Abstract
Background
Viscosupplementation of the synovial liquid, by intra-articular injection of hyaluronic acid, is a widely used symptomatic treatment in knee osteoarthritis. Besides products designed for multi-injections (typically 3–5 injections at 1-week intervals), special interest is being given to single-injection products that offer specific advantages such as the reduction of the number of visits to the doctor and the number of invasive interventions with their associated risks. However, a question remains about the efficacy of these monoinjections, compared with the multi-injections regimens.
Methods
A postmarket, prospective, multicenter, open study (ART-ONE 75), was performed with the single-injection product Arthrum 2.5% (3 mL, 75 mg hyaluronic acid) (LCA Pharmaceutical, Chartres, France), on 214 patients with knee osteoarthritis. Patients were followed at 30, 60, 120, and 180 days. The average patient profile at inclusion was age 62.9 years, 56% were women, Kellgren-Lawrence (KL) grade I through III (46% Kellgren-Lawrence status III), body mass index 27.2, and 4 years osteoarthritis anteriority. A post hoc comparison was performed using a single intra-articular injection placebo (326 patients, pooled from 3 randomized controlled trials) providing a similar patient profile.
Results
The main criterion was the variation from baseline of the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index, pain subscale (A) score (range 0-100), at 60 days, which was reduced by 28.9 (17.4) for the intent-to-treat population (199 patients), 28.0 (17.8) for the per protocol at inclusion population (175 patients), and by 27.7 (16.8) for the per protocol completed population (143 patients). The secondary and accessory criteria included WOMAC A score at the other times, WOMAC B (stiffness) score, WOMAC C (function) score, quality of life, and handicap at each follow-up time. All indexes were significantly improved and continued to improve at the end of the study. The therapeutic assessment at 180 days showed more than 75% of patients were satisfied with pain reduction, mobility improvement, and reduction in taking analgesics and nonsteroidal anti-inflammatory drugs. The percentage of patients defined as Outcome Measures in Rheumatology Clinical Trials–Osteoarthritis Research Society International Standing Committee for Clinical Trials Response Criteria Initiative (OMERACT-OARSI) responders was >86%, from 60 days onward. The overall tolerance was good, without any serious adverse event. The result of the post hoc comparison for the WOMAC A score showed an effect size from 0.33 (95% CI 0.15–0.51) at 60 days to 0.65 (95% CI 0.45–0.85) at 180 days (P < 0.001), versus injected placebo (saline solution), which is clinically relevant in favor of Arthrum 2.5%.
Conclusions
The present study suggests the clinical efficacy of a single intra-articular injection of 3 mL intra-articular hyaluronic acid solution containing 75 mg high molecular weight (>2 MDa) native hyaluronic acid.
Key words: hyaluronic acid, intra-articular, knee osteoarthritis, viscosupplementation, pain & function, handicap
Introduction
Osteoarthritis (OA) is a major disease, affecting a large part of the population aged 40 years and older. In France, the prevalence of OA is estimated at 17%, which would be 9 to 10 million people with OA.1 OA is characterized by pain and limitation of movement and is confirmed by the progressive degradation of the cartilage, which can be observed by radiography, to relate the technique and other imaging techniques.
Knee OA is particularly painful and debilitating. In the United States, the radiologic prevalence of knee OA has been estimated at 3.8% of the whole population.1 Today no treatment exists to restore osteoarthritic cartilage, so the treatments are symptom based—pharmacologic or nonpharmacologic—and eventually, surgery may be considered for joint replacement.
Among the knee OA treatments, viscosupplementation of the joint with intra-articular (IA) injections of hyaluronic acid (HA) has been widely and successfully used since receiving approval in Japan and Italy in 1987-1988. The concept of IA HA is to re-establish the properties of the synovial liquid because HA concentration is lowered inside the pathologic OA joint compared with a normal, healthy joint. Because IA HA is locally administered, the risk of systemic adverse events is low, and viscosupplementation is considered a safe treatment. The benefits of IA HA are reduction of pain and improvement of functional mobility with consequences for handicap and quality-of-life scores.
For knee OA, the IA HA treatment generally consists of a succession of injections (3–5), at 1-week intervals, performed by a specialist doctor. Since 2004, a single-injection IA HA treatment has been proposed (3 mL, 60 mg) as an alternative to the multi-injections (currently 3 × 2 mL, 20 mg each), dispensing the same amount of HA. A single injection would provide several practical and economic advantages by reducing the number of visits to the doctor, the number of invasive procedures, and the associated risks.
The purpose of this study was to assess the clinical efficacy of a single IA HA injection (3 mL, 75 mg).
Methods
Objectives of the study
The primary objective of the study was to evaluate the clinical efficacy on pain of 1 single IA injection of 3.0 mL HA solution, in the symptomatic treatment of OA, with a follow-up period of 2 months.
The secondary objectives were to assess the evolution of the pain, function, tolerance, and handicap of the patients with OA, during the 6-month period of follow-up. Complementary objectives were to assess the capability of the IA HA product to reduce analgesic and nonsteroidal anti-inflammatory drug (NSAID) consumption and to analyze the influence on patients’ daily life (ie, quality of life).
Product studied
The viscosupplementation product studied was Arthrum 2.5% (LCA Pharmaceutical, Chartres, France) (also called Arthrum visc 75), an HA solution presented in a sterile, prefilled (with 3 mL product) glass syringe. Each syringe contains 75 mg of a highly purified HA obtained by bacterial fermentation of a Streptococcus equi species, free of proteins from animal origin, with an average molecular weight of 2.4 MDa for the finished product. The product Arthrum 2.5% has been Conformité Européenne (CE) marked since 2009, and at the end of 2015 more than 80,000 units had been delivered.
Study design and ethics statement
The routine postmarket study ART-ONE 75 was designed as multicenter, open, prospective, and observational. At the time this study was launched (April 2014), it was not considered in France as an interventional study because the assignment of the Arthrum 2.5% treatment, was done before the recruitment. Also, the fact that the same person could be both the prescriber and the investigator was not regarded here as a major risk of bias because all these investigators were currently prescribing Arthrum 2.5% in their routine practice and because they never received any substantial advantage from their participation to the study.
This study was entirely carried out in France, in conformity with French laws and regulations for clinical research, with particular reference to informing the patients, collecting their consents (Code de Santé Publique, articles L.1122-1–L.1122-2), and data disclosure and storage. Before the start of the study, the protocol, the case report form, the information sheet, and the patient consent form were submitted to the French advisory committee of research in health (CCTIRS). As the study was non-interventional and did not interfere with or modify the patient healthcare routine, no authorization from an ethical committee and no registration with health authorities were required.
Prescription and acceptance of the treatment Arthrum 2.5% by the patient was undertaken before any request to participate to the study. Then the patients were individually informed about the study, and they were only included after agreeing to participate, which was confirmed by their written consent (Figure 1). They were also free to cancel their participation at any time. The injection of the product was performed at the end of the inclusion visit, or later. The inclusion visit fees were covered by the national health system, meaning that for the nonparticipating patients, the Arthrum 2.5% treatment was then administrated on the current practice base, outside the study. The 4 control visits fees and the time spent by the investigators for the data management were covered by the sponsor of the study. For the participating patients, the advantage was limited to the free supply of Arthrum 2.5% (1 syringe), and the counterpart was their commitment to come for the 4 control visits.
