Abstract
Background
Sex differences in obesity and related diseases are well established. Gonadal hormones are a major determinant of these sex differences. However, sex differences in body size and composition are evident prior to exposure to gonadal hormones, providing evidence for gonadal-independent contributions attributable to the XX or XY sex chromosome complement. Large-scale genetic studies have revealed male/female differences in the genetic architecture of adipose tissue amount and anatomical distribution. However, these studies have typically neglected the X and Y chromosomes.
Scope of the review
Here we discuss how the sex chromosome complement may influence obesity, lipid levels, and inflammation. Human sex chromosome anomalies such as Klinefelter syndrome (XXY), as well as mouse models with engineered alterations in sex chromosome complement, support an important role for sex chromosomes in obesity and metabolism. In particular, the Four Core Genotypes mouse model—consisting of XX mice with either ovaries or testes, and XY mice with either ovaries or testes—has revealed an effect of X chromosome dosage on adiposity, hyperlipidemia, and inflammation irrespective of male or female gonads. Mechanisms may include enhanced expression of genes that escape X chromosome inactivation.
Major conclusions
Although less well studied than effects of gonadal hormones, sex chromosomes exert independent and interactive effects on adiposity, lipid metabolism, and inflammation. In particular, the presence of two X chromosomes has been associated with increased adiposity and dyslipidemia in mouse models and in XXY men. The enhanced expression of genes that escape X chromosome inactivation may contribute, but more work is required.
Keywords: Genetics, Gonadal hormones, Adipose tissue, Sex chromosome anomalies, X chromosome inactivation, Mouse models
Highlights
-
•
Sex differences are influenced by both gonadal type and sex chromosome complement.
-
•
Males and females differ in adipose tissue distribution and expansion.
-
•
X chromosome dosage may be one factor underlying sex differences in adiposity.
-
•
X inactivation escapee genes are expressed at higher levels in XX vs. XY cells.
1. Sexual dimorphism in disease
The study of sex differences in physiology has gained traction as it pertains to understanding male/female differences in disease susceptibility. Women more often than men suffer from autoimmune diseases (e.g., systemic lupus erythematosus, scleroderma, Sjogren's syndrome), as well as osteoporosis, Alzheimer's disease, and clinical depression [1], [2], [3], [4], [5], [6]. Autism occurs at higher rates in boys than girls, and sex differences are also observed in neurological diseases such as Parkinson's disease and schizophrenia, to name a few [7], [8], [9]. In cardiovascular disease and stroke, there are differences between the sexes in age of onset, disease pathology, and mortality [10].
Some sex differences in human disease are directly related to the distinct sex chromosome complement in females (XX) and males (XY). Males are more commonly afflicted with X-linked diseases such as color blindness, Duchenne muscular dystrophy, and hemophilia. Women may be protected from (or experience attenuated severity of) these recessive disorders, because they possess two X chromosomes [11]. The random inactivation of one X chromosome in female cells during early development silences the X chromosome carrying a mutant gene in approximately half of female cells, allowing the exclusive expression of a wild-type gene in those cells. An extreme example of the influence of XX versus XY genotype on disease severity is Rett syndrome, which results from mutation in the MECP2 gene on the X chromosome. Females afflicted with Rett mutations experience progressive neuro-developmental deficiencies leading to impaired learning, communication, coordination, and other brain functions. By contrast, males with MECP2 mutations typically die in utero or in infancy [12].
Here we will discuss how the sex chromosome complement may influence factors that underlie metabolic disturbances such as obesity, dyslipidemia, and inflammation. It is well known that gonadal hormones have strong effects on fat storage and susceptibility to related diseases such as cardiovascular disease and type 2 diabetes [13]. Since female gonads are typically found together with XX chromosomes, and male gonads with XY chromosomes, the independent roles of gonadal hormones and sex chromosomes has not been appreciated. However, human sex chromosome anomalies such as Klinefelter syndrome (XXY) and Turner syndrome (XO), as well as mouse models with engineered alterations in sex chromosome complement, support an important role for sex chromosomes in obesity and metabolism.
2. Sex differences in human adiposity and obesity
Adipose tissue serves as an efficient energy storage depot. It also has active roles in fatty acid uptake from circulating lipoproteins produced in response to a meal, and in the regulated release of fatty acids for use by other tissues between meals or during physical activity. There are key differences between males and females in adipose tissue distribution, with men accumulating greater amounts of visceral adipose tissue and women typically having greater fat accumulation in subcutaneous (gluteal–femoral) depots [14], [15]. Sex-specific fat distribution is influenced by several factors, including diet and hormonal status [16]. One contributor to sex bias in adipose tissue distribution may be the rate of direct fatty acid uptake by tissues, a process that occurs independently of lipoprotein lipase (the enzyme responsible for liberation of fatty acids from lipoproteins). Direct fatty acid uptake is higher in the gluteal–femoral depot in women and in the abdominal depot in men [17].
Both overall fat mass and visceral fat accumulation are strongly associated with the development of cardiovascular disease, stroke, hypertension, and insulin resistance [15], [18], [19]. Standard measurements for fat accumulation in humans include body mass index (weight as a function of height), which reflects whole body adiposity, and waist-to-hip ratio, which provides an indication of fat distribution, with the waist measurement as a proxy for visceral fat and the hip measurement for gluteal fat [19]. Seminal studies performed in the 1980's provided evidence that overall adiposity, as well as subcutaneous fat mass, have a heritability of approximately 30% [20]. This estimate was corroborated by a 1990 study in Caucasian male twins that showed a 31% heritability of waist-to-hip ratio, while a more recent population-based study estimated heritability of the same trait at 39% [21], [22]. Some estimates indicate that heritability of fat distribution is greater in women than in men [19]. Fat distribution and heritability also differ across ethnic groups [23], [24]. One approach to identify the genes contributing to sex differences in adipose tissue accumulation and distribution is genome-wide association studies (GWAS) in large human cohorts. This approach types genetic variants across the genome and correlates their occurrence with a trait to identify loci that are associated. GWAS performed in hundreds of thousands of people have identified more than 100 genetic loci that harbor common genetic variants that influence adiposity [25], [26], [27]. Importantly, at least 17 loci that are associated with body mass index have also been identified in GWAS for type 2 diabetes [25].
Analysis of accumulated GWAS data has revealed a distinct genetic architecture for loci affecting adiposity in males and females. For example, a meta-analysis of more than 50 GWAS studies with waist-to-hip ratio (adjusted for total fat) in more than 200,000 individuals identified 49 loci, 20 of which showed sex-specific effects, with 19 of these having stronger effects in women [28]. These loci represent a rich resource for the identification of sex-biasing genetic factors for body composition and fat distribution, although at present, these loci together account for only a few percent of the genetic variance in adiposity. Much work remains to be done, including the identification of the causal variants at each locus and their mechanism of action to influence adiposity. Of note, these analyses did not take into account loci on the X or Y chromosomes, leaving a gap in our knowledge regarding how genetic variations on X and Y may contribute to observed sex differences in adiposity. The following sections describe studies outside of GWAS that have informed about the role of the X and Y chromosomes in adiposity and metabolic disease.
3. Sex differences in adipose tissue expansion
It has been suggested that adipocytes in gluteal–femoral depots (and other subcutaneous depots) confer better metabolic health because of the ability to expand to store more fat by recruiting new adipocytes [16], [29], [30]. It has previously been thought that male mice exhibit greater diet-induced fat mass expansion (in both visceral and subcutaneous depots) than females, and this is partly due to effects of sex hormones [31], [32], [33]. However, a recent study demonstrates that in C57BL/6J mice, the sex differences in diet-induced weight gain depend strongly on the age of the mice when fed a high fat diet. In juvenile mice (aged 6 weeks), feeding a high-fat diet for 3 months led to greater percent weight gain in males than females. However, in adult mice (aged 31 weeks), the trend was reversed, and females gained substantially greater percent body weight in response to high-fat diet [34].
One potential contributor to sex differences in adipose tissue expansion is the numbers of adipocyte precursor cells (pluripotent stem cells that may differentiate into adipocytes, chondrocytes or osteoblasts) in mouse gonadal or subcutaneous fat depots. On a low-fat chow diet, female C57BL/6J mice have more adipocyte precursor cells than males in gonadal (visceral) and inguinal (subcutaneous) fat pads [35], [36], [37]. When fed a high-fat diet (45% calories as fat), female mice showed increased adipocyte precursor cells and mature adipocytes in gonadal fat, but males did not increase mature fat cells in the gonadal fat pad [36]. Other studies, which employed pulse-labeling or lineage tracing to follow the fate of proliferating adipocyte progenitor cells, inferred that male gonadal fat exhibits hyperplasia in response to a high-fat diet, whereas females exhibit adipocyte hyperplasia in both gonadal and subcutaneous fat depots [38], [39]. The sex-specific patterns were reversed by ovariectomy in female mice, or estrogen administration in male mice, suggesting a role for gonadal hormones [37]. These studies have proved valuable, but additional studies of adipocyte recruitment and turnover in fat depots of both sexes are needed to clarify discrepancies between studies that have used distinct methodologies and to provide additional details.
4. Gonadal and chromosomal influences on adiposity
Gonadal hormones have major effects on fat storage and related diseases, as evidenced by comparisons of pre-menopausal and post-menopausal women. After menopause and reduction in the levels of estrogen and other gonadal hormones, women typically experience increased fat storage in abdominal depots, and an increased occurrence of cardiovascular diseases, hyperlipidemia, insulin resistance, and hypertension [14], [40], [41], [42], [43]. However, short-term reduction in estrogen levels (4 weeks) did not lead to altered fat storage, although it did affect post-prandial circulating triglyceride levels [44]. By contrast, short-term (4 weeks) suppression of testosterone levels in men led to increased storage of meal-derived fatty acids in the gluteal–femoral fat depot [45]. The effects of gonadal hormones on fat depot development and metabolism are likely influenced by numerous variables, including hormone levels and estrogen and androgen receptor levels [46].
Beyond the well-established roles of gonadal hormones, accumulating evidence supports a key role for the sex chromosomes in the determination of sex differences in adiposity. The presence of XX chromosomes in females and XY chromosomes in males determines the development of ovaries or testes, respectively [47]. Specifically, the SRY (sex-determining region Y) gene present on the Y chromosome encodes a transcription factor that initiates testes development in XY embryos, and subsequent elaboration of testicular hormones. Lack of an SRY gene in an embryo (due to XX chromosomes or deletion of the SRY gene) leads to the development of ovaries and production of ovarian hormones. Sex differences in body composition are evident prior to exposure to gonadal hormones. For example, during human and mouse embryonic development, male fetuses show a larger size than female fetuses even before gonadal differentiation [48], [49]. Children continue to exhibit sex differences in body weight at birth, with male babies typically weighing more than female babies, in part due to greater body length and larger head circumference [50]. Male/female differences in height, weight, lean mass, and total body fat mass persist in childhood, prior to puberty [51].
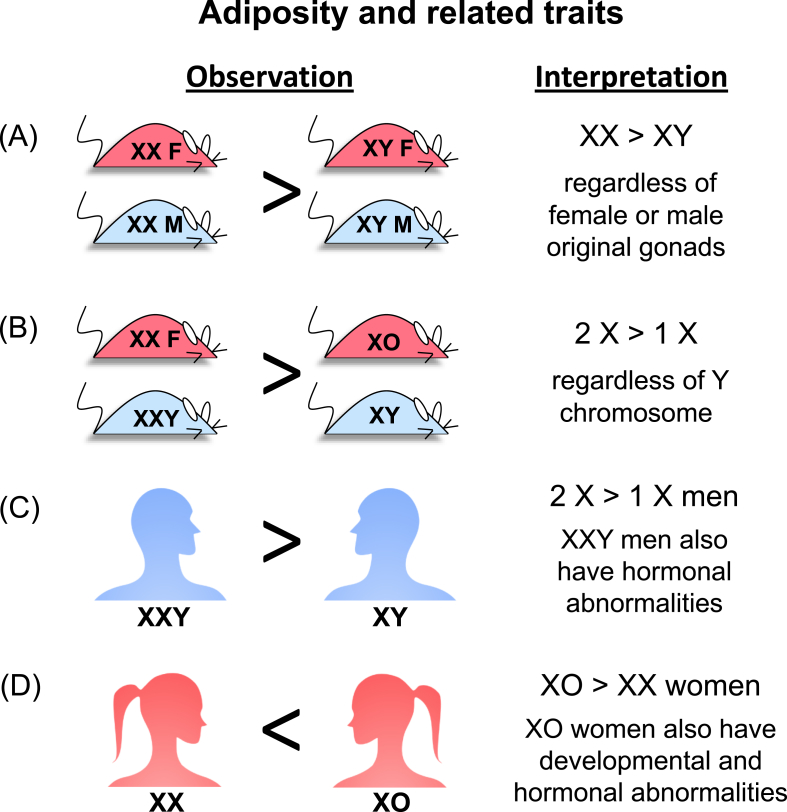
One approach to study effects of sex chromosome complement is to assess adiposity and related traits in individuals with Klinefelter syndrome (XXY) or Turner syndrome (XO). Klinefelter syndrome is the most common sex chromosome disorder, occurring in approximately 1 in 660 births, and is a frequent cause of infertility and hypogonadism in men [52]. These men typically present with a eunuchoid body habitus, small testes, hypergonadotrophic hypogonadism, and infertility [53]. Individuals with Klinefelter have been shown to have an almost five-fold higher incidence of developing metabolic syndrome [54], defined as the occurrence of at least three of the following: abdominal obesity, elevated fasting glucose levels, elevated triglyceride levels, reduced high-density lipoprotein levels, and hypertension [55]. XXY men also have increased risk of insulin resistance and type 2 diabetes [56], [57]. It is possible that the increased risk for metabolic syndrome and insulin resistance in XXY individuals is secondary to increased abdominal obesity, which, in turn, may stem from hypogonadism and low testosterone levels in XXY men [54], [56], [58]. Importantly, studies of prepubertal XXY boys (mean age < 11) identified increased body fat mass and/or waist circumference prior to an influence of altered gonadal hormone levels at puberty [59], [60]. This suggests that XXY sex chromosome complement itself may affect adiposity and related metabolic traits.
Turner syndrome (TS) is the most common sex chromosome abnormality affecting females, with an incidence of 1 in 2500–3000 live births (8). TS women are characterized by short stature, a webbed neck, and sexual infantilism with gonadal dysgenesis [61], [62]. TS women also have congenital heart defects, which may predispose to heart disease that develops independent of metabolic factors [63]. Relevant to metabolic disease, XO women have dramatically reduced gonadal hormone levels [64]. Since estrogens and progestins in normal pre-menopausal women confer protection against abdominal obesity and insulin resistance and promote fat storage in gluteal–femoral fat pads [40], the low levels of these hormones may obscure any direct effects of the XO chromosome complement on metabolism. Consistent with low gonadal hormone levels, TS women have a four-fold increase in risk for type 2 diabetes and a three-fold increase in overall mortality [65]. A potential role for early fetal programming has been proposed to contribute to the metabolic abnormalities in TS women, which is consistent with low birth weight and high body weight in adult life [66]. Other data show a role for sex chromosome number, as described below.
The confounding effect of aberrant gonadal hormone levels in XO women makes it difficult to reach conclusions about the influence of the sex chromosome complement on metabolic homeostasis in these individuals. From an experimental perspective, it would be valuable to compare the effects of a single X chromosome in TS women to individuals with two X chromosomes on a background of similarly low gonadal hormones. The latter condition is approximated in XX women with primary ovarian insufficiency (POI) and corresponding low gonadal hormone levels. A comparison of lipid profiles in women with TS and POI (age- and body mass-matched) two weeks after halting estrogen replacement therapy showed that TS women had a more atherogenic lipid profile [67]. Because both TS and POI women had reduced gonadal hormone contributions compared to normal women, and were matched for lifestyle and body composition, investigators have hypothesized