Abstract
Background:
Perioperative enteral nutrition (EN) enriched with immune-modulating substrates is preferable for patients undergoing major abdominal cancer surgery. In this study, perioperative EN enriched with immune-modulating nutrients such as arginine, glutamine, and omega-3 fatty acids was evaluated for its anti-inflammatory efficacy in patients with gastric adenocarcinoma or gastrointestinal stromal tumor (GIST) receiving curative surgery.
Materials and Methods:
This prospective, randomized, double-blind study recruited 34 patients with gastric adenocarcinoma or gastric GIST undergoing elective curative surgery. These patients were randomly assigned to the study group, receiving immune-modulating nutrient-enriched EN, or the control group, receiving standard EN from 3 days before surgery (preoperative day 3) to up to postoperative day 14 or discharge. Laboratory and inflammatory parameters were assessed on preoperative day 3 and postoperative day 14 or at discharge. Adverse events (AEs) and clinical outcomes were documented daily and compared between groups.
Results:
No significant differences were observed between the two groups in selected laboratory and inflammatory parameters, or in their net change, before and after treatment. AEs and clinical outcomes, including infectious complications, overall complications, time to first bowel action, and length of hospital stay after surgery, were comparable between treatment groups (all P > 0.05).
Conclusion:
Immune-modulating nutrient-enriched EN had no prominent immunomodulation effect compared with that of standard EN.
KEY WORDS: Arginine, gastric cancer, glutamine, immune-modulating nutrients, omega-3 fatty acids
Introduction
With the increasing incidence in the recent years, cancer is the leading cause of death in Taiwan. Gastric cancer accounted for approximately 3.80% of all malignancies and 5.0% of all malignancy-related mortality in 2013, resulting in the sixth most common cancer in males and the fifth in females. Histologically, the most common type is adenocarcinoma (89.7%), followed by gastrointestinal stromal tumor (GIST) (4.6%) (http://www.mohw.gov.tw).
The main treatment recommended for both adenocarcinoma and GIST is surgical resection. Early enteral nutrition (EEN) after gastrointestinal (GI) surgery improves not only the quality of life (QOL)[1] but also nutritional status and clinical outcomes. EEN results in higher postoperative serum albumin and prealbumin levels,[2,3] and furthermore, EEN shortens the time to first bowel action[4] and the length of hospital stay (LOS).[5] Regarding postoperative complications, EEN reduces the rates of both noninfectious and infectious complications.[6]
Nutrition plays a major role in many aspects of cancer development and treatment. A total of 20%–40% of cancer-related deaths may result from malnutrition instead of disease itself.[7,8] Proper nutrition intervention restores the nutrition stores of cancer patients and prevents from their body weight loss, releasing from nausea or constipation, maintaining bowel function, and improving QOL. In contrast, no or inadequate nutrition supplement leads to malnutrition, increases both the incidence and severity of treatment side effects and the risk of infection, and in the end, reducing the rates of survival.[9] The incidence of malnutrition in patients with cancer ranges from 40% to 80%, especially in patients with GI tract cancer, in which bowel function is usually impaired and oral food ingestion is usually not sufficient owing to cancer-related symptoms. Therefore, nutritional supplements are indicated for patients who do not meet their energy needs through oral food ingestion. Evidence has indicated that enteral nutrition (EN) is superior to parenteral nutrition (PN) and that it is also easier and associated with fewer catheter-related complications.[10]
Immune-modulating substances such as arginine, glutamine, omega-3 fatty acids, nucleic acids, and antioxidants can modulate immune and inflammatory processes in burn or trauma and major surgery and improve clinical outcomes. Arginine is a non-essential amino acid that plays a role in the synthesis of nitric oxide, which regulates gene expression and stimulates cell-mediated immunity. However, when arginine depletes, cells generate superoxide, instead of nitric oxide and superoxide leads to cell injury as a result.
Glutamine is mainly synthesized from the catabolism of branch-chained amino acids in skeletal muscle and is the most plentiful non-essential amino acid in the human body, which is a preferable energy source for rapidly proliferating lymphocytes and enterocytes.[11] As a result, glutamine is a conditionally essential amino acid while excessive tissue demand of glutamine overcomes glutamine production to meet the increasing requirement during major surgery, trauma, or other catabolic stress situations,[12] and in turn contributes to infection, bowel function impairment, weight loss, and muscle wasting.[13] Omega-3 polyunsaturated fatty acids (PUFAs) compete with omega-6 PUFAs, to produce less thrombotic and inflammatory prostaglandins, leukotrienes, and thromboxanes.[14] Therefore, n-3 PUFAs regulate pro- and anti-inflammatory cytokine gene expression and modulate inappropriate proinflammatory activities.
The aim of this study is to determine whether an immune-modulating nutrient-enriched EN diet can improve nutritional status and reduce postoperative infection and surgery-induced immune suppression in patients with gastric cancer or GIST undergoing major surgery.
Materials and Methods
The protocol was approved by the local ethics committees (KMUHIRB-2011-05-01(II)) and was performed in accordance with Declaration of Helsinki of 1975 as revised in 1996. Written informed consent was obtained from patients before the study was conducted. The study was registered at clinicaltrials.gov under the identification code ID: NCT03123432 (www.clinicaltrials.gov/ct2/show/NCT03123432) and trial name: Immune-modulating Nutrients in Perioperative Patients with Gastric Cancer.