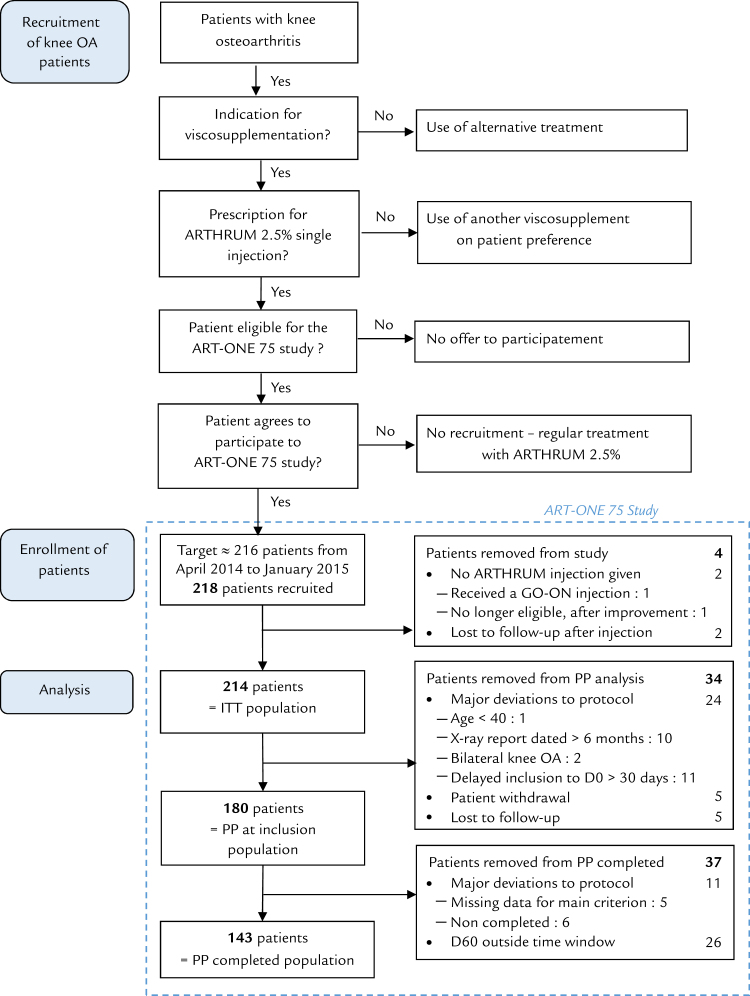
Figure 1.
Study flowchart. ITT = intention to treat; OA = osteoarthritis; PP = per protocol. *LCA Pharmaceutical, Chartres, France.
The study was supervised by an independent scientific committee from the start of the design of the study until the final statistical report. The investigators were all specialist medical doctors practicing in France: rheumatologists, orthopedic surgeons, or physical medicine and rehabilitation doctors. They were in charge of all interactions with the patients, from the recruitment to the last follow-up visit. Assistance monitoring was provided by the sponsor to control in real-time each case report form completion. After completion of data collection, every paper form was definitively made anonymous by removal of the full name of the patient—which was only present on a special removable label—keeping only the 3 first letters of each last name and first name, for the rest of the document. Each patient consent was kept inside a cover attached to the case report form, as a legal requirement, to remain sealed at all times, unless by a special request from the sponsor.
Then, the statistical analysis was performed by an independent clinical research organization. Only anonymous data were computerized, and the study was submitted for eventual review from the Commission Nationale de l’Informatique et des Libertés (CNIL).
Selection of patients
The inclusion and noninclusion criteria are shown in Table 1. These criteria were having met the recommendations of European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO)2 for the indication of IA HA to older patients with confirmed OA diagnosis. However, with real-world conditions, some deviations to the protocol could be accepted upon approval by the scientific committee.
Table 1.
Inclusion and noninclusion criteria.
| Inclusion or exclusion | Criteria |
|---|---|
| Inclusion | Patient man or woman, aged 40 years or older |
| With a unilateral knee osteoarthritis: | |
| |
| Able to understand the development of the study and to give written consent | |
| Geographically stable for the duration of the study | |
| Covered by the French health system (Sécurité Sociale) | |
| Exclusion | Inflammatory arthritis |
| Progressive infection of the studied knee | |
| Anterior viscosupplement treatment within the prior year | |
| Intra-articular corticosteroid in the studied knee within the prior 3 mo | |
| Known hypersensitivity to hyaluronic acid or to similar activity ingredients | |
| Anticoagulant treatment in place | |
| Insulin-dependent/type I diabetes | |
| Pregnant or breastfeeding women | |
| Patient under guardianship or curatorship or under legal protection | |
| Patient presently participating in another clinical research study | |
KL: Kellgren Lawrence; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Main criterion
The primary objective of the study was evaluated using as main criterion the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale A. The WOMAC A comprised 5 items, each assessed with a Likert scale questionnaire (5 levels, starting from 0 = no pain, 1 = minimal pain, 2 = moderate pain, 3 = severe pain, and up to 4 = extreme pain). In the absence of a comparison group, a significant variation from baseline was considered as a good clinical indicator if achieved at day 60, a relatively short time after the injection. After day 60, continuous improvement was still expected. The main criteria analysis was made for both intention to treat (ITT) and PP (per protocol) populations.
From score results a comparison with baseline was performed, from effect size (ES), 95% CI, and P value calculations. To quantitatively assess these results, we refer to Miller et al3 who proposed results for the standardized mean difference (SMD) versus baseline for pain index: SMD = 1.37 (95% CI 1.12–1.61) at 4 to 13 weeks.
Secondary criteria
In addition to the main criterion, secondary criteria were including the subscale scores of the WOMAC index: WOMAC A (pain) at other times than day 60, and WOMAC C (function, 17 items), with the 5-level Likert scale. Therefore globally, pain and functional capability were assessed from the inclusion day (day 0) to day 30, day 60, day 120, and day 180 follow-up visits for the ITT and for the PP populations.
Miller et al3 also reported SMD = 1.14 (95% CI 0.89–1.39) versus baseline results (P < 0.001), for pain at 14 to 26 weeks. Similarly, they reported for function: SMD = 1.16 (95% CI 0.99–1.34) at 4 to 13 weeks and SMD = 1.07 (95% CI 0.84–1.30) at 14 to 26 weeks. These reference results were used to assess ES calculated versus baseline, for WOMAC A and WOMAC C.
As secondary criteria for the ITT population, WOMAC B (stiffness, 2 items) score was assessed. Then the WOMAC index (global score, 24 items) was calculated.
Accessory criteria
Quality of life (was assessed from the influence on patient activity (Agence nationale d'accréditation et d'évaluation en santé (ANAES): 3 questions, 6-level scale), relative to the influence on walking, working (including daily activities), and on sleeping.
Handicap was assessed, both by the investigating doctor and the patients, as a major consequence of the OA disease. Functional handicap affects the whole population and was analyzed first. Then the professional handicap—concerning specifically the active fraction of the population—was analyzed separately, whenever applicable. Functional and professional handicaps were assessed with the 5-level scale, either by the patient or by the investigator. The assessment of functional handicap by the patient was considered a major result, and an expression of the overall patient assessment.
A therapeutic efficacy assessment was made from day 30 to day 180 with a 5-level scale, for pain reduction, mobility improvement, and reduction of drug consumption (analgesics and NSAIDs). Clinical tolerance (local and general) and safety of the treatment were assessed by the investigator. Side effects and adverse events were recorded.
The accessory criteria were assessed on ITT population, grouping all patients having received the treatment with a minimum of follow-up: all available scores were taken without restriction.
Complement analysis
Outcome measures in rheumatoid arthritis clinical trials– Osteoarthritis Research Society International (OMERACT-OARSI) responders4 were calculated for each population at any follow-up time, from day 30 to day 240. For the pain, function, and overall patient assessment, we used the score results obtained for WOMAC A, WOMAC C, and functional handicap (patient assessment), respectively.
The predictive factors indicated by this study were identified as age, body mass index, and the radiologic grades (Kellgren-Lawrence [KL] I, II, and III). Age and body mass index are considered as continuous variables. To explore the predictive factors, the OMERACT-OARSI results were stratified first into “responders” and “nonresponders” and then by KL grade (I+II vs III).
Population size
The minimum number of patients was estimated at N = 116 from the bilateral test formula:
where α = 0.025, β = 0.05, σ = 0.097, and Δ = 0.19. The values of σ (minimal perceptible clinical improvement) and Δ (standard deviation) were respectively determined from Ehrich et al5 and Mazières et al,6 with respect to the WOMAC A (pain subscale).
To anticipate the loss of patients (30%), deviations at inclusion (30%), and potential reluctance for the use of a nonreimbursed product (10%)—even if offered through the study—the recruitment objective was increased to 216.
Amendments to initial protocol
The timing given by the initial protocol was too strict regarding the delay between the inclusion visit and the injection (the window was extended from 30 to 90 days), and regarding the dates of follow-up. The reference times were redefined and the windows were extended to 15 to 44 days for day 30, 45 to 89 days for day 60, 90 to 149 days for day 120, and 150 to 209 days for day 180. Consequently, the visits were attributed at their real time, and the final visits that were delayed from 210 to 270 days have been analyzed separately in an additional 240 days window. The other amendment was for radiograph reports made beyond the 6-month limit by just a few days, which have been accepted.
However, in the PP completed analysis, the 30-day delay to injection and the narrow time windows were restored, to limit the risk of a potential selection bias.
