Abstract
Hamelia patens, is a plant traditionally used to treat a variety of conditions among the Huastec people of Mexico. The objective of this study is to characterize the phenolic content and critically examine the antimicrobial activity of leaf extracts H. patens, obtained by maceration, Soxhlet and percolation, using ethanol as 70% solvent. Phenolic compounds are characterized by liquid chromatography, coupled to a High Resolution Mass Spectrometry, and the antimicrobial activity was studied from the inhibitory effect of each extract for Escherichia coli, Staphylococcus aureus, Salmonella typhi and S. paratyphi, and by the Minimum Bactericidal Concentration, the percentage of activity and the Index of Bacterial Susceptibility of each extract. The phenolic compound identified in different concentrations in the three extracts was epicatechin. The extracts obtained by the three methods had antimicrobial activity, however, there was no significant difference (p < 0.05) between the Minimum Bactericidal Concentration of the extracts obtained by maceration, percolation and Soxhlet. The results of this study contribute to the body of knowledge on the use of extracts in controlling microorganisms with natural antimicrobials.
Keywords: Antibacterial activity, Hamelia patens, Polyphenols, HPLC–MS
Introduction
From ancient times, man has taken refuge in natural remedies to find a cure for their diseases. The plants used for this purpose are known as medicinal plants, as they contain chemical compounds that have biological properties which are beneficial to health. These properties include, antimicrobial activity attributed to phenolic compounds and essential oils present in different parts of the plant.1 Hamelia patens belongs to the family of the Rubiaceae,2 and is traditionally used to treat skin conditions and the antimicrobial and antioxidant activity of its phenolic compounds have been studied.3 Ríos and Aguilar4 reported that H. patens is a plant used in traditional Mexican medicine which are associated antimicrobial, analgesic and anti-inflammatory effects. Although some authors,5, 6 have reported therapeutic properties such as antidarrheal, intestinal antispasmodic, anemia wound, healing, and antidiabetic for H. patens, in the Huasteca Potosina region in Mexico, it is a common practice to use it to wash wounds, so it is of interest to know if it also has antimicrobial activity. Prieto et al.7 obtained extracts of plants belonging to this family, using the maceration method with two types of solvents; ethyl ether and petroleum ether, it was reported that the former had better antibacterial antifungal activity. Furthermore, Piña et al.8 reported that the rumberina and palmirina, which are secondary metabolites isolated from leaves of H. patens, showed inhibitory activity on Leishmania mexicana promastigotes. Although, most studies on the antibacterial activity of this plant have been made from the extracts obtained with organic solvents, the aqueous extracts have also shown some bactericidal activity.9
Most studies on the antimicrobial activity of H. patens have focused more on the effect of solvent used, and less on the method used to obtain the extracts. A knowledge of whether the approach or method used to obtain the extracts can affect the content and biological activity of chemical compounds, such as polyphenols, leading to the possibility of more efficient resources laboratory where it works best with plant extracts, will enable one to choose which to allow, that will bring about better extraction of the active compounds in the medicinal plants. The Minimum Bactericidal Concentration (MBC) is defined as the minimum concentration of antimicrobial which eliminates more than 99.9% of microorganisms viable after a set incubation time,10 so it is a reliable parameter for evaluating the antimicrobial properties of a substance. Therefore, this paper aims to characterize the phenolic content and study the antimicrobial activity of leaf extracts of H. patens, obtained by three different extraction techniques, Maceration (ME), Soxhlet (SE) and Percolation (PE).
Material and methods
Sample preparation
Scientific name voucher: Hamelia patens Jacq. HPHP-SP-102; leaves of this specimen were collected from different household backyards located in the Huasteca, northeast of Mexico. Latitude: 99°01′ W; longitude: 21°59′ N; meters above sea level: 74. The plant was identified by the taxonomist responsible and curator for the “Isidro Palacios” Herbarium of the Autonomous University of San Luis Potosí, and was deposited in this. Those leaves which do not have physical damage were selected. Selected leaves were then blanched in order to inactivate the polyphenol oxidase enzyme of the leaves in order to confirm that the guayacol test inactivation was performed.11 Blanched leaves H. patens were dried in a conventional oven (Linderberg/Blue, USA) at 55 °C for 48 h. The moisture content was also determined according to the methodology of the AOAC.12
Obtaining extracts
Extracts were obtained by the methods of Maceration, Soxhlet and Percolation, as described below: Classical method of Maceration; 20 g of dried leaves of H. patens were placed in a flask and 200 mL of 70% ethanol was added. The solution was then stirred for 24 h. Soxhlet method; 20 g of dried powdered leaves were placed in 200 mL of 70% ethanol. Soxhlet equipment (Barnstead/Lab-line Multi Heater Unit Extraction) was used to analyze the solution at a temperature of 60 °C for 4 h continuously. Percolation method. 20 g of leaves of H. patens powder were placed in a percolation system and 1 L of 70% ethanol was passed through the sample. The extracts obtained by the three methods were filtered using filter paper 125 mm, No. 3 (Whatman brand) and concentrated under reduced pressure in a rotary evaporator (brand BUGI) pressure to remove the solvent. The extracts were resuspended in distilled water and sterilized by membrane filtration with pore 45 μm (Whatman brand) and stored at 4 °C until use. From the stock solutions of each extract, other concentrations to be determined by MBC and measure their antimicrobial activity were prepared.
Characterization of phenolic compounds
H. patens extracts were analyzed using a system of high resolution liquid chromatography coupled with mass spectrometry (HPLC-ESI-MS). The HPLC consisted of an autosampler (Varian ProStar 410, USA), a ternary pump (Varian ProStar 230I, USA) and a PDA detector (Varian ProStar 330, USA) set at 280 nm. For mass spectrometric analysis, mass spectrometer ion trap (Varian 500-MS) equipped with an electrospray ion source was used. The column used (C18 5 μm, 150 mm × 2.0 mm) was maintained at 30 °C while the mobile phase was acetic acid 3% (A) and acetonitrile (B). The flow used was 0.3 mL/min with an injection volume of 5 μL sample. The B gradient elution were: initial 3%; 9% 5 min; 15 min 16%; 45 min 50%. Thereafter, the column was washed and reconditioned. All effluent (0.3 mL/min) was injected directly into the source of the mass spectrometer, without division. All experiments were carried out in negative mode [M−H]−1. Nitrogen was used as nebulizer gas and helium as a buffer gas. The parameters of the ion source were: voltage (3.5 kV), capillary voltage (90.0 V) and temperature (350 °C). Data were collected and processed using the MS Workstation software (V 6.9).
Some standard of phenolic compounds were used during chromatography such as pyrogallol, gallic acid, resorcinol, chlorogenic acid, methyl gallate, coumaric acid, catechin, (−)-epicatechin, procyanidins (B1, B2 and C1), 2-hidroxycinamic acid, ellagic acid, quercetin and cinnamic acid, all were purchased from Sigma Aldrich.
Antimicrobial activity
To study the antibacterial activity of the three extracts, MBC and its inhibitory effect on S. aureus ATCC 35556, E. coli ATCC 25922, S. typhi ATCC 14028 and S. paratyphi ATCC 9150 were determined. The microorganisms used in this study were provided by the Ciudad Valles Sanitary Jurisdiction. From the revitalized strains, the inoculum was prepared as described by Cockerill et al.13 The percentage of activity of each extract and the rate of bacterial susceptibility were also calculated.
Minimum Bactericidal Concentration (MBC)
MBC was used to determine the effect of ethanolic extracts of H. patens on different bacterial species. It was performed according to the methodology described by Chiong et al.14 Series of 10 tubes with 2 mL of nutrient broth were prepared; the first of each series contained a double concentration of broth nutrient and other single concentration. From a stock solution, 60 mg/mL of each extract was added to the first tube of each series then, 2 mL transferred to the next tube, and so on to give concentrations of 30, 15, 7.5, 3.75, 1.85 and 0.9 mg/mL of each extract. Each tube was inoculated with 100 μL (1 × 108 CFU) of the bacterial suspension and were incubated at 35 °C for 24 h. Two concentrations used as controls were also conducted using distilled water as negative control and Moxifloxacin, a broad-spectrum antibiotic, as positive control. After the incubation period, a roast of the last tube of the series did not show turbidity and inoculation in appropriate media culture was taken: Baird Parker for Staphylococcus aureus, McConkey agar for Escherichia coli and Salmonella-Shigella agar for Salmonella typhi and S. paratyphi. The media were incubated for 24 h at 37 °C. All tests were performed in triplicate. The technique used allows to know the minimum inhibitory concentration of the extract, which corresponds to the dilution in which turbidity is no longer observed, however, when inoculating this dilution in specific media without extract, the microorganisms can not develop, indicates that they have dead, so that the tube with the lowest bactericidal concentration – the maximum dilution in the technique –, will correspond to the MBC.
Inhibitory effect
The inhibitory effect of each extract was measured from the zones of inhibition observed when using the disk diffusion technique 100 μL (1 × 108 CFU) of bacteria was inoculated with the agar surface Muller Hinton. Thereafter, six filter paper discs (brand Whatman) of 6.0 mm in diameter were placed and sprayed with 10 μL of each of the extracts at different concentrations and with the substances used as control. All tests were performed in triplicate.
Percentage of activity
The percentage of activity of each extract (Eq. (1)) indicates the total antimicrobial potency of the extract in particular.15
| (1) |
Index bacterial susceptibility (IBS)
The IBS (Eq. (2)) shows the number of microorganisms susceptible to extract, assessing ranges from 0 (resistance extract all samples) to 100 (susceptible to whole extract).15
| (2) |
Results
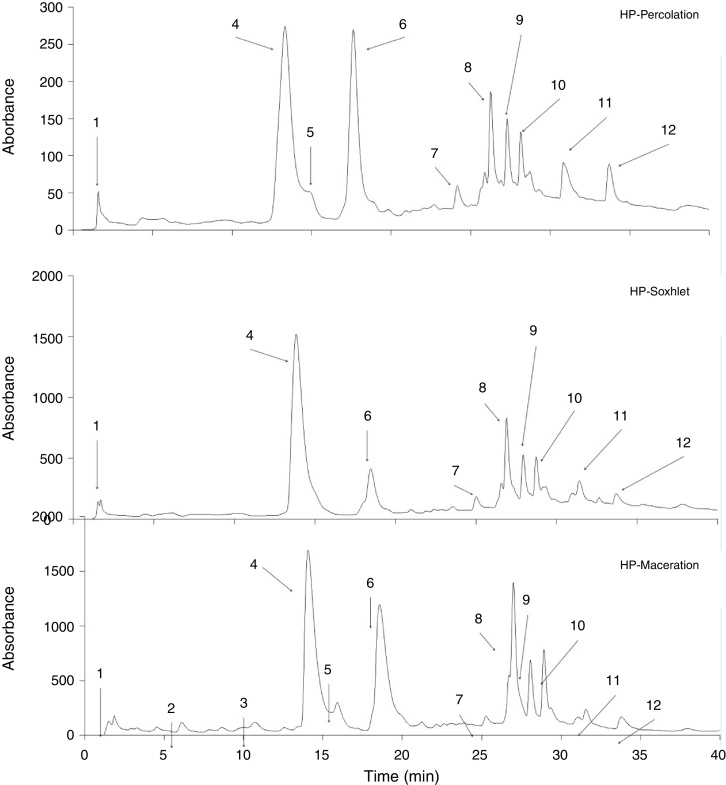
Fig. 1 shows the chromatogram of the compounds identified in each extract. Twelve different compounds in extracts of H. patens were identified. The compounds present depended on the type of extraction method used. Compounds identified as 2 and 3 (Fig. 1) were only present in the extracts obtained by maceration. While the compound identified as 5 was absent in the extract obtained by Soxhlet. Compounds 2, 3 and 5 were identified as hydroxycinnamic acid, catechin, and procyanidin B2 (Table 1). The main controlling compound in the extracts obtained by the three extraction techniques was compound No. 4 (Fig. 1) identified as chlorogenic acid (Table 1). The phenolic compound present in the different concentrations of the three extracts was epicatechin (6) (Table 1).
Fig. 1.
HPLC chromatogram of extracts of leaves Hamelia patens obtained by percolation (A), Soxhlet (B) and maceration (C).
Table 1.
Characterization of compounds found in extracts of leaves of H. patens.
| Peak | Time of retention (min) | [M−H]−1 | Molecular weight | Tentative identification |
|---|---|---|---|---|
| 1 | 1.6 | 191 | 192 | Quinic acid |
| 2 | 6.1 | 353 | 354 | Hydroxycinnamic acid |
| 3 | 10.7 | 289 | 290 | Catechin |
| 4 | 14 | 353 | 354 | Caffeoylquinic acid |
| 5 | 15.9 | 577 | 578 | Procyanidin B2 |
| 6 | 18.6 | 289 | 290 | (−)-Epicatechin |
| 7 | 25.3 | 451 | 452 | (+)-Catechin 3-O-glucose |
| 8 | 26.9 | 609 | 610 | Quercetin 3-O-rutinoside |
| 9 | 28 | 593 | 594 | Kaempferol 3-O-rutinoside |
| 10 | 28.9 | 497 | 498 | No identification |
| 11 | 31.6 | 451 | 452 | 3-Hydroxyphloretin 2′-O-Glucosid |
| 12 | 33.8 | 573 | 574 | No identification |
The MBC is the lowest concentration of an antimicrobial for inhibiting the growth of a microorganism agent, and is considered an essential parameter to check the sensitivity of a bacteria to an antibacterial.16 A higher MBC value for a specific microorganism indicates a lower antimicrobial agent employed capacity. For this reason, it is one of the most reliable techniques to determine the antimicrobial properties of a substance. Table 2 shows the MBC H. patens extracts obtained by the three extraction techniques against four pathogenic bacteria. By setting the MBC for H. patens extracts obtained by different extraction methods, it found out their potential as a antimicrobial agent for pathogenic bacteria studied. Extracts of maceration and Soxhlet CBI presented the largest percentage relative to the positive control (Moxifloxacin). Moreover, the extract obtained by percolation showed MBC values very similar to those obtained by the positive control reflecting its greater potential as an antimicrobial agent for the bacteria studied.
Table 2.
Minimum Bactericidal Concentration (MBC) of the three extracts of H. patens obtained by different techniques against pathogenic bacteria studied.
| Bacteria | Extraction methods |
||
|---|---|---|---|
| Maceration |
Soxhlet |
Percolation |
|
| Minimum Bactericidal Concentration (mg/mL) | |||
| E. coli | 4.0 | 7.5 | 3.5 |
| S. aureus | 12.0 | 8.0 | 3.5 |
| S. typhi | 2.0 | 3.5 | 3.0 |
| S. paratyphi | 10.0 | 7.5 | 3.5 |
In general the three extracts had a 75% efficiency for S. aureus and S. typhi, 50% for S. paratyphi and 100% for E. coli. In the three extracts obtained by different methods the percentage activity was 100% and the Index of Bacterial Susceptibility (IBS) was 75%.
Discussion
According to the HPLC chromatograms in the presence of three extracts most phenolic compounds identified was observed. The main compound identified in H. patens plant was chlorogenic acid, which belongs to the class of cinnamate and is widely distributed in nature. Chlorogenic acid encompasses a family of cinamoilquinicos trans esters. These compounds have been reported to possess a wide range of biological properties which highlights the antimicrobial activity.17 Interestingly, the extracts obtained by percolation generally present a higher antimicrobial activity against bacteria studied. Unlike maceration and Soxhlet extracts, percolation extract possess greater amount of the compounds identified as epicatechin, which has also been reported to possess antimicrobial properties and forms a precursor polymer formation called condensed tannins, which in some compounds are reported as antimicrobial and sometimes recalcitrant.18 Therefore, the antimicrobial activity could be evaluated, widely linked to these two compounds, chlorogenic acid and epicatechin.
In determining the MBC for H. patens extracts which were obtained by different methods, it was found as an antimicrobial potential for the bacteria studied. Although the MBC of the three extracts were different for each of the microorganisms, no significant difference existed between them (p < 0.05). Sauceda and Nereyda1 mention that the variables that affect the extraction process, regardless of the scale of production or type of end product are: agitation, state of division of matter, extraction time, nature of the solvent, temperature and pH. The contact time of the solvent with the plant allows diffusion of metabolites solvent to yield better results when time is greater; the temperature can increase the rate of extraction because it favors the solubility, however high temperatures can affect the stability of the compounds. Moreover, agitation promotes greater extraction while efficiency decreases because of diffusion resistance, eliminating the fluid film covering the surface of the solid at rest and, finally, reducing particle is of great importance, because it increases the contact area and decreases the time required for the extraction, especially for low porosity solid.19
The results obtained in this study are similar to those of Villavicencio and Perez20 who report that H. patens is more active in E. coli to S. aureus. Yasunaka et al.21 also claim that H. patens has a MBC lower for E. coli to S. aureus. Furthermore, Cervantes and Gonzalez22 showed that aqueous and ethanol extracts of H. patens had greater antimicrobial activity against S. aureus about E. coli, and both extracts, the ethanol was most effective.
The fact that the MBC are less attributable to increased susceptibility of the bacteria to the active compounds present in the extract, or because the way that the extract was obtained, allowed increased extraction of these compounds. The MBC of the extracts varied, not only in terms of the extraction method, but also depending on the bacteria studied. It was observed that the MBC of EP, was less for E. coli, S. aureus and S. paratyphi, S. typhi that the MBC required when using the MS and ES.
Cáceres et al.23 state that the wetting of the raw material increases the porosity of the cell wall and cell facilitates the diffusion of extractables outwardly of plant cells, whereby the percolation process may be more efficient because it increases the contact time of the feedstock with the solvent and drag the active compounds and related sample solvent are obtained, allowing complete extraction of the active ingredients of the plant.
When obtaining the temperature handled at >50 °C, which could affect the biologically active compounds present in the plant; it was recalled that the other methods of extraction are performed at <50 °C. Although the dissolution of extractables is facilitated by the increased temperature, many active ingredients are thermolabile and can be fully or partially destroyed at elevated temperatures.24
The extracts obtained by the three methods showed 100% of antimicrobial activity and IBS was of 75%. However, it is considered that the MBC of the extracts of H. patens are high because Cockerill et al.13 reported that an extract is considered with antimicrobial activity, MBC should be <500 μg/mL. Although MBC of extracts H. patens are high, its antimicrobial activity must be recognized, the conditions traditionally used by the population as a poultice, may be able to enhance the activity of the active compounds present in the plant. The yield of the extracts obtained by maceration, percolation and Soxhlet was 25, 44.5 and 24% respectively. The results obtained in this study prove that a higher percentage yield in obtaining the extract is not an indicator of increased antibacterial activity. Gyawali et al.25 report that active compounds present in the plants have antimicrobial activity, which is well documented, although the mechanisms of action are not clear, however due to the resistance of microorganisms to traditional antibiotics, the application of antimicrobials natural resources of plant origin currently have a greater application.
Conclusions
H. patens extracts obtained by Maceration, Percolation and Soxhlet had antimicrobial activity, which was attributed to its content of phenolic compounds, due to the presence of epicatechin. However, the extract obtained by percolation had the lowest MBC and greater inhibitory effect on the studied bacteria, than those obtained by Maceration and Soxhlet.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank to Taxonomist José García Pérez, Responsible and curator of the “Isidro Palacios” Herbarium of the Autonomous University of San Luis Potosí, for their support for the identification of the plant under study
Associate Editor: Luis Henrique Guimarães
References
- 1.Sauceda R., Nereyda E. Uso de agentes antimicrobianos naturales en la conservación de frutas y hortalizas. Ra Ximhai. 2011;7:153–170. [Google Scholar]
- 2.Sotelo D., Casas F., Camelo M. Borojó (Borojoa patinoi): fuente de polifenoles con actividad antimicrobiana. Rev Fac Quím Farm. 2009;17:329–336. [Google Scholar]
- 3.Ahmad A., Pandurangan A., Singh N., Ananad P. A mini review chemistry and biology of Hamelia patens (Rubiaceae) Pharmacogn J. 2012;29:1–4. [Google Scholar]
- 4.Rios M., Aguilar G. Alcaloides indólicos, terpenos, esteroles y flavonoides de las hojas de Hamelia patens Jacquin (Rubiaceae) Rev Cubana Plant Med. 2006;11(1):1–5. [Google Scholar]
- 5.Alonso-Castro A.J., Maldonado-Miranda J.J., Zárate-Martínez A. Medicinal plants used in the Huasteca Potosina, Mexico. J Ethnopharmacol. 2012;143(1):292–298. doi: 10.1016/j.jep.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 6.Cruz E.C., Andrade-Cetto A.J. Ethnopharmacological field study of the plants used to treat type 2 diabetes among the Cakchiquels in Guatemala. J Ethnopharmacol. 2015;159:238–244. doi: 10.1016/j.jep.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Prieto R., Patiño L., Lesmes L., Cuca S. Estudio fitoquímico de hojas de Uncaria guianensis y evaluación de actividad antibacteriana. Acta Amaz Univ Nac Colombia. 2011;41:303–310. [Google Scholar]
- 8.Piña V.S., Cárdenas M., Suárez A.I. Estudios de Toxicidad sobre Artemisia salina de los alcaloides aislados en las hojas de Hamelia patens, Facultad de Farmacia. Universidad Central de Venenzuela. Acta Cient Estud. 2003;1:66. [Google Scholar]
- 9.Rodríguez H. EUNA; San José, Costa Rica: 2000. La utilidad de las plantasmedicinales; pp. 61–62. [Google Scholar]
- 10.Ingraham J.L., Ingraham C.A. vol. 2. Editorial Reverté, S.A.; 1997. p. 495. (Introducción a la Microbiología). [Google Scholar]
- 11.Meyer R., Gaetano P., Olmos C.U., Figueroa J.M. Editorial Trillas; México: 1984. Control de calidad de productos agropecuarios. 35p. [Google Scholar]
- 12.AOAC. Official methods of analysis of AOAC International, vol. 16. Virginia, USA; 2014. p. 1.
- 13.Cockerill F.R., III, Wikler M.A., Alder J. vol. 32. CLSI (Clinical and Laboratory Standards Institute); Wayne, PA: 2012. (Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standards – Eleventh Edition). 58p. [Google Scholar]
- 14.Chiong R., Betancourt A., Cuza C.M.C., Álvarez J., Jiménez D. Instituto Nacional de Higiene, Epidemiología y Microbiología; La Habana, Cuba: 1985. Pruebas microbiológicas para evaluar la efectividad bacteriana de desinfectantes químicos. Sección de microbiología sanitaria. [Google Scholar]
- 15.López-Malo A., Palou E., Mickey P., Michael D. Antimicrobials in Food. third ed. CRC Press; 2005. Methods for activity assay and evaluation of results; pp. 659–680. [Google Scholar]
- 16.Struthers K., Westran R. Masson S.A.; México: 2005. Bacteriología Clínica; p. 192. [Google Scholar]
- 17.Machado E.M.S., Rodríguez R., Teixeira J., Mussatto S. Growth of fungal strains on coffee industry residues with removal of polyphenolic compounds. Biochem Eng J. 2012;60:87–90. [Google Scholar]
- 18.Aguilera C.A., Augur C., Prado B.L.A., Favela T.E., Aguilar C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl Microbiol Biotechnol. 2008;78:189–199. doi: 10.1007/s00253-007-1276-2. [DOI] [PubMed] [Google Scholar]
- 19.Fenaroli G. CRC Press; 2000. Fenaroli's Handbook of Flavor Ingredients. [Google Scholar]
- 20.Villavicencio M.N., Pérez B.E. Universidad Autónoma del Estado de Hidalgo; 2010. Vegetación e inventario de la flora útil de la huasteca y la zona Otomí-Tepehua de Hidalgo. Área académica de Biología. Available from: http://repositoriodigital.academica.mx/jspui/bitstream/987654321/41934/1/Ciencia_Universitaria_01_003.pdf. [Google Scholar]
- 21.Yasunaka K., Abe F., Nagayama A. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J Ethnopharmacol. 2005;2:293–299. doi: 10.1016/j.jep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes B., González A. Tesis de Licenciatura Universidad Autónoma de San Luis Potosí; México: 2007. Efecto bactericida de Hamelia patens sobre S. aureus y E coli. [Google Scholar]
- 23.Cáceres A., Barrios M., Morales C. Actividad Biocida de plantas detectadas por etnobotánica y bioprospección en la reserva de la biósfera de Sierra de las Minas. Cienc Tecnol. 2001;6:23–47. [Google Scholar]
- 24.Meléndrez L.B. Universidad de San Carlos de Guatemala; 2011. Evaluación de las propiedades fisicoquímicas y fitoquímicas de la fracción lipídica en la semilla del fenogreco (Trigonella foenumgraecum L.) obtenida a nivel laboratorio utilizando el método de extracción por decocción. Available from: http://issuu.com/fiusac/docs/tesario_2011. [Google Scholar]
- 25.Gyawali R., Hayek S.A., Ibrahim S.A. Handbook of Natural Antimicrobials for Food Safety and Quality. Ed. Elsevier; 2015. Plants extracts as antimicrobials in food products: types; pp. 31–47. [chapter 2] [Google Scholar]