In this prospective, randomized, double-blind study, patients with gastric adenocarcinoma or gastric GIST age ≥20 years who underwent elective radical subtotal or total gastrectomy from June 2011 to June 2016 were screened for eligibility. The following patients were excluded: those with liver disease (total bilirubin >2.5 mg/dL), renal disease (creatinine >1.5 mg/dL or hemodialysis requirement), heart failure (the New York Heart Association functional class > III, or stroke history), severe hypoalbuminemia (albumin <2.5 g/dL), or clinical illness (Karnofsky performance status less than 60); those who were overweight [body mass index (BMI) >30 kg/m2], exhibited drug abuse or chronic alcoholism, had life-threatening disease, or underwent emergent surgery; those with infection or bowel obstruction; those who were pregnant or lactating; those who had received chemotherapy within 14 days of the initiation of the trial; those who had received immunosuppressive therapy or had immunological diseases recently, had already participated in another clinical study with an investigational drug or an investigational medical device within a month of the initiation or during the study; and those hypersensitive to casein, fish oil, soybean, or corn oil. Before study initiation, a randomization sequence was computer-generated (GraphPad statistical software; GraphPad, USA) with two blocks of 15 patients. Patients from each block were 1:1 allocated by an independent investigator. Based on randomization sequence, another investigator assigned patients to either the study group or the control group, and these patients received study medication that was identical in appearance. All investigators and staff members, except for the aforementioned independent investigator, were blinded to patient assignment until the end of the study. Based on randomization sequence, emergency envelopes were provided to the study centers to break blinding if reasonable suspicion of harm to the participants due to the investigational EN was expressed. The emergency envelopes were provided in two sets: one was installed at the ward and the other at the pharmacy of the hospital.
Patients received oral feeding with an ordinary diet plus 400 mL/day (400 kcal/day) of the interventional diet for 3–5 days before curative surgery for gastric adenocarcinoma or gastric GIST. The composition of feeding diet is summarized in Table 1. Jejunostomy for tube feeding was routinely performed in patients undergoing radical resection for gastric adenocarcinoma. On postoperative day 3, EN was initiated with 5% glucose in water at a rate of 20 mL/h either through the jejunostomy tube in patients with gastric adenocarcinoma or through a nasogastric tube in patients with gastric GIST. On postoperative day 4, patients received a semi-liquid diet plus 400 mL/day (400 kcal/day) of the interventional diet. From postoperative days 5–14 or to discharge whichever occurred first, 1200 mL/day (1200 kcal/day) of the interventional diet was administered, and an oral soft diet was also administered if no postoperative complications developed and if oral feeding was not prohibited.
Table 1.
Composition of feeding diet
| Immunomodulating nutrient-enriched diet | Standard diet | |
|---|---|---|
| Calories/1200 mL | 1200 kcal/1200 mL | 1200 kcal/1200 mL |
| Osmolarity, mOsm/L | 400 | 375 |
| Protein, %kcal | 25 | 22 |
| Glutamine, %protein/g | 10/7.5 | - |
| Arginine, %protein/g | 20/15 | - |
| Fat, %kcal | 25 | 25 |
| Fish oil, %fat/g | 20/6.96 | - |
| Carbohydrate, %kcal | 50 | 53 |
The primary objective of this study was to evaluate the effect of immune-modulating nutrient-enriched diets on postoperative inflammatory response. Inflammatory markers including interleukin (IL)-6, C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) were used to assess inflammatory processes. Blood tests for glucose, albumin, prealbumin, blood urine nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, triglycerides (TGs), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), sodium (Na), and leukocyte count were also conducted. We measured these levels and collected demographic data before intervention and on day 14 or at discharge.
Vital signs, clinical course, concomitant medication, concomitant procedure, complications, adverse events (AEs), and severe AEs (SAEs) were recorded daily from the day of intervention to postoperative day 14 or to discharge, whichever occurred first. The last visit of patients was at discharge from the hospital or 14 days after surgery, whichever occurred first. AEs/SAEs and clinical outcomes, including postoperative complications, time to first bowel action, and LOS after surgery, were assessed until the discharge of patients from hospital.
To derive the sample size (1:1 allocation), the primary endpoint, statistical significance level, and power were specified. According to the preliminary data of our patients undergoing curative surgery for gastric cancer, the CRP level on day 14 was expected to be 81.47 ± 31.62 mg/dL, and the immune-modulating nutrient-enriched diet was expected to decrease proinflammatory cytokine by 40%. We had previously conducted a two-sided testing of independent means. A standard statistical significance level of 5% and power of 80% were chosen for rejecting the null hypothesis if the alternative hypothesis was correct. Sample size calculation was performed with software program nQuery version 5.0. Therefore, sample size resulted in 15 patients at least. Taking a drop-out rate of 20%, the sample size was at least 18 patients per study arm, that is, a total of 36 patients were enrolled into the study.
The results were compared using Mann–Whitney U test or Kruskal–Wallis test for categorical unpaired data. Wilcoxon rank-sum test or Friedman test was used to analyze paired data. Fisher's exact test was used to compare dichotomous variables. Pearson's Chi-square test was used to analyze nominal variables. McNemar's test was used to analyze paired categorical data. The means were compared using a two-sample test and using analysis of variance (ANOVA) or linear regression, as appropriate. However, for all aforementioned inferential analysis methods, the center effect was not considered when comparing one treatment with another. Therefore, ANOVA incorporating the center effect and Cochran–Mantel–Haenszel test stratified by the center effect were applied to replace the two-sample t-test and Fisher's exact test. For efficacy analyses and part of safety analyses (including laboratory data and vital sign data), considering the effect of baseline data on the endpoints, analysis of covariance was applied when comparing one treatment mean with another, with their respective baseline as covariates. Baseline data were defined as the data obtained before the first administration of treatment before surgery. Endpoints were defined as the net change in post-treatment data from baseline data. Statistical analyses were conducted using SPSS 18.0 (SPSS, Chicago, IL, USA). P values less than 0.05 were statistically significant.