Statistical analysis
The case report forms (paper) were collected and the data acquisition performed with Clinsight 7.0 (Ennov, France) in duplicate, by 2 separate operators. The data collected were compared by the data manager. Then the quality control of the clinical research organization determined that the error rate was <1% on a sample of (n + 1)0.5 files. After the data were accepted they were frozen, and statistical analysis was performed with SAS version 9.3 software (SAS Institute Inc, Cary, NC).
The quantitative variables were described by their mean, SD, median, extreme values (minimum and maximum), and the population measured. The qualitative variables were described by the frequency (n [%]) of their modalities. The comparison tests were described by the mean of the differences in score, SD, completed with the 95% CI, ES, and P value (obtained from t test, z test, Wilcoxon signed-rank test, or χ2 test for the qualitative variables). The significance threshold was set at 5% except for the main criteria (α = 2.5%). Populations with missing data have been described for each measure. No missing data were replaced.
The final analysis was completed by the calculation of the OMERACT-OARSI responders,4 and from there by an assessment of the predictive factors.
Post hoc placebo comparison
Our assessment of the clinical efficacy from a baseline comparison had several limits because the main criterion was defined at day 60—a relatively short time—rather than for a longer period, and because the reference results vs baseline were only available from 1 meta-analysis.3
In the absence of a control group within this open study, the comparison was attempted with a placebo group obtained from more published reference studies. Reference studies for single IA injection of HA in knee OA are relatively scarce compared with the multi-injection studies, because the concept of single injection was introduced only in 2004. A systematic review of published studies in the treatment of knee OA, identified 4 controlled double-blind randomized trials comparing a single injection of IA HA to a single injection of placebo (saline solution). Each of these trials reported comparative results for the WOMAC A (pain) at various follow-up times, up to 3 or 6 months. Results from WOMAC C (function) were also reported by 3 of these trials. The trials were published by Strand et al,7 Chevalier et al,8 Altman et al,9 and Hangody et al,10 The first 3 are reference-quality studies, because they have been selected in several recent meta-analyses.3., 11., 12., 13., 14. The fourth study by Hangody et al10 was published in 2017 and was too recent for any of these selections.
After selecting the reference trial, the placebo arm of each study was evaluated. The first step was to match the patient profile of the reference studies to our own study: combining randomized controlled trial placebo arms targeted to reach the same KL distribution as our study. The second step was to pool these selected placebo results and to calculate the average scores with SD and the number of patients at each follow-up time. For example, we planned to make curves showing the placebo score variations from baseline at day 30, day 60, day 90, and day 180 for the WOMAC A and for the WOMAC C. The third step was the comparison of placebo results with the results from our study, superimposing the curves on the same graph and calculating the ES and P value at the different time points.
The post hoc placebo comparison was performed on the ITT population only, first because comparison studies refer on ITT results, close to the real life, and second because Arthrum 2.5% results were nearly identical between ITT and PP populations, so a double analysis was not justified.
Results
Population and patient profile
A total of 218 patients were recruited in France from April 2014 to January 2015, by 48 investigators participating in the study: 37 rheumatologists, 4 orthopedic surgeons, and 7 doctors of physical medicine and rehabilitation.
The populations recruited and selected are described by the flow-chart (Figure 1). Two hundred sixteen patients received the treatment, but 2 of them were lost immediately after, and therefore could not be analyzed. Two hundred fourteen patients are in the ITT (and tolerance) population. Due to the number of deviations, it was decided to double the PP analysis, with 180 patients in the PP population (at inclusion) after removal of the patients whose injection was delayed more than 30 days, and 143 in the PP population (completed studies) after restoring of the initial small time windows. This was done, to better assess the incidence of these deviations on the results.
The patient profiles at inclusion are shown in Table 2.
Table 2.
Characteristics of patients at inclusion (N = 218).
| Characteristic | Result |
|---|---|
| Age, y | |
| Mean (SD) | 62.9 (12.6) |
| Min–Max | 24–88 |
| Sex, n (%) | |
| Men | 95 (43.6) |
| Women | 123 (56.4) |
| KL radiologic grade of knee osteoarthritis, n (%) | |
| Grade I | 33 (15.2) |
| Grade II | 85 (39.2) |
| Grade III | 99 (45.6) |
| Weight, kg (SD) | 76.5 (14.7) |
| Height, m (SD) | 1.675 (0.092) |
| Body mass index (SD) | 27.2 (4.3) |
| Anteriority of knee osteoarthritis, y (SD) (%) | 4.14 (5.40) (100.0%) |
| ≤1 y (n = 83) | 0.56 (0.29) (39.0%) |
| ≥1 year (n = 130) | 6.42 (5.85) (61.0%) |
| Medical history, n(%) | |
| Metabolic | 50 (22.9) |
| Cardiovascular | 63 (28.9) |
| Gastrointestinal | 27 (12.4) |
| Neurologic | 11 (5.0) |
| Surgical and rehabilitation history, n (%) | |
| Surgical intervention on the knee | 62 (28.4) |
| Arthroscopic examination | 36 (16.5) |
| Therapeutic arthroscopy | 41 (18.8) |
| Rehabilitation postsurgery | 44 (20.2) |
| Pain and function scores (0–100 scale) | |
| WOMAC subscale A (SD) | 50.3 (15.6) |
| WOMAC subscale B (SD) | 47.3 (22.1) |
| WOMAC subscale C (SD) | 42.4 (17.9) |
| WOMAC total score (SD) | 44.4 (16.5) |
KL = Kellgren-Lawrence; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
For the ITT population, after visit reports were made according to each redefined window, the number of participants analyzed was found to be 207 at day 30, 199 at day 60, 180 at day 120, 183 at day 180, and 21 at day 240 (after removal of 2 patients seen at a later time; ie, at day 330 and day 427). However, due to the limited subpopulation, results at day 240 are mostly indicative.
WOMAC: Main and secondary criteria
WOMAC A
At any time, the improvement of the WOMAC A subscale score was significant (P < 0.001) for both ITT and PP populations (Table 3), without difference between these 3 populations.
Table 3.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale A results, with main criterion.
| WOMAC A Pain (base 100) | Time, d | n n | Variation (SD) | Effect size (95% CI) | P value |
|---|---|---|---|---|---|
| Intention to treat population | 30 | 207 | 22.3 (20.0) | 1.11 (0.97–1.25) | < 0.001 |
| 60*,† | 199 | 28.9 (17.4) | 1.66 (1.52–1.80) | < 0.001 | |
| 120 | 180 | 30.7 (17.8) | 1.72 (1.57–1.87) | < 0.001 | |
| 180‡ | 183 | 33.6 (17.4) | 1.93 (1.79–2.07) | < 0.001 | |
| 240§ | 23 | 31.9 (21.4) | 1.38 (0.95–1.81) | < 0.001 | |
| Per protocol population at inclusion | 30 | 176 | 22.4 (18.9) | 1.18 (1.04–1.33) | < 0.001 |
| 60*,† | 175 | 28.0 (17.8) | 1.57 (1.42–1.72) | < 0.001 | |
| 120 | 161 | 29.9 (18.4) | 1.63 (1.47–1.78) | < 0.001 | |
| 180‡ | 162 | 33.0 (19.3) | 1.71 (1.55–1.86) | < 0.001 | |
| 240§ | 19 | 33.7 (22.8) | 1.47 (1.02–1.92) | < 0.001 | |
| Per protocol population completed | 30 | 139 | 22.7 (18.5) | 1.22 (1.06–1.39) | < 0.001 |
| 60*,† | 143 | 27.7 (16.8) | 1.65 (1.48–1.81) | < 0.001 | |
| 120 | 118 | 31.6 (17.0) | 1.85 (1.67–2.03) | < 0.001 | |
| 180‡ | 120 | 33.7 (18.0) | 1.87 (1.69–2.05) | < 0.001 | |
Main criterion is in boldface type.
Comparable result by Miller et al:3 standard mean difference = 1.37 (95% CI, 1.12–1.61) for pain at 4 to 13 weeks.
Comparable result by Miller et al:3 standard mean difference = 1.14 (95% CI, 0.89–1.39) for pain at 14 to 26 weeks.
Due to reduced population, results at 240 days are indicative, but confirm extended time stability.
For the main criterion, the improvement from baseline of the WOMAC A at day 60 was 28.9 (17.4), which is important (more than 55%) for the ITT population, 28.0 (17.8) for the PP at inclusion population, and 27.7 (16.8) for the PP completed population. In both cases, the ES was > 1.55. Therefore, Arthrum 2.5% compared favorably with the results from Miller et al,3 SMD = 1.37 (95% CI 1.12–1.61), at 4 to 13 weeks.
