Genetics and dominance hierarchy of S haplotypes in Brassica
Sporophytic self-incompatibility (SSI) in Brassicaceae is governed by the single Mendelian ‘S’ locus consisting of multiple alleles, referred as S haplotypes (Bateman 1955; Ockendon 1974). So far, more than 100 S-alleles have been reported in Brassica campestris (Nou et al. 1991, 1993), 50 in Brassica oleracea (Brace et al. 1994; Ockendon 1974, 2000; Nasrallah 1997), and 34 S-alleles in Raphanus sativus L. (Sampson 1957b; Karron et al. 1990). The S haplotypes exhibit complex inheritance and show dominant, co-dominant, and recessive allele interactions (Bateman 1955; Brugiere et al. 2000), and thus, four types of S-allele interactions (Types I, II, III, and IV) have been reported in Brassica (Mackay 1997; Hoser-Krauze 1979; Wallace 1979). Depending upon the SI phenotype, the Brassica S-alleles have been categorized into two classes viz. class-I haplotypes and class-II haplotypes (Nasrallah et al. 1991; Nasrallah and Nasrallah 1993). The high-activity alleles (class I) determining strong self-incompatibility are placed relatively high on the dominance scale, in which on average, development of 0–10 pollen tubes occurs per stigma on self-pollination. On the other hand, the low-activity alleles, exhibiting competitive and recessive interactions in pollen (class II), have weak incompatibility phenotype in which 10–30 pollen tubes develop per self-pollinated stigma (Nasrallah et al. 1991; Sobotka et al. 2000). In case of SSI, all the pollen grains produced on self-incompatible plant exhibit same incompatibility reaction irrespective of S-allele (constitution) assigned to particular pollen grain in the process of male gamete formation. The activity of S-alleles in stigma and pollen of heterozygous plants is based upon the result of complex dominant/recessive allelic interactions (Thompson and Taylor 1966).
The dominance hierarchy of S haplotypes, which is the key feature of SSI in Brassica (Bateman 1955; Thompson and Taylor 1966; Hatakeyama et al. 1998b), relies on their relative genetic behavior to other S haplotypes in stigma and pollen of heterozygous plants (Thompson and Taylor 1966; Ockendon 1975). Dominance relationships have been investigated in several species of Brassicaceae, like B. campestris (Haruta 1962; Hatakeyama et al. 1998b), kale (Thompson and Taylor 1966, 1971), Brussels sprout (Ockendon 1973, 1975), cabbage (Wallace 1979; Negi and Vidyasagar 2008), and Sinapis arvensis (Stevens and Kay 1989). The dominance relationships between three S-alleles (S1, S2, and S3) can be linear and non-linear. When S1 > S2 (S1 dominant to S2), S2 > S3 (S2 dominant to S3), and S1 is also dominant to S3 (S1 > S3), there is linear dominance (S1 > S2 > S3), in contrast, if S1 is not dominant to S3, it is non-linear dominance relationship (Thompson and Taylor 1966). For example, Hatakeyama et al. (1998a, b), Shiba et al. (2002), and Kakizaki et al. (2003) reported linear dominance relationship between four S haplotypes (S44 > S60 > S40 > S29) in self-incompatible Brassica rapa pollen. Genetic analysis of dominance relationships in Brassicaceae unveiled following characteristic features viz. (1) co-dominance is more common than dominance/recessiveness; (2) frequent occurrence of dominance/recessiveness in pollen in contrast to stigma; (3) dominance relationships between pollen and stigma are distinct; and (4) occurrence of non-linear dominance relationships in stigma is more common as compared to pollen (Thompson and Taylor 1966; Ockendon 1975; Hatakeyama et al. 1998b; Shiba et al. 2002). On the basis of these genetic features, the molecular mechanisms of dominance relationships between S haplotypes were revealed.
Genes governing self-incompatibility response in Brassica
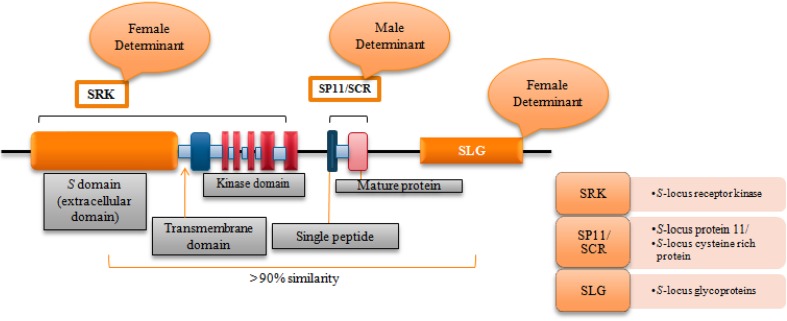
The cell-to-cell communication of sporophytic self-incompatibility (SSI) system in Brassicaceae is governed by single polymorphic ‘Mendelian S locus’ (Bateman 1955). The molecular genetic analysis revealed that ‘S locus’ is multigene, multiallelic highly complex locus, which spans many kilobase pairs, and encompasses various physically linked polymorphic genes that co-segregate with SI phenotype (Yu et al. 1996; Boyes et al. 1997; Suzuki et al. 1999; Casselman et al. 2000; Sobotka et al. 2000). Genomic analysis and physical mapping of S locus in Brassica have identified three polymorphic physically linked loci (Fig. 1) viz. S-receptor kinase (SRK) (Female determinant) (Stein et al. 1991), S-locus cysteine-rich protein/S-locus protein 11 (SCR/SP11) (Pollen ligand) (Suzuki et al. 1999; Takayama et al. 2000), and S-locus glycoprotein (SLG) (Female determinant) (Nasrallah et al. 1985; Suzuki et al. 1995). Besides these polymorphic linked genes, the other candidate genes playing role in downstream SI-signaling pathway in Brassicaceae have been identified (Fig. 2). The identification of first S-locus gene in Brassica oleracea as S-specific stigmatic proteins, termed S-locus glycoprotein (SLG) (Nasrallah and Wallace 1967; Nasrallah et al. 1985; Suzuki et al. 1995) attributed to initiation of molecular investigation on elucidating the mechanism of self-incompatibility in Brassica. It is highly expressed specifically in stigmatic papillar cells as revealed by in situ hybridization of SLG transcripts (Nasrallah et al. 1988) and GUS (GUS: β-glucuronidase) reporter gene system assay of SLG promoter sequence (Sato et al. 1991). In the variant S-homozygotes of Brassica also S-locus glycoproteins were explored (Nishio and Hinata 1977; Nishio and Kusaba 2000). There is abundance of SLG proteins in stigma papilla cells, which is the site of self-incompatibility reaction (Nasrallah et al. 1985, 1987). Identification, cloning, and sequencing of S-locus glycoproteins (SLG) have been done (Nasrallah et al. 1985, 1987), along with determination of amino-acid sequence of SLG by direct sequencing of protein (Takayama et al. 1987). Subsequently, in different Brassica crops such as Brassica oleracea, Brassica rapa, and Raphanus sativus, this sequence information enabled the sequencing of various S haplotypes of SLG (Chen and Nasrallah 1990; Kusaba et al. 1997; Sakamoto et al. 1998). For secretion to the outside of cells, S-locus glycoproteins (SLG) consist of a hydrophobic signal peptide at the N-terminus, various N-glycosylation sites and 12 conserved cysteine residues (Kitashiba and Nasrallah 2014).
Fig. 1.
Structure of S locus comprising three tightly linked polymorphic loci: SRK, SCR/SP11, and SLG. SRK and SLG share high sequence identity and act as female determinants of Brassica SI response. On interaction with pollen ligand (SCR/SP11), the kinase domain of SRK gets activated and signal is transduced to stigma papilla cell and causes self-pollen rejection (Nasrallah 1997; Watanabe et al. 2012; Kitashiba and Nishio 2009)
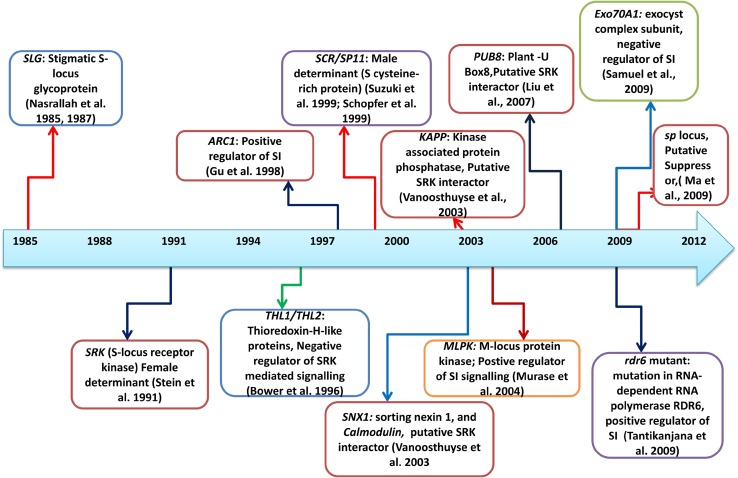
Fig. 2.
Timeline of identification of SI-signaling components in Brassica
The identification of SLG led to the isolation of another S-locus gene, S-locus receptor kinase (SRK) having extracellular transmembrane S domain, depicting serine/threonine kinase activity (Stein et al. 1991). Both genes, SLG and SRK, known as female determinants of SI response in Brassica, exhibit high polymorphism (Stein et al. 1991; Kusaba et al. 1997) and are expressed particularly at the mature stigmatic surface (Sobotka et al. 2000). However, some transcripts of SLG and SRK have been diagnosed in anther tissue, but detection of both proteins could be done only in stigmatic surface (Stein et al. 1996; Delorme et al. 1995). The SRK gene encompasses three domains: an extracellular S domain, which is the center for recognition of pollen ligand gene and shares high degree of sequence similarity to SLG gene; the other domain which passes through the plasma membrane is a transmembrane domain (encoding exon 2) and an intracellular kinase domain (exons 4–7), which plays role in signal transduction in stigma cells (Takayama and Isogai 2005; Edh et al. 2009). The female determinant, SRK gene consists of 12 conserved cysteine residues in the Brassicaceae (Jung et al. 2013) and its coding region spans a length of 2.6 kb which is divided by six introns (Sato et al. 2002).
SLG gene encodes a 55 kDa glycoprotein secreted into the stigmatic papillar cell wall, is about 1.3 kb in length, and contains no introns (Sobotka et al. 2000; Jung et al. 2013). SLG and extracellular domain (S domain) of SRK derived from the same S-allele shares about 90% nucleotide sequence homology and in some cases exceeds 98% (Watanabe et al. 1994; Hatakeyama et al. 1998c). The consistent genetic experiment indicates the essentiality of both stigmatic proteins for the operation of SI response (Toriyama et al. 1991; Nasrallah et al. 1992; Takasaki et al. 1999) and suggesting that SLG work as the co-receptor of male determinant and important for SRK stabilization (Dixit et al. 2000; Takasaki et al. 2000; Hiscock and Allen 2008; Watanabe et al. 2003). Although the essentiality of SLG for the activation of SI response is more equivocal and has been questioned (Cabrillac et al. 1999; Nishio and Kusaba 2000; Silva et al. 2001), in particular, after the report of high level of class-II type SLG proteins expression in naturally self-compatible line of B. oleracea (Gaude et al. 1995). In an experiment, Suzuki et al. (2000) reported the occurrence of frame-shift mutation and non-sense mutation in Brassica oleracea SLG haplotypes, S-18 and S-60 respectively. Sato et al. (2002), observed lack of SLG protein in S-24 haplotype of Brassica oleracea and S-32, S-33, and S-36 haplotypes in Brassica rapa. The absence of SLG gene also recorded in another self-incompatible species of Brassicaceae, Arabidopsis lyrata (Kusaba et al. 2001; Goubet et al. 2012). These results indicate the dispensability of SLG female determinant in SI reaction and essentiality of SRK proteins rather than SLG in playing a key role in the SI reaction of Brassica. The evolution of SLG stigmatic protein has been suggested to be related to SRK gene duplication (Tantikanjana et al. 1993). The other S-locus glycoprotein (SLG)-related genes (SLR1, SLR2, and SLR3) have been identified, which shows no genetic linkage to S locus, but exhibit sequence similarity with S-locus glycoprotein (Scutt et al. 1990; Boyes et al. 1991; Watanabe et al. 1992; Cock et al. 1995; Tantikanjana et al. 1996; Hatekeyama et al. 1998c). Of these, SLR2 has been shown to exhibit extensive sequence similar to SLGs detected from pollen-recessive haplotypes (Tantikanjana et al. 1996). Depending upon the degree of sequence similarity among SLGs and dominance relationships among their respective S haplotypes, SLGs were grouped into two classes (Nasrallah and Nasrallah 1993). The class-I SLG haplotypes show dominance in pollen, whereas SLG haplotypes in class II show recessiveness.