Results
After screening visits, a total of 46 patients who met the inclusion criteria agreed to participate in the study. Three patients were screening failures; thus, 43 patients were randomized to the immune-modulating nutrient-enriched diet group (n = 22) and the standard diet group (n = 21), respectively. Figure 1 shows the randomization of patients to the two treatment groups. A total of five patients withdrew before surgery, and only received study medication for a day. Four patients were also withdrawn from this trial and did not receive all scheduled EN [Table 2]. The remaining 34 patients who received treatment diets and fulfilled all eligibility criteria completed this trial and were therefore included in the per-protocol population.
Figure 1.
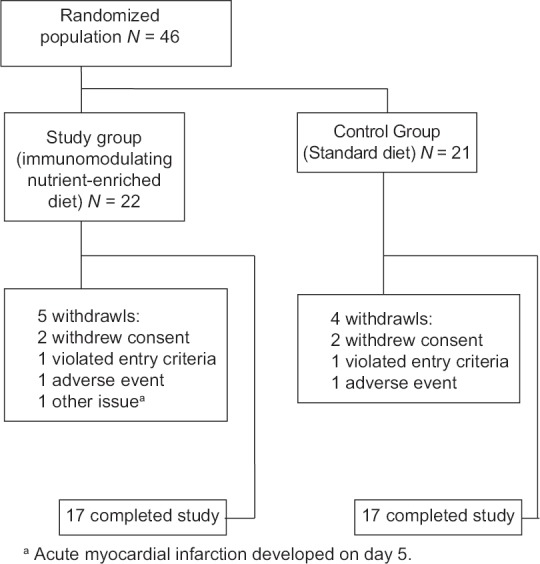
Diagram of randomization of patients with gastric cancer to receive an immune-modulating nutrient-enriched diet or a control standard diet
Table 2.
List of patients who were excluded from per-protocol population
| Treatment | Reason for premature termination | Treatment duration (days) |
|---|---|---|
| Study group (immunomodulating nutrient-enriched diet) | Patient withdrew consent | 1 |
| Patient withdrew from the study due to acute myocardial infarction occurring on day 5 | 8 | |
| Patient withdrew from the study due to nausea and floating | 2 | |
| Patient withdrew from the study because of unresectable tumor encountered during operation | 3 | |
| Patient withdrew consent | 1 | |
| Control group (standard diet) | Patient refused operation | 1 |
| Patient withdrew consent | 1 day | |
| Patient withdrew from the study due to nausea and floating | 2 days | |
| Patient withdrew consent | 1 day |
The demographic characteristics of all patients are summarized in Table 3. The demographic data were comparable between groups; no difference was observed in concomitant medications or concomitant procedures between the test groups. The parameters were similar between treatment groups during the study period [Table 4]. In both study and control groups, a statistically significant reduction in BMI, albumin, prealbumin, cholesterol, and HDL and a statistically significant increase in leukocyte count, IL-6, and CRP were detected after treatment (all P < 0.05). Among the aforementioned parameters, albumin, prealbumin, and HDL were lower and IL-6 and CRP were higher than normal range after treatment. Moreover, a significant increase in TG and a reduction in LDL in the control group and a significant reduction in Na in the study group were found. Finally, considering EN (i.e. study and control groups together), BMI, albumin, prealbumin, cholesterol, LDL, HDL, and Na significantly decreased and leukocyte count, IL-6, and CRP significantly increased after curative surgery for gastric cancer. BMI, albumin, prealbumin, and LDL were lower than the normal value, whereas IL-6 and CRP were higher.
Table 3.
Demographic data in our studied patients
| Study group (N=17) | Control group (N=17) | P (95% CI) | |
|---|---|---|---|
| Sex | |||
| Male/female | 9/8 | 13/4 | 0.151 |
| Age (years) | 60.24±12.26 | 62.0±9.8 | 0.646 (−9.52; 5.99) |
| BMI (kg/m2) | 24.21±2.91 | 23.03±3.49 | 0.292 (−1.06; 3.43) |
| Tumor staging | 0.875 | ||
| Study group (N=17) | Control group (N=17) | Total | |
| UICC Stage I | 7 (41.2%) | 7 (41.2%) | 14 (41.2%) |
| Stage II | 2 (11.8%) | 3 (17.6%) | 5 (14.7%) |
| Stage III | 8 (47.1%) | 7 (41.2%) | 15 (44.1%) |
CI: confidence interval; BMI: body mass index; UICC: Union of International Cancer Control
Table 4.