As secondary criteria, with WOMAC A at longer observation times, ES always compared favorably to Miller et al,3 SMD = 1.14 (95% CI 0.89; 1.39), at 14 to 26 weeks.
WOMAC C
As secondary criterion, the WOMAC C function subcale score variations from baseline, were given for the ITT and PP populations at each follow-up time (Table 4). In all cases, the improvement was important and always progressed from day 30 to day 240. Scores from day 60 and later, showed ES always >1.30. This result compared favorably with those of Miller et al:3 SMD = 1.16 (95% CI 0.99–1.34) at weeks 4 to 13, and SMD = 1.07 (95% CI 0.84–1.30) at weeks 14 to 26.
Table 4.
Secondary criteria results.
| WOMAC (base 100) | Time, d | n | Variation (SD) | Effect size (CI 95%) | P value |
|---|---|---|---|---|---|
| WOMAC subscale C | 30 | 185 | 18.3 (18.9) | 0.97 (0.83–1.11) | < 0.001 |
| ITT population | 60* | 181 | 23.4 (17.3) | 1.36 (1.22–1.50) | < 0.001 |
| 120† | 160 | 25.6 (17.0) | 1.51 (1.36–1.66) | < 0.001 | |
| 180† | 165 | 27.6 (17.5) | 1.58 (1.44–1.72) | < 0.001 | |
| 240 | 17 | 29.8 (13.1) | 2.27 (1.84–2.70) | < 0.001 | |
| WOMAC subscale C | 30 | 159 | 17.9 (16.9) | 1.06 (0.91–1.22) | < 0.001 |
| PP population (at inclusion) | 601 | 159 | 22.2 (16.9) | 1.31 (1.16–1.47) | < 0.001 |
| 1202 | 145 | 24.4 (16.8) | 1.45 (1.29–1.62) | < 0.001 | |
| 1802 | 148 | 26.2 (18.4) | 1.42 (1.26–1.59) | < 0.001 | |
| 2403 | 15 | 30.9 (21.4) | 1.45 (0.94–1.95) | < 0.001 | |
| WOMAC subscale C | 30 | 128 | 18.2 (17.1) | 1.06 (0.89–1.24) | < 0.001 |
| PP population (completed) | 601 | 131 | 22.3 (16.1) | 1.39 (1.22–1.56) | < 0.001 |
| 1202 | 109 | 25.8 (15.9) | 1.63 (1.44–1.82) | < 0.001 | |
| 1802 | 114 | 26.5 (18.4) | 1.44 (1.26–1.63) | < 0.001 | |
| WOMAC subscale B | 30 | 208 | 21.2 (22.0) | 0.96 (0.82–1.10) | < 0.001 |
| ITT population | 60 | 200 | 27.3 (19.1) | 1.43 (1.29–1.57) | < 0.001 |
| 120 | 181 | 31.5 (18.6) | 1.70 (1.55–1.85) | < 0.001 | |
| 180 | 182 | 32.4 (18.7) | 1.74 (1.60–1.88) | < 0.001 | |
| 2403 | 21 | 30.6 (22.1) | 1.38 (0.95–1.81) | < 0.001 | |
| WOMAC index ITT population | 30 | 185 | 19.5 (18.6) | 1.05 (0.91–1.19) | < 0.001 |
| 60 | 180 | 25.0 (16.6) | 1.50 (1.36–1.64) | < 0.001 | |
| 120 | 160 | 27.1 (16.6) | 1.63 (1.48–1.78) | < 0.001 | |
| 180 | 164 | 29.2 (17.0) | 1.72 (1.58–1.39) | < 0.001 | |
| 2403 | 17 | 31.0 (14.1) | 2.19 (1.76–2.62) | < 0.001 | |
ITT = intention to treat; PP = per protocol; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
‡Due to reduced population, results at 240 days are indicative, but confirm extended time stability.
Comparable result by Miller et al:3 standard mean difference = 1.16 (95% CI, 0.99–1.34) for function at 4 to 13 weeks
Comparable result by Miller et al:3 standard mean difference = 1.07 (95% CI, 0.84–1.30) for function at 14 to 26 weeks.
WOMAC B and WOMAC global score
As secondary criteria for the ITT population, WOMAC B, and calculated WOMAC global index score were also improved along the follow-up times.
Accessory criteria
Quality of life
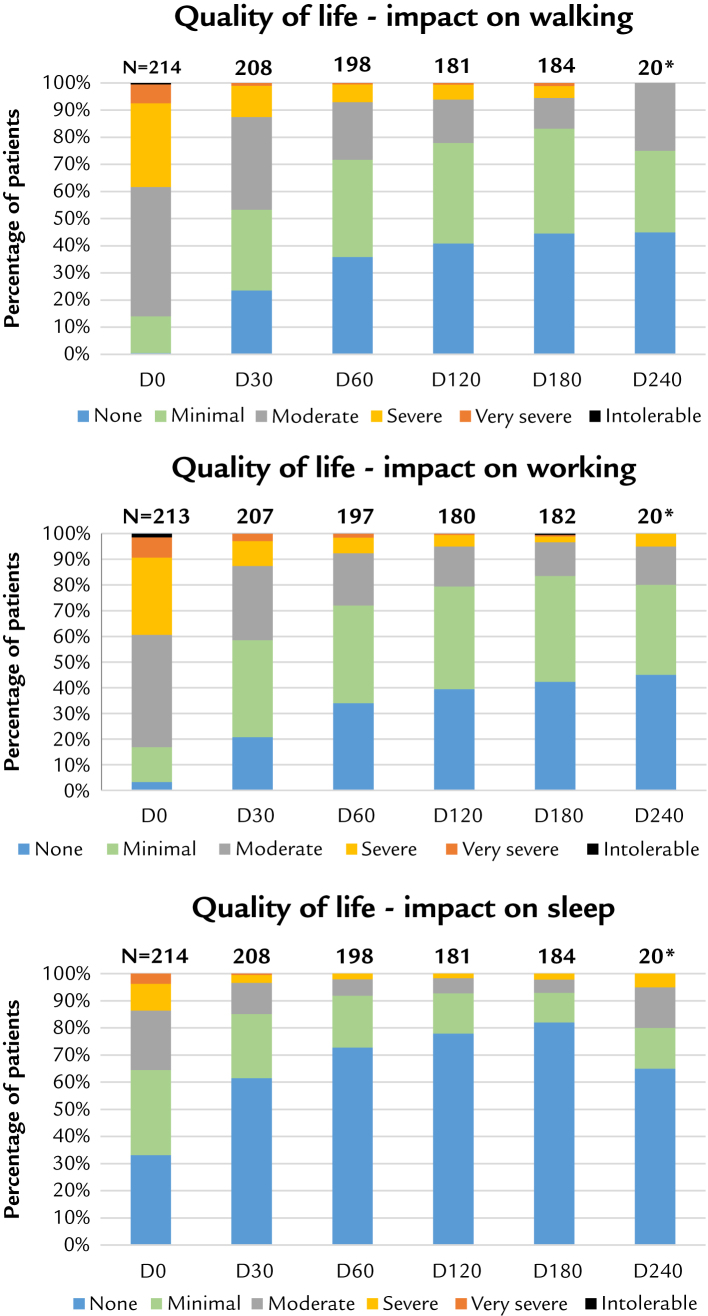
Results for quality of life are given by the graphs (Figure 2) for each of the 3 questions. We represented the distribution of quality scores (6 levels, from “none” (or "no") influence, to “intolerable”). The cumulated low scores (“none” + “minimal”) starting from <20% at day 0 for walking or working, increased with time, and reached >70% at day 60, and then further increased. Reciprocally, the cumulated high scores (“severe” + “very severe” + “intolerable”) starting from >40% progressively reduced to <10%, at the same observation times. For sleeping, the potential for improvement was smaller starting from <65%, but >90% was reached from day 60 to day 180. All these balanced variations, shifting the population in the direction of improvement, indicate a positive correlation for the efficacy of the product to improve the quality of life.
Figure 2.
Quality of life. D = day. *Indicative result at 240 days.
