Summary
The dentate gyrus (DG) is crucial for behaviorally discriminating similar spatial memories, predicting that dentate gyrus place cells change (“remap”) spatial tuning (“place fields”) for memory discrimination. This prediction was never tested, although DG place cells remap across similar environments without memory tasks. We confirm this prior finding but find that DG place fields do not remap across spatial tasks that require DG-dependent memory discrimination. Instead of remapping, place-discriminating discharge is observed transiently amongst DG place cells, particularly when memory discrimination is most necessary. The DG network may signal memory discrimination by expressing distinctive sub-second network patterns of co-firing at memory discrimination sites. This involves increased coupling of discharge from place cells and interneurons, as was observed during successful but not failed behavioral expression of memory discrimination. Instead of remapping, these findings indicate that memory discrimination is signaled by sub-second patterns of correlated discharge within the dentate network.
Keywords: Dentate gyrus, place cells, remapping, memory discrimination, pattern separation
eTOC blurb
van Dijk and Fenton report that dentate gyrus place cells signal memory discrimination not by remapping, but by variable sub-second patterns of coordinated place cell discharge marked by enhanced discharge coupling between excitatory and inhibitory neurons during memory discrimination.
Introduction
Hippocampus is crucial for discriminating between memories (Gilbert et al., 1998; Yassa and Stark, 2011), for navigating space (Maguire et al., 1998; Morris et al., 1982; O’Keefe and Nadel, 1978), and the discharge of hippocampus place cells represents locations (O’Keefe, 1976; Wilson and McNaughton, 1993). Although place cell discharge is unreliable in time (Fenton and Muller, 1998), the discharge reliably localizes to specific regions of space called the cell’s place field (O’Keefe, 1976). Place fields relocate when the environment changes sufficiently, a process known as “spatial remapping” (Muller and Kubie, 1987) or alternatively, they maintain their locations but systematically change firing rates in what is called “rate remapping” (Hayman et al., 2003; Leutgeb et al., 2005b). Both forms of remapping have been associated with memory discrimination (Alme et al., 2014; Colgin et al., 2008; Wills et al., 2005), and the dominant cognitive map theory predicts place fields will remap across conditions requiring distinct place memories (O’Keefe and Nadel, 1978).
Theories of hippocampus memory computation assert the DG is specialized for discriminative functions such that DG outputs are more distinctive than the corresponding inputs, what is called “pattern separation” (Marr, 1971; O’Reilly and McClelland, 1994; Treves and Rolls, 1994). As predicted, compromising DG function impairs difficult memory discriminations in modestly distinct environments (Burghardt et al., 2012; Gilbert et al., 2001; Kheirbek et al., 2013; Lee et al., 2005; McHugh et al., 2007; Nakashiba et al., 2012). In addition, DG principal cells consisting of granule cells and mossy cells remap in response to sufficiently large environmental changes (Danielson et al., 2017; GoodSmith et al., 2017; Leutgeb et al., 2007; Neunuebel and Knierim, 2014; Senzai and Buzsaki, 2017), but the extent to which these observations indicate pattern separation and whether they are relevant to memory discrimination is not established.
Indeed, DG place cells have been characterized but not during an explicit memory discrimination task that requires intact DG function (Danielson et al., 2017; GoodSmith et al., 2017; Leutgeb et al., 2007; McHugh et al., 2007; Neunuebel and Knierim, 2014; Senzai and Buzsaki, 2017). To critically test the prediction that DG remapping mediates memory discrimination we recorded DG neurons while mice on a rotating arena perform DG-dependent memory discriminations in active place avoidance tasks that require avoiding a shock zone (Burghardt et al., 2012; Kheirbek et al., 2013; Park et al., 2015). The rotation dissociates the environment into two spatial reference frames and reveals that distinct frame-specific patterns of ensemble place representations alternate in the ensemble spike time series (Fenton et al., 1998; Kelemen and Fenton, 2010). We designed experiments that manipulated the demand for DG-dependent memory discriminations and compared the characteristics of DG neural discharge across the conditions of high and low demand to evaluate whether changes in firing fields specifically accompany DG-dependent memory discrimination. Instead of time-averaged changes in spatial tuning associated with remapping, we find that changing DG-dependent memory discrimination modulates dynamic patterns of correlated DG discharge. Test statistics are provided in the main text except those from comparisons of data in figures, which are reported in the corresponding figure legends.
Results
DG place field remapping across a modified environment
We began by conducting a standard remapping experiment that compares hippocampus place fields across different versions of an environment, which is standardly interpreted to require different spatial memories of the different conditions (GoodSmith et al., 2017; Leutgeb et al., 2005a; Lever et al., 2002; Muller and Kubie, 1987; Wills et al., 2005). The experimental design assumes steady state physical and behavioral conditions, although variations in hidden cognitive variables can potentially cause transient discharge variations that are not captured by the session-averaged properties of firing fields (Fenton et al., 2010; Johnson et al., 2009; Johnson and Redish, 2008; Kelemen and Fenton, 2013; Singer et al., 2010) reviewed in (Karlsson and Frank, 2008).
We recorded cells across three versions of an environment (standard, 90° cue relocation, and wall removal; Fig. 1A, Fig. S1). While we focused on recording DG place cells, we also evaluated a subset of place cells from CA3 and CA1 in these conditions. These DG place cells can be granule cells or mossy cells, or more likely a mixture with granule cells being about 2/3 as likely as mossy cells to exhibit spatial firing (GoodSmith et al., 2017; Sasaki et al., 2018). This comparison confirmed that place cells in DG (45 place cells of 133 cells in 5 mice) are more sensitive to environmental changes than those in Ammon’s horn (place cells from CA3 n = 7 and CA1 n = 13, of 42 cells in 3 mice) (Leutgeb et al., 2007). The majority of DG (64%) and CA (75%) place cells had a single place field during the initial recording in the standard environment (test of proportions z = 1.6, p = 0.1). The number of place fields scaled with the 2-fold increase in area from the circular (standard and cue-relocated environments) to the wall removal conditions, with the DG cells scaling more (DG circular: 1.48±0.19 fields; removed: 2.70±0.20 fields; CA circular 1.19±0.28 fields; removed 2.0+0.30 fields; region: F1,64.2 = 4.12, p = 0.05; condition: F1, 64.2=17.1, p=10−4, interaction: F1, 64.2 = 0.67, p = 0.42 post-hoc: removed DG > circular DG and CA; circular CA = removed CA), as previously reported for rat place cells (Fenton et al., 2008; Park et al., 2011). Other discharge properties were also similar for the DG and CA principal cell populations (Table S1). DG place cell firing rate map stability was less than in CA and the stability of both populations was changed by wall removal but not by cue relocation (Fig. 1B). Rate remapping in DG and CA place cells did not differ across the environment manipulations (region: F1,172=0.16, p=0.68; manipulation: F2,172 = 2.8, p = 0.064; region x manipulation: F2,172 = 0.2, p= 0.81). The magnitude of the firing rate changes in the primary place field also did not differ across manipulations or regions (Fig. 1C).
Figure 1. DG place field remapping across a modified environment.
A) Example DG (left) and CA1 (right) firing rate maps of mice exploring three versions of an environment: a transparent cylindrical enclosure with cue cards in a square box with a cue card (standard), the same cylindrical enclosure rotated 90° while the box remained stable (relocated) and the square box by itself (cylinder removed). Each unit’s average concatenated tetrode waveform for each session is placed above each rate map. B) Firing rate map stability measured by correlating rate maps of each cell across a pair of conditions. We define three comparison manipulations: replication = [standard-standard (illustrated), rotated-rotated, removed-removed], relocation = [standard-relocated] and removal = [standard-removed (illustrated), relocated-removed]. Stability of place cells in Ammon’s horn (CA) is higher than those in DG. Stability is lower for the removal manipulation than for replication and relocation manipulations (region: F1,172 =15.1, p = 0.0001; manipulation effect F2,172=12.4, p<0.0001; interaction: F2,172, p=0.77). C) Left: Log field rates and rate changes for the first versus second trial of each manipulation. The magnitude of field rate changes did not differ between manipulations nor regions (region: F1,164 = 3.75, p = 0.06, manipulation: F2,164 = 0.37, p = 0.69, interaction: F2,164 = 0.8, p = 0.45). These results confirm that DG place cells are sensitive to changes in the environment and that DG shows less firing rate map stability than CA. See also Figure S1. Bar graphs represent mean ± SEM. * signifies regional differences, # signifies manipulation differences.