Paired t-test of laboratory data before and after intervention
| Study group (N=17) | Control group (N=17) | Study and control group (N=34) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | P (95% CI) | M1 | M2 | P (95% CI) | M1 | M2 | P (95% CI) | |
| BMI | 24.21±2.91 | 23.15±2.54 | <0.001 (0.72; 1.39) | 23.03±3.49 | 22.2±3.28 | <0.001 (0.63; 1.25) | 23.69±3.24 | 22.69±2.92 | <0.001 (0.79; 1.22) |
| Glucose (mg/dL) | 105.35±22.64 | 107.71±14.23 | 0.731 (−16.60; 11.89) | 103.18±17.69 | 111.0±22.03 | 0.256 (−21.90; 6.25) | 104.26±20.04 | 109.35±18.34 | 0.284 (−14.60; 4.42) |
| Albumin (g/dL) | 4.07±0.55 | 3.45±0.396 | <0.001 (0.36; 0.88) | 4.01±0.42 | 3.33±0.47 | <0.001 (0.43; 0.92) | 4.04±0.48 | 3.39±0.43 | <0.001 (0.48; 0.82) |
| Prealbumin (mg/dL) | 25.91±7.31 | 14.56±6.0 | <0.001 (8.79; 13.91) | 22.06±7.91 | 12.80±4.17 | <0.001 (6.35; 12.7) | 23.98±7.75 | 13.68±5.17 | <0.001 (8.44; 12.17) |
| BUN (mg/dL) | 13.54±2.82 | 12.85±3.65 | 0.429 (−1.11; 2.49) | 13.72±4.70 | 10.90±3.26 | 0.039 (0.17; 5.48) | 13.63±3.82 | 11.87±3.55 | 0.029 (0.19; 3.32) |
| Creatinine (mg/dL) | 0±0.81±0.25 | 1.05±1.50 | 0.506 (−1.00; 0.51) | 0.86±0.20 | 0.78±0.18 | 0.026 (0.01; 0.16) | 0.84±0.22 | 0.92±1.06 | 0.664 (−0.44; 0.19) |
| AST (U/L) | 26.82±12.77 | 33.94±27.19 | 0.298 (−21.16; 6.92) | 29.82±13.28 | 36.12±17.13 | 0.146 (−15.03; 2.44) | 28.32±12.92 | 35.03±22.40 | 0.090 (−14.52; 1.11) |
| ALT (U/L) | 25.82±12.0 | 31.71±29.30 | 0.419 (−20.91; 9.15) | 22.59±7.93 | 25.18±13.91 | 0.402 (−8.96; 3.79) | 24.21±10.73 | 28.44±22.83 | 0.273 (−11.97; 3.50) |
| Bilirubin (mg/dL) | 0.77±0.43 | 0.77±0.42 | 0.903 (−0.33; 0.29) | 0.73±0.30 | 0.89±0.70 | 0.334 (−0.42; 0.15) | 0.75±0.36 | 0.83±0.57 | 0.444 (−0.28; 0.12) |
| TG (mg/dL) | 110.65±73.47 | 115.41±39.39 | 0.671 (−28.10; 18.57) | 85.88±39.23 | 107.94±24.43 | 0.009 (−37.72; −6.40) | 98.26±59.34 | 111.68±32.50 | 0.054 (−27.04; 0.21) |
| Cholesterol (mg/mL) | 185.18±73.47 | 148.47±30.09 | 0.002 (15.98; 57.43) | 154.94±39.87 | 129.41±25.28 | 0.003 (10.04; 41.02) | 170.06±40.67 | 138.94±29.02 | <0.001 (18.73; 43.50) |
| LDL (mg/mL) | 110.52±38.24 | 96.90±26.47 | 0.067 (−1.06; 28.30) | 93.82±28.82 | 77.46±24.32 | 0.028 (2.02; 30.70) | 102.17±34.41 | 87.18±26.91 | 0.004 (5.28; 24.70) |
| HDL (mg/mL) | 46.08±9.49 | 28.57±6.43 | <0.001 (12.64; 22.38) | 43.01±14.94 | 30.01±17.79 | 0.032 (1.28; 24.72) | 44.54±12.42 | 29.29±13.19 | <0.001 (9.20; 21.31) |
| Na (mEq/L) | 139.47±1.74 | 137.53±2.21 | 0.011 (0.50; 3.38) | 139.24±2.05 | 138.88±2.06 | 0.619 (−1.12; 1.83) | 139.35±1.87 | 138.21±2.21 | 0.028 (0.13; 2.16) |
| Leukocyte (103/µL) | 5.48±1.52 | 8.16±3.08 | <0.001 (−3.82; −1.57) | 5.92±1.72 | 8.0±2.97 | 0.019 (−3.77; −0.38) | 5.70±1.62 | 8.09±2.98 | <0.001 (−3.35; −1.42) |
| IL-6 (pg/mL) | 2.79±2.77 | 19.22±23.34 | 0.008 (−28.01; −4.86) | 4.84±5.97 | 25.92±39.07 | 0.025 (−39.17; −2.99) | 3.81±4.71 | 22.57±31.87 | 0.001 (−28.94; −8.58) |
| CRP (mg/dL) | 2.93±6.08 | 61.28±49.33 | <0.001 (−83.23; −33.46) | 2.96±3.01 | 56.41±41.69 | <0.001 (−74.59; −32.30) | 2.95±4.72 | 58.84±45.04 | <0.001 (−71.35; −40.44) |
| TNF-α (pg/mL) | 0.31±0.88 | 0.47±1.26 | 0.255 (−0.44; 0.13) | 0.20±0.29 | 0.18±0.35 | 0.871 (−0.20; 0.24) | 0.25±0.65 | 0.32±0.90 | 0.414 (−0.24; 1.39) |
CI: confidence interval; M1: mean of before intervention, 3-5 days before surgery; M2: mean of after intervention, 14 days after surgery or at discharge; BMI: body mass index; BUN: blood urine nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TG: triglyceride; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; TNF-α: tumor necrosis factor-α
All laboratory parameters, except for cholesterol, which was significantly higher in the study group (185.18 ± 73.47 vs. 154.94 ± 39.87, P = 0.028), were comparable at baseline, and no statistically significant difference was observed in the perioperative net changes between groups [Table 5]. A total of eight patients experienced treatment-related AEs, namely three (16.7%) versus five (27.8%) patients in the study versus control group, respectively [Table 6]. No significant difference was observed (P = 0.691). These AEs included bloating in one patient in each group, which resulted in patients’ withdrawal from the study in the end. The remaining AEs were diarrhea that could be easily managed by adjustment of osmolarity of the feeding diet. No death occurred in this study.