Handicap
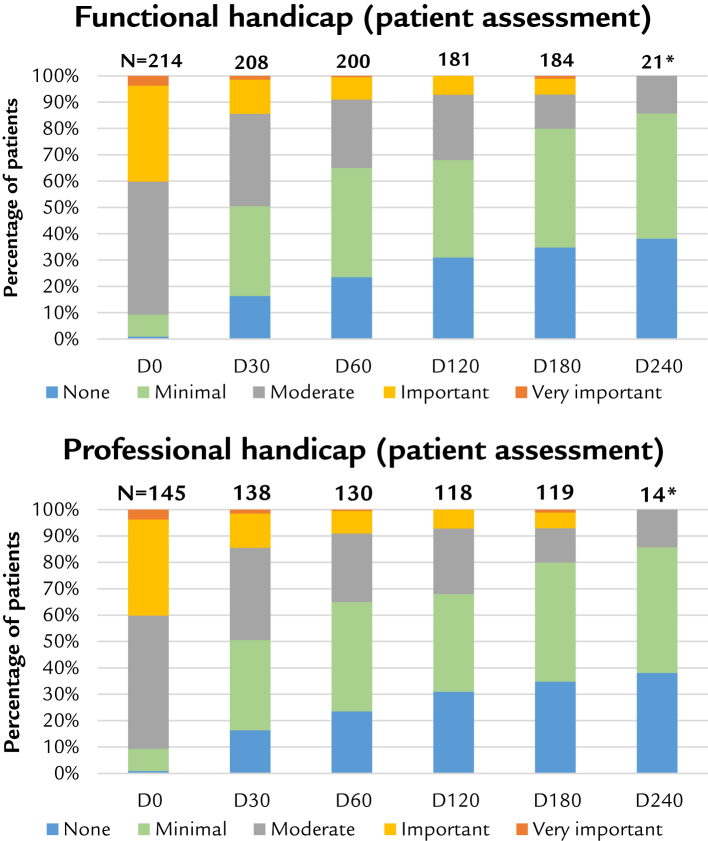
Results for handicap shown by graphs (Figure 3) are only those reported by patients because the difference has been found to be minor when compared with the results given by doctors.
Figure 3.
Handicap. D = day. *Indicative result at 240 days.
We represented the distribution of quality scores (5 levels, from disadvantage/disability levels of “none,” to “very important”). With regard to quality of life, the population has been shifted in the direction of improvement, with a strong reduction in patient handicap. Both functional and professional handicap have been improved, allowing satisfactory activity, with positive consequences for quality of life.
Therapeutic efficacy assessment
Results for the 4 questions are given in percentage of patients (“satisfied” + “very satisfied”), as assessed by patients or doctors (Table 5).
Table 5.
Therapeutic assessment: Rate of satisfaction.
| Assessment by percentage (satisfied + very satisfied) | Day | ||||
|---|---|---|---|---|---|
| 30 | 60 | 120 | 180 | 240 | |
| Pain reduction | |||||
| n | 208 | 199 | 182 | 183 | 21 |
| Patient (%) | 61.1 | 68.8 | 72.0 | 75.4 | 76.2 |
| Doctor (%) | 63.2 | 72.4 | 74.2 | 80.3 | 81.0 |
| Mobility improvement | |||||
| n | 208 | 198 | 181 | 182 | 21 |
| Patient (%) | 61.1 | 66.7 | 72.9 | 76.4 | 81.0 |
| Doctor (%) | 64.4 | 68.7 | 73.5 | 80.8 | 85.7 |
| Reduction of analgesics | |||||
| n | 191 | 182 | 170 | 167 | 19 |
| Patient | 66.0 | 69.8 | 74.7 | 76.6 | 73.7 |
| Doctor | 67.5 | 72.0 | 75.9 | 79.6 | 84.2 |
| Reduction of nonsteroidal anti-inflammatory drugs | |||||
| n | 177 | 166 | 157 | 155 | 16 |
| Patient (%) | 66.7 | 71.1 | 76.4 | 781. | 81.3 |
| Doctor (%) | 70.1 | 75.9 | 77.7 | 80.6 | 87.5 |
The percentages of unsatisfied patients (not shown here) are consequently reduced. A slow but continuous improvement is observed, for each of the 4 criteria.
Safety
There were 26 patients who reported local reactions to the treatment.
For 19 (8.8% of the population), these events were minor local reactions, starting the day of injection and spontaneously resolved within 1 to 3 days (eg, pain at the site of injection, redness, itching, or swelling sometimes with a small effusion).
Among the remaining 7 patients, postinjection pain on walking was reported (interpretation of a persisting pain on walking during the first weeks following the treatment should be considered with care because the maximal clinical efficacy of IA HA is typically delayed over 30 days) and with 4 patients this lasted from 7 to 14 days (1.9% of the population). A case of pruritus lasting 36 days postinjection was described by 1 patient (the patient with pruritus was physically active, climbing 70 stairs on average per day; at the end of the study, this patient was very satisfied with the IA HA treatment). The cause of the pruritus has not been identified. Delayed pain (starting at 12 or 14 days postinjection, which spontaneously resolved during the next 4 days) was reported by 2 patients (one of the final 2 patients was removed from the study at day 60 after diagnosis of a chondrocalcinosis not visible at inclusion. After biological analysis, this event was not considered related to Arthrum 2.5% injection).
No general or serious adverse event has been reported in this study.
OMERACT-OARSI responders and predictive factors
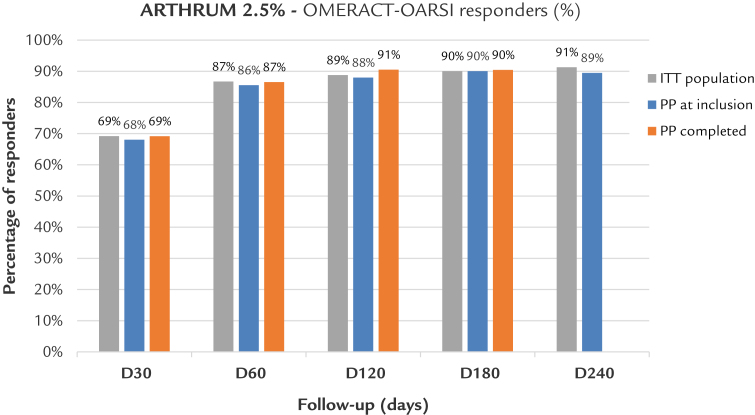
Results demonstrate a high rate of patients responding to the treatment (over 85% by day 60), with a continuous improvement throughout the follow-up period, as shown by the graph in Figure 4. The compared graphs confirm the similarity between the ITT and the 2 PP populations.
Figure 4.
Osteoarthritis Research Society International Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT-OARSI) Response Criteria Initiative responders. Arthrum; LCA Pharmaceutical, Chartres, France. D = day; ITT = intention to treat; PP = per protocol.
Note: Because, the population is reduced at 240 days, results are only indicative.
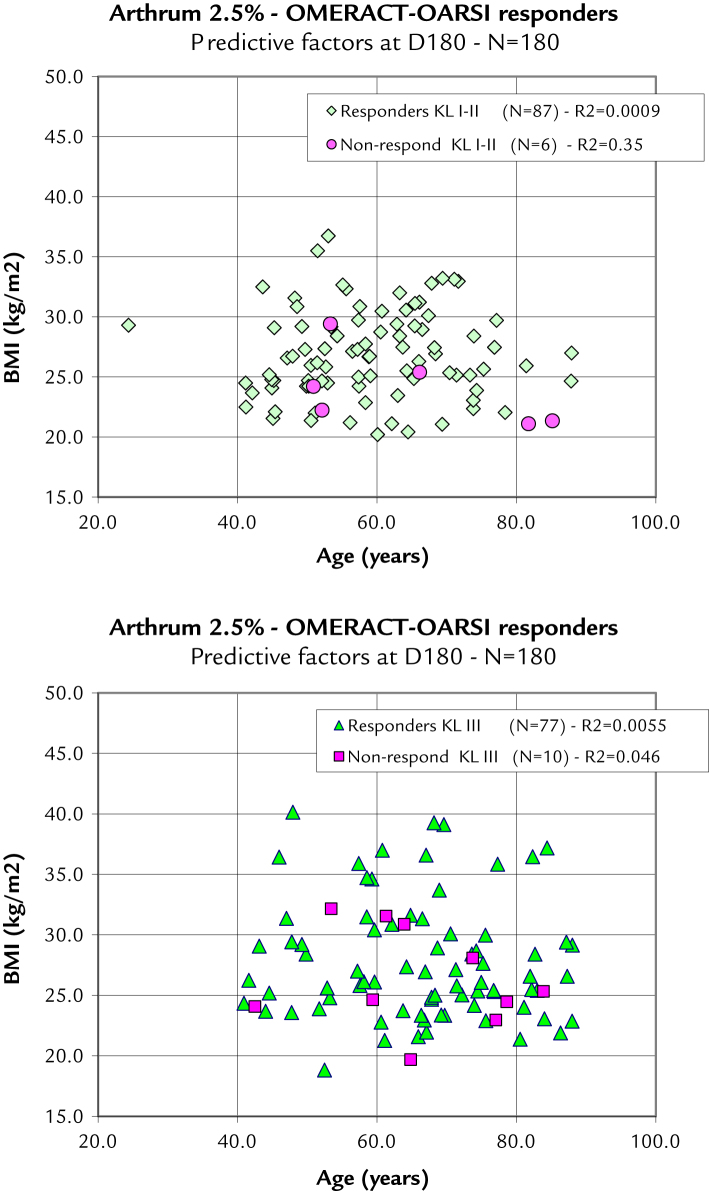
To assess the predictive factors on the ITT population, a direct comparison with age did not show any differences, and neither did a comparison with body mass index, meaning than the probability of achieving a successful response from the treatment always remains high, regardless of the combination of age, body mass index, and KL grade. This result is illustrated as 2 scatter plots, splitting the KL grades (Figure 5). From these graphs, the nonresponders are quite uniformly distributed among the responders, and no evidence of the influence of predictive factors can be detected.