Optogenetic verification of a DG-dependent memory discrimination task
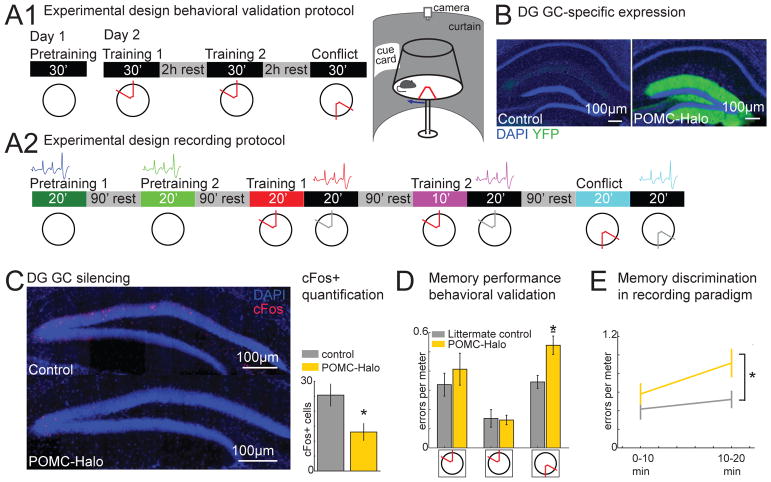
Before testing the prediction that place fields change with memory discrimination demand using an active place avoidance paradigm, we confirmed that the memory discrimination task depends on DG function, as previously reported (Burghardt et al., 2012; Kheirbek et al., 2013). The basic task requires a mouse on a rotating circular disk-shaped arena to avoid being in a 60° sector that is designated a shock zone (Fig. 2A). The zone is unmarked and is defined by its stable location with respect to the room. The animal must actively avoid the region because the arena rotation can transport the mouse into the shock zone. The behavioral protocol has three phases (Fig. 2A). During the first, pretraining, there is no shock and the mouse can learn and become familiar with the environment. During the two trials of the training phase, shock occurs upon entering the shock zone and the mice express a conditioned avoidance, typically preferring to spend their time opposite the shock zone. The third phase is called conflict; the shock zone is relocated 180° and nothing changes in the environment except where shock is delivered. Since shock is unmarked there is nothing that is physically different about the environment on any of the pretraining, training, or conflict trials except during the exact moments of shock which constitutes ~1% of a mouse’s experience of the environment. Cre+ POMC-Halorhodopsin mice expressing the inhibitory opsin in DG granule cells (Fig. 2B) were used to test that the task is DG-dependent. All mice received laser illumination during the behavioral protocol and mice were initially naïve to the arena and place avoidance. The illumination silences granule cells in the Cre+ but not Cre− littermates, and this was confirmed by post-conflict cFos immunostaining (Fig. 2C). The effect of optogenetic silencing of DG function on active place avoidance was evaluated in two cohorts. The cohorts differed in that the behavioral protocol for one was designed only to evaluate the role of DG function in the task (Cre+ = 7, Cre− = 8; Fig. 2A1) and the protocol for the other cohort (n’s = 7) was modified to be identical to the protocol that was used for the electrophysiological recording (Fig. 2A2). This modified protocol was used to facilitate electrophysiological data collection; the protocol was completed in one day, and two instead of one pretraining trials allowed baseline estimation of spatial firing stability. Furthermore, to avoid difficulties with shock-related noise, shock artifacts, and shunting shock current to electrodes, the electrophysiological recordings were only made during 20-min shock-off trials that immediately followed the training and conflict sessions with shock on (Fig. 2A2).
Figure 2. Optogenetic verification of a DG-dependent memory discrimination task.
A) top: Behavioral protocol to evaluate the task is DG dependent. Day 1: 30-minute pretraining trial on the rotating arena. Day 2: mice learn in two 30-minute initial training trials (2 hours apart) to avoid a shock zone that is stationary with respect to the room. During the conflict trial the shock zone is relocated 180° and mice learn to distinguish between the current and previous shock zone locations, neither of which are marked. bottom: Behavioral protocol to evaluate whether the 1-day recording paradigm is also DG dependent. Day 1: two pretraining sessions on the rotating arena, followed by two training sessions, then memory discrimination is tested in the conflict trial in which the shock zone is relocated 180°. Mice were trained and immediately after the training or conflict trials the shock was turned off and single units were recorded. Rest in the home cage between trials was 90 min. B) Expression of halorhodopsin is limited to DG GCs in Cre+ POMC-Halorhodopsin mice and is not expressed in Cre- littermate controls. C) Cre+ POMC-Halorhodopsin mice show fewer c-Fos+ GCs labeled during the conflict trial (t = 2.7, p = 0.04). D) No differences exist between protocol type and data were combined. Mice with light-silenced granule cells learn avoidance at similar rates as control mice, but after the shock zone relocation, Cre+ POMC-Halorhodopsin mice are deficient in discriminating between the old and new shock zone location confirming that intact DG function is necessary for memory discrimination on the active place avoidance task (3-way genotype x protocol x session ANOVA: no effects of protocol: F1,25 = 1.93, p = 0.18 or genotype: F1,25 = 3.22, p = 0.08; but effects of condition: F2,24 = 28.98, p = 10−7 and the genotype x session interaction: F2,24 = 4.03, p = 0.03; all other interactions were not significant F2,24’s ≤ 2.19, p’s ≥ 0.13; post-hoc tests confirmed Cre+ worse than Cre− only on the conflict trial. Errors/m is measured to allow comparisons between the two different protocols. E) In the recording protocol, during the shock off session of the conflict trial mice with light-silenced cells continue to make more errors than controls (genotype effect: F1,12 = 4.82, p < 0.05; time point: F1,12 = 3.55, p = 0.08; genotype x time point: F1,12 = 0.97, p = 0.34). Bar graphs represent mean ± SEM. * indicates significantly different from all other manipulations.
Task performance in the two protocols could not be distinguished by 3-way genotype x protocol x condition ANOVA, so the data were combined (Fig. 2D). Importantly, the Cre+ mice were worse on the conflict session but not during the other sessions, confirming prior work (Burghardt et al., 2012; Kheirbek et al., 2013) that DG cells are not essential for learning to avoid the initial location of shock, but are essential for avoiding shock on the conflict trial when the shock is relocated 180° (Fig. 2D). Avoidance when the shock zone is initially added requires discriminating between memories of the pretraining session when there is no shock and the training session when shock is in the initial location. Making this distinction appears not to critically depend on DG function, whereas discriminating between the initial and current location of shock on the conflict session depends on discriminating between memory for the initial and current locations of shock, and DG function. The impairment during the conflict trial is consistent with a memory discrimination deficit, and it furthermore coincided with increased effort to avoid the initial shock zone location in Cre+ mice. Indeed, compared to the Cre− mice, the Cre+ mice spent significantly more time in the quadrant of the arena that was counter-clockwise to the conflict shock zone (t27 = 2.33, p < 0.03), which on the clockwise rotating arena is the preferred region for avoiding both the initial and the conflict shock zones. The demand for memory discrimination during the training sessions when shock is initially added is henceforth considered to be “low” because it is a categorical, qualitative distinction and the demand for memory discrimination during the conflict session when the shock is relocated is considered by comparison, “high” because the distinction is quantitative, between the currently experienced locations of shock and the remembered locations. Because illumination both directly and indirectly silences DG cells this demonstrates the DG is specifically necessary for conflict memory discrimination (Burghardt et al., 2012; Kheirbek et al., 2013). The impaired memory discrimination performance of Cre+ mice persisted during the period of time after shock was turned off, when electrophysiological recordings were performed in subsequent experiments (Fig. 2E), consistent with prior reports of normal extinction learning after ablation of DG neurogenesis (Burghardt et al., 2012). Optogenetically silencing granule cells directly can also rapidly and poly-synaptically activate other granule cells and so silence mossy cells; this is because granule cells excite inhibitory interneurons as well as mossy cells that excite distal inhibitory interneurons (see Fig. S1 in Senzai and Buzsaki, 2017).
DG-dependent memory discrimination is not selectively accompanied by dentate firing field remapping
We then addressed the central question by testing whether DG place cell firing rate maps (n = 42 place cells of 113 cells in 6 wild-type mice) change across conditions that differ in the demand for memory discrimination (Fig. 3). We recorded from DG place cells using the identical protocol to the optogenetic validation (Fig. 2A2). The mice were initially naïve to the arena and active place avoidance; they learned and performed the various phases of the protocol similar to the mice in the optogenetic experiments that confirmed which task variants require intact dentate function. Place avoidance learning was normal when the shock was on (Fig. 3B top) and the conditioned avoidance was maintained during the shock-off trials when cells were recorded (Fig. 3B bottom). No differences in running speed were observed between the training and conflict conditions (F2,13 = 1.93, p = 0.18; data not shown). The measures of spatial firing quality did not differ across the pretraining, initial training, and conflict training trials (Table S2). Importantly, firing rate map stability also did not differ across the “replication manipulation” (pretraining 1 vs. pretraining 2, initial training 1 vs. initial training 2), across the “shock addition” manipulation (pretraining vs. initial training) and across the “shock relocation” manipulation (initial training vs. conflict training; Fig. 3C). Note that analysis of the shock addition and the shock replication manipulations used data collected during both the training 1 and training 2 sessions, and that the conclusions were unchanged when only training 1 data were considered. Although place conditioning changed spatial behavior, firing rate map stability in the replication and relocation comparisons also did not differ (Fig. 3C). These estimates of stability were similar to the estimates across the replication control manipulation of the environment (Fig. 1B) for DG place cells (Wilcoxon ranksum = 1958, z = 0.19, p > 0.85) but were weaker compared to CA place cells (Wilcoxon ranksum = 700, z = 2.43, p < 0.02). The magnitude of DG place cell firing rate changes (H2,117 =14.4, p<0.001) and place field-specific rate changes were greater in response to the shock-added and shock-relocated manipulations than across the replication manipulations (Fig. 3D). This pattern of replication < shock addition = shock relocated was also observed in the putative inhibitory neurons (H2,57 = 8.97, p = 0.01). These findings demonstrate DG firing rates are sensitive to changes in task contingency, consistent with episodic encoding (Leutgeb et al., 2005b). Note however that these single-cell estimates of neural pattern discrimination were not specific to DG-dependent memory discrimination.
Figure 3. DG-dependent memory discrimination is not selectively accompanied by dentate firing field remapping.
A) Example DG firing rate maps from two mice during each trial. Each unit’s average concatenated tetrode waveform for each session is placed above each rate map. Dwell time maps show the time spent in each pixel for each session. B) Top: Mice learned to avoid in the first training trial and maintained avoidance memory during the second training trial. Errors in the conflict trial initially increased then quickly decreased as shown by the within-trial learning curve in the inset (condition: F2,4 =18.2, p=0.01, training 2< training 1 and conflict). Inset: pretraining and conflict learning curves. Bottom: The mice expressed the conditioned avoidance during the shock-off recording sessions that followed the shock-on trials, and performance during the shock-on and shock-off trials were statistically indistinguishable as assessed by 2-way shock-status x condition ANOVA (shock status: F1,9.1 =3.29, p=0.10; condition: F2,18.2 = 5.22, p = 0.02; interaction: F2,18.2 = 0.15, p = 0.86). Inset: pretraining and conflict learning curves. Note that analysis of errors/m moved leads to the same conclusions (shock status: F1,9 = 4.40, p = 0.07; condition: F2,18 = 5.59, p = 0.01; interaction: F2,18 = 1.45, p = 0.26). C) Firing rate map stability did not differ between the replication (pretraining-pretraining [illustrated], training 1-training 2), shock addition (pretraining- training [illustrated]) and shock relocation (training-conflict [illustrated]) manipulations (H2,117 = 1.02, p = 0.60) and was no different from the replication condition in Fig 1b. Firing rate map stability was indistinguishable in the replication and relocation comparisons (Wilcoxon ranksum = 1756, z = 0.96, p = 0.34). D) Left: Field rates and rate changes for the first vs. second trial of each manipulation. Place cells with high firing rates decreased firing while place cells with low firing rates increased firing. Right: The magnitude of field rate remapping is greater for the shock addition and shock relocation manipulations compared to the replication manipulation (H2,117 = 9.32, p =0.01). Bar graphs represent mean ± SEM. * indicates different as shown by lines; * indicates significantly different from all other manipulations.