Table 5.
Various laboratory data before and after intervention between the two studied groups
| Study group (N=17) | Control group (N=17) | Difference1,2 (95% CI) | P | |
|---|---|---|---|---|
| BMI | ||||
| M1 | 24.21±2.91 | 23.03±3.49 | 1.18 (−1.06; 3.43) | 0.292 |
| M2 – M1 | −1.06±0.65 | −0.94±0.58 | −0.12 (−0.55; 0.32) | 0.597 |
| Glucose (mg/dL) | ||||
| M1 | 105.35±22.64 | 103.18±17.69 | 2.18 (−12.02; 3.43) | 0.757 |
| M2 – M1 | 2.35±27.70 | 7.82±27.37 | −5.47 (−24.71; 13.77) | 0.567 |
| Albumin (g/dL) | ||||
| M1 | 4.07±0.55 | 4.01±0.42 | 0.06 (−0.28; 0.40) | 0.729 |
| M2 – M1 | −0.62±0.51 | −0.68±0.47 | 0.06 (−0.29; 0.40) | 0.745 |
| Prealbumin (mg/dL) | ||||
| M1 | 25.91±7.31 | 22.06±7.91 | 3.86 (−1.47; 9.18) | 0.150 |
| M2 – M1 | −11.35±4.98 | −9.26±5.66 | −2.09 (−5.82; 1.63) | 0.260 |
| BUN (mg/dL) | ||||
| M1 | 13.54±2.82 | 13.72±4.70 | −0.19 (−2.90; 2.52) | 0.888 |
| M2 – M1 | −0.69±3.50 | −2.82±5.16 | 2.14 (−0.95; 5.22) | 0.168 |
| Creatinine (mg/dL) | ||||
| M1 | 0.81±0.25 | 0.86±0.20 | −0.05 (−0.21; 0.10) | 0.499 |
| M2 – M1 | 0.24±1.47 | −0.09±0.14 | 0.33 (−0.40; 1.06) | 0.366 |
| AST (U/L) | ||||
| M1 | 26.82±12.77 | 29.82±13.28 | −3.00 b(−12.10; 6.10) | 0.507 |
| M2 – M1 | 7.12±27.31 | 6.29±16.98 | 0.82 (−15.06; 16.71) | 0.917 |
| ALT (U/L) | ||||
| M1 | 25.82±12.0 | 22.59±7.93 | 3.24 (−4.29; 10.76) | 0.387 |
| M2 – M1 | 5.88±29.23 | 2.59±12.40 | 3.29 (−12.39; 18.98) | 0.672 |
| Bilirubin (mg/dL) | ||||
| M1 | 0.77±0.43 | 0.73±0.30 | 0.04 (−0.23; 0.30) | 0.787 |
| M2 – M1 | 0.02±0.58 | 0.13±0.54 | −0.112 (−0.52; 0.29) | 0.564 |
| TG (mg/dL) | ||||
| M1 | 110.65±73.47 | 85.88±39.23 | 24.76 (−16.38; 65.91) | 0.229 |
| M2 – M1 | 4.76±45.38 | 22.06±30.45 | −17.29 (−44.29; 9.70) | 0.201 |
| Cholesterol (mg/mL) | ||||
| M1 | 185.18±73.47 | 154.94±39.87 | 30.26 (3.52; 56.95) | 0.028 |
| M2 – M1 | −36.71±10.30 | −25.53±30.13 | −11.18 (−36.04; 13.68) | 0.367 |
| LDL (mg/mL) | ||||
| M1 | 110.52±38.24 | 93.82±28.82 | 16.70 (−6.96; 40.36) | 0.160 |
| M2 – M1 | −13.62±28.55 | −16.36±27.88 | 2.74 (−16.98; 22.46) | 0.779 |
| HDL (mg/mL) | ||||
| M1 | 46.08±9.49 | 43.01±14.94 | 3.08 (−5.67; 11.82) | 0.479 |
| M2 – M1 | −17.51±9.47 | −13.0±22.80 | −4.51 (−16.71; 7.69) | 0.457 |
| Na (mEq/L) | ||||
| M1 | 139.47±1.74 | 139.24±2.05 | 0.24 (−1.09; 1.56) | 0.720 |
| M2 – M1 | −1.94±2.79 | −0.35±2.87 | −1.59 (−3.57; 0.39) | 0.112 |
| Leukocyte (103/µL) | ||||
| M1 | 5.48±1.52 | 5.92±1.72 | −0.44 (−1.58; 0.69) | 0.433 |
| M2 – M1 | 2.70±2.19 | 2.07±3.29 | 0.62 (−1.33; 2.57) | 0.522 |
| IL-6 (pg/mL) | ||||
| M1 | 2.79±2.77 | 4.84±5.97 | −2.05 (−5.31; 1.21) | 0.209 |
| M2 – M1 | 16.44±22.51 | 21.08±35.19 | −4.64 (−25.27; 16.00) | 0.650 |
| CRP (mg/dL) | ||||
| M1 | 2.93±6.08 | 2.96±3.01 | −0.03 (−3.38; 3.32) | 0.986 |
| M2 – M1 | 58.35±48.41 | 53.44±41.12 | 4.90 (−26.48; 36.28) | 0.752 |
| TNF-α (pg/mL) | ||||
| M1 | 0.31±0.88 | 0.20±0.29 | 0.12 (−0.34; 0.57) | 0.607 |
| M2 – M1 | 0.16±0.54 | −0.02±0.43 | 0.17 (−0.17; 0.51) | 0.310 |
CI: confidence interval; M1: mean of before intervention, 3–5 days before surgery; M2: mean of after intervention, 14 days after surgery or at discharge; BMI: body mass index; BUN: blood urine nitrogen; AST: aspartate aminotransferase; ALT: alanine aminotransferase; TG: triglyceride; LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; TNF-α: tumor necrosis factor-α
Table 6.