Figure 5.
Outcome Measures in Rheumatoid Arthritis Clinical Trials-Osteoarthritis Research Society International (OMERACT-OARSI) Response Criteria Initiative responders stratified with predictive factors. In these 2 scatter plots, each point represents a patient as a function of his age and body mass index (BMI). The distribution of the OMERACT-OARSI responders and nonresponders is random and does not indicate any of these predictive factors to be a determinant for the success or failure of the viscosupplementation with Arthrum 2.5% (LCA Pharmaceutical, Chartres, France). D = day; KL = Kellgren-Lawrence radiologic osteoarthritis scale.
Post hoc placebo comparison
Pooled placebo selection
The patient profile of each placebo arm of the randomized controlled trials are slightly different, although each describes a population of patients with knee OA, around age 60 years, with KL grade I through III, and an average body mass index between 25 and 30 (Table 6). Other factors such as sex or OA anteriority, were also described. To have the best fit with the Arthrum 2.5% patient KL profile (Table 2), it was necessary to exclude the Altman et al14 study, because 26% of patients included were KL grade IV. The pooled remaining studies (those of Chevalier et al,8 Strand et al,7 and Hangody et al10 = 326 patients total) had a strong comparative KL profile (χ2 = 0.86; P = 0.65) to Arthrum 2.5%. For the body mass index (the second critical factor), it was not possible to find a balance between groups, so we accepted the difference of 2.0. The limitation of the Strand study was the 3-month duration, and it contained no WOMAC C results: in these cases, the placebo group was reduced (n = 198 patients), but the KL profile remained nearly unchanged (χ2= 1.12; P = 0.57), and the age difference became insignificant (P = 0.070).
Table 6.
Placebo comparator: Patient profile comparison.
| Characteristics of patients at inclusion | Chevalier et al8 | Strand et al7* | Hangody et al10 | Pooled placebo† | Arthrum 2.5%‡ | Matching the groups§ |
|
|---|---|---|---|---|---|---|---|
| Difference | Statistics | ||||||
| Population, n | 129 | 128 | 69 | 326 | 218 | ||
| Age, y | |||||||
| Mean (SD) | 62.5 (9.2) | 60.3 (10.0) | 58.0 (9.0) | 60.7 (9.5) | 62.9 (12.6) | –2.2 (10.8) | P = 0.019 |
| Sex, n (%) | |||||||
| Men | 41 (32) | 51 (40) | 18 (26) | 110 (34) | 95 (44) | –10% | |
| Women | 88 (68) | 77 (60) | 51 (74) | 216 (66) | 123 (56) | 10% | |
| KL radiologic grade of knee osteoarthritis, n (%) | |||||||
| Grade I-II | 51 (39) | 65 (51) | 55 (80) | 171 (52) | 118 (54) | –2% | χ2 = 0.86 |
| Grade III | 78 (60) | 63 (49) | 14 (20) | 155 (48) | 99 (46) | 2% | |
| Grade IV | 1 (1) | 0 | 0 | 1 (0) | 0 | 0% | P = 0.65 |
| Body mass index | |||||||
| Mean (SD) | 29.8 (5.7) | 28.7 (3.8) | 29.1 (4.5) | 29.2 (4.8) | 27.2 (4.3) | 2.0 (4.6) | p < 0.001|| |
| Anteriority of knee osteoarthritis, y | |||||||
| Mean (SD) | 5.8 (5.4) | – | – | 5.8 (5.4) | 4.1 (5.4) | 1.7 | |
KL= Kellgren-Lawrence.
Strand was only effective for the Western Ontario and McMaster Universities Osteoarthritis Index pain subscore A, from day 30 to day 90. With Chevalier and Hangody alone (N = 198), there was no incidence on the Kellgren-Lawrence (radiologic osteoarthritis scale profile matching (χ2 = 1.12; P = 0.57) and the age criteria passed (P = 0.070).
LCA Pharmaceutical (Product group studied), Chartres, France.
Matching the pooled placebo group with the Arthrum 2.5% group.
The body mass index difference (2.0 more for placebo) was accepted as both groups were in the same body mass index category (overweight).
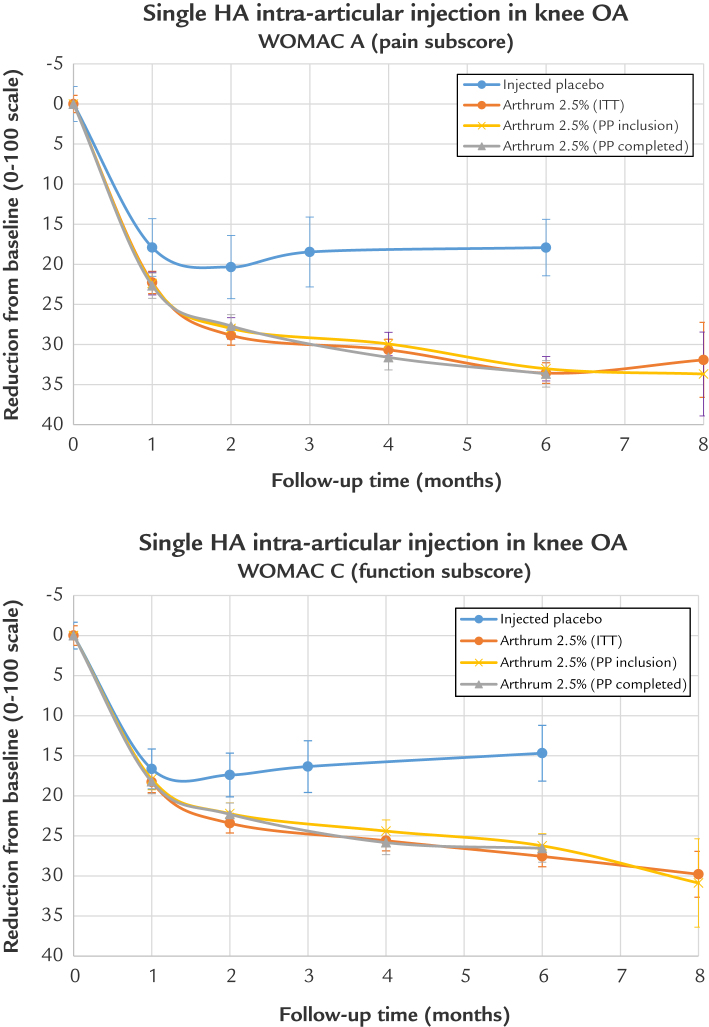
Results of Arthrum 2.5% versus IA placebo
The variations of the IA placebo from baseline are presented graphically (Figure 6) and compared with Arthrum 2.5% for the WOMAC A and for the WOMAC C, with the follow-up time (x-axis) extended to 8 months. The trend of the treatment results is illustrated. These graphs confirm that the clinical effect of the injected placebo is important, with a duration persisting up to 6 months (and possibly more); it is notable that the IA placebo has an important and long-lasting effect, > 20 on the 0 to 100 scale. The Arthrum 2.5% curves are well superimposed for the ITT and PP populations.
Figure 6.
WOMAC A and WOMAC C : Arthrum 2.5% vs placebo. Indicative result at month 8. HA = hyaluronic acid; ITT = intention to treat; OA = osteoarthritis; PP = per protocol; WOMAC= Western Ontario and McMaster Universities Osteoarthritis.