Dentate place cell ensembles transiently and purposefully discriminate places in distinct spatial frames
We next examined these DG data by analyzing the conjoint discharge of cells on sub-second time scales. After not observing changes in firing field properties that correspond to changes in memory discrimination demand, we began to examine whether other features of DG place cell discharge changed with memory discrimination demand. We were motivated because the assumptions of the preceding session-averaged analyses of single-cell firing (Figs. 1,3) contrast with the strong non-stationarity (Carr and Frank, 2012; Fenton et al., 2010; Fenton and Muller, 1998; Ferbinteanu et al., 2011; Gothard et al., 2001; Gothard et al., 1996; Gupta et al., 2010; Huxter et al., 2003; Jackson and Redish, 2007; Redish et al., 2000; Shapiro and Ferbinteanu, 2006; Singer et al., 2010) and ensemble place coding properties (Dupret et al., 2010; Harris et al., 2003; O’Neill et al., 2008; Park et al., 2011; Pastalkova et al., 2008; Pfeiffer and Foster, 2015; Wikenheiser and Redish, 2015; Wilson and McNaughton, 1993) that are reported for hippocampal spiking dynamics. We thus considered a different form of neural pattern discrimination that can be measured on a moment-to-moment basis. The rotation of the arena dissociates the environment into two distinct spatial frames. One is stationary, defined by room-anchored cues and the other is rotating, defined by local arena-anchored cues (Fenton et al., 1998). To solve this task the mouse has to discriminate between room-based spatial information and arena-based spatial information. This is essential in the vicinity of the shock zone to avoid shock that is defined by room-frame information, and not by arena-frame information. Spatial frame-specific positional discharge was estimated by separately computing momentary positional information (Ipos) in each spatial frame for the place cell ensemble (Kelemen and Fenton, 2010; Olypher et al., 2003), and computing ΔIpos, the difference between room-frame and arena-frame Ipos each 133 ms (Fig. 4A). Like place cell firing rate fluctuations (Fenton et al., 2010), the correlation between ΔIpos and running speed explains little of the variance (r2=0.69%, p < 0.001). Room information dominates arena information more often than vice versa during pretraining but this preference disappears when shock is added or relocated (Fig 4B) even though room information must be used to avoid shock (Bures et al., 1997; Fenton and Bures, 2003). During the ~1 s before mice avoid or enter the initial location of shock (Fig. 4C) ΔIpos is positive (i.e. room-preferring) and larger than when the mice are in the corresponding area 180° away in the potentially safest zone (Fig. 4C). The prevalence of room-preferring information followed the shock zone relocation on the conflict trial (Fig. 4C), even though time-averaged firing rate maps did not change (Fig. 3). We observed a similar preference for room-specific discharge in the vicinity of the current shock zone when we examined the locations of frame-specific Ipos values (Fig. 4D), instead of their differences. These findings show increased likelihood of room-frame location-specific discharge near the room-defined shock zone, and lower likelihood opposite it. Thus, within a single session, these observations demonstrate discrimination of neural representations of location in DG discharge; the discrimination is both dynamic and purposeful insofar that it corresponds to the need for discriminative place avoidance memory preferentially near the locations of shock.
Figure 4. Dentate place cell ensembles transiently and purposefully discriminate places in distinct spatial frames.
A1) Upper left shows a schematic representation of the rotating arena and stationary room frames, defined by arena cues (blue stars) and room cues (red stars), respectively. Room frame firing rate maps of a seven DG place cell ensemble. A2) Room frame (top panel) and arena frame (second panel) ensemble Ipos over time of the place cell ensemble depicted in A1, as well as ΔIpos (third panel) and ensemble firing rate (bottom panel). A3) Example of an ensemble’s ΔIpos time series during pretraining, training and conflict trials and the corresponding frame-specific Ipos histograms. B) Room information dominates arena information more often in pretraining trials, but the room frame preference disappears when shock is added or relocated (spatial frame: F1,10.6 = 2.0, p = 0.18, condition: F2,19.8 = 0.3, p = 0.78; frame x condition: F2,19.8 = 4.9, p = 0.018). C) Left: example of ΔIpos time series (top) during a 2-min period and the mouse’s concurrent avoidance behavior shown as distance from the shock zone (bottom). Right: After training or conflict trials, ΔIpos is positive (room-dominating) and larger during ~1s before active avoidance (defined as a local minimum in the distance to a shock zone time series) compared to when mice are in the safe zone that is the corresponding region on the opposite side of the arena or compared to when mice are close to that shock zone in trials that it was safe (condition: F2,18.5 = 0.8, p = 0.47; shock zone: F1,9.9 = 3.3, p = 0.10; condition x shock zone: F2,18.5 = 11.4, p = 0.0006). D) Summary maps of the proportion of time mice were in the room-dominant state during training (top) and conflict (bottom) trials show a preference for signaling room-frame locations close to the relevant shock zone. These training and conflict maps are not correlated (r = −0.02, p = 0.67). The bar graphs quantify the comparison of room and arena positional information within 4.5 cm of the relevant shock zone normalized by the overall Ipos and compared to other trial conditions. During training trials room-frame positional information is significantly higher near the training shock zone than during pretraining or conflict trials (F2,8.9 = 8.7, p = 0.008). During conflict trials, room positional information is significantly higher near the relocated shock zone than during pretraining and training trials (F2,9.8 = 9.0, p = 0.006). Bar graphs represent mean ± SEM. * indicates different as shown by lines; * indicates significantly different from all other manipulations.
Sub-second coordination within the network of DG cells mirrors likelihood of DG-dependent memory discrimination
Next, we investigated whether the sub-second coordination of DG spike trains changes across the trials requiring different levels of memory discrimination. The distributions of spike train correlations (τ) between pairs of DG place cells measured at the 133-ms time scale of the Ipos estimates do not differ in the three trial types (H2,381 = 1.57, p = 0.46, Fig. S2). Nor do they change systematically from one manipulation to another (Figs. 5A and S2A). Standardized firing rates were computed during 5-s intervals that identified passes through firing fields (Fenton et al., 2010; Fenton and Muller, 1998; Jackson and Redish, 2007). The distributions of standardized rates did not differ across the pretraining, training, and conflict sessions (F2,9149 = 0.31; p=0.73). Overdispersion of the standardized rates in the pretraining sessions (var = 5.2) was similar to the overdispersion in rat area CA1 (Fenton et al., 2010; Jackson and Redish, 2007). Overdispersion during pretraining was also indistinguishable from during training (var = 5.74; F4189,3169 = 1.10; p = 0.17), and conflict (var = 5.63; F1791,3169 = 1.08; p = 0.63) sessions (also when the unit of analysis was a single cell with at least 15 passes, F2,178 = 0.44; p=0.64). Similar to time-averaged measures of spatial firing (Fig. 3), single cell estimates of short time-scale spiking dynamics did not differ across the trial types (Fenton et al., 2010; Fenton and Muller, 1998; Jackson and Redish, 2007).
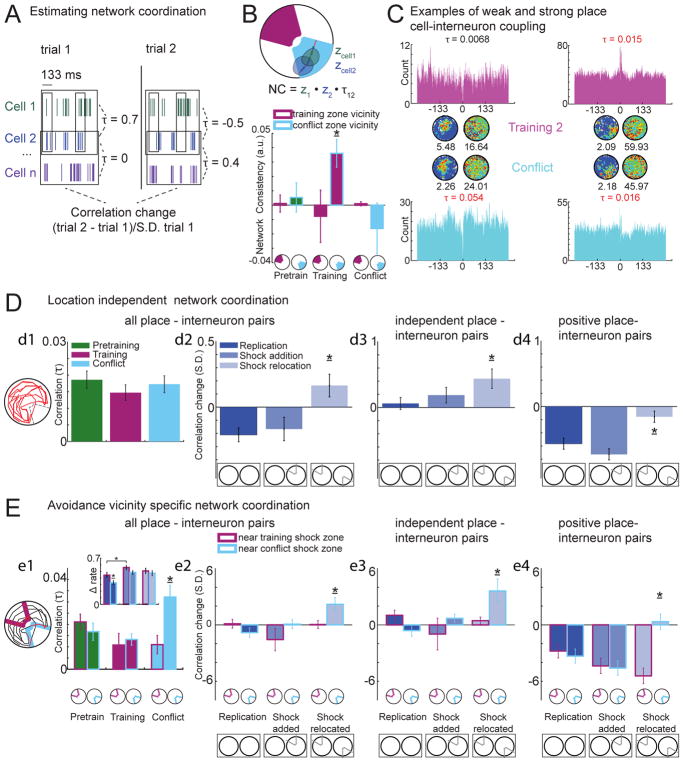
Figure 5. Sub-second coordination within the network of DG cells mirrors likelihood of DG-dependent memory discrimination.