AEs and clinical outcomes between the two studied groups
| Study group (N=17) | Control group (N=17) | P | |
|---|---|---|---|
| AE | |||
| Bloatinga | 1 (5.6%) | 1 (5.6%) | 1.000 |
| Diarrhea | 2 (11.8%) | 4 (23.5%) | 0.656 |
| Total | 3 (16.7%) | 5 (27.8%) | 0.691 |
| Infectious complications | 0 (0%) | 2 (11.8%) | 0.485 |
| Non-infectious complications | 2 (11.8%) | 5 (29.4%) | 0.398 |
| Overall complications | 2 (11.8%) | 6 (35.3%) | 0.225 |
| Time to first bowel action (h) | 57.88±16.51 | 56.53±18.46 | 0.823 |
| LOS (day) | |||
| Total population | 15.59±18.20 | 14.65±8.67 | 0.849 |
| Population without complications | 11.00±1.36 | 12.18±7.67 | 0.562 |
aReason for two patients withdrawing from the study. AE: adverse event; LOS: length of hospital stay after surgery
Complications were classified into infectious and noninfectious [Table 6]. Infectious complications were sepsis in one patient and intra-abdominal abscess in another patient in the control group, and no infectious complications developed in the study group. Noninfectious complications were postoperative bleeding in one patient and delayed gastric emptying in five patients in the control group. In contrast, wound dehiscence developed in one patient and delayed gastric emptying in one patient in the study group. In the control group, one patient developed concomitant sepsis and delayed gastric emptying and another patient experienced concomitant postoperative bleeding and delayed gastric emptying. Despite more concomitant complications and a higher number of complications in the control group, no statistically significant difference was observed in infectious (0% vs. 11.8%, P = 0.485), noninfectious (11.8% vs. 29.4%, P = 0.398), or all complications (11.8% vs. 35.3%, P = 0.225) between the two treatment groups. Time to first bowel action and LOS after surgery were comparable between the two groups, but no significant differences were observed (P = 0.823).
Discussion
EEN improves QOL, the rate of infectious complications, time to first bowel action, and LOS. The European Society for Clinical Nutrition and Metabolism guidelines for surgery recommend that oral intake should be initiated within hours of surgery in patients undergoing colon resection; however, the timing of EN after upper GI surgery is not clearly defined.[15] However, an increasing number of recent randomized controlled trials (RCTs) have administered EN on postoperative day 1 in patients undergoing proximal GI surgery and demonstrated a positive correlation with clinical outcomes.[16,17] Even so, EEN was achieved in 52% of patients undergoing total gastrectomy for gastric cancer[6] and in 63% of patients undergoing pancreaticoduodenectomy.[17] Considering impaired postoperative gastric emptying and subsequent vomiting after diet intake, EN was started on postoperative day 3 in this study, which was initiated in 2011.
Patients scheduled to undergo major abdominal surgery usually cannot maintain adequate oral intake owing to symptoms of anorexia, bloating, nausea, vomiting, bleeding, and epigastralgia and are potentially malnourished before surgery. After surgery, their nutritional status may worsen, because surgery induces stress metabolism, leading to increased morbidity and mortality. Nonetheless, appropriate nutritional intervention may remedy the deficiencies and may improve immune function. In addition to providing energy, an immune-modulating nutrient-enriched diet may modulate inflammation, cellular immune function, and oxidative stress response in patients with critical illness[18] and those undergoing major open GI surgery.[19]
In a study conducted by Ma et al.,[20] omega-3 PUFA-enriched parenteral fat emulsion was administered to patients with gastric or colorectal cancer undergoing minimally invasive surgery, and the findings demonstrated that proinflammatory factors including IL-6, CRP, and TNF-α returned to normal level on postoperative day 7. In contrast, in this study, open surgery was performed for gastric cancer, and the findings revealed that IL-6, CRP, and TNF-α significantly increased after surgery in both study and control groups, and inflammatory response was mild. The results may be attributed to open surgery, instead of minimally invasive surgery, and more extensive dissection for the involved lymph nodes of gastric adenocarcinoma. Furthermore, immune-modulating nutrients did not exert prominent anti-inflammatory and immunomodulatory effects on clinical outcomes of infectious, noninfectious, and overall complications; time to first bowel action; and LOS of elective gastric surgery, consistent with the aforementioned previous study, in which omega-3 PUFA-enriched parenteral fat emulsion had no immunomodulation effect. However, lower rates of infectious, noninfectious, and overall complications were observed in the study group than in the control group. This finding might be because of the relatively small sample size. Thus, larger clinical trials should be conducted to provide definite conclusions.