The S-receptor kinase (SRK) is essential for SI response as loss of the function experiments of SRK were reported to result in breakdown of self-incompatibility (Goring et al. 1993; Nasrallah et al. 1994; Sato et al. 2002), and furthermore, the importance of SRK in SI response was also supported by the genetic analysis of self-compatible variants of cabbage (Brassica oleracea var. capitata L.) and oilseed rape (Nasrallah et al. 1994; Goring et al. 1993). The expression level of SLG gene in self-compatible variants of both the species was found to be normal, in both the species mutations led to the lack of SRK transcripts or to the generation of truncated transcripts; thus, no functional SRK can occur in both the variants. These findings suggest that SRK gene alone plays role in SI reaction. On the other hand, the role of SLG in stimulating SI response might be restricted to some S haplotypes exhibiting extensive homology between SLG and SRK, for example, S-29 haplotype in Brassica rapa.
After the identification of female determinant, SRK, it took a long gap of about 8 years for the detection of male determinant (SCR/SP11) of SI reaction, when two different group of researchers by employing the cloning and sequencing of Brassica S-locus region, reported the isolation of another polymorphic gene expressed in anther tapetum (Suzuki et al. 1999; Schopfer et al. 1999). The male determinant was first identified in Brassica rapa as S9-haplotype-specific gene expressed in anther tapetum in an SLG/SRK flanking region of S9-haplotype (Suzuki et al. 1999) and named S-locus protein 11 (SP11). Concurrently, in Brassica rapa itself, Schopfer et al. (1999), independently identified the different allele of the same gene in the corresponding region of S8-haplotype as potential pollen ligand of SRK, and named it as SCR (S-locus cysteine-rich protein). SCR is basic cysteine-rich protein and is predicted to have N-terminal signal peptide (Schopfer et al. 1999). The SCR/SP11 locus is adjacent to locus of female determinants (SLG/SRK) and sequence comparison of mature paternal SCR/SP11 protein demonstrated that SCR/SP11 exhibit extensive allelic diversity within species (with less than 50% amino-acid similarity shared by variants) (Sato et al. 2002; Okamoto et al. 2004; Kitashiba and Nasrallah 2014). The SCR/SP11 variants have C1–C8 conserved cysteine residues, a glycine residue between C1 and C2 cysteines, and an aromatic amino-acid residue between C3 and C4 cysteines (Schopfer et al. 1999; Takayama et al. 2000; Watanabe et al. 2000; Kitashiba and Nasrallah 2014). All eight cysteines play role in intramolecular disulphide bonds (Takayama et al. 2001). In addition, the gain-of-function experiments, for example, GUS reporter analysis of promoter region of SCR/SP11 (Schopfer and Nasrallah 2000), RNA gel blot and in situ hybridization of transcripts of SCR/SP11 protein (Takayama et al. 2000; Kusaba et al. 2002), also revealed that SCR/SP11 gene is specifically expressed in anther tapetal cells at early developmental stages and in the microspores later (Takayama et al. 2000; Shiba et al. 2001). Hence, SCR/SP11 act as direct pollen ligand for SRK specifically (Watanabe et al. 2003; Shimosato et al. 2007). Jung et al. (2012) also proved the essentiality of SP11/SCR gene as potential ligand for self-incompatibility response in Brassica. They obtained the self-compatible Brassica rapa line with the RNAi-mediated gene silencing of S60-haplotype of SCR/SP11 of cv. ‘Osome’. Male determinant (SCR/SP11) has been proved to exhibit high allelic diversity (19.5–94% amino-acid identity) (Watanabe et al. 2003; Hiscock and Mclnnis 2003), and so far, 22 alleles, encoding small basic cysteine-rich proteins, of SCR/SP11 have been reported in Brassica species like, B. rapa, B. oleracea var. botrytis and oilseed rape (Bi et al. 2000; Watanabe et al. 2000; Shiba et al. 2001; Hiscock 2002). Despite having high sequence variability, all SCR male determinants form a typical defensin-like 3D structure comprising three β-sheets and one α-helix (Mishima et al. 2003; Chookajorn et al. 2004; Yamamoto and Nishio 2014). Most of the sequence diversity in male determinant SRK exists within its receptor domain having hyper-variability regions for S specificity (Hiscock and Tabah 2003; Nasrallah 2002; Watanabe et al. 2003; Hiscock and Mclnnis 2003). The DNA sequence is now available in the NCBI database for the 20, 23, 2, and 10 SCR variants of Brassica oleracea, Brassica rapa, Brassica napus, and Raphanus sativus, respectively (http://www.ncbi.nlm.nih.gov/unigene) (Naithani et al. 2013).
Other players of SI response in Brassica
In addition to male and female determinants of SI reaction in Brassica, some other downstream signaling factors acting as positive or negative regulator of self-incompatibility in Brassicaceae have been identified (Fig. 2). Although, the exact signaling pathway of some molecules is yet obscure. Below, we have provided a brief account of other genes regulating SI response in Brassica.
THL1 and THL2
The yeast-two-hybrid assay in the SRK kinase domain has been instrumental in the identification of various SRK interacting proteins. Employing this approach, Bower et al. (1996) reported the identification of two thioredoxin-h-like proteins THL1 and THL2, as putative SRK interactor using SRK910 protein kinase domain of B. napus as bait. When the SRK activating pollen coat protein consisting self-SCR is absent, the THL1 protein keeps SRK receptor in inhibited state and autophosphorylation of receptor is impeded (Cabrillac et al. 2001). Thus, THL1 and THL2 are potential negative regulator of Brassica SI response. Furthermore, the antisense suppression of THL1/THL2 mRNA in the stigmas of Brassica napus cv. Westar, resulted in the low level of constitutive pollen inhibition response typical to Brassica SI rejection response (Haffani et al. 2004).
MLPK, ARC1, and Exo70A1
A recessive mod mutation of the modifier (m) gene, which leads to elimination of Brassica self-incompatibility response, facilitated to the discovery of cytoplasmic protein kinase, M-locus protein kinase (MLPK) encoded by the mod locus (Murase et al. 2004). In case of Brassica rapa, the two distinct transcript isoforms of MLPK (MLPKf1 and MLPKf2) are generated through alternate transcription initiation sites (Murase et al. 2004; Kakita et al. 2007a). The positional cloning of M locus from a self-compatible line ‘Yellow Sarson’ of B. rapa revealed that MLPK is having serine/threonine protein kinase activity, and membrane localization of MLPK by different mechanisms restores the self-pollen rejecting ability of mm papilla cells, confirming MLPK as essential positive regulator of Brassica self-incompatibility (Murase et al. 2004; Kakita et al. 2007a). The transcript MLPKf1 localizes to papillae cell membrane via N-terminal myristoylation motif, while localization of other transcript MLPKf2 depends upon its N-terminal hydrophobic region (Kakita et al. 2007a). Recently, the genome-wide survey and synteny analysis revealed the identification of new MLPKf1 homologous gene MLPKn1 in Brassica oleracea and both shares as high as 84.3% sequence identity (Gao et al. 2016). Both, B. oleracea and B. rapa genomes, contain three MLPK homologous genes, BoMLPKf1/2, BoMLPKn1, Bol008343n and BrMLPKf1/2, BraMLPKn1, Bra040929, respectively (Gao et al. 2016).
Arm-repeat-containing protein 1 (ARC1) was identified as another positive regulator of SI response in Brassica by employing yeast-2-hybrid approach with the SRK kinase domain as bait (Gu et al. 1998). ARC1 is a stigma-specific plant U-Box protein having E3 ubiquitin ligase activity and is phosphorylated by SRK–MLPK complex in vitro (Gu et al. 1998; Stone et al. 2003; Samuel et al. 2008; Yee and Goring 2009). Thus, ARC1 shows high specificity to SRK and interacts with female determinant in phosphorylation-dependent manner (Stone et al. 1999). The role of ARC1 in SI response has been proved by different studies, suggesting ARC1 as potential player of self-incompatibility reaction. Stone et al. (1999) reported partial breakdown of SI response in transgenic Brassica with the antisense ARC1 and this breakdown was attributed to incomplete silencing or the activity of another component of SI reaction (Stone et al. 1999). Recently, ARC1 was also discovered in the self-incompatible species Arabidopsis lyrata, playing role in self-pollen rejection (Indriolo et al. 2012). Furthermore, the strong self-incompatible phenotypes were obtained in self-compatible A. thaliana, with the introgression of ARC1 of B. napus or A. lyrata with SCRb–SRKb into the Col-0 or Sha ecotypes of A. thaliana (Indriolo et al. 2014). Ubiquitination has been proved to control many processes in plant system including inhibition of self-pollen in self-incompatibility response of Brassica (Indriolo and Goring 2014). A new compatibility factor, termed negative regulator of SI response in Brassica, ‘Exo70A1’, was identified in Brassica and Arabidopsis by using yeast-2-hybrid analysis with ARC1 as bait (Samuel et al. 2009). Exo70A1 has been suggested to be the substrate for ARC1’s ubiquitination mediated degradation pathway degrading the proteins of compatible reaction in the SI response (Samuel et al. 2009). The antisense knockdown of Exo70A1 gene using RNA interference (RNAi) technique in the stigma of self-compatible B. napus cultivar, ‘Westar’ resulted reduction in pollen grains count on the stigmatic surface after pollination (Samuel et al. 2009). Furthermore, the control of Exo70A1 by SLR1 promoter (Franklin and Centre 1996) resulted in partial breakdown of self-incompatibility in the SI line of B. napus, ‘W1’ (Samuel et al. 2009). The Exo70A1 has been suggested to play role in pollen grains hydration, germination, and then pollen tube penetration (Samuel et al. 2009). Eventually, it was suggested that Exo70A1 protein acts at the intersection of two cellular pathways, where it is essential in the stigma for the acceptance of compatible pollen in both Arabidopsis and Brassica and is negative regulator of self-incompatibility in Brassica.
JDP1
By employing yeast-2-hybrid approach against cDNA library from stigma of ornamental kale (Brassica oleracea var. acephala), another protein was identified recently by Lan et al. (2015), which is J domain protein 1 (JDP1) that interacts with ARC1. JDP1, a member of heat shock protein 40 (Hsp40) family, having 344 amino acids is a 38.4-kDa protein. The N-terminus of JDP1 (JDP1–68) and C-terminus of JDP1 (JDP169–344) contains a J and X domain, respectively (Lan et al. 2015). The C-terminus of JDP1 is sufficient for interaction with ARC1 and Tyr8 in the JDP1 N-terminal region is the specific site for JDP1 and ARC1 interaction. However, the exact role of JDP1–ARC1 complex in regulating the SI response in Brassica is yet to be elucidated.