Results of the comparison (Table 7) demonstrate a significant advantage of Arthrum 2.5% treatment for the WOMAC A (pain subscale score) from day 30 (P = 0.013) onward (P < 0.001). At day 60, day 90, and day 180, respectively, the mean ES was 0.33 (95% CI, 0.15–0.51], 0.52 (95% CI, 0.34–0.70), and 0.65 (95% CI, 0.45–0.85), for the WOMAC A. Similarly, at 90 days and 180 days, respectively, ES = 0.34 (95% CI, 0.13–0.55 and ES = 0.48 (95% CI, 0.27–0.69) for the WOMAC C (function subscale score). These results are clinically relevant.
Table 7.
Placebo comparison results.
| Time | Arthrum 2.5% |
IA Placebo |
Difference (SD) | Effect size (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|
| Variation/0 d (SD) | N | Variation/0 d (SD) | n | |||||
| WOMAC A | 30 | 22.3 (20.0) | 207 | 17.9 (19.6) | 326 | 4.39 (19.8) | 0.22 (0.05 to 0.39) | 0.013 |
| Pain (base 100) | 60 | 28.9 (17.4) | 199 | 22.2 (21.7) | 326 | 6.70 (20.2) | 0.33 (0.15 to 0.51) | < 0.001 |
| 90* | 30.7 (17.8) | 180 | 19.2 (24.3) | 326 | 11.49 (22.3) | 0.52 (0.34 to 0.70) | < 0.001 | |
| 180 | 33.6 (17.4) | 183 | 21.0 (21.2) | 198 | 12.61 (19.5) | 0.65 (0.45 to 0.85) | < 0.001 | |
| WOMAC C | 60 | 23.4 (17.3) | 181 | 21.3 (18.4) | 198 | 2.09 (17.9) | 0.12 (–0.08 to 0.32) | 0.26 |
| Function (base 100) | 90* | 25.6 (17.0) | 160 | 19.4 (19.0) | 198 | 6.17 (18.1) | 0.34 (0.13 to 0.55) | 0.001 |
| 180 | 27.6 (17.5) | 165 | 18.0 (21.6) | 198 | 9.57 (19.8) | 0.48 (0.27 to 0.69) | < 0.001 | |
WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Results for Arthrum 2.5% (LCA Pharmaceutical, Chartres, France) obtained at 120 days have been used in the comparison at 90 days with placebo, as justified from the stability of the measures (see Figure 6).
For the IA placebo control group, the percentage of patients classified as OMERACT-OARSI responders was estimated at 54.6% at 90 days by Strand et al7 and at 55.9% at 180 days by Chevalier et al.8 This confirms the efficacy of Arthrum 2.5%, which achieved percentages more than 86% at same follow-up times (Figure 4).
Discussion
During 2014-2015, our study was launched and finalized without the need of authorization from an ethical committee. Nowadays, this study would be considered interventional, because regulation changed in November 2016 in France, to meet same rules as in the United States. During the study itself, no claim or problem of any kind was reported in relation with this design. Regarding the weakness of this design, we believe that at inclusion time, all care was taken to eliminate the risk of an improper assignment of the Arthrum 2.5% treatment for any pretext. Our target was to explore at best the available data, obtained in close to real world conditions. Several points are discussed below.
The main objective of the study was to determine whether a single IA injection of 3 mL HA can be clinically effective for the treatment of knee OA. Our results suggest that this objective has been confirmed from findings of important clinical improvement in both pain and in function, which was maintained during the follow-up period of the study of 6 months and more.
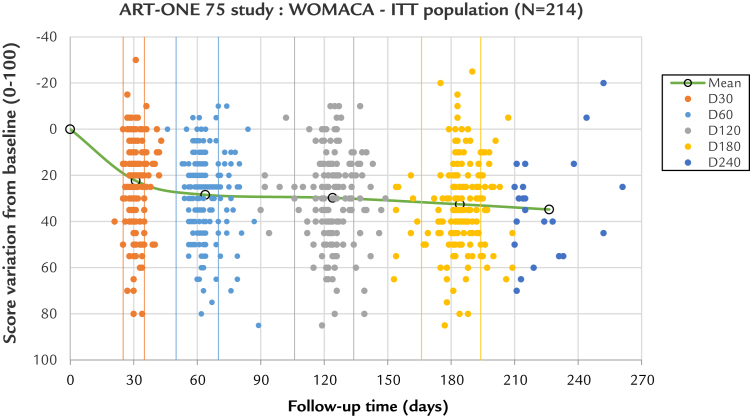
To assess precisely whether any incidence on results was induced by the use of large time windows, a scatter plot (Figure 7) was proposed for the WOMAC A at each observation time. The conclusion is that no evidence of change to the result can be detected.
Figure 7.
Incidence of large versus small time windows. The scatter plot represents each patient observation as a point, giving the Western Ontario and McMaster Universities Osteoarthritis Index pain subscale A score variation to day 0 (y-axis) as a function of the exact time for each visit (x-axis). Each visit is color coded from day 0 to day 240. The small windows limits are represented by vertical lines, in same colors. For each group from day 30 to day 180, most of the points are within tolerances. For the points that are outside, most are above the upper limit, illustrating the tendency for an increased delay for the control visits by practitioners. There is a wide distribution of scores from very high (around 80) to negative (around –20), meaning that some patients have their pain totally relieved and a few others are worse. The points are distributed in horizontal lines every 5 score units, just because the Likert scale has been used (21 scores on the whole scale 0–100): This is not an artifact. One advantage of the reclassification within large windows is that no overlapping of the visits was possible. The center of gravity of each group (average score / average time) is represented by a circle, and the line linking them compares with the previous graph (see Figure 6). This line, which is flat above day 60, confirms the score stability with time. A small time shift can be seen between the average and the target time, which is not significant. The removal of all scores obtained outside the time limits cannot alter or improve the clinical results, confirming that the acceptance of these scores does not create any bias. The extra day 240 group has an average close to day 226, meaning 7.5 months rather than 8 months. D = day; ITT = intention to treat.
Our post hoc comparison with injected placebo is probably an unusual method. However, we took maximum care in the definition and selection of our placebo comparison arm. We used the WOMAC, which has been the most universally used index in the field of knee OA for pain and function for many years. The validation of this index has been carefully made, and we consider it to be the most reliable tool, allowing the comparison and association of results from different studies, in a good scientific context.
There are limits to the comparability of the groups with our method generated by different doctors, investigating different patients, in different countries. However, the majority of the patients selected here for the placebo arm were recruited in France and in Hungary. We have determined that the comparability was statistically acceptable (placebo vs Arthrum 2.5%), on the basis of patient age (60.7 vs 62.9 years) and radiologic KL grade I or II (52% vs 54%). We accepted the difference for body mass index (29.2 vs 27.2) considering that both groups belonged to the same body mass index category of overweight patients. Other factors such as sex (66% vs 56% women) and the anteriority (5.8 vs 4.1 years), are not necessarily recognized as determinants for knee OA disease. There were large differences in WOMAC scores at inclusion, but the improvement assessment is based on variations not on absolute scores.
The ES of our pooled placebo versus baseline has been found to be high for pain: ES = 1.02 (95% CI, 0.68–1.36) at day 60, and ES = 0.99 (95% CI, 0.70–1.28) at day 180. These placebo results are higher than those found by Altman et al14 in a meta-analysis dedicated to the IA placebo used in viscosupplementation studies: for pain, comparing placebo versus no treatment SMD = 0.75 (95% CI 0.65–0.85) at ≤90 days (25 study arms with IA placebo), and SMD = 0.70 (95% CI 0.54–0.86) at 180 to 360 days (15 study arms with IA placebo). Altman et al14 concluded that, “Pain relief observed with IA saline should prompt health care providers to consider the additional effectiveness of current IA treatments that use saline comparators in clinical studies, and challenges of identifying IA saline as a ‘placebo.’ ” The importance of the effect of the IA placebo is also demonstrated in another network meta-analysis. Bannuru et al15 quantified the importance of this clinical effect of the IA placebo ES = 0.29 over oral placebo, ranking the IA placebo higher than paracetamol (ie, acetaminophen) ES = 0.18, in a classification of knee OA treatment, and ranked IA HA at first position, ES = 0.63, for efficacy against pain in knee OA. Because the results for our IA placebo arm are not underestimated, they cannot lead to an overestimation of Arthrum 2.5% results, thereby eliminating the risk of a potential bias in favor of Arthrum 2.5%.