Clinical evidence has indicated that in patients with critical illness, immune-modulating nutrients reduced infectious complications, time on mechanical ventilation, and lengths of ICU and hospital stay.[21,22] In contrast to patients with critical illness involving hyperinflammatory status, the effect of immune-modulating nutrients might be masked in patients undergoing elective major gastric surgery, and the resulting measurable effects on the inflammatory response may be mild. However, a recent RCT demonstrated that compared with high-protein EN, immune-modulating nutrient-enriched EN did not improve infectious complications and clinical outcomes in patients with critical illness; thus, such EN may be harmful and lead to increased adjusted mortality at 6 months.[23]
The doses of immune-modulating nutrients may play an important role; however, there is a lack of consensus for doses in the guidelines. In a study conducted by De Luis et al.,[24] a higher dose of arginine (18.9 vs. 12.3 vs. 5.7 g/day) tube-fed for approximately 10 days postoperatively reduced LOS and fistula complication but not infectious complications in postsurgical oral and laryngeal cancer patients. The dose of arginine given in this study was 5 g/day for 3–5 days before surgery and 15 g/day for 5–14 days after operation or until discharge, and there was no significant difference in LOS, infectious, or non-infectious complications instead. In the Reducing Deaths Due to Oxidative Stress trial, enteral glutamine at 30 g/day and parenteral glutamine at 0.35 g/kg ideal body weight/day were administered to mechanically ventilated, critically ill patients for a maximum of 28 days, until discharge from the ICU or death. The trial reported that glutamine supplement increased mortality rates without reducing infection rates.[25] Compared to the aforementioned study, the dose of studied glutamine was relatively low (7.5 g/day) and infection rates did not reduce, either. Similarly, in the EDEN-OMEGA study, a daily intake of 16.2 g of omega-3 fatty acids and γ-linoleic acid was supplemented enterally in patients with acute lung injury (6.96 g/day of enteral fish oil supplemented in this study), but that study was prematurely closed due to failure to reach the primary endpoint of reducing ventilator-free days, with higher 60-day in-hospital death rates.[26] The inflammatory responses of the involved patients (elective surgery vs. critical ill) and the doses of investigational immune-modulating nutrients contribute to the heterogeneity of study results. Correspondingly, this study used lower doses of immune-modulating nutrients and might not have been sufficiently powered to draw significant conclusions. The detection of significant doses and effects of immune-modulating nutrients might require additional larger clinical trials.
Surgical trauma induces a catabolic response (i.e., hypercatabolism) and impaired lipid tolerance as well.[27] In this study, BMI, albumin, prealbumin, cholesterol, LDL, and HDL significantly decreased postoperatively in both groups. The depletion was mild even with perioperative EN supplement and reflected hypercatabolism after major surgery for gastric cancer. Although patients were administered at least 1200 kcal of daily caloric supplement (plus oral intake), this appeared to be inadequate for patients with gastric malignancy undergoing gastrectomy. Particularly, the destruction of vagus nerve, decreased gastric volume, and poor appetite are frequently encountered in such patients, which may decrease oral intake. The postoperative TG levels were elevated in both groups, and the degrees of elevation were mild and within normal ranges.
The remaining laboratory parameters and AE incidence were comparable between both treatment groups. Bloating and diarrhea were possibly treatment-related. Bloating – because of which one patient in each group withdrew from the study – was not encountered after surgery, and this might be due to tube feeding, which avoided gastric emptying. Diarrhea was the main AE, and most episodes of diarrhea were mild and ceased spontaneously and could be managed using antimotility agents. No death was recorded in this study.
Overall, the duration of intervention of immune-modulating nutrients was relatively short and the sample size was less than ideal that might be underpowered and contributory to negative results. Despite the limitations mentioned above, this study revealed that immune-modulating nutrients had less immune-modulatory effects than expected in gastric cancer patients undergoing elective surgery, in which inflammation might be only mild compared to critically ill patients. Besides, the catabolic response after major operation for gastric cancer was greater than 1200 kcal supplemented enterally in this study with evidences of significant depletion of albumin, prealbumin, and BMI.
Conclusion
The effects of immune-modulating nutrient-enriched EN on laboratory parameters were comparable with those of the standard formula. Immune-modulating nutrient-enriched EN had no significant anti-inflammatory effect in patients undergoing elective surgery for gastric cancer and resulted in mild inflammation, as a small-population study in short-term perioperative use of immune-modulating nutrient-enriched EN with some limitations mentioned above that might be a part of the causes. Surgery for gastric malignancy induces a catabolic response that requires more intensive nutrition support.
Financial support and sponsorship
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 107-2321-B-037-003) and Kaohsiung Medical University Hospital (KMUH102-2M23, KMUH106-6R32, KMUH106-6M28, KMUH106-6M29, KMUH106-6M30, KMUH106-6M31, KMUHS10522, KMUHS10505, KMUHS10518, KMUHGCRC2016002, KMUHS10601, KMUHS10608, KMUHA10664). In addition, this study was supported by the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, R.O.C. and Grant by Kaohsiung Medical University aim for the top University, grant no. KMU-S105011.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Immune-modulating nutrient-enriched enteral diet was provided by Taiwan Otsuka Pharmaceutical Co., Ltd.
References
- 1.Kim AR, Cho J, Hsu YJ, Choi MG, Noh JH, Sohn TS, et al. Changes of quality of life in gastric cancer patients after curative resection: A longitudinal cohort study in Korea. Ann Surg. 2012;256:1008–13. doi: 10.1097/SLA.0b013e31827661c9. [DOI] [PubMed] [Google Scholar]
- 2.Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. Impact of early enteral and parenteral nutrition on prealbumin and high-sensitivity C-reactive protein after gastric surgery. Genet Mol Res. 2015;14:7130–5. doi: 10.4238/2015.June.29.6. [DOI] [PubMed] [Google Scholar]
- 3.Shu XL, Kang K, Gu LJ, Zhang YS. Effect of early enteral nutrition on patients with digestive tract surgery: A meta-analysis of randomized controlled trials. Exp Ther Med. 2016;12:2136–44. doi: 10.3892/etm.2016.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong L, Han Y, Zhang H, Zhao Q, Liu J, Yang J, et al. Effect of early oral feeding on short-term outcome of patients receiving laparoscopic distal gastrectomy: A retrospective cohort study. Int J Surg. 2014;12:637–9. doi: 10.1016/j.ijsu.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 5.Boelens PG, Heesakkers FF, Luyer MD, van Barneveld KW, de Hingh IH, Nieuwenhuijzen GA, et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: Prospective, randomized, controlled trial. Ann Surg. 2014;259:649–55. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 6.Sierzega M, Choruz R, Pietruszka S, Kulig P, Kolodziejczyk P, Kulig J. Feasibility and outcomes of early oral feeding after total gastrectomy for cancer. J Gastrointest Surg. 2015;19:473–9. doi: 10.1007/s11605-014-2720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer. 2010;102:966–71. doi: 10.1038/sj.bjc.6605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wie GA, Cho YA, Kim SY, Kim SM, Bae JM, Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26:263–8. doi: 10.1016/j.nut.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Zheng HL, Lu J, Li P, Xie JW, Wang JB, Lin JX, et al. Effects of preoperative malnutrition on short- and long-term outcomes of patients with gastric cancer: Can we do better? Ann Surg Oncol. 2017;24:3376–85. doi: 10.1245/s10434-017-5998-9. [DOI] [PubMed] [Google Scholar]
- 10.Elke G, van Zanten AR, Lemieux M, McCall M, Jeejeebhoy KN, Kott M, et al. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit Care. 2016;20:117. doi: 10.1186/s13054-016-1298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MH, Kim H. The roles of glutamine in the intestine and its implication in intestinal siseases. Int J Mol Sci. 2017;18:pii. doi: 10.3390/ijms18051051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wischmeyer PE. Glutamine: Mode of action in critical illness. Crit Care Med. 2007;35:S541–4. doi: 10.1097/01.CCM.0000278064.32780.D3. [DOI] [PubMed] [Google Scholar]
- 13.Windle EM. Glutamine supplementation in critical illness: Evidence, recommendations, and implications for clinical practice in burn care. J Burn Care Res. 2006;27:764–72. doi: 10.1097/01.BCR.0000245417.47510.9C. [DOI] [PubMed] [Google Scholar]
- 14.McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE, et al. Anti-inflammatory omega-3 endocannabinoid epoxides. Proc Natl Acad Sci U S A. 2017;114:E6034–43. doi: 10.1073/pnas.1610325114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. ESPEN Guidelines on Enteral Nutrition: Surgery including organ transplantation. Clin Nutr. 2006;25:224–44. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Chung HK, Hwang HK, Kim JK, Yoon DS. Postoperative nutritional effects of early enteral feeding compared with total parental nutrition in pancreaticoduodectomy patients: A prosepective, randomized study. J Korean Med Sci. 2012;27:261–7. doi: 10.3346/jkms.2012.27.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perinel J, Mariette C, Dousset B, Sielezneff I, Gainant A, Mabrut JY, et al. Early enteral versus total parenteral nutrition in patients undergoing pancreaticoduodenectomy: A randomized multicenter controlled trial (Nutri-DPC) Ann Surg. 2016;264:731–7. doi: 10.1097/SLA.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 18.Hegazi RA, Wischmeyer PE. Clinical review: Optimizing enteral nutrition for critically ill patients – A simple data-driven formula. Crit Care. 2011;15:234. doi: 10.1186/cc10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg. 2012;255:1060–8. doi: 10.1097/SLA.0b013e318252edf8. [DOI] [PubMed] [Google Scholar]
- 20.Ma CJ, Wu JM, Tsai HL, Huang CW, Lu CY, Sun LC, et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr J. 2015;14:9. doi: 10.1186/1475-2891-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001;286:944–53. doi: 10.1001/jama.286.8.944. [DOI] [PubMed] [Google Scholar]
- 22.Montejo JC, Zarazaga A, Lopez-Martinez J, Urrutia G, Roque M, Blesa AL, et al. Immunonutrition in the intensive care unit. A systematic review and consensus statement. Clin Nutr. 2003;22:221–33. doi: 10.1016/s0261-5614(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 23.van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: A randomized clinical trial. JAMA. 2014;312:514–24. doi: 10.1001/jama.2014.7698. [DOI] [PubMed] [Google Scholar]
- 24.De Luis DA, Izaola O, Terroba MC, Cuellar L, Ventosa M, Martin T. Effect of three different doses of arginine enhanced enteral nutrition on nutritional status and outcomes in well nourished postsurgical cancer patients: A randomized single blinded prospective trial. Eur Rev Med Pharmacol Sci. 2015;19:950–5. [PubMed] [Google Scholar]
- 25.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–97. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]
- 26.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–81. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patino JF, de Pimiento SE, Vergara A, Savino P, Rodriguez M, Escallon J. Hypocaloric support in the critically ill. World J Surg. 1999;23:553–9. doi: 10.1007/pl00012346. [DOI] [PubMed] [Google Scholar]