KAPP, Snx1, and Calmodulin
Besides above-stated positive and negative regulators of Brassica SI response, KAPP (kinase-associated protein phosphatase), sorting-nexin1 (Snx1), and calmodulin have been reported to be other putative interactors of SRK (Braun et al. 1997; Vanoosthuyse et al. 2003). KAPP is a membrane-anchored type 2C protein phosphatase, which by its kinase interaction domain interacts with phosphorylated receptor-like kinase (RLK) of plants (Braun et al. 1997; Shah et al. 2002; Vanoosthuyse et al. 2003; Manabe et al. 2008). Furthermore, KAPP is suggested to inactivate the functioning of RLK by dephosphorylation process (Tichtinsky et al. 2003), and thus, different genetic studies speculate KAPP to be a negative regulator of RLK pathways (Williams et al. 1997; Stone et al. 1998; Vanoosthuyse et al. 2003; Manabe et al. 2008). Eventually, it was suggested that Brassica female determinant SRK interact with Brassica homolog of KAPP and this interaction leads to dephosphorylation of SRK, indicating downregulation of SRK proteins by KAPP (Vanoosthuyse et al. 2003). Thus, SRK acts as a substrate for phosphatase activity of KAPP. Brassica homolog KAPP protein extracted from Brassica stigma cDNA library shares 80.1% amino-acid identity with Arabidopsis KAPP (Vanoosthuyse et al. 2003). The other two proteins, Snx1 and calmodulin, also interact with SRK and these have been reported to downregulate RLK in animals. The interaction of calmodulin and kinase domain of SRK was confirmed by calmodulin-Sepharose affinity binding approach (Vanoosthuyse et al. 2003). It was reported that calmodulin is not phosphorylated in this calmodulin–SRK interaction and has no direct effect on SRK kinase activity, implicating that calmodulin does not act as a substrate for SRK kinase activity. Furthermore, the Brassica sorting nexin homolog (BoSNX1) was isolated as SRK29 kinase domain interactor via yeast-2-hybrid screening (Vanoosthuyse et al. 2003). The genome-wide sequence analysis showed great similarity of Brassica SNX1 with Arabidopsis and Human SNX1 protein. The BoSNX1 was suggested to interact with kinase domain of SRK indicating that SNX1 regulate many plant receptor kinase (PRK) signaling pathways.
rdr6 mutation enhancing SI
Using transgenic self-incompatible A. thaliana model and mutants screening, a recessive mutation was identified in the RNA-dependent RNA polymerase rdr6 producing trans-acting short interfering RNA (ta-siRNA), which results in exsertion of stigma and enhancing SI response, without simultaneous increase in level of SRK transcripts. This stigma exsertion in the rdr6 mutant background is attributed to pistil elongation due to S-locus receptor kinase (SRK) catalytic activity (Tantikanjana et al. 2009). Thus, trans-acting short interfering RNA pathways regulate the potential positive regulators of SI response and pistil development depicting dual role of SRK, in SI response and pistil development owing to coordinate evolution of these mechanisms.
Proteomics in understanding of SI
There are a few other different stigmatic proteins and have also been identified in Brassicaceae with respect to SI response. Proteomics approaches have been widely used in unravelling the SI response in Brassica and have extended our understanding of this enigma to great extent. The proteome research in SI has progressed from the use of isoelectric focusing in last three decades to the current third generation technique of comparative isobaric tag for relative and absolute quantification (iTRAQ) (Sankaranarayanan et al. 2013a, b). The role of multiple modifier genes in weakening the self-incompatibility or causing pseudoincompatibility has been reported by genetic studies of transgenic self-incompatible A. thaliana (Nasrallah et al. 2004; Boggs et al. 2009b). Samuel et al. (2011), utilized three-dimensional difference gel electrophoresis technique (2-D DIGE) and compared the stigmatic proteins of self-incompatible Brassica napus at 0 and 60 min after self-pollination and observed that tubulin and microtubule network were linked to SI reaction. They further observed the breakdown of microtubule network (MT) in the compatible pollinations, which was suggested to be mediated by exocyst component, Exo70A1, leading to successful fertilization and seed set. Recently, Wang and associates (2014) identified 25 protein spots with distinct activity in self-incompatible and compatible lines of non-heading Chinese cabbage by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF/TOF MS) and peptide mass fingerprinting (PMF), out of which 22 protein spots were confidently established. Then, by employing quantitative RT-PCR technique, mRNA levels of the corresponding genes were identified and suggested that UDP–sugar pyrophosphorylase could have role in sucrose degradation to affect pollen germination and growth. Furthermore, they also suggested the role of glutathione S transferases in pollen grains’ maturation, and affecting the pollen fertility and concurrently, the senescence-associated cysteine protease could be related to self-pollen recognition of non-heading Chinese cabbage (Wang et al. 2014). Thus, there are several lines of evidence which support the role of multiple signaling pathways in Brassica SI response (Tantikanjana et al. 2010).
Molecular mechanism and interaction of Brassica SI-signaling components
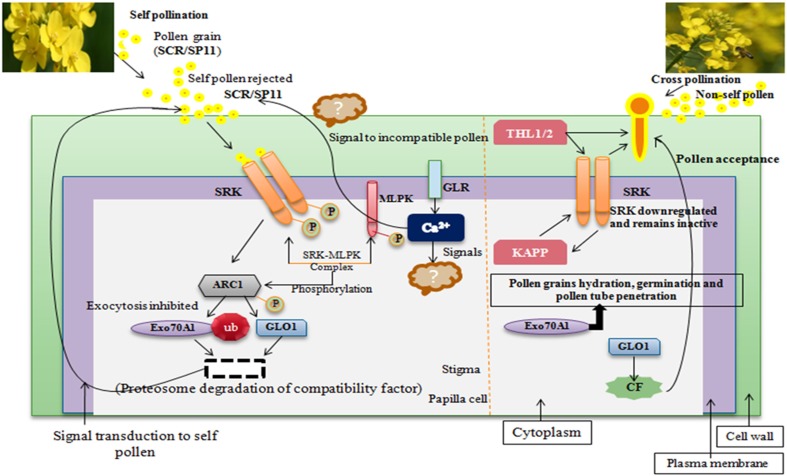
A schematic model of interaction of Brassica SI-signaling components is presented in Fig. 3. When there is self-pollination, the pollen ligand (SCR/SP11) and receptor (SRK) interaction from the same S haplotype induces autophosphorylation of S-receptor kinase in an S-haplotype-specific manner (Takayama et al. 2001; Kachroo et al. 2001, 2002; Watanabe et al. 2012). The autophosphorylated SRK elicit a signal transduction cascade in stigma papilla cells that results in the pollen rejection and inhibition of pollen tube penetration into the epidermal cell wall of stigma and induces SI response (Kao and McCubbin 2000; Ivanov and Gaude 2009; Watanabe et al. 2012; Nasrallah and Nasrallah 2014; Yamamoto and Nishio 2014). In contrast, when there is cross pollination, the pollen ligand (SCR) can neither bind nor activate SRK kinase domain and no pollen rejection signaling cascade is triggered, hence leads to development and penetration of pollen tube into the stigma papillae. It is the extracellular S-domain region of SRK which comprises hypervariable subdomains for haplotype-specific pollen ligand (SCR/SP11) binding (Kemp and Doughty 2007; Shimosato et al. 2007; Boggs et al. 2009a; Jung et al. 2013). Despite the identification of both ligand and receptor, the downstream mechanism behind the SRK signal transduction was not understood. To elucidate the SRK-signaling cascade, various SRK interactors were identified and characterized (Gu et al. 1998; Stone et al. 1999, 2003; Cabrillac et al. 2001; Murase et al. 2004; Kakita et al. 2007a, b; Leducq et al. 2014). Two thioredoxin-h-like proteins, THL1 and THL2 (Bower et al. 1996; Cabrillac et al. 2001), are putative interactor of SRK which maintain SRK in an inactive state in unpollinated stigmas (Cabrillac et al. 2001; Haffani et al. 2004; Jung et al. 2013). Furthermore, MLPK (M-locus protein kinase) was identified as another positive interactor of SRK (Murase et al. 2004). The kinase domain of female determinant, SRK, interacts with MLPK and forms SRK–MLPK complex. This SRK–MLPK complex induces signal transduction to stigma papilla cell and causes self-pollen rejection (Gao et al. 2016). Thus, MLPK act as positive regulator of SI response. Another positive regulator of SI response is ARC1 (Gu et al. 1998; Stone et al. 2003; Samuel et al. 2008), which shows high specificity to SRK. When ARC1 interact with SRK, the SRK–MLPK complex induces phosphorylation of ARC1 via kinase domain of SRK. This phosphorylated ARC1 interact with Exo70A1, which is putative component of exocyst complex (Samuel et al. 2009). This phosphorylated ARC1 degrade the Exo70A1 and inhibit exocytosis. Thus, it is speculated that these phosphorylated pathways prevent or degrade compatibility factors secretion to stigma and result in inhibition of pollen tube penetration (Ivanov et al. 2010; Lan et al. 2015). Another protein, a kinase-associated protein phosphatase (KAPP), also interacts with phosphorylated SRK kinase domain (Braun et al. 1997) and leads to dephosphorylation of SRK, thus downregulating the SRK. Hence, KAPP act as a negative player of self-incompatibility response. Sankaranarayanan et al. (2015) reported ‘glyoxalase 1 (GLO1)’, as another compatibility factor which is downregulated by ARC1-mediated reaction in SI-signaling pathway. GLO1 is a compatible protein in stigma required for pollination to occur and its suppression leads to reduction in compatibility, and thus leads to SI response. On the other hand, the over expression of GLO1 induces partial breakdown of self-incompatibility, as reported in self-incompatible stigma of B. napus (Sankaranarayanan et al. 2015). Recently, the role of cytoplasmic Ca2+ was reported in self-incompatibility reaction in Brassicaceae (Iwano et al. 2015; Franklin-Tong 2015). When there is self-pollination, incompatible pollen lands on stigma and the ligand–receptor interaction lead to increase in cytoplasmic Ca2+ in stigma papilla cells. Therefore, stigma papilla cells use this cytoplasmic calcium influx to give signal to incompatible pollen to inhibit hydration, germination and pollen tube growth on stigmatic surface. However, it is still obscure that how this cytoplasmic Ca2+ influx in stigma papilla cells triggers signal transduction to incompatible pollen which results in self-pollen rejection and ultimately leads to self-incompatibility response.
Fig. 3.
Proposed schematic model of interaction of SI-signaling components in Brassicaceae. SCR/SP11 S-locus cysteine-rich protein/S-locus protein 11 (Suzuki et al. 1999; Takayama et al. 2000); SRK S-receptor kinase (Stein et al. 1991); SLG S-locus glycoprotein (Nasrallah et al. 1985; Suzuki et al. 1995); THL1/THL2 thioredoxin-h-like proteins (Cabrillac et al. 2001); ARC1 armadillo-repeat containing protein 1 (Gu et al. 1998); MLPK M-locus protein kinase (Murase et al. 2004); Exo70A1 Exocyst subunit exo70 family protein A1 (Samuel et al. 2009); KAPP Kinase-associated protein phosphatase (Braun et al. 1997; Vanoosthuyse et al. 2003); GLO1 Glyoxylase 1 (Sankaranarayanan et al. 2015); GLR glutamate-like receptor (Iwano et al. 2015); CF compatibility factors for compatible pollination (Sankaranarayanan et al. 2015)
Molecular genetics of dominance relationships between S haplotypes
In the stigma of self-incompatible Brassica, the dominance relationships between S haplotypes are determined by stigmatic female determinant, S-receptor kinase (SRK) by post-transcriptional regulation (Hatakeyama et al. 2001). Regarding dominance relationships on pollen side, the pollen ligand S-locus protein 11 (SP11)/S-locus cysteine-rich protein (SCR) from pollen-recessive S haplotypes have not been observed yet. Although, it has been suggested that a set of SLG and SRK Class-II S haplotypes is present in pollen-recessive S haplotypes, while a set of class-I S haplotypes is present in pollen-dominant S haplotypes (Nasrallah and Nasrallah 1993; Shiba et al. 2002). The class-II SLGs pollen-recessive S haplotypes have been found in Brassica rapa (S29, S40, S44, S60) (Hatakeyama et al. 1998c; Takasaki et al. 2000) and Brassica oleracea (S2, S5, S15) (Chen and Nasrallah 1990; Scutt and Croy 1992; Cabrillac et al. 1999). Shiba et al. (2002) and Kakizaki et al. (2003) suggested that dominance/recessive relationships of S haplotypes in Brassica pollen are regulated by transcription of pollen ligand SP11/SCR. They observed significant reduction in the expression (mRNAs) of recessive pollen ligand SP11 in the anther tapetum cells of S-heterozygous plants, while dominant SP11 predominantly expressed. Furthermore, Kakizaki et al. (2003) depicted the role of epigenetic regulation, as the dominance/recessiveness of S44, S40, and S60 S haplotypes could be altered. Furthermore, the role of DNA methylation, which is instrumental in modifying eukaryotic genomes and altering gene expression, was confirmed in governing dominance relationship between S-alleles by Shiba et al. (2006). They reported de novo DNA methylation at the promoter region of recessive allele-specific pollen ligand SP11 in anther tapetum before initiation of its transcription. Thus, suggested the role of DNA methylation in silencing of recessive allele-specific gene SP11, hence controlling dominance relationship between S haplotypes in Brassica. This DNA methylation of promoter regions of recessive pollen ligand SP11 is activated by 24-nucleotide trans-acting small non-coding RNA (sRNA) produced by dominant allele and causes mono-allelic gene silencing (Tarutani et al. 2010; Durand et al. 2014). Recently, Yasuda et al. (2016) put forward a ‘polymorphic dominance modifiers’ model as the mechanism responsible for dominance hierarchy of S haplotypes produced by receiving polymorphic sRNA (small RNA) and their targets. They proved that single polymorphic sRNA, ‘Smi2’ (SP11 methylation inducer 2), and its polymorphic targets govern the linear dominance relationship of class-II S haplotypes (S44 > S60 > S40 > S29) in Brassica rapa. Hence, as the genetic elements, SP11 methylation inducer (Smi) and SP11 methylation inducer 2 (Smi2) correspond to ‘dominance modifiers’ in Brassica rapa and control the dominance hierarchy of S haplotypes (Tarutani et al. 2010; Yasuda et al. 2016).
Molecular markers and S-haplotype identification
The genetic mechanism of SSI has been commercially utilized for hybrid seed production in Brassicaceae. Thus, the discrimination of S-alleles is useful not only for the study of self-incompatibility, but also for the commercial hybrid seed production. Traditionally, S haplotypes have been identified by controlled pollination with S-allele tester lines, followed by fluorescence microscopic observation of pollen tube growth through stigma or by seed set data. The technique of counting seed set data is labor and time consuming, though the fluorescent microscopy is relatively fast, but it is essential to have the plants in flowering stage. In addition, the ideal plant selection having recessive traits, such as SI, is often difficult and thus a potential barrier in accelerating plant breeding. In this regard, the use of molecular marker technology for the S-haplotype identification is advantageous that it could be done on young plants and no need to have the S-allele plants in flowering phase (Brace et al. 1994). Hence, the development and identification of molecular markers linked with self-incompatible genes are of utmost value for geneticist and plant breeders to discriminate between class-I and class-II S haplotypes (Havlickova et al. 2014). Different kinds of molecular markers have been reported for the identification of S haplotypes in Brassica species by different groups of researchers (Table 1), although all the S haplotypes have not been identified yet.
Table 1.
Molecular markers for S-haplotype identification in Brassica species
| Brassica crop | S haplotype Types/SI Gene | Type of marker | Population used | References |
|---|---|---|---|---|
| Brassica oleracea L. | SLG, SRK and SLR | PCR-RFLP | B. oleracea homozygous S-allele lines | Brace et al. (1994) |
| Chinese cabbage | Two Class-I SRK haplotypes (P1, P2) and Two Class-II SRK haplotypes (P3, P4) | PCR-RFLP | 24 DH lines | Tingting et al. (2013) |
| Broccoli, cabbage | Class-II and Class-I SLG alleles | PCR-RFLP | 31 F1 hybrid cultivars of broccoli and cabbage | Sakamoto et al. (2000) |
| Radish | Class-I and Class-II SLG/SRK haplotypes | PCR-RFLP | 24 homozygous breeding lines belong to 10 S haplotypes | Lim et al. (2002) |
| Chinese cabbage, B. rapa ssp. chinensis var. purpurea, cauliflower, cabbage, mustard | Class-I and Class-II SLG Class-I and Class-II SRK |
PCR-RFLP | 72 genotypes of 5 Brassica vegetables | Wang et al. (2007) |
| B. rapa | Class-I and Class-II SLG haplotypes | PCR-RFLP | F1 and open pollinated cultivars of B. rapa | Sakamoto and Nishio (2001) |
| Broccoli | Class-I and Class II SRK Class-I and Class-II SCR |
PCR based gene specific SRK and SCR/SP11 primers | 18 DH lines | Yu et al. (2014) |
| Cabbage, broccoli | Class-I and Class-II SRK | PCR-CAPS marker | Inbred lines of cabbage and broccoli | Park et al. (2002) |
| Brassica napus | SLG, SLR | PCR-CAPS | F2 population and B. napus SI lines | Mohring et al. (2005) |
| Brassica napus | Class-I and Class-II SRK, SLG, SP11 | CAPS, SCAR | F2 population of SI and self-compatible lines | Zhang et al. (2008) |
Maintenance of S-allele lines
The genetic mechanism of sporophytic self-incompatibility has been proved effective for commercial hybrid seed production in Brassica crops across the world (Wang et al. 2007; Koprna et al. 2005; Hamid et al. 2010; Kucera et al. 2006). For the continuous development of F1 hybrids using SI phenomenon, the maintenance of parental S-allele lines is one of the basic requirements (Singh and Vidyasagar 2012). The maintenance of SI lines is a costly affair. In the course of time, the number of techniques has been identified and utilized commercially to overcome the SI reaction for the successful maintenance of parental S-allele lines. The method being followed usually for the large-scale seed production is manual sib mating in bud stage or bud pollination (Cabin et al. 1996; Hamid et al. 2010). However, bud pollination is time consuming, tedious, and labor intensive method. The other techniques being adopted are exposing the plants to high concentration of CO2 (3–5%) and relative humidity (100%) in air-tight growth chamber for a period of 8–24 h (Palloix et al. 1985; Nikura and Matsuura 2000; Kwun et al. 2004), chemical treatments such as plant lectin (Sharma et al. 1984), phytohormones (Matsubara 1985), KOH (Tatebe 1968), and common salt (NaCl) solution sprays (Wang et al. 2012; Singh and Vidyasagar 2012). The other alternative methods being suggested are electric aided pollination (Roggen et al. 1972), thermally aided pollination (Roggen and Van Dijk 1976), steel-brush pollination (Roggen and Van Dijk 1972), pollen coat extracts (Roggen 1975), high-temperature treatments (Gonai and Hinata 1971), double pollination, use of mixed pollen, and delayed pollination (Kakizaki 1930). The efficiency of these methods depends upon the level of self-incompatibility, activity of S-alleles, genetic background, flower age, type of plant, species, and incompatibility system. All these treatments lead to temporary breakdown of self-incompatibility in Brassica.
The in vitro tissue culture technique can accelerate the F1 hybrid breeding programme in B. oleracea vegetables by employing this technique in maintenance of parental inbred lines of F1 hybrids. Different researchers have reported the use of in vitro tissue culture techniques for the maintenance and multiplication of SI lines in Brassica (Sanghera et al. 2012; Bhatia et al. 2013).
Recently, Lao et al. (2014) carried out the physiological and genetic analysis of CO2-induced breakdown of SI in Brassica rapa. With the use of X-ray microanalysis, they suggested that self-incompatibility breakdown in S-allele line was accompanied by significant accumulation of calcium (Ca) at the pollen–stigma interface. The genetic analyses using F1 and F2 progeny of a cross between CO2-sensitive × CO2-insensitive line suggested that CO2 sensitivity is a semi-dominant and quantitative trait, which is under the control of more than one gene. Furthermore, two major loci, BrSIO1 and BrSIO2, were identified using QTL analyses, which act additively in overcoming SI response during CO2 treatment (Lao et al. 2014).
Recently, Tantikanjana and Nasrallah (2015) suggested a new alternative technique, ligand-mediated cis-inhibition of receptor signaling for the control of SI mechanism in Brassica. The use of cis-SCR transgenes, or SRK off switches, is suggested to have practical implication for both maintenance of parental S-allele lines and commercial hybrid seed production. The cis-inhibition of SRK is mediated by allele-specific ligand–receptor interaction (Tantikanjana and Nasrallah 2015). The SRK receptor is entrapped in the endoplasmic reticulum by the stigma-expressed SCR and thus inhibits the exact targeting of SRK to the plasma membrane, where receptor–ligand interaction takes place.
Conclusion
As outlined in this review, the Brassica SI response is controlled by multiple signaling pathways and is a complex phenomenon. The mechanism of SSI is most interesting phenomenon in the angiosperms which promote out-breeding in plants. The molecular genetics and physiology of SI pathway in Brassicaceae have been extensively studied. As we presented, much progress has been made towards the understanding of downstream complex SI-signaling pathways. However, still, there are many aspects which have not been elucidated yet and current research is going on side by side to understand the downstream SI-signaling events occurring in stigmatic papillae following the pollination. The use of proteomic approach has also been reported to better understand the mechanism of SI response in Brassica vegetables and potential accumulating pistil proteins have been identified especially in Chinese cabbage. Recently, the role of glutamate receptor-like channel (GLR) gene mutants in mediating enhancement of cytoplasmic Ca2+ influx in stigma papilla cells was reported, which transmit signal to incompatible pollen and causes self-pollen rejection. However, this signaling pathway via cytoplasmic Ca2+ to incompatible pollen is yet to be elucidated. Then, further elucidation of interaction of GLR and Ca2+ with other Brassica SI-signaling components will help in understanding other molecular pathways involved in Brassica self-incompatibility. Furthermore, the other genomics and next generation sequencing (NGS) approaches can be extended to different Brassicaceae crops for the identification of candidate proteins functioning in the SI-signaling pathways. In addition, the downstream molecular events involved in overcoming or temporary breakdown of SI response in Brassica with various techniques are still not elucidated. With the progress in use of advanced biotechnological, physiological, and molecular approaches, it would be possible to elucidate the multiple complex mechanisms involved in SI response and it will further enhance our understanding of multiple Brassica SI-signaling pathways to new heights in near future. In addition, it will act as a catalyst to boost the F1 hybrid seed production of Brassicaceae crops.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest with respect to this paper.
References
- Adamson RM. Self and cross-incompatibility in early round-headed cabbage. Can J Plant Sci. 1965;45:493–497. [Google Scholar]
- Bateman AJ. Self-incompatibility systems in angiosperms. I. Theory. Heredity. 1952;6:285–310. [Google Scholar]
- Bateman AJ. Self-incompatibility systems in angiosperms III Cruciferae. Heredity. 1955;9:52–68. [Google Scholar]
- Bhatia R, Parkash C, Dey SS, Chandresh C, Bhardwaj V. In vitro propagation of a self-incompatible cabbage line ‘Sel 5’. Indian J Hortic. 2013;70:364–368. [Google Scholar]
- Bi Y-M, Brugière N, Cui Y, Goring DR, Rothstein SJ. Ttransformation of Arabidopsis with a Brassica SLG/SRK region and ARC1 gene is not sufficient to transfer the self-incompatibility phenotype. Mol Gen Genet. 2000;263:648–654. doi: 10.1007/s004380051213. [DOI] [PubMed] [Google Scholar]
- Boggs NA, Dwyer KG, Nasrallah ME, Nasrallah JB. In vivo detection of residues required for ligand selective activation of the S-locus receptor in Arabidopsis. Curr Biol. 2009;19:786–791. doi: 10.1016/j.cub.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs NA, Nasrallah JB, Nasrallah ME. Independent S-locus mutations caused self-fertility in Arabidopsis thaliana. PLoS Genet. 2009;5:e1000426. doi: 10.1371/journal.pgen.1000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower MS, Matias DD, Fernandes-Carvalho E, et al. Two members of Thioredoxin-h family interact with the kinase domain of a Brassica S locus receptor kinase. Plant Cell. 1996;8:1641–1650. doi: 10.1105/tpc.8.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Chen CH, Tantikanjana T, Esch JJ, Nasrallah JB. Isolation of a second S-locus related cDNA from Brassica oleracea: genetic relationships between the S-locus and two related loci. Genetics. 1991;127:221–228. doi: 10.1093/genetics/127.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Nasrallah ME, Vrebalov J, Nasrallah JB. The self-incompatibility (S) haplotypes of Brassica contain highly divergent and rearranged sequences of ancient origin. Plant Cell. 1997;9:237–247. doi: 10.1105/tpc.9.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace J, King GJ, Ockendon DJ. A molecular approach to the identification of S-alleles in Brassica oleracea. Sex Plant Reprod. 1994;7:203–208. [Google Scholar]
- Braun DM, Stone JM, Walker JC. Interaction of the maize and Arabidopsis kinase interaction domains with a subset of receptor-like protein kinases: implications for transmembrane signaling in plants. Plant J. 1997;12:83–95. doi: 10.1046/j.1365-313x.1997.12010083.x. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL. Pollen cytology and incompatibility mechanisms in plants. J Hered. 1957;48:271–277. [Google Scholar]
- Brugiere N, Cui Y, Bi Y, Arnoldo M, Jackman L, Rothstein SJ. Molecular genetics of self-incompatibility in Brassica napus. Ann Bot. 2000;85:133–139. [Google Scholar]
- Cabin RJ, Evans AS, Jennings DL, Marshall DL, Mitchell RJ, Sher AA. Using bud pollinations to avoid self-incompatibility; implications from studies of three mustards. Can J Bot. 1996;74:285–289. [Google Scholar]
- Cabrillac D, Delorme V, Garin J, Ruffio-Chable V, Giranton JL, Dumas C, Gaude T, Cock JM. The S15 self-incompatibility haplotype in Brassica oleracea includes three S gene family members expressed in stigmas. Plant Cell. 1999;11:971–986. doi: 10.1105/tpc.11.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrillac D, Cock JM, Dumas C, Gaude T. The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature. 2001;410:220–223. doi: 10.1038/35065626. [DOI] [PubMed] [Google Scholar]
- Casselman AL, Vrebalov J, Conner JA, Singal A, Giovanni J, Nasrallah ME, Nasrallah JB. Determining the physical limits of Brassica S-locus by recombinational analysis. Plant Cell. 2000;12:23–24. doi: 10.1105/tpc.12.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LA, Goring DR. Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J Exp Bot. 2010;61:1987–1999. doi: 10.1093/jxb/erq021. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Swarup V. Self-incompatibility in Indian cauliflower. Eucarpia Cruciferae Newslett. 1984;9:25–27. [Google Scholar]
- Chen CH, Nasrallah JB. A new class of S sequence defined by a pollen recessive self-incompatibility allele of Brassica oleracea. Mol Gen Genet. 1990;222:241–248. doi: 10.1007/BF00633824. [DOI] [PubMed] [Google Scholar]
- Chookajorn T, Kachroo A, Ripoll DR, Clark AG, Nasrallah JB. Specificity determinants and diversification of the Brassica self-incompatibility pollen ligand. Proc Natl Acad Sci USA. 2004;101:911–917. doi: 10.1073/pnas.2637116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock JM, Stanchev B, Delorme V, Croy RRD, Dumas C. SLR3: a modified receptor kinase gene that has been adapted to encode a putative secreted glycoprotein similar to the S-locus glycoprotein. Mol Gen Genet. 1995;248:151–161. doi: 10.1007/BF02190796. [DOI] [PubMed] [Google Scholar]
- de Nettancourt D. Incompatibility and incongruity in wild and cultivated plants. Berlin: Springer; 2001. [Google Scholar]
- Delorme V, Giranton JL, Hatzfeld Y, Friry A, Heizmann P, Ariza MJ, Dumas C, Gaude T, Cock JM. Characterization of the S-locus genes, SLG and SRK, of the Brassica S3 haplotype: identification of a membrane-localized protein encoded by the S-locus receptor kinase gene. Plant J. 1995;7:429–440. doi: 10.1046/j.1365-313x.1995.7030429.x. [DOI] [PubMed] [Google Scholar]
- Dickinson HG, Lewis D. Cytochemical and ultrastructural differences between intraspecific compatible and incompatible pollinations in Raphanus. Proc R Soc Lond B Biol Sci. 1973;183:21–38. [Google Scholar]
- Dixit R, Nasrallah ME, Nasrallah JB. Post transcriptional maturation of the S receptor kinase of Brassica correlates with co-expression of the S-locus glycoprotein in the stigmas of two Brassica strains and in transgenic tobacco plants. Plant Physiol. 2000;124:297–311. doi: 10.1104/pp.124.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E, Meheust R, Soucaze M, Goubet PM, Gallina S, Poux C, et al. Dominance hierarchy arising from the evolution of a complex small RNA regulatory network. Science. 2014;346:1200–1205. doi: 10.1126/science.1259442. [DOI] [PubMed] [Google Scholar]
- Edh K, Widen B, Ceplitis A. The evolution and diversification of S-locus haplotypes in Brassicaceae family. Genetics. 2009;181:977–984. doi: 10.1534/genetics.108.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund AF, Swanson R, Preuss D. Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell. 2004;16:84–97. doi: 10.1105/tpc.015800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman CJ, Dickinson HG. The role of the exine coating in pollen-stigma interactions in Brassica oleracea L. New Phytol. 1990;114:511–518. doi: 10.1111/j.1469-8137.1990.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Franklin TM, Centre JI. SLR1 function is dispensable for both self-incompatible rejection and self-compatible pollination processes in Brassica. Sex Plant Reprod. 1996;9:203–208. [Google Scholar]
- Franklin-Tong N. Calcium signaling in Brassica. Nat Plants. 2015;1:15129. doi: 10.1038/nplants.2015.129. [DOI] [PubMed] [Google Scholar]
- Gao Q, Shi S, Liu Y, Pu Q, Liu X, Zhang Y, Zhu L. Identification of a novel MLPK homologous gene MLPKn1 and its expression analysis in Brassica oleracea. Plant Reprod. 2016;29:239–250. doi: 10.1007/s00497-016-0287-5. [DOI] [PubMed] [Google Scholar]
- Gaude T, Rougier M, Heizmann P, Ockendon DJ, Dumas C. Expression level of SLG is not correlated with the self-incompatibility phenotype in class II S haplotype of Brassica oleracea. Plant Mol Biol. 1995;27:1003–1014. doi: 10.1007/BF00037027. [DOI] [PubMed] [Google Scholar]
- Gonai H, Hinata K. The effect of temperature on pistil growth and the expression of self-incompatibility in cabbage. Jpn J Breed. 1971;21:195–198. [Google Scholar]
- Goring DR, Glavin DL, Schafer U, Rothstein SJ. An S receptor kinase gene in self-compatible Brassica napus has a 1-bp deletion. Plant Cell. 1993;5:531–553. doi: 10.1105/tpc.5.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubet PM, Berges H, Bellec A, Prat E, Helmstetter N, Mangenot S, Gallina S, Holl AC, Fobis-Loisy I, Vekemans X, Castrick V. Contrasted patterns of molecular evolution in dominant and recessive self-incompatibility haplotypes in Arabidopsis. PLoS Genet. 2012;8:e 1002495. doi: 10.1371/journal.pgen.1002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu T, Mazzurco M, Sulaman W, Matias DD, Goring DR. Binding of an arm repeat protein to the kinase domain of the S-locus receptor kinase. Proc Natl Acad Sci USA. 1998;95:382–387. doi: 10.1073/pnas.95.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffani YZ, Gaude T, Cock JM, Goring DR. Antisense suppression of thioredoxin h mRNA in Brassica napus cv. Westar pistils causes a low level constitutive pollen rejection response. Plant Mol Biol. 2004;55:619–630. doi: 10.1007/s11103-004-1126-x. [DOI] [PubMed] [Google Scholar]
- Hamid A, Maqsood A, Shah AS, Monakhos GF. Self- incompatibality and seed setting through heterogamic bud pollination and autogamic pollination of flowers as affected by location of bud/flower on plant, air temperature and time of pollination in self-incompatible inbred lines of head cabbage. Sarhad J Agric. 2010;26:1–5. [Google Scholar]
- Haruta T (1962) Studies on the genetics of self and cross-incompatibility in cruciferous vegetables. Research Bulletin No 2, Takii PI. Breeding Experiment Station, Kyoto, Japan
- Hatakeyama K, Takasaki T, Watanabe M, Hinata K. High sequence similarity between SLG and the receptor domain of SRK is not necessarily involved in higher dominance relationships in stigma in self-incompatible Brassica rapa L. Sex Plant Reprod. 1998;11:292–294. [Google Scholar]
- Hatakeyama K, Watanabe M, Takasaki T, Ojima K, Hinata K. Dominance relationships between S-alleles in self-incompatible Brassica campestris L. Heredity. 1998;80:241–247. [Google Scholar]
- Hatakeyama K, Takasaki T, Watanabe M, Hinata K. Molecular characterization of S locus genes, SLG and SRK, in pollen-recessive self-incompatibility haplotypes of Brassica rapa L. Genetics. 1998;149:1587–1597. doi: 10.1093/genetics/149.3.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama K, Takasaki T, Suzuki G, Nishio T, Watanabe M, Isogai A, Hinata The S receptor kinase gene determines dominance relationships in stigma expression of self-incompatibility in Brassica. Plant J. 2001;26:69–76. doi: 10.1046/j.1365-313x.2001.01009.x. [DOI] [PubMed] [Google Scholar]
- Havlickova L, Jozova E, Klima M, Kucera V, Curn V. Detection of self-incompatible oilseed rape plants (Brassica napus L.) based on molecular markers for identification of the class I S haplotype. Genet Mol Biol. 2014;37:556–559. doi: 10.1590/s1415-47572014000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ. Pollen recognition during the self-incompatibility response in plants. Genome Biol. 2002;3:004.1–004.6. doi: 10.1186/gb-2002-3-2-reviews1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock SJ, Allen AM. Diverse cell signaling pathways regulate pollen-stigma interactions: the search for consensus. New Phytol. 2008;179:286–317. doi: 10.1111/j.1469-8137.2008.02457.x. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Mclnnis SM. Pollen recognition and rejection during the sporophytic self-incompatibility response: Brassica and beyond. Trends Plant Sci. 2003;8:1360–1385. doi: 10.1016/j.tplants.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Hiscock SJ, Tabah DA. The different mechanisms of sporophytic self-incompatibility. Philos Trans R Soc Lond B Biol Sci. 2003;358:1037–1045. doi: 10.1098/rstb.2003.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoser-Krauze J. Inheritance of self-incompatibility and the use of it in the production of F1 hybrids of cauliflower. Genetica Polonica. 1979;20:341–367. [Google Scholar]
- Igic B, Lande R, Kohn JR. Loss of self-incompatibility and its evolutionary consequences. Int J Plant Sci. 2008;169:93–104. [Google Scholar]
- Indriolo E, Goring DR. A conserved role for the ARC1 E3 ligase in Brassicaceae self-incompatibility. Front Plant Sci. 2014;5:181. doi: 10.3389/fpls.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E, Tharmapalan P, Wright SI, Goring DR. The ARC1 E3 ligase gene is frequently deleted in self-compatible Brassicaceae species and has a conserved role in Arabidopsis lyrata self-pollen rejection. Plant Cell. 2012;24:4607–4620. doi: 10.1105/tpc.112.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indriolo E, Safavian D, Goring DR. The ARC1 E3 ligase promotes two different self-pollen avoidance traits in Arabidopsis. Plant Cell. 2014;26:1525–1543. doi: 10.1105/tpc.114.122879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov R, Gaude T. Endocytosis and endosomal regulation of the S-receptor kinase during the self-incompatibility response in Brassica oleracea. Plant Cell. 2009;21:2107–2117. doi: 10.1105/tpc.108.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov R, Fobis-Loisy I, Gaude T. When no means no: guide to Brassicaceae self-incompatibility. Trends Plant Sci. 2010;15:387–394. doi: 10.1016/j.tplants.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Iwano M, Ito K, Fujii S, Kakita M, et al. Calcium signaling mediates self-incompatibility response in the Brassicaceae. Nat Plants. 2015;1:15128. doi: 10.1038/nplants.2015.128. [DOI] [PubMed] [Google Scholar]
- Johnson AG. Problems in breeding and seed production of hybrid slots. Commer Grow. 1972;24:749–750. [Google Scholar]
- Jung HJ, Ahmed NU, Park JI, Kang KK, Hur Y, Lim YP, Nou IS. Development of self-compatible B. rapa by RNAi-mediated S-locus gene silencing. PLoS One. 2012;7:e49497. doi: 10.1371/journal.pone.0049497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Ahmed NU, Park JI, Chung MY, Cho YG, Nou IS. Molecular genetic aspects of self-incompatibility in Brassicaceae. Plant Breed Biotechnol. 2013;1:205–217. [Google Scholar]
- Kachroo A, Schopfer CR, Nasrallah ME, Nasrallah JB. Allele-specific receptor–ligand interactions in Brassica self-incompatibility. Science. 2001;293:1824–1826. doi: 10.1126/science.1062509. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Nasrallah ME, Nasrallah JB. Self-incompatibility in Brassicaceae: receptor–ligand signaling and cell-to-cell communication. Plant Cell. 2002;14:227–238. doi: 10.1105/tpc.010440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakita M, Murase K, Iwano M, Matsumoto T, Watanabe M, Shiba H, Isogai A, Takayama S. Two distinct forms of M locus protein kinase localize to the plasmamembrane and interact directly with S-locus receptor kinase to transduce self-incompatibility signaling in Brassica rapa. Plant Cell. 2007;19:3961–3973. doi: 10.1105/tpc.106.049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakita M, Shimosato H, Murase K, Isogai A, Takayama S. Direct interaction between S-locus receptor kinase and M-locus protein kinase involved in Brassica self-incompatibility signaling. Plant Biotechnol. 2007;24:185–190. [Google Scholar]
- Kakizaki Y. Studies on genetics and physiology of self- and cross-incompatibility in the common cabbage. Jpn J Bot. 1930;5:133–208. [Google Scholar]
- Kakizaki T, Takada Y, Ito A, Suzuki G, Shiba H, Takayama S, Isogai A, Watanabe M. Linear dominance relationship among four class-II S-haplotypes in pollen side determined by the expression of SP11 in Brassica self-incompatibility. Plant Cell Physiol. 2003;44:70–75. doi: 10.1093/pcp/pcg009. [DOI] [PubMed] [Google Scholar]
- Kanno T, Hinata K. An electron microscopic study of the barrier against pollen tube growth in self-incompatible Cruciferae. Plant Cell Physiol. 1969;10:213–216. [Google Scholar]
- Kao TH, Mccubbin AG. How flowering plants discriminate between self and non-self pollen to prevent inbreeding. Proc Natl Acad Sci USA. 1996;93:12059–12065. doi: 10.1073/pnas.93.22.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao TH, Mccubbin AG. A social stigma. Nature. 2000;403:840–841. doi: 10.1038/35002702. [DOI] [PubMed] [Google Scholar]
- Karron JD, Marshall DL, Olilveras DM. Numbers of sporophytic self-incompatibility alleles in populations of wild radish. Theor Appl Genet. 1990;79:457–460. doi: 10.1007/BF00226152. [DOI] [PubMed] [Google Scholar]
- Kemp BP, Doughty J. S cysteine-rich (SCR) binding domain analysis of the Brassica self incompatibility S-locus receptor kinase. New Phytol. 2007;175:619–629. doi: 10.1111/j.1469-8137.2007.02126.x. [DOI] [PubMed] [Google Scholar]
- Kitashiba H, Nasrallah JB. Self-incompatibility in Brassicaceae crops: lessons for interspecific incompatibility. Breed Sci. 2014;64:23–37. doi: 10.1270/jsbbs.64.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitashiba H, Nishio T. Self-incompatibility. In: Gupta SK, editor. Biology and breeding of crucifers. New York: CRC Press, Taylor and Francis Group; 2009. pp. 99–112. [Google Scholar]
- Koprna R, Kucera V, Kolovrat O, Vyvadilova M, Klima M. Development of self-incompatible lines with improved seed quality in winter oilseed rape (Brassica napus L.) Czech J Genet Plant Breed. 2005;41:105–111. [Google Scholar]
- Kroh M. An electron microscopic study of the behaviour of Cruciferae pollen after pollination. In: Linskens HF, editor. Pollen physiology and fertilisation. Amsterdam: North Holland; 1964. pp. 221–224. [Google Scholar]
- Kucera V, Chytilova V, Vyvadilova M, Klima M. Hybrid breeding of cauliflower using self-incompatibility and Cytoplasmic male sterility. HortScience. 2006;33:148–152. [Google Scholar]
- Kusaba M, Nishio T, Satta Y, Hinata K, Ockendon D. Striking similarity in inter- and intra-specific comparisons of class I SLG alleles from Brassica oleracea and Brassica campestris: implications for the evolution and recognition mechanism. Proc Natl Acad Sci USA. 1997;94:7673–7678. doi: 10.1073/pnas.94.14.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Dwyer K, Henderahot J, Verbalov J, Nasrallah JB, Nasrallah ME. Self-incompatibility in the genus Arabidopsis, characterization of the S-locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell. 2001;13:627–643. [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Tung CW, Nasrallah ME, Nasrallah JB. Monoallelic expression and dominance interactions in anthers of self-incompatible Arabidopsis lyrata. Plant Physiol. 2002;128:17–20. [PMC free article] [PubMed] [Google Scholar]
- Kwun M, Choi Y, Yoon H, Park BS, Kang BJ, Chung YY. Expression analysis of the pistil genes in controlling self-incompatibility in Brassica campestris by CO2 gas using microarray. Mol Cells. 2004;18:94–99. [PubMed] [Google Scholar]
- Lan Y, Yang J, Cao M, Wang Y, Kawabata S, Li Y. Isolation and characterization of a J domain protein that interacts with ARC1 from ornamental kale (Brassica oleracea var. acephala) Plant Cell Rep. 2015;34:817–829. doi: 10.1007/s00299-015-1744-6. [DOI] [PubMed] [Google Scholar]
- Lao X, Suwabe K, Niikura S, Kakita M, Iwano M, Takayama S. Physiological and genetic analysis of CO2-induced breakdown of self-incompatibility in Brassica rapa. J Exp Bot. 2014;65:939–951. doi: 10.1093/jxb/ert438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. Population genetics of the homomorphic self-incompatibility polymorphisms in flowering plants. Ann Bot. 2000;85:221–226. [Google Scholar]
- Leducq JB, Gosset CC, Gries R, Calin K, Schmitt E, Castric V, Vekemans X. Self-Incompatibility in Brassicaceae: identification and characterization of SRK-like sequences linked to the S-Locus in the tribe Biscutelleae. Genes Genom Genet. 2014;4:983–992. doi: 10.1534/g3.114.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SH, Cho HJ, Lee SJ, Cho YH, Kim BD. Identification and classification of S haplotypes in Raphanus sativus by PCR-RFLP of the S-locus glycoprotein (SLG) gene and the S locus receptor kinase (SRK) gene. Theor Appl Genet. 2002;104:1253–1263. doi: 10.1007/s00122-001-0828-6. [DOI] [PubMed] [Google Scholar]
- Mackay CR. A diallel cross method for the recognition of S-allele homozygote in turnip (Brassica campestris L. ssp. rapifera) Heredity. 1997;38:201–208. [Google Scholar]
- Manabe Y, Bressan RA, Wang T, Li F, Koiwa H, Sokolchik I, Li X, Maggio A. The Arabidopsis kinase-associated protein phosphatase regulates adaption to Na+ stress. Plant Physiol. 2008;146:612–622. doi: 10.1104/pp.107.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara S. Overcoming the self-incompatibility of Raphanus sativus by application of amino acids, vitamins and phytohormones. J Jpn Soc Hortic Sci. 1985;54:46–57. [Google Scholar]
- Mishima M, Takayama S, Sasaki K, Jee JG, et al. Structure of the male determinant factor for Brassica self-incompatibility. J Biol Chem. 2003;278:36389–36395. doi: 10.1074/jbc.M305305200. [DOI] [PubMed] [Google Scholar]
- Mohring S, Horstmann V, Esch E. Development of a molecular CAPS marker for the self-incompatibility locus in Brassica napus and identification of different S alleles. Plant Breed. 2005;124:105–110. [Google Scholar]
- Murase K, Shiba H, Iwano M, Che FS, Watanabe M, Isogai A, Takayama S. A membrane-anchored protein kinase involved in Brassica self-incompatibility signaling. Science. 2004;303:1516–1519. doi: 10.1126/science.1093586. [DOI] [PubMed] [Google Scholar]
- Naithani S, Dharmawardhana P, Nasrallah JB. SCR. In: Kastin AJ, editor. Handbook of biologically active peptides. 2. Cambridge: Elsevier, Academic Press; 2013. pp. 58–66. [Google Scholar]
- Nasrallah JB. Evolution of the Brassica self-incompatibility locus: a look into S-locus gene polymorphisms. Proc Natl Acad Sci USA. 1997;94:9516–9519. doi: 10.1073/pnas.94.18.9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah JB. Recognition and rejection of self in plant reproduction. Science. 2002;296:305–308. doi: 10.1126/science.296.5566.305. [DOI] [PubMed] [Google Scholar]
- Nasrallah JB, Nasrallah ME. Pollen-stigma signaling in the sporophytic self-incompatibility response. Plant Cell. 1993;5:1325–1335. doi: 10.1105/tpc.5.10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah JB, Nasrallah ME. S-locus receptor kinase signaling. Biochem Soc Trans. 2014;42:313–319. doi: 10.1042/BST20130222. [DOI] [PubMed] [Google Scholar]
- Nasrallah ME, Wallace DH. Immunogenetics of self-incompatibility in Brassica oleracea L. Heredity. 1967;38:201–208. [Google Scholar]
- Nasrallah JB, Kao TH, Goldberg ML, Nasrallah ME. A cDNA clone encoding an S-locus specific glycoprotein from Brassica oleracea. Nature. 1985;318:263–267. [Google Scholar]
- Nasrallah JB, Kao TH, Chen CH, Goldberg ML, Nasrallah ME. Amino-acid sequence of glycoproteins encoded by three alleles of the S-locus of Brassica oleracea. Nature. 1987;326:617–619. [Google Scholar]
- Nasrallah JB, Yu S-M, Nasrallah ME. Self-incompatibility genes of Brassica oleracea: Expression, isolation, and structure. Proc Natl Acad Sci USA. 1988;85:5551–5555. doi: 10.1073/pnas.85.15.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah JB, Nishio T, Nasrallah ME. The self-incompatibility genes of Brassica: expression and use in genetic ablation of floral tissues. Ann Rev Plant Physiol. 1991;42:393–422. [Google Scholar]
- Nasrallah ME, Kandasamy MK, Nasrallah JB. A genetically defined trans-acting lovcus regulates S-locus function in Brassica. Plant J. 1992;2:497–506. [Google Scholar]
- Nasrallah JB, Rundle SJ, Nasrallah ME. Genetic evidence for the requirement of Brassica S-locus receptor kinase in the self-incompatibility response. Plant J. 1994;5:373–384. [Google Scholar]
- Nasrallah ME, Liu P, Sherman-Broyles S, Boggs NA, Nasrallah JB. Natural variation in expression of self-incompatibility in Arabidopsis thaliana: implications for the evolution of selfing. Proc Natl Acad Sci USA. 2004;101:16070–16074. doi: 10.1073/pnas.0406970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi S, Vidyasagar Inheritance studies of self-incompatibility in low chill requiring genotypes of cabbage (Brassica oleracea L. var. capitata) for boltized flowering. Indian J Genet Plant Breed. 2008;68:204–207. [Google Scholar]
- Nikura S, Matsuura S. Genetic analysis of the reaction level of self-incompatibility to a 4% CO2 gas treatment in the radish (Raphanus sativus L.) Theor Appl Genet. 2000;101:1189–1193. [Google Scholar]
- Nishio T, Hinata K. Analysis of S-specific proteins in stigma of Brassica oleracea L. by isoelectric focusing. Heredity. 1977;38:391–396. [Google Scholar]
- Nishio T, Kusaba M. Sequence diversity of SLG and SRK in Brassica oleracea L. Ann Bot. 2000;85:141–146. [Google Scholar]
- Nou IS, Watanabe M, Isogai A, Shiozawa A, Hinata K. Variation of S-alleles and S-glycoproteins in a naturalized population of self-incompatible Brassica campestris L. Jpn J Genet. 1991;66:227–239. [Google Scholar]
- Nou IS, Watanabe M, Isuzugawa K, Isogai A, Hinata K. Isolation of S-alleles from a wild population of Brassica campestris L. at Balcesme. Turkey and their characterization by S-glycoproteins. Sex Plant Reprod. 1993;6:71–78. [Google Scholar]
- Ockendon DJ. Pollen tube growth and site of incompatibility reaction in Brassica oleracea. New Phytol. 1972;71:519–522. [Google Scholar]
- Ockendon DJ. Selection for high self-incompatibility in inbred lines of Brussels sprouts. Euphytica. 1973;22:503–509. [Google Scholar]
- Ockendon DJ. Distribution of self-incompatibility alleles and breeding structure of open pollinated cultivars of Brussel sprouts. Heredity. 1974;33:159–171. [Google Scholar]
- Ockendon DJ. Dominance relationships between S-alleles in the stigma of Brussels sprouts (Brassica oleracea var. gemmifera) Euphytica. 1975;24:165–172. [Google Scholar]
- Ockendon DJ. The S-allele collection of Brassica oleracea. Acta Hortic. 2000;539:25–30. [Google Scholar]
- Odland ML, Noll CJ. The utilization of cross compatibility and self-incompatibility in the production of F1 hybrid cabbage. Proc Am Soc Hortic Sci. 1950;55:391–402. [Google Scholar]
- Okamoto S, Sato Y, Sakamoto K, Nishio T. Distribution of similar self-incompatibility (S) haplotypes in different genera, Raphanus and Brassica. Sex Plant Reprod. 2004;17:33–39. [Google Scholar]
- Palloix A, Herve Y, Knox RB, Dumas C. Effect of CO2 and relative humidity on self-incompatibility in cauliflower, Brassica oleracea. Theor Appl Genet. 1985;70:628–633. doi: 10.1007/BF00252288. [DOI] [PubMed] [Google Scholar]
- Park JI, Lee SS, Watanabe M, Takahata Y, Nou IS. Identification of S-alleles using polymerase chain reaction-cleaved amplified polymorphic sequence of the S-locus receptor kinase in inbreeding lines of Brassica oleracea. Plant Breed. 2002;121:192–197. [Google Scholar]
- Pearson OH. Breeding plants of the cabbage group. Calif Agric Exp Stn Bull. 1932;532:3–22. [Google Scholar]
- Roberts IN, Stead AD, Ockendon DJ, Dickinson HG. Pollen stigma interactions in Brassica oleracea. Theor Appl Genet. 1980;58:241–246. doi: 10.1007/BF00265173. [DOI] [PubMed] [Google Scholar]
- Roberts IN, Gaude TC, Harrod G, Dickinson HG. Pollen-stigma interactions in Brassica oleracea; a new pollen germination medium and its use in elucidating the mechanism of self-incompatibilty. Theor Appl Genet. 1983;65:231–238. doi: 10.1007/BF00308074. [DOI] [PubMed] [Google Scholar]
- Roggen H. Stigma application of an extract from rape pollen (Brassica napus L.) effects self-incompatibility in Brussels sprouts (Brassica olerace L. var. gemmifera DC) Incompat Newslett. 1975;6:80–86. [Google Scholar]
- Roggen H, Dijk Van AJ. Breaking incompatibility of Brassica oleracea L. by steel-brush pollination. Euphytica. 1972;21:424–425. [Google Scholar]
- Roggen H, Dijk Van AJ. ‘Thermally aided pollination’: a method of breaking self-incompatibility in Brassica oleracea L. Euphytica. 1976;25:643–646. [Google Scholar]
- Roggen HPJR, Dijk AJ, Van Dorsman C. ‘Electric aided pollination’: a method of breaking incompatibility in Brassica oleracea L. Euphytica. 1972;21:181–184. [Google Scholar]
- Sakamoto K, Nishio T. Distribution of S haplotypes in commercial cultivars of Brassica rapa. Plant Breed. 2001;120:155–161. [Google Scholar]
- Sakamoto K, Kusaba M, Nishio T. Polymorphism of the S-locus glycoprotein gene (SLG) and the S-locus related gene (SLR1) in Raphanus sativus L. and self-incompatible ornamental plants in the Brassicaceae. Mol Gen Genet. 1998;258:397–403. doi: 10.1007/s004380050747. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Kusaba M, Nishio T. Single-seed PCR-RFLP analysis for the identification of S haplotypes in commercial F1 hybrid cultivars of broccoli and cabbage. Plant Cell Rep. 2000;19:400–406. doi: 10.1007/s002990050747. [DOI] [PubMed] [Google Scholar]
- Sampson DR. The genetics of self and cross-compatibility in Brassica oleracea. Genetics. 1957;42:253–263. doi: 10.1093/genetics/42.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson DR. The genetics of self-incompatibility in radish. J Heredity. 1957;48:26–29. [Google Scholar]
- Samuel MA, Mudgil Y, Salt JN, Delmas F, Ramachandran S, Chilelli A, Goring DR. Interactions between the S-domain receptor kinases and AtPUB-ARM E3 ubiquitin ligases suggest a conserved signaling pathway in Arabidopsis. Plant Physiol. 2008;147:2084–2095. doi: 10.1104/pp.108.123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Chong YT, Hassen KE, Aldea-Brydges MG, Stone SL, Goring DR. Cellular pathways regulating responses to compatible and self-incompatible pollen in Brassica and Arabidopsis stigmas intersect at Exo70A1, a putative component of the exocyst complex. Plant Cell. 2009;21:2655–2671. doi: 10.1105/tpc.109.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Tang W, Jamshed M, Northey J, Patel D, Smith D, Siu KW, Muench DG, Wang ZY, Goring DR. Proteomic analysis of Brassica stigmatic proteins following the self-incompatibility reaction reveals a role for microtubule dynamics during pollen response. Mol Cell Proteom. 2011;10:M1111.011338. doi: 10.1074/mcp.M111.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera GS, Hussian W, Singh G, Parray GA. Molecular perspective to understand the phenomenon of self-incompatibility and its utilization in crop plants. Inroads. 2012;1:166–177. [Google Scholar]
- Sankaranarayanan S, Jamshed M, Samuel MA. Proteomics approaches advance our understanding of plant self-incompatibility response. J Proteom Res. 2013;12:4717–4726. doi: 10.1021/pr400716r. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Jamshed M, Samuel MA. Degradation of glyoxalase 1in Brassica napus stigma leads to self-incompatibility response. Nat Plants. 2013;1:15185. doi: 10.1038/nplants.2015.185. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Jamshed M, Samuel MA. Degradation of Glyoxalase I in Brassica napus stigma leads to self-incompatibility response. Nat Plants. 2015;21:15185. doi: 10.1038/nplants.2015.185. [DOI] [PubMed] [Google Scholar]
- Sato T, Thorsness MK, Kandasamy MK, Nishio T, Hirai M, Nasrallah JB, Nasrallah ME. Activity of an S locus gene promoter in pistils and anthers of transgenic Brassica. Plant Cell. 1991;3:867–876. doi: 10.1105/tpc.3.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Nishio T, Kimura R, Kusaba M, Suzuki T, Hatakeyama K, Ockendon D, Satta Y. Co-evolution of the S-locus genes, SRK, SLG and SCR/SP11 in Brassica oleracea and Brassica rapa. Genetics. 2002;162:931–940. doi: 10.1093/genetics/162.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah JB. Self-incompatibility. Prospects for a novel putative peptide-signaling molecule. Plant Physiol. 2000;124:935–939. doi: 10.1104/pp.124.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopfer CR, Nasrallah ME, Nasrallah JB. The male determinant of self-incompatibility in Brassica. Science. 1999;286:1697–1700. doi: 10.1126/science.286.5445.1697. [DOI] [PubMed] [Google Scholar]
- Scutt CP, Croy RRD. An S5 self-incompatibility allele-specific cDNA sequence from Brassica oleracea shows high homology to the SLR2 gene. Mol Gen Genet. 1992;232:240–246. doi: 10.1007/BF00280002. [DOI] [PubMed] [Google Scholar]
- Scutt CP, Gates PJ, Gatehouse JA, Boulter D, Croy RRD. A cDNA encoding an S-locus specific glycoprotein from Brassica oleracea plants containing the S5 self-incompatibility allele. Mol Gen Genet. 1990;220:409–413. doi: 10.1007/BF00391746. [DOI] [PubMed] [Google Scholar]
- Shah K, Russinova E, Gadella TW, Jr, Willemse J, De Vries SC. The Arabidopsis kinase-associated protein phosphatase controls internalization of the somatic embryogenesis receptor kinase 1. Genes Dev. 2002;16:1707–1720. doi: 10.1101/gad.220402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Bajaj M, Shivanna KR. Overcoming self-incompatibility through the use of lectins and sugars in Petunia and Eruca. Ann Bot. 1984;55:139–141. [Google Scholar]
- Shen JX, Wang HZ, Fu TD, Tian BM. Cytoplasmic male sterility with self-incompatibility, a novel approach to utilizing heterosis in rapeseed (Brassica napus L.) Euphytica. 2008;162:109–115. [Google Scholar]
- Shiba H, Takayama S, Iwano M, Shimosato H, Funato M, et al. A pollen coat protein SP11/SCR, determines the pollen S-specificity in the self-incompatibility of Brassica species. Plant Physiol. 2001;125:2095–2103. doi: 10.1104/pp.125.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H, Iwano M, Entani T, Ishimoto K, Shimosato H, Che FS, Satta Y, Ito A, Takada Y, Watanabe M, Isogai A, Takayama S. The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level. Plant Cell. 2002;14:491–504. doi: 10.1105/tpc.010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba H, Kakizaki T, Iwano M, Tarutani Y, Watanabe M, Isogai A, Takayama S. Dominance relationships between self-incompatibility alleles controlled by DNA methylation. Nat Genet. 2006;38:297–299. doi: 10.1038/ng1734. [DOI] [PubMed] [Google Scholar]
- Shimosato H, Yokota N, Shiba H, Iwano M, Entani T, Che FS, Watanabe M, Isogai Takayama S. Characterization of the SP11/SCR high-affinity binding site involved in self/nonself recognition in Brassica self-incompatibility. Plant Cell. 2007;19:107–117. doi: 10.1105/tpc.105.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NF, Stone SL, Christie LN, Sulaman W, Nazarian KAP, Burnett LA, Arnoldo MA, Rothstein SJ, Goring DR. Expression of the S receptor kinase in self-compatible Brassica napus cv. Westar leads to the allele-specific rejection of self-incompatible Brassica napus pollen. Mol Genet Genom. 2001;265:552–559. doi: 10.1007/s004380100446. [DOI] [PubMed] [Google Scholar]
- Singh S, Vidyasagar Effect of common salt (NaCl) sprays to overcome the self-incompatibility in the S-allele lines of Brassica oleracea var. capitata L. SABRAO J Breed Genet. 2012;44:339–348. [Google Scholar]
- Singh PK, Pandey V, Singh M, Sharma SR. Genetic improvement of cauliflower. Veg Sci. 2013;40:121–136. [Google Scholar]
- Sobotka R, Sakova L, Curn V. Molecular mechanisms of self-incompatibility in Brassica. Curr Issues Mol Biol. 2000;2:103–112. [PubMed] [Google Scholar]
- Stein JC, Howlett B, Boyes DC, Nasrallah ME, Nasrallah JB. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus Brassica oleracea. Proc Nat Acad Sci USA. 1991;88:8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JC, Dixit R, Nasrallah ME, Nasrallah JB. SRK, the stigma specific S-locus receptor kinase of Brassica, is targeted to the plasma membrane in transgenic tobacco. Plant Cell. 1996;8:429–445. doi: 10.1105/tpc.8.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JP, Kay QON. The number, dominance relationships and frequencies of self-incompatibility alleles in a natural population of Sinapsis arvensis L. in South Wales. Heredity. 1989;62:199–205. [Google Scholar]
- Stone JM, Trotochaud AE, Walker JC, Clark SE. Control of meristem development by CLAVATA1 receptor kinase and kinase-associated protein phosphatase interactions. Plant Physiol. 1998;117:1217–1225. doi: 10.1104/pp.117.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Arnold M, Goring DR. A breakdown of Brassica self-incompatibility in ARC1 antisense transgenic plants. Science. 1999;286:1729–1731. doi: 10.1126/science.286.5445.1729. [DOI] [PubMed] [Google Scholar]
- Stone SL, Anderson EM, Mullen RT, Goring DR. ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self-incompatible Brassica pollen. Plant Cell. 2003;15:885–898. doi: 10.1105/tpc.009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout AB. Fertility in Cichorium intybus. The sporadic occurrence of self-fertile plants among the progeny of self-sterile planrs. Am J Bot. 1917;4:375–395. [Google Scholar]
- Sulaman W, Arnoldo MA, Yu K, Tulsieram L, Rothstein SJ, Goring DR. Loss of callose in the stigma papillae does not affect the Brassica self-incompatibility phenotype. Planta. 1997;203:327–331. [Google Scholar]
- Suzuki G. Recent progress in plant reproduction research: the story of the male gametophyte through to successful fertilization. Plant Cell Physiol. 2009;50:1857–1864. doi: 10.1093/pcp/pcp142. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Watanabe M, Toriyama K, Isogai A, Hinata K. Molecular cloning of members of the S-multigene family in self-incompatible Brassica campestris. Plant Cell Physiol. 1995;36:1273–1280. [PubMed] [Google Scholar]
- Suzuki G, Kai N, Hirose T, Fukui K, Nishio T, Takayama S, Isogai A, Watanabe M, Hinata K. Genomic organization of the S locus: identification and characterization of genes in SLG/SRK region of S9 haplotype of Brassica campestris (syn. rapa) Genetics. 1999;153:391–400. doi: 10.1093/genetics/153.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Kusaba M, Matsushita M, Okazaki K, Nishio T. Characterization of Brassica S-haplotypes lacking S-locus glycoprotein. FEBS Lett. 2000;482:102–108. doi: 10.1016/s0014-5793(00)02048-2. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Watanabe M, Toriyama K, Isogai A, Hinata K. Introduction of SLG (S-locus glycoprotein) alters the phenotype of endogenous S-haplotype, but confers no new S haplotype specificity in Brassica rapa L. Plant Mol Biol. 1999;40:659–668. doi: 10.1023/a:1006274525421. [DOI] [PubMed] [Google Scholar]
- Takasaki T, Hatakeyama K, Suzuki G, Watanabe M, Isogai A, Hinata K. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature. 2000;403:913–916. doi: 10.1038/35002628. [DOI] [PubMed] [Google Scholar]
- Takayama S, Isogai A. Self-incompatibility in plants. Annu Rev Plant Biol. 2005;56:467–489. doi: 10.1146/annurev.arplant.56.032604.144249. [DOI] [PubMed] [Google Scholar]
- Takayama S, Isogai A, Tsukamoto C, Ueda YK, Hinata KO, Suzukhi A. Sequences of S-glycoproteins, products of the Brassica campestris self-incompatibility locus. Nature. 1987;326:102–105. [Google Scholar]
- Takayama S, Shiba H, Iwano M, Shimosato H, Che FS, Kai N, Watanabe M, Suzuki G, Hinata K, Isogai A. The pollen-determinant of self-incompatibility in Brassica campestris. Proc Natl Acad Sci USA. 2000;97:1920–1925. doi: 10.1073/pnas.040556397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Shimosato H, Shiba H, Funato M, Che FS, Watanabe M, Iwano M, Isogai A. Direct ligand–receptor complex interaction controls Brassica self-incompatibility. Nature. 2001;413:534–538. doi: 10.1038/35097104. [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah JB. Ligand-mediated cis-inhibition of receptor signaling in the self-incompatibility response of the Brassicaceae. Plant Physiol. 2015;169:1141–1154. doi: 10.1104/pp.15.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Stein JC, Chen CH, Nasrallah JB. An alternative transcript of the S locus glycoprotein gene in a class II pollen recessive self-incompatibility haplotype of Brassica oleracea encodes a membrane-anchored protein. Plant Cell. 1993;5:657–666. doi: 10.1105/tpc.5.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Nasrallah JB. The Brassica S gene family: molecular characterization of the SLR2 gene. Sex Plant Reprod. 1996;9:107–116. [Google Scholar]
- Tantikanjana T, Rizvi N, Nasrallah ME, Nasrallah JB. A dual role for the S-locus receptor kinase in self-incompatibility and pistil development revealed by an Arabidopsis rdr6 mutation. Plant Cell. 2009;21:2642–2654. doi: 10.1105/tpc.109.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantikanjana T, Nasrallah ME, Nasrallah JB. Complex networks of self-incompatibility signaling in the Brassicaceae. Curr Opn Plant Biol. 2010;13:520–526. doi: 10.1016/j.pbi.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Tarutani Y, Shiba H, Iwano M, Kakizaki T, Suzzuki G, Watanabe M, Isogai A, Takayama S. Trans-acting small RNA determines dominance relationships in Brassica self-incompatibility. Nature. 2010;466:983–986. doi: 10.1038/nature09308. [DOI] [PubMed] [Google Scholar]
- Tatebe T. Studies on physiological mechanism of self-incompatibility in Japanese radish. II. Breakdown of self-incompatibility by chemical treatments. J Jpn Soc Hortic Sci. 1968;37:43–46. [Google Scholar]
- Thompson KF. Self-incompatibility in marrow stem kale Brassica oleracea var. acephala. I. Demonstration of sporophytic system. J Genet. 1957;55:45–60. [Google Scholar]
- Thompson KF, Taylor JP. Identical S-alleles in different botanical varieties of Brassica oleracea. Nature. 1965;208:306–307. [Google Scholar]
- Thompson KF, Taylor JP. Non-linear dominance relationships between S-alleles. Heredity. 1966;21:345–362. [Google Scholar]
- Thompson KF, Taylor JP. Self-compatibility in kale. Heredity. 1971;27:459–471. [Google Scholar]
- Tichtinsky G, Vanoosthuyse V, Cock JM, Gaude T. Making inroads into plant receptor kinase signalling pathways. Trends Plant Sci. 2003;8:231–237. doi: 10.1016/S1360-1385(03)00062-1. [DOI] [PubMed] [Google Scholar]
- Tingting G, Li W, Aifen Z, Tongkun L, Jianjun W, Xilin H, Ying L. S haplotypes in double-haploid lines of non-heading Chinese cabbage (Brassica campestris L. ssp. chinensis Makino) Indian J Genet Plant Breed. 2013;73:400–404. [Google Scholar]
- Toriyama K, Stein JC, Nasrallah ME, Nasrallah JB. Transformation of Brassica oleracea with an S-locus gene from Brassica campestris changes the self-incompatibility phenotype. Theor Appl Genet. 1991;81:769–776. doi: 10.1007/BF00224988. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse V, Tichtinsky G, Dumas C, Gaude T, Cock JM. Interaction of calmodulin, a sorting nexin and kinase-associated protein phosphatase with the Brassica oleracea S locus receptor kinase. Plant Physiol. 2003;133:919–929. doi: 10.1104/pp.103.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagar (1981) Self-incompatibility and S-alleles in Indian cauliflower. PhD. Thesis, PG School, IARI, New Delhi
- Wallace DH. Interactions of S-alleles in sporophytically controlled self-incompatibility of Brassica. Theor Appl Genet. 1979;54:193–201. doi: 10.1007/BF00267707. [DOI] [PubMed] [Google Scholar]
- Wallace DH, Nasrallah ME. Pollination and sereological procedures for isolating incompatibility genotypes in the crucifers. Cornell Univ Agric Exp Stn Memoir. 1968;406:23. [Google Scholar]
- Wang T, Li H, Lu Y, Zhang J, Ye Z. Identification and distribution of S haplotypes in Brassica vegetables from China. Sci Hortic. 2007;112:271–277. [Google Scholar]
- Wang L, Hou X, Zhang A, Li Y. Effect of NaCl in overcoming self-incompatibility in non-heading Chinese cabbage (Brassica campestris ssp. chinensis Makino) studied by fluorescent microscopy. Acta Hortic. 2012;932:127–132. [Google Scholar]
- Wang L, Peng H, Ge T, Liu T, Hou X, Li Y. Identification of differentially accumulating pistil proteins associated with self-incompatibility of non-heading Chinese cabbage. Plant Biol. 2014;16:49–57. doi: 10.1111/plb.12016. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Nou IS, Takayama S, Isogai A, Suzuki A, Takeuchi T, Hinata K. Variations in and inheritance of NS-glycoprotein in self-incompatible Brassica campestris L. Plant Cell Physiol. 1992;33:343–351. [Google Scholar]
- Watanabe M, Takasaki T, Toriyama K, Yamakawa S, Isogai A, Suzuki A, Hinata K. A high degree of homology exists between the protein encoded by SLG and the S receptor domain encoded by SRK in self-incompatible Brassica campestris L. Plant Cell Physiol. 1994;335:1221–1229. doi: 10.1093/oxfordjournals.pcp.a078716. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ito A, Takada Y, Ninmiya C, et al. Highly divergent sequences of the pollen self-incompatibility (S) gene in class-I S haplotypes of Brassica campestris (syn. rapa) L. FEBS Lett. 2000;473:139–144. doi: 10.1016/s0014-5793(00)01514-3. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Takayama S, Isogai A, Hinata K. Recent progress on self-incompatibility research in Brassica species. Breed Sci. 2003;53:199–208. [Google Scholar]
- Watanabe M, Suwabe K, Suzuki G. Molecular genetics, physiology and biology of self-incompatibility in Brassicaceae. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:519–535. doi: 10.2183/pjab.88.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts LE. Investigations breeding. I. Studies on self-incompatibility. Euphytica. 1963;12:330–340. [Google Scholar]
- Watts LE. Investigations into the breeding system of cauliflower. II. Adaptation of the system to inbreeding. Euphytica. 1965;14:67–77. [Google Scholar]
- Williams RW, Wilson JM, Meyerowitz EM. A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway. Proc Natl Acad Sci USA. 1997;94:10467–10472. doi: 10.1073/pnas.94.19.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Nishio T. Commonalities and differences between Brassica and Arabidopsis self-incompatibility. Hortic Res. 2014;1:14054. doi: 10.1038/hortres.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Wada Y, Kakizaki T, Tarutani Y, Miura-Uno E, Murase K, Fujii S, Hioki T, Shimoda T, Takada Y, Shiba H, Takasaki-Yasuda T, Suzuki G, Watanabe M, Takayama S. A complex dominance hierarchy is controlled by polymorphism of small RNAs and their targets. Nat Plants. 2016;3:16206. doi: 10.1038/nplants.2016.206. [DOI] [PubMed] [Google Scholar]
- Yee D, Goring DR. The diversity of plant U-box E3 ubiquitin ligases: from upstream activators to downstream target substrates. J Exp Bot. 2009;60:1109–1121. doi: 10.1093/jxb/ern369. [DOI] [PubMed] [Google Scholar]
- Yu K, Schafer U, Glavin TL, Goring DR, Rothstein SJ. Molecular characterization of the S locus in two self-incompatible Brassica napus lines. Plant Cell. 1996;8:2369–2380. doi: 10.1105/tpc.8.12.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HF, Zhao ZQ, Sheng XG, Wang JS, Gu HH, Chen JS, Xu YJ. Identification of S-haplotypes in DH-lines of broccoli (Brassica oleracea L. var. italic) J Hortic Sci Biotechnol. 2014;89:430–434. [Google Scholar]
- Zhang X, Ma C, Fu T, Li Y, Wang T, Chen Q, Tu J, Shen J. Development of SCAR markers linked to self-incompatibility in Brassica napus L. Mol Breed. 2008;21:305–315. [Google Scholar]