All HAs cannot be clinically equivalent because many effects of IA HA have been described16 as being dose-dependent or molecular weight-dependent, and the market offers a variety of HA products of different origins, concentrations and molecular weights. There are limits to the efficacy of these; for example, there is an ultra-high molecular weight HA obtained by cross-linking, which claims a longer residence time, but without real clinical advantage, because this product may have lost the capability to bind to cell receptors such as CD44.17 Clinical results of the IA HA single injection Arthrum 2.5%, are high compared with competitors, and this is explained by the plentiful supply of HA (75 mg), combined with unmodified native HA obtained from biofermentation and featuring high molecular weight (>2 MDa).
Other products of the Arthrum range, using the same HA at other concentrations and volumes, have shown their clinical efficacy in treating OA. A single IA injection of 3 mL HA (Coxarthrum, LCA Pharmaceutical, Chartres, France) to treat hip OA has been shown to be effective during a 12-month period.18., 19. A regimen of 3 injections, each of 2 mL (40 mg), for knee OA (Arthrum H 2%, LCA Pharmaceutical, Chartres, France) has been shown to be as effective as Hyalgan (Hyalgan, Fidia Farmaceutici, Abano Terme, Italy) (a reference IA HA that is distributed worldwide, including the United States), in a double-blind randomized controlled trial.20 The OMERACT-OARSI percentages of responders were high with Arthrum H 2%, with 78.7% at 90 days and 85.0% at 180 days. A post hoc analysis of the predictive factors21 did not reveal any influence of factors on the results, which was similar to Arthrum 2.5%. In a pharmacoeconomic study22 comparing IA HA to conventional treatment with NSAIDs only, WOMAC A variations from 0 to 180 days were 49.9 – 27.6 = 22.3 with Arthrum H 2%. Finally, the results obtained with Arthrum H 2% (3 injections), and with Arthrum 2.5% (single injection) are quite similar in terms of pain index variation from baseline or from OMERACT-OARSI responders. This supports the concept that a single injection of IA HA may be as efficient as a treatment with 3 injections.
Conclusions
The present study suggests the clinical efficacy of a single IA injection of 3 mL viscoelastic solution containing 75 mg of high molecular weight (2.4 MDa) native HA.
Conflicts of Interest
Drs BARON, FLIN, PORTERIE and DESPAUX have declared they have no conflict of interest for this study or with LCA Pharmaceutical.
P VINCENT, as employee and shareholder of LCA Pharmaceutical, has declared his interest.
Acknowledgments
The study was sponsored and entirely founded by LCA Pharmaceutical, Chartres, France.
There was neither contribution from the government, nor by any other organization.
Dr Baron, as member of the scientific committee of the study ART-ONE 75, has participate to the design of this study (protocol, amendments, control of reports...). He was one of the investigators too.
He has participate to each step of the redaction of the article from the beginning on the base of the statistical report made by the clinical research organization (CRO), to the final correction of the latest version.
Drs Flin, Porterie and Despaux, were investigators during the study ART-ONE 75. Their participation to the manuscript was limited to their review and advice, before each submission to the editor.
P Vincent, as sponsor representative, has followed the statistical analysis of the study made by the CRO. He made the first conceptualization of the article, with Dr Baron, and later most of the synthesis of the data presented in the manuscript.
As corresponding author, P Vincent has also participate to the answers to the reviewers and to the subsequent corrections, up to the final version.
References
- 1.Société Française de Rhumatologie : Livre blanc 2006, chapitre 3 : Epidémiologie des maladies rhumatismales www.rhumatologie.asso.fr/05-Bibliotheque/Livre-Blanc/C6-e
- 2.Bruyere O., Cooper C., Pelletier J.P., Branco J., Luisa Brandi M., Guillemin F. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2014;44(3):253–263. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Miller L.E., Block J.E. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: Systematic review and meta-analysis of randomized, saline-controlled trials. Clinical medicine insights Arthritis and musculoskeletal disorders. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pham T., Van der Heidje D., Altman R.D., Anderson J.J., Bellamy N., Hochberg M. OMERACT-OARSI Initiative: Osteoarthritis Research Society International set of responder criteria for clinical trials revisited. Osteoarthritis and Cartilage. 2004;12:389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Ehrich E.W., Davies G.M., Watson D.J., Bolognese J.A., Seidenberg B.C., Bellamy N. Minimal Perceptible Clinical Improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessment in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641. Nov. [PubMed] [Google Scholar]
- 6.Mazieres B., Bard H., Ligier M., Bru I., d'Orsay G.G., Le Pen C. Medicoeconomic evaluation of hyaluronic acid for knee osteoarthritis in everyday practice: the MESSAGE study. Joint Bone Spine. 2007;74:453–460. doi: 10.1016/j.jbspin.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Strand V., Baraf H.S.B., Lavin P.T., Lim S., Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis and Cartilage. 2012;20(5):350–356. doi: 10.1016/j.joca.2012.01.013. May. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier X., Jerosch J., Goupille P., Van Dijk N., Luyten F.P., Scott D.L., Bailleul F., Pavelka K. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomized, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis. 2010;69:113–119. doi: 10.1136/ard.2008.094623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altman R.D., Akermark C., Beaulieu A.D., Schnitzer T. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis and Cartilage. 2004;12:642–649. doi: 10.1016/j.joca.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Hangody L., Szody R., Lukasik P., Zgadzaj W., Lenart E., Dokoupilova E., Bichovsk D., Berta A., Vasarhelyi G., Ficzere A., Hangody G., Stevens G., Szendroi M. Intra-articular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage. 2017 doi: 10.1177/1947603517703732. sagepubcom/ journalsPermission.nav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutjes A.W.S., Jüni P., da Costa B.R., Trelle S., Nüesch E., Reichenbach S. Viscosupplementation for Osteoarthritis of the Knee. A systematic review and meta-analysis. Annals of Internal Medicine. 2012;157(3):180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. [DOI] [PubMed] [Google Scholar]
- 12.Colen S., Bekerom Van den, Mulier M., Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis. A systematic review and meta-analysis wirh emphasis on the efficacy of different products. Biodrugs. 2012;26(4):257–268. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Bannuru R.R., Natov N.S., Obadan I.E., Dasi U.R., Schmid C.H., McAlindon T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis – meta-analysis. Osteoarthritis and Cartilage. 2011;19:611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altman R.D., Devji T., Bhandari M., Fierlinger A., Niazi F., Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Seminars in Arthritis and Rheumatism. 2016;46:151–159. doi: 10.1016/j.semarthrit.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Bannuru R.R., Schmid C.H., Kent D.M., Vaysbrot E.E., Wong J.B., McAlindon T.E. Comparative Effectiveness of Pharmacologic Interventions for Knee Osteoarthritis - A Systematic Review and Network Meta-analysis. Annals of Internal Medicine. 2015;162(1):46–55. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 16.Moreland L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis res Ther. 2003;5:54–67. doi: 10.1186/ar623. http://arthritis-research.com/content/5/2/54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitanzo P., Sennett B. Hyaluronans: Is Clinical Effectiveness Dependent on Molecular Weight? Am J Orthop (Belle Mead NJ) 2006;35:421–428. [PubMed] [Google Scholar]
- 18.Rivera F. Single intra-articular injection of high molecular weight hyaluronic acid for hip osteoarthritis (Coxarthrum) J.Orthopaed Traumatol. 2015 doi: 10.1007/s10195-015-0381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rivera F. Viscosupplementation for pain management on hip osteoarthritis (Coxarthrum) – Poster at 15th EFORT Congress. London, UK. June 2014:4–6. [Google Scholar]
- 20.Germonville T., Prudat M., Vincent P. Pain Care in Knee Osteoarthritis by Intra-Articular injections of Hyaluronic Acid (ARTHRUM® H 2.0 %). A Randomized double-blind Controlled Trial versus another Hyaluronic Acid (HYALGAN®) Minerva Ortopedica e Traumatologica. 2015;66(6):235–253. [Google Scholar]
- 21.Hilliquin P. Facteurs prédictifs de succès ou d’échec de la viscosupplémentation. Réflexions Rhumatologiques. 2017;188(21):43–45. [Google Scholar]
- 22.Thomas T., Amouroux F. Vincent P/ Intra articular hyaluronic acid in the management of knee osteoarthritis: Pharmaco-economic study from the perspective of the national health insurance system. PLoS ONE. 2017;12(3):e0173683. doi: 10.1371/journal.pone.0173683. [DOI] [PMC free article] [PubMed] [Google Scholar]