A) Schematic of how pair-wise spike train correlation changes are estimated (for panels D2–4 and E2–4). Kendall’s correlation (τ) at 133 ms resolution is computed for all possible cell pair spike trains. The difference between the τ values for a pair of trials is normalized by the standard deviation of τ for that cell pair during the first trial. B) Top: cartoon of the momentary location-specific discharge of two neurons with overlapping place fields. Their momentary discharge relationship as the mouse passes across the firing fields (z1 · z2) is compared to their overall tendency to discharge together (τ1,2), and is measured as network consistency (NC = z1 · z2 · τ1,2) Bottom: Network consistency increases opposite the shock zone after training and decreases when the shock is relocated (shock zone: F1,1038 = 1.2, p = 0.28, condition: F2,1038 = 1.5, p = 0.22, shock zone x manipulation interaction F2,1038 = 3.2, p = 0.04). This indicates location-specific co-firing is less consistent at sites of memory discrimination. C) Firing rate maps of two example place cell-interneuron cell pairs and their cross-correlograms. (left) An uncorrelated cell pair during the training trial becomes correlated during the conflict trial, whereas (right) a positively correlated cell pair remains positively correlated. D1) No differences in correlations across conditions for place cell-interneuron pairs (H2,435 = 1.92, p = 0.38). D2) During shock-relocation, place cell-interneuron pairs become more positively correlated compared to the replication and shock addition manipulations (F2,435 = 7.1, p = 10−4). D3) The changes during shock-relocation, are driven by the cell pairs that were initially independent becoming more positively correlated (H2,236 = 8.1, p < 0.017) while D4) the initially positively correlated pairs do not change. The initially-positive cell pairs decrease correlations during the replication and shock addition manipulations (H2,280 = 24.3, p = 10−6). E) Changes in correlated firing were specific to the vicinity of the shock zone. E1) Correlations are increased only when the animal is close to the relocated shock zone during the conflict trial (shock zone: F1,806 = 1.9, p = 0.15; condition: F2,806 = 2.6, p = 0.11; zone x condition: F2,806 = 6.7, p = 0.0013). Inset: Rate remapping in the vicinity of the shock zones increases similarly with shock addition and relocation and does not mirror Kendall correlations or correlation changes: shock zone x manipulation ANOVA (shock vicinity: F1,245 = 6.93 p = 0.009; manipulation: F2,245 = 8.96 p = 10−4; shock vicinity x manipulation: F2,245 = 0.68, p = 0.51). E2–4: The correlation increases demonstrated above in panels D2–4 only occur when the animal is close to the relocated shock zone, when DG-dependent memory discrimination is most likely E2) all cell pairs: shock zone: F1,746 = 3.8, p = 0.05; manipulation: F2,746 = 6.3, p = 0.002; shock zone x manipulation: F2,746 = 5.5, p = 0.0044; E3) independent cell pairs: shock zone: F1,593= 1.3, p = 0.3, manipulation: F2,593 = 4.4, p = 0.01, shock zone x manipulation F2,593 = 6.9, p = 0.0011; E4) positively-correlated cell pairs: shock zone: F1,245 = 7.7, p = 0.006; manipulation: F2,245 = 2.9, p < 0.06, shock zone x manipulation: F2,245 = 11.5, p = 10−5. The post-hoc tests on the data in panels E2–4 confirm that after shock relocation, the correlations near the relocated shock zone increase compared to during the other manipulations and compared to the opposite zone. See also Figure S2. Bar graphs represent mean ± SEM. * indicates significantly different from all other manipulations.
We further considered the short time-scale spiking dynamics of place cell pairs. The momentary discharge of place cells with overlapping firing fields can characteristically covary positively so that when the mouse passes through the firing fields both cells fire more or less than predicted by their firing fields, or the cell pairs can covary negatively so when one fires excessively the other does not, or they can discharge independently so that the firing variations of one cell do not predict the other’s (Fenton, 2015; Kelemen and Fenton, 2012; Talbot et al., 2018). To evaluate the dynamic interactions amongst the network of cells, we investigated the momentary firing rate variations of pairs of place cells as the mouse crossed overlapping firing fields (z1 and z2) and asked whether they were consistent with the overall short-time scale discharge coupling between the cell pair (τ1,2; see schematic in Fig. 5B top). Because τ1,2 did not vary across conditions, we computed network consistency (NC = z1 · z2 · τ1,2), which is maximal when the momentary firing rates of two cells with overlapping firing fields covary similar to the overall spike train correlation of the cell pair; network consistency is minimal when the rate covariance is opposite to the spike train correlation. Although overall network consistency was positive and similar across the pretraining, training, and conflict sessions (F2,2523 = 1.3; p = 0.27), the requirement for memory discrimination predicts greater pattern distinctiveness and thus lower network consistency, particularly in the vicinity of the shock zone when memory discrimination is most required. During pretraining, before shock was ever experienced, network consistency was similar when the mouse was in the vicinity of the future shock zone and the opposite corresponding region of the arena (Fig. 5B bottom). During training trials, network consistency increased opposite the shock zone and network consistency in the same location decreased when the shock was relocated 180° in the conflict trial. While the increased network consistency opposite the initial shock zone was not predicted, the reduced network consistency in this location when shock is relocated is predicted by the greater demand for memory discrimination in the relocated shock zone vicinity (Fig. 5B bottom). The hypothesis that distinct network states manifest for distinct memories can also explain the observed increase of network consistency opposite the initial shock zone if the mice consistently considered it safe and distant from shock. This pattern of change in network consistency cannot simply be due to the mice spending more or less time in the shock zones because network consistency was unchanged across the session types in the vicinity of the initial location of shock, whether or not it was a neutral, avoided, of preferred region of the environment. These data suggest that unlike session-averaged features of place cell discharge like the location of place fields, the sub-second discharge coordination within the network of DG place cells systematically varies across time and locations and these distinctive network states are associated with increased demand for memory discrimination and may be sensitive to rapid changes with learning experience (Bittner et al., 2015; Cheng and Frank, 2008).
Increased place cell-inhibitory neuron coactivity at sites of memory discrimination
To explore how distinctive network states might transiently arise, we investigated whether the task manipulations alter discharge correlations between place cells and inhibitory neurons. Inhibitory networks may be important for memory discrimination (Buzsaki, 2010; Danielson et al., 2017; Jinde et al., 2012; Park et al., 2015), and network consistency, perhaps in part because high levels of inhibition promote pattern separation in mature granule cells (Marin-Burgin et al., 2012). The distributions of place cell – interneuron discharge correlations did not differ across trials (Fig 5D1). In contrast, the correlated firing of the individual cell pairs differed across the three types of manipulation; the changes were higher across the shock-relocation manipulation (Fig. 5D2) that requires memory discrimination and relies on intact DG function (Fig. 2). This increase in place cell – interneuron co-firing was observed generally, most place cells increased co-firing with at least one interneuron (83% of place cells for the shock-addition (z = 1.15, p = 0.2) and 92% for the shock-change (z = 2.3, p = 0.03) comparisons relative to 75% of place cells for the replication comparison). These findings also hold for 20 ms and 250 ms timescales (Fig. S2B, C).
Because changes in correlated firing may not be homogeneous within a network of neurons (Harris, 2005; Okun et al., 2015; Olypher et al., 2006), the pairs were sub-classified for further analysis. During pretraining, cell pairs were significantly positively correlated (81 pairs, 53%), significantly negatively correlated (15 pairs; 10%), or independent (56 pairs, 37%) according to statistical criteria. After shock-relocation, the correlations amongst the initially independent place cell-interneuron pairs increased (Fig. 5D3) whereas positively correlated pairs did not change, although they decreased with the replication and shock addition manipulations (Fig. 5D4). Changes were not observed amongst the initially negatively correlated pairs (H2,73 =4.28, p = 0.12). These changes in correlated firing were specific to the vicinity of the shock zone, where memory discrimination is most required. The correlation increases are larger after shock relocation when the mouse is close to the currently to-be-avoided zone than when it is close to the previous shock zone location (Fig 5E2–4). These location-specific differences were not detected for the other manipulations. In accord with these changes, during the conflict trial, we observed higher correlations near the current shock zone compared to its prior location (Fig 5E1). These differences between the locations were not observed when corresponding random samples of intervals were compared, nor were the location-specific differences observed in the pretraining or training trials. These correlations were not related to running speed (r2 = 0.004, p>0.11) or acceleration (r2= −0.00004, p>0.84), but were related to place cell (r2 = 0.02 p <10−4) and interneuron (r2 = 0.02 p <10−3) firing rates in the vicinity of the shock zones, although there were no measurable differences in firing rates across the conditions (zone vicinity: F1, 293 = 3.01, p = 0.08; condition: F2, 293 = 0.64, p = 0.53; interaction: F2, 293 = 0.26, p = 0.77). We also investigated if firing rate changes near and far from the shock zones changed depending on the proximity to the shock location, because where the mice visit alters with the recent presence of shock (Fig. 5E1 inset). Place cell firing rates changed when shock was added; the changes near and far from the shock location were indistinguishable. Similarly, relocating the shock zone to the opposite part of the environment caused firing rates to change just as much near and far from the shock zone; the changes were similar to the effects of adding shock and not easily explained by the differential sampling of the environment in the presence of shock. Importantly, the pattern of correlations (Fig. 5E1) and correlation changes (Fig. 5E2–4) across the three manipulations do not correspond to the pattern of firing rate changes in the corresponding shock zone vicinities (Fig. 5E1 inset). Accordingly, firing rate changes cannot easily explain the observed correlation changes, and provide no evidence that rate remapping is associated with potentially different demands for memory discrimination at the different shock zone locations. Together, these data indicate that spike train temporal coupling increases between the firing of DG place cells and inhibitory neurons, specifically when the animal needs to perform DG-dependent memory discrimination to avoid the location of shock.
Increased place cell-inhibitory neuron coactivity before successful but not failed place avoidance
Finally, to evaluate the behavioral relevance of the changes in coupling between spike trains, we examined whether the increased coupling between the discharge of place cell-interneuron pairs depended of whether or not mice expressed place avoidance, and by inference memory discrimination (Fig. 6A). Indeed, the increased place cell – interneuron spike train correlations were only observed during the conflict trial before the mice successfully avoided the shock zone (Fig. 6B); this increase was not detected before the mice entered the shock zone (Fig. 6C), nor did it correspond to the concurrent variations in firing rates (Fig. 6 insets). These findings strongly support the idea that memory discrimination is expressed transiently, on the time scale of seconds or less, and that the neural representation of the discrimination is more saliently expressed by the conjoint discharge of DG place cells and interneurons than it is by the session-averaged spatial firing properties that are estimated from place cell firing fields.
Figure 6. Increased place cell-inhibitory neuron coactivity before successful but not failed place avoidance.
A) PC-INT correlations were measured during the 4 s before successful and failed place avoidance. The behavioral episodes were identified in the time series of the mouse’s distance from the shock zone. Local minima in the time series that are at least 75% of the average distance from the shock zone identified successful avoidance (if the minimum was not in the shock zone) or failed avoidance (if the minimum was in the shock zone). B) PC-INT correlations increased during successful avoidances in the conflict trial (shock zone: F1,297 = 2.5, p = 0.11; condition: F2,546.8 = 0.61, p = 0.54; zone x condition: F2,546.8 = 3.8, p = 0.02). Inset: Unlike the correlation increases, rate changes do not differ between shock zone vicinities but do differ across manipulations (shock zone vicinity: F1,235 = 0.64, p = 0.42; manipulation F2,235 = 4.46 p = 0.01; interaction: F2,235 = 0.41, p = 0.66, posthoc: shock addition in conflict zone vicinity > replication in both zone vicinities). C) No PC-INT correlation increases are present during failed avoidance. Inset: Rate changes differ between zone vicinities and manipulations unlike the correlation values (shock zone vicinity: F1,245 = 3.94, p = 0.048; manipulation F2,245 = 14.81 p < 0.0001; interaction: F2,245 = 1.13, p = 0.33. posthoc: replication conflict zone vicinity < all. Replication training zone vicinity = shock addition conflict zone vicinity < shock addition training zone vicinity and shock relocation both zone vicinities). Bar graphs represent mean ± SEM. * indicates significantly different from all other manipulations.
Discussion
We evaluated hypotheses of how the dentate gyrus contributes to memory discrimination (Colgin et al., 2008; Leutgeb et al., 2007; Neunuebel and Knierim, 2014; Wills et al., 2005) by investigating the changes in DG place cell firing across different behavioral episodes that included a test demonstrated to require DG-dependent memory discrimination (Fig. 2A; Burghardt et al., 2012; Kheirbek et al., 2013). Contrary to expectations that time-averaged spatial discharge patterns (firing field arrangements) correspond to distinct spatial memories (Leutgeb et al., 2005a; Wills et al., 2005), we did not observe any form of firing field rearrangement (“remapping”) that was specific to the memory discrimination trial (Fig. 3C, D), even though spatial remapping of DG place cell responses to spatial cue manipulations was apparent (Fig. 1B; Leutgeb et al., 2007). Firing rates overall as well as in the primary place fields varied with changed task contingencies (Fig. 3D), which has been called rate remapping. Thus, the DG place cell responses to cue manipulations replicate prior reports and establish that it was possible to detect remapping with our techniques. In contrast, place fields did not selectively remap with the memory discrimination test. Although rate remapping was not specific to the DG-dependent memory discrimination test, it is nonetheless possible that rate remapping contributes to the discrimination itself. These findings are in opposition to expectations that spatial remapping underlies spatial memory discrimination (Leutgeb et al., 2005a; Leutgeb et al., 2005b; Wills et al., 2005), but resembles how the place cell network responds to displaced objects; by changing which object-coding cells co-fire with which place cells, none of which remap (Muller and Kubie, 1987; Rivard et al., 2004). Instead of memory discrimination being associated with remapping, we found that changed demand for DG-dependent memory discrimination associates with reduced network consistency: weaker correspondence between momentary co-firing amongst cell pairs with overlapping spatial tuning and overall sub-second discharge correlations (Fig. 5B). The memory task-related variations in network consistency and the corresponding modulation of excitatory-inhibitory cell pair coupling suggest that memory discrimination task-related network representations in DG place cell firing are distinguished by sub-second interactions between excitatory discharge signaling spatial information and local control of that discharge by interneurons (Buzsaki, 2010; Danielson et al., 2017), possibly as a consequence of learning-related synaptic plasticity (Hashimotodani et al., 2017). Because the set of pair-wise correlations within a network estimates the overall network state (Schneidman et al., 2006) the present observations demonstrate that changes in memory discrimination demand are associated with distinctive DG functional states that are defined by dynamic and coordinated excitatory-excitatory and excitatory-inhibitory interactions. Like the requirement for memory discrimination, the coordinated discharge interactions were dynamic, and could be best estimated by analysis of momentary activity rather than by session-averaged measurements. The findings (Fig. 5 and 6) indicate that the sub-second co-firing of dentate place cells and interneurons transiently increases during the moments that mice are most likely to be making memory discriminations that require dentate function, pointing to greater interneuron-driven control of dentate network function during dentate-dependent memory discriminations (Fig. S2D). In fact, these increases were not observed during the episodes when the mice failed to demonstrate memory discrimination by entering the conflict shock zone (Fig. 6), adding to the evidence that these changes in DG network dynamics are associated with memory discrimination rather than extinction and/or surprise at the absence of shock. These distinctive memory-associated patterns of network discharge presumably feedforward to CA3 to implement memory discrimination, and other mnemonic functions of the hippocampal system (Lee and Jung, 2017; Sasaki et al., 2018). This network view is based on recordings that do not discriminate between granule and mossy cells in the DG, which may have distinctive extracellular discharge properties (Danielson et al., 2017; GoodSmith et al., 2017; Neunuebel and Knierim, 2012; Senzai and Buzsaki, 2017). Mossy cells reside in the hilus of the DG and receive focal input from local granule cells (Amaral, 1978; Scharfman et al., 1990). In addition to exciting granule cells directly, mossy cells excite DG inhibitory neurons broadly along the dorso-ventral axis that then provide strong global inhibition onto granule cells and may consequently establish the lateral-inhibition component of the functional architecture for a competitive network (Amari, 1977), specialized for discriminating input patterns and network states (Scharfman, 2016; Sloviter and Lomo, 2012). Correspondingly, ablating mossy cells causes hyperexcitability in granule cells and impaired pattern separation (Jinde et al., 2012) and according to a recent computational model, loss of mossy cell excitatory drive onto granule cells does not affect pattern separation, while loss of the mossy cell driven inhibition of granule cells impairs pattern separation (Danielson et al., 2017). While the present physiological findings cannot distinguish between differential roles of granule cells and mossy cells in memory discrimination, nonetheless a crucial contribution of these DG cells was demonstrated using optogenetic silencing (Fig. 2B; Kheirbek et al., 2013). We were unable to functionally discriminate between the DG place cells recordings by dividing them into distinct classes on the basis of features that may discriminate granule cells and mossy cells, including firing field number, firing rate, spike width and preferred theta phase (GoodSmith et al., 2017; Senzai and Buzsaki, 2017). Nonetheless, the findings point to a network perspective that focuses on the interactions amongst granule, mossy, and inhibitory cells to emphasize the network state of the DG rather than the isolated contribution of single cell classes. Future work will do well to define the integrated roles of these classes by determining if specific cell classes make particular contributions that merit distinctive functional classification. Future work should also evaluate the distinctiveness of inputs in comparison to the DG place cell output to critically evaluate the notions of pattern separation that have been assigned to these cells.
In summary, substantial evidence from behavioral tests have established an important role of the DG in memory discrimination, which we find is associated with changes in the network state of sub-second interactions amongst excitatory and inhibitory cells within the DG, rather than by the spatial tuning of DG place cells measured across the time scales of minutes, much longer than the time scale of memory discrimination and decision.
STAR METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| c-Fos Anti-Rabbit primary antibody | Millipore | ABE457; RRID:AB_2631318 |
| 594 nm Goat Anti-Rabbit Alexa Fluor secondary antibody | ThermoFisher Scientific | R37117, RRID:AB_2556545 |
| Vectashield mounting medium with DAPI | VectorLabs | H-1200 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Isoflurane | Henry Schein Animal Health | 029405 |
| Ketofen (Ketoprofen) | Henry Schein Animal Health | 005487 |
| Metabond | Parkell | S398, S371, S396 |
| Grip cement | Dentsply | N/A |
| Deposited Data | ||
| DATA to be deposited on CRCNS.org | CRCNS.org. | |
| Experimental Models: Organisms/Strains | ||
| Mouse: Cre-POMC-Halorhodopsin | Kheirbek et al., 2013 | |
| Mouse: B6.FVB-Tg(Pomc-cre)1Lowl/J | Jackson Laboratories | RRID:IMSR_JAX:010714 |
| Mouse: B6;129S-Gt(ROSA)26Sortm39(CAG-hop/EYFP)Hze/J | Jackson Laboratories | RRID:IMSR_JAX:014539 |
| Mouse: C57/B6J | Jackson Laboratories | RRID:IMSR_JAX:000664 |
| Software and Algorithms | ||
| MATLAB | Mathworks | www.mathworks.com |
| WClust | A.A. Fenton | www.github.com/FentonLab |
| JMP version 12 | SAS Institute Inc, NC | www.jmp.com |
| Data acquisition software: dacqUSB | Axona Ltd, St.Albans, UK | V1.2.2 |
| Data acquisition software: Tracker | BioSignal Group, Corp, MA | V2.37 |
| Data analysis software: Track Analysis | BioSignal Group, Corp, MA | V2.52 |
| Other | ||
| 593.5 nm DPSS/Diode Laser System | OEM laser systems, UT | YL-593-00100-CWM-SD-05-LED-F |
| 17μm tungsten wires | California Fine Wire | M253670 |
| 1.25mm zirconia ferrules | Thorlabs | CFLC230-10 |
| Optic fiber | Thorlabs | FT200EMT |
| Stainless Steel Bone screw | FHC | 40-77-8 |
| Axona recording system | Axona Ltd, St Albans, UK | N/A |
| Avoidance Apparatus | BioSignal Group, Corp, MA | N/A |
| Vaseline Petroleum Jelly Original | Vaseline US | www.vaseline.us |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to the Lead Contact André Fenton (afenton@nyu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Nine C57BL/6 and twenty-nine Cre-POMC-Halorhodopsin (14 Cre+ and 15 Cre−) male adult mice were used. Cre-POMC-Halorhodopsin mice were obtained by crossing B6.FVB-Tg(Pomc-cre)1Lowl/J x B6;129S-Gt(ROSA)26Sortm39(CAG-hop/EYFP)Hze/J. Mice were group-housed prior to implantation and single-housed afterwards in a room with a 12 hour light/dark cycle (lights on at 7am) with ad libitum access to food and water. All procedures and care adhered to protocols approved by New York University Animal Welfare Committee, which follow NIH guidelines.
METHOD DETAILS
Surgery
Mice were anesthetized with isoflurane (3% for induction 1.5–1.75% maintenance) for surgical procedures. Ketoprofen post-surgical analgesic was administered for three days after surgery. A minimum of 2 weeks passed before experimental procedures began.
Optic Fiber implantation
Bilateral optic fibers (0.125 diameter optic fibers (NA 0.37) in 1.25mm zirconia ferrules) were surgically implanted at AP: −1.8, ML: ±1.25, DV: −1.55 using C&B Metabond to attach the ferrules to the skull.
Electrode implantation
Custom Versadrives (Neuralynx, Bozeman, MT) with 4 independently movable tetrodes (17μm tungsten wires, gold plated to ~120 kΩ impedance at 1 kHz) were surgically implanted to center bregma coordinates ML:1.25, AP: −1.5, DV: −1.2. Sterile vaseline covered the tetrode shafts and C&B Metabond and Grip Cement (Dentsply) was used to attach the microdrive to the skull and bone screw. A bone screw was placed at ML:−3, AP +0.5 relative to bregma, and served as electrical ground. Mice recovered from surgery for at least 1 week before lowering of the tetrodes. Tetrodes were advanced slowly through CA1 and past the hippocampal fissure, determined by LFPs, until dentate single units were identified.
Recording set up and single unit analysis
A unity-gain preamplifier configured as a source-follower was connected to the mouse and the signals were conducted to a commercial recording system (Axona Ltd., St. Albans, U.K.). The signals were filtered (300 Hz – 7 kHz), amplified 2000 – 8000 times, and digitized at 48 kHz for recording presumptive action potentials using the dacqUSB system (Axona Ltd., St. Albans, U.K.). Local-field potentials were filtered (0.1 – 200 Hz), amplified (100–1000 times) and digitized (2 kHz). The position of the mouse was determined 30 times a second by a video tracking system that identified the position of two infrared LEDs that were attached to the preamplifier (Tracker, Bio-Signal Group Corp., Acton, MA). The LEDs appeared different sizes in the video image so they could be reliably discriminated. The spatial resolution at which the location-specific discharge of cells was analyzed is 1.5 cm/pixel. Action potential waveforms were analyzed offline using custom Wclust software (A.A. Fenton). Single unit discrimination quality was objectively assessed using IsoI metrics (Neymotin et al., 2011); average IsoIbackground = 6.52 ± 0.15 bits; average IsoInearest neighbour = 7.58 ± 0.23 bits. Firing rate maps were computed by dividing the number of action potentials that a cell discharged in a location by the amount of time the mouse was detected in that location. Unitary waveforms were assigned to putative cell classes (place cells, non-spatial principal neurons, inhibitory interneurons) according to the following features: waveform width, firing rate, firing rate map information content, coherence, and % active pixels. Furthermore, the concurrent LFP was filtered in the theta range (4–12 Hz) and the phase determined by MATLAB’s implementation of the Hilbert transform [hilbert()]. Each unit’s action potential probability density was computed as a function of the theta phase and the peak was used to characterize the cell’s discharge. Based on these characteristics the place cells were split up into two and three putative differential classes (granule cells, mossy cells, immature granule cells) by the k-means clustering algorithm. When the data from each of the putative cell classes were separately run through all analyses, no differences between these putative groups were found and thus in this report remain combined.
For the environmental changes experiment (Fig. 1) we recorded from both DG (45 place cells) and CA3/1 regions (20 place cells). Because of limited numbers of cells from Ammon’s horn we grouped CA3/1 place cells together. Of the 20 CA cells 7 were from CA3, and 13 from CA1. The discharge characteristics of CA3 and CA1 place cells were compared for the circular environments and did not differ (each cell contributed a single value that was the average for multiple recordings). The place cell recordings from Ammon’s horn were compared to the properties of DG place cells (Table S1). Note that, recordings during the active place avoidance paradigm (Figures 3–6) only pertain to DG cells (42 place cells), since this study focused on whether DG place cells remap to signal memory discrimination, we also analyzed concurrent recordings from 21 putative DG interneurons. Six place cells of one mouse could no longer be confidently isolated after the second training session, so that conflict analyses were not performed on this animal making the conflict dataset 36 DG place cells from 5 mice (see Table S2).
Behavioral Procedures
Place cell responses to environmental manipulations
Seven mice implanted with tetrodes explored three versions of an environment during electrophysiological data acquisition: 1) Standard: a transparent 40-cm diameter circular enclosure with three distinctive cards on the wall. The enclosure was placed within a 50 × 50 cm square box also with another distinctive card on centered on one wall; 2) Rotated: the same circular enclosure in the box with all cards on the circular enclosure rotated 90°; 3) Removed: the square box by itself after removing the circular enclosure. Three comparison conditions were evaluated by comparing place cell properties across a pair of recordings conditions: 1) Replication compared standard-standard, rotated-rotated, and removed-removed conditions; 2) Rotation compared standard-rotated conditions; and 3) Removal compared standard-removed, and rotated-removed conditions.
Active place avoidance
A commercial active place avoidance apparatus and software system was used (Bio-Signal Group, Corp., Acton, MA). Mice were trained on the active place avoidance task in a black-curtained room with distinctive visual cues on the curtains. For optogenetic silencing, a 593.5 nm laser (OEM laser systems) was attached to the implanted optic fibers. Peak laser power in the brain was 15 mW. On day 1, mice explored the rotating disk during a 30-minute pretraining trial with no shock. On day 2 mice learned to avoid computer-controlled 0.2 mA constant current, 60 Hz, 500 ms foot shocks in a 60° zone during two 30-minute training trials (2 hours apart). Foot shock was triggered if the mouse was in the shock zone for 500 ms and shock continued every 1500 ms until the mouse left the zone. The shock zone was stationary with respect to the room (Cimadevilla et al., 2001). On the third trial of day 2 the mice were trained in a conflict trial with the shock zone relocated 180°. Mice were euthanized and perfused 105 minutes after the start of the conflict trial and the brains were extracted and prepared for immunohistochemistry (see below).
For optogenetic silencing, seven transgenic POMC-Halorhodopsin that express the light sensitive chloride channel halorhodopsin selectively in DG granule cells and eight littermate controls received the active place avoidance training in a “behavioral validation protocol,” as described above. Additionally, another cohort of mice (7 Cre+ and 7 Cre−) were trained to confirm the role of DG cells in a modified “recording protocol” that was used for the electrophysiological recordings, as detailed below. To directly compare performance of the mice in the two protocols, place avoidance performance was compared during the first portion of each behavioral validation trial because this portion corresponded to the shorter trial time of the recording protocol. To minimize the influence of potential differences in locomotion due to different trial durations, place avoidance performance was assessed as the number of errors per distance walked (see Fig. 2).
Six mice implanted with tetrodes performed the active place avoidance task in the recording protocol that consisted of five sessions that took place during one day with 90 min rest periods after each session. In two 20-min pretraining sessions, the mice explored the rotating disk while the action potential discharge of DG units were recorded. Next, in a 20-min training session mice learned to avoid a 60° shock zone. Immediately after the training phase with shock on, shock was turned off to avoid electrical artifacts, and a 20-min recording session with no shock began, during which the mice continued to avoid the shock zone. Immediately after the recording session, the shock was turned on for a 5-min reminder session to discourage the mice from extinguishing the avoidance. The second training trial began after a 90-min rest in the home cage. The mice had a 10-min training trial with the shock on, after which there was a 20-min recording trial with shock off. After another 90-min rest, the mice received conflict training for 20 min with the shock zone relocated 180°. Immediately after the conflict training there was a 20-min recording with shock off.
Place avoidance behavior was analyzed by TrackAnalysis software (Bio-Signal Group Corp., Acton, MA). The end-point measures we used were the number of errors (shock zone entries), number of errors per meter, speed and time spent in each arena quadrant.
Firing rate stability
Spatial remapping was estimated by the firing rate map stability, measured as Fischer’s z-transformation of the Pearson correlation. The correlation was computed for the corresponding pixels from the firing rate maps from the two recordings. The measure was taken to be the maximum correlation after allowing for one map to move with respect to the other a maximum of 2 pixels in the x and y dimensions. This ensured that small changes due to experimental error were minimized. The active place avoidance trials produce spatial behavior and single unit discharge in two spatial frames. Firing rate maps from both spatial frames were analyzed and the higher correlation was taken as the estimate for map stability. If there were multiple trial correlations for a given manipulation (e.g. standard 1 - rotated, standard 2 - rotated) the average correlation was used to estimate the value for each cell.
Firing rate remapping was estimated as the difference between a cell’s average rates in two trials divided by the maximum of the two rates. Place field rate remapping was estimated similarly; the field firing rate in each session was defined by a “field mask”, pixels that comprised the largest place field in the first trial of the day. We also calculated the corresponding change in the out-of-field rate by estimating the average rate in all the “non-mask” pixels that did not include the field mask. Thus separate measures of spatial remapping, rate remapping, firing field remapping, and out-of-field remapping were evaluated.
Positional information
Positional information (Ipos) was calculated for each place cell at 133 ms temporal resolution (Kelemen and Fenton, 2010; Olypher et al., 2003):
Ipos=|p(n|x)log2(p(n|x)/(p(n))|, where p(n|x) is the probability of observing n action potentials in 133 ms at location x, and p(n) is the probability of observing n action potentials in 133 ms independent of location. Ipos during the arena rotation was frame specific because it was separately computed for positions in the room and arena spatial frames.
Ensemble ΔIpos was calculated by independently summing the room frame and the arena frame Ipos time series of cells in an ensemble and subtracting the two frame-specific Ipos sums at each 133 ms time step, such that a positive value indicated a momentary preference for room frame information and a negative value indicated an arena frame preference (Kelemen and Fenton, 2010). During each trial, the total amounts of time room- and arena-preferring ensemble discharge were observed was estimated as the total time ΔIpos was positive and negative, respectively. Local minima of the mouse’s distance to the nearest edge of the shock zone were calculated and selected if they were closer to the shock zone than 50% of the animal’s average positions. The Ipos values during the ~1 s prior to the minima estimated frame-specific discharge during an active avoidance. This was calculated for each mouse as the average of the Ipos values preceding each minimum. This average was computed for the vicinity of each shock zone location on each trial (even when there was no shock zone as in pretraining trials). Summary maps of the proportion of time that discharge was room-preferring in each pixel were calculated for each mouse and averaged over all the animals. To estimate the frame-preference of spatial discharge in the vicinity of the shock zone, the room and arena Ipos values were calculated separately and averaged for those times during which the mice were within 3 pixels (4.5 cm) of the shock zones or in the shock zone itself.
Cell pair spike train discharge correlations
Kendall’s correlation was calculated for all possible place cell pairs in an ensemble and for all place cell-interneuron pairs (interneuron-interneuron pair numbers per animal were not sufficient for analysis). The correlation was computed at the 20 ms, 133 ms, and 250 ms time scales; the data from the 133 ms time scale are reported in the main text and the conclusions were the same for the other time scales. How a cell pair’s discharge correlation changed across two recordings was estimated by subtracting the correlation in one trial from the correlation of that cell pair in the other trial. The difference was divided by the standard deviation of the correlation to avoid non-linear estimates of correlation changes due to correlations being near zero. To estimate the standard deviation of a cell-pair’s discharge, the discharge correlation was calculated six times after dividing the earlier trial into six periods. The standard deviation was based on the six correlation estimates. Kendall’s correlation was also calculated for the time intervals that the mouse was within 3 (4.5 cm) pixels of the shock zones but not in the zones themselves. To check for correlations between speed and acceleration of the animal and Kendall’s tau, a rolling window of ~1 sec was used to calculate speed and acceleration for each animal and trial. Then Kendall’s tau was calculated for each animal for each trial in ~6 sec bins (the average number of seconds that was used to calculate tau correlations in Fig. 5). The average speed and acceleration in each ~6 seconds was then correlated with Kendall’s tau at that time point.
Local minima of the mouse’s distance to the nearest edge of the shock zone were calculated and selected if they were closer to the shock zone than 75% of the animal’s average position. Correlations were calculated during the 4 seconds prior to the minima and calculated separately for minima that were in the shock zone (errors) and those that were outside the shock zone (avoidances).
Overdispersion
Overdispersion measures the reliability of a place cell’s spatial discharge (Fenton and Muller, 1998). It assumes that the session-averaged firing rate map is an accurate prediction of the momentary discharge of a cell as the subject moves across a firing field. The expected firing during a pass through a firing field is determined on the basis of the Poisson assumption, by summing the product of the rate (rx) and the duration (tx) in each location (x) of the N locations that define the pass: . Then for each pass we can compute the standardized rate as:
. If z is much more or less than expected from the session-averaged firing rate map, the variance of the distribution of z’s will exceed two standard deviation units, and then the cell’s discharge is overdispersed (Jackson and Redish, 2007; Olypher et al., 2002). Standardized rates were computed as in (Fenton et al., 2010) by computing z for each 5-s episode, during which exp was greater than the average firing of the cell. Only passes with locations (x) that had been sampled more than 0.67 s were considered.
Network Consistency
Network consistency measures the reliability of a pair of place cells’ spatial co-firing. It assumes that the cell pair’s session-wide correlated discharge is an accurate prediction of the momentary co-firing of the cell pair as the subject moves across the overlapping firing fields of the two cells. The standardized firing of each cell of the pair along the pass (z1 and z2) is computed as described for overdispersion, above. Kendall’s correlation (τ1,2) was used to estimate the overall tendency of the cell pair to co-fire during a short time interval (e.g. 133 ms). Network Consistency was computed as:
NC = z1 · z2 · τ1,2. The product of the two standardized rates during an individual pass was used as an independent estimate of how the discharge of the cell pair covaries. If the momentary discharge across passes covaries like the overall correlation for the cell pair then NC is maximized and if the momentary discharge covaries opposite to the overall correlation, then NC is minimized. As in computing overdispersion, the standardized rate on the pass for each cell estimates the momentary variation of firing from the session-averaged expectation:
. If the expected rate was smaller than the session-averaged rate of that cell then the pass was excluded to ensure that only passes through a place field were evaluated (Fenton et al., 2010). To estimate zone specific network consistency, we added an additional constraint by only calculating the standardized rates on passes through firing fields in the vicinity of each potential shock zone (including the surrounding 6 cm). Only passes through the shock zone vicinity that were longer than 3 s were considered.
Histology
Mice were anesthetized with a sodium pentobarbital solution (100mg/kg, i.p.) and perfused with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (transgenic and control mice) or 10% formalin (electrophysiology mice). The brains were removed and post-fixed overnight. To identify the electrode tracks, mice heads with the brain partially exposed were put in formalin solution overnight, after which the brains were removed and post-fixed for another day. After post-fixing, the brains were cryoprotected in 30% sucrose 0.1 M phosphate buffer solution. Sections (50 μm) through the hippocampus were stained by cresyl violet to locate the electrode tracks. The sections were 35 μm for the transgenic and control mice and the tissue was stained with c-Fos Anti-Rabbit primary antibody (1:8000; from Millipore) and 594 nm Goat Anti-Rabbit Alexa Fluor (1:500) secondary for fluorescent microscopy. The sections were mounted with Vectashield mounting medium with DAPI (Vector Labs). Images were acquired on an Olympus VS120 Virtual Slide Microscope with a 20x 0.75 NA objective and composite images of either DAPI and eYFP immunofluorescence (excitation wavelengths 455 nm and 518 nm; Fig 2B) or DAPI and c-Fos immunofluorescence (excitation wavelengths: 455 nm and 580 nm; Fig 2C) are displayed. To estimate neuronal activity during the conflict trial c-Fos+ cells were counted in six slices per animal.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analyses were performed using custom-written Matlab code (2015B; 2017A, Mathworks) and JMP software (Version 12, SAS Institute, NC). One-way, two-way and three-way (repeated measures) ANOVAs were used as appropriate. When data were not normally distributed or the group variances were unequal, one of the following non-parametric tests was used, as appropriate: two-way ANOVA on ranks, Kruskal-Wallis or Welch’s one-way ANOVA. Fisher’s LSD were performed for post-hoc pair-wise comparisons. Statistical analyses are reported in the figure legends or in the RESULTS section, when not corresponding to a figure. The significance level is set to 0.05 and all tests are two-tailed.
DATA AND SOFTWARE AVAILABLITY
The datasets generated and analyzed during the current study are available in the Collaborative Research in Computational Neuroscience – Data sharing repository at (hylink to be added).
Data analysis code is available on GitHub at https://github.com/FentonLab/Mcode_DG-MemoryDiscrimination-NeuronMS.git.
Supplementary Material
Highlights.
Dentate gyrus-dependent memory discrimination does not require place cell remapping
Dentate neural correlates of memory discrimination are transient, lasting seconds
Sub-second dentate network discharge correlations signal memory discrimination
Dentate excitatory-inhibitory coupling is increased at memory discrimination sites
Acknowledgments
Supported by NIH grant R01AG043688. We acknowledge Younghun Lim and Zejia Angel Yu for help with histology. We thank Edith Lesburgueres, Eun-Hye Park and Dino Dvorak for sharing their expertise. We are grateful to Gyorgy Buzsaki, René Hen, and Helen Scharfman for discussions, guidance and comments on the manuscript.
Footnotes
Declaration of Interests
The authors declare no competing interests.
Author contributions
Conceptualization: MvD and AAF; Methodology: MvD and AAF; Software: MvD and AAF; Validation: MvD and AAF; Formal Analysis: MvD; Investigation: MvD; Writing - Original Draft: MvD and AAF; Writing - Review & Editing: MvD & AAF; Visualization: MvD; Supervision: AAF; Funding acquisition: AAF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alme CB, Miao C, Jezek K, Treves A, Moser EI, Moser MB. Place cells in the hippocampus: eleven maps for eleven rooms. Proc Natl Acad Sci U S A. 2014;111:18428–18435. doi: 10.1073/pnas.1421056111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern. 1977;27:77–87. doi: 10.1007/BF00337259. [DOI] [PubMed] [Google Scholar]
- Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci. 2015;18:1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bures J, Fenton AA, Kaminsky Y, Rossier J, Sacchetti B, Zinyuk L. Dissociation of exteroceptive and idiothetic orientation cues: effect on hippocampal place cells and place navigation. Philos Trans R Soc Lond B Biol Sci. 1997;352:1515–1524. doi: 10.1098/rstb.1997.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MF, Frank LM. A single microcircuit with multiple functions: state dependent information processing in the hippocampus. Curr Opin Neurobiol. 2012;22:704–708. doi: 10.1016/j.conb.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Frank LM. New experiences enhance coordinated neural activity in the hippocampus. Neuron. 2008;57:303–313. doi: 10.1016/j.neuron.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Fenton AA, Bures J. New spatial cognition tests for mice: passive place avoidance on stable and active place avoidance on rotating arenas. Brain Res Bull. 2001;54:559–563. doi: 10.1016/s0361-9230(01)00448-8. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Danielson NB, Turi GF, Ladow M, Chavlis S, Petrantonakis PC, Poirazi P, Losonczy A. In Vivo Imaging of Dentate Gyrus Mossy Cells in Behaving Mice. Neuron. 2017;93:552–559 e554. doi: 10.1016/j.neuron.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci. 2010;13:995–1002. doi: 10.1038/nn.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA. Coordinating with the “Inner GPS”. Hippocampus. 2015;25:763–769. doi: 10.1002/hipo.22451. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Bures J. Navigation in the moving world. In: Jeffery K, editor. The Neurobiology of Spatial Behaviour. Oxford: Oxford University Press; 2003. [Google Scholar]
- Fenton AA, Kao HY, Neymotin SA, Olypher AV, Vayntrub Y, Lytton WW, Nandor L. Unmasking the CA1 ensemble place code by exposures to small and large environments: more place cells and multiple, irregularly-arranged, and expanded place fields in the larger space. J Neurosci. 2008;28:11250–11262. doi: 10.1523/JNEUROSCI.2862-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck-Santini PP, Zinyuk LE, Kubik S, Bures J, Poucet B, Muller RU, Olypher AV. Attention-like modulation of hippocampus place cell discharge. J Neurosci. 2010;30:4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Muller RU. Place cell discharge is extremely variable during individual passes of the rat through the firing field. Proc Natl Acad Sci U S A. 1998;95:3182–3187. doi: 10.1073/pnas.95.6.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Wesierska M, Kaminsky Y, Bures J. Both here and there: simultaneous expression of autonomous spatial memories in rats. Proc Natl Acad Sci U S A. 1998;95:11493–11498. doi: 10.1073/pnas.95.19.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Shirvalkar P, Shapiro ML. Memory modulates journey-dependent coding in the rat hippocampus. J Neurosci. 2011;31:9135–9146. doi: 10.1523/JNEUROSCI.1241-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, DeCoteau WE. Memory for spatial location: role of the hippocampus in mediating spatial pattern separation. J Neurosci. 1998;18:804–810. doi: 10.1523/JNEUROSCI.18-02-00804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- GoodSmith D, Chen X, Wang C, Kim SH, Song H, Burgalossi A, Christian KM, Knierim JJ. Spatial Representations of Granule Cells and Mossy Cells of the Dentate Gyrus. Neuron. 2017;93:677–690 e675. doi: 10.1016/j.neuron.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Hoffman KL, Battaglia FP, McNaughton BL. Dentate gyrus and ca1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J Neurosci. 2001;21:7284–7292. doi: 10.1523/JNEUROSCI.21-18-07284.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD. Neural signatures of cell assembly organization. Nat Rev Neurosci. 2005;6:399–407. doi: 10.1038/nrn1669. [DOI] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Nasrallah K, Jensen KR, Chávez AE, Carrera D, Castillo PE. LTP at Hilar Mossy Cell-Dentate Granule Cell Synapses Modulates Dentate Gyrus Output by Increasing Excitation/Inhibition Balance. Neuron. 2017;95:928–943 e923. doi: 10.1016/j.neuron.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman RM, Chakraborty S, Anderson MI, Jeffery KJ. Context-specific acquisition of location discrimination by hippocampal place cells. Eur J Neurosci. 2003;18:2825–2834. doi: 10.1111/j.1460-9568.2003.03035.x. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O’Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Redish AD. Network dynamics of hippocampal cell-assemblies resemble multiple spatial maps within single tasks. Hippocampus. 2007;17:1209–1229. doi: 10.1002/hipo.20359. [DOI] [PubMed] [Google Scholar]
- Jinde S, Zsiros V, Jiang Z, Nakao K, Pickel J, Kohno K, Belforte JE, Nakazawa K. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012;76:1189–1200. doi: 10.1016/j.neuron.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Fenton AA, Kentros C, Redish AD. Looking for cognition in the structure within the noise. Trends Cogn Sci. 2009;2:55–64. doi: 10.1016/j.tics.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Measuring distributed properties of neural representations beyond the decoding of local variables — implications for cognition. In: Holscher C, Munk MHJ, editors. Mechanisms of information processing in the Brain: Encoding of information in neural populations and networks. London: Cambridge University Press; 2008. pp. 95–119. [Google Scholar]
- Karlsson MP, Frank LM. Network dynamics underlying the formation of sparse, informative representations in the hippocampus. J Neurosci. 2008;28:14271–14281. doi: 10.1523/JNEUROSCI.4261-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS Biol. 2010;8:e1000403. doi: 10.1371/journal.pbio.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. The organization of neuronal discharge on timescales of milliseconds and seconds is related to the spatial response properties of hippocampal neurons. 1. New York: Springer-Verlag; 2012. [Google Scholar]
- Kelemen E, Fenton AA. Key features of human episodic recollection in the cross-episode retrieval of rat hippocampus representations of space. PLoS Biol. 2013;11:e1001607. doi: 10.1371/journal.pbio.1001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, Zeng H, Fenton AA, Hen R. Differential Control of Learning and Anxiety along the Dorsoventral Axis of the Dentate Gyrus. Neuron. 2013;77:955–968. doi: 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lee JW, Jung MW. Separation or binding? Role of the dentate gyrus in hippocampal mnemonic processing. Neuroscience & Biobehavioral Reviews. 2017;75:183–194. doi: 10.1016/j.neubiorev.2017.01.049. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005a;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, Moser MB. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005b;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335:1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. Spatial firing correlates of physiologically distinct cell types of the rat dentate gyrus. J Neurosci. 2012;32:3848–3858. doi: 10.1523/JNEUROSCI.6038-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron. 2014;81:416–427. doi: 10.1016/j.neuron.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neymotin SA, Lytton WW, Olypher AV, Fenton AA. Measuring the Quality of Neuronal Identification in Ensemble Recordings. J Neurosci. 2011;31:16398–16409. doi: 10.1523/JNEUROSCI.4053-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- O’Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat Neurosci. 2008 doi: 10.1038/nn2037. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Okun M, Steinmetz NA, Cossell L, Iacaruso MF, Ko H, Bartho P, Moore T, Hofer SB, Mrsic-Flogel TD, Carandini M, Harris KD. Diverse coupling of neurons to populations in sensory cortex. Nature. 2015;521:511–515. doi: 10.1038/nature14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olypher AV, Klement D, Fenton AA. Cognitive disorganization in hippocampus: a physiological model of the disorganization in psychosis. J Neurosci. 2006;26:158–168. doi: 10.1523/JNEUROSCI.2064-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olypher AV, Lansky P, Fenton AA. Properties of the extra-positional signal in hippocampal place cell discharge derived from the overdispersion in location-specific firing. Neuroscience. 2002;111:553–566. doi: 10.1016/s0306-4522(01)00586-3. [DOI] [PubMed] [Google Scholar]
- Olypher AV, Lansky P, Muller RU, Fenton AA. Quantifying location-specific information in the discharge of rat hippocampal place cells. J Neurosci Methods. 2003;127:123–135. doi: 10.1016/s0165-0270(03)00123-7. [DOI] [PubMed] [Google Scholar]
- Park E, Dvorak D, Fenton AA. Ensemble Place Codes in Hippocampus: CA1, CA3, and Dentate Gyrus Place Cells Have Multiple Place Fields in Large Environments. PLoS ONE. 2011;6:e22349. doi: 10.1371/journal.pone.0022349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EH, Burghardt NS, Dvorak D, Hen R, Fenton AA. Experience-Dependent Regulation of Dentate Gyrus Excitability by Adult-Born Granule Cells. J Neurosci. 2015;35:11656–11666. doi: 10.1523/JNEUROSCI.0885-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. PLACE CELLS. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science. 2015;349:180–183. doi: 10.1126/science.aaa9633. [DOI] [PubMed] [Google Scholar]
- Redish AD, Rosenzweig ES, Bohanick JD, McNaughton BL, Barnes CA. Dynamics of hippocampal ensemble activity realignment: time versus space. J Neurosci. 2000;20:9298–9309. doi: 10.1523/JNEUROSCI.20-24-09298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivard B, Li Y, Lenck-Santini PP, Poucet B, Muller RU. Representation of objects in space by two classes of hippocampal pyramidal cells. J Gen Physiol. 2004;124:9–25. doi: 10.1085/jgp.200409015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Piatti VC, Hwaun E, Ahmadi S, Lisman JE, Leutgeb S, Leutgeb JK. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nature Neuroscience. 2018 doi: 10.1038/s41593-017-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The enigmatic mossy cell of the dentate gyrus. Nat Rev Neurosci. 2016;17:562–575. doi: 10.1038/nrn.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Schneidman E, Berry MJ, 2nd, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440:1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzai Y, Buzsaki G. Physiological Properties and Behavioral Correlates of Hippocampal Granule Cells and Mossy Cells. Neuron. 2017;93:691–704 e695. doi: 10.1016/j.neuron.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Ferbinteanu J. Relative spike timing in pairs of hippocampal neurons distinguishes the beginning and end of journeys. Proc Natl Acad Sci U S A. 2006;103:4287–4292. doi: 10.1073/pnas.0508688103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J Neurosci. 2010;30:11586–11604. doi: 10.1523/JNEUROSCI.0926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Lomo T. Updating the lamellar hypothesis of hippocampal organization. Front Neural Circuits. 2012;6:102. doi: 10.3389/fncir.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot ZN, Sparks FT, Dvorak D, Curran BM, Alarcon JM, Fenton AA. Normal CA1 Place Fields but Discoordinated Network Discharge in a Fmr1-Null Mouse Model of Fragile X Syndrome. Neuron. 2018 doi: 10.1016/j.neuron.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD. Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr Opin Neurobiol. 2015;32:8–15. doi: 10.1016/j.conb.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends Neurosci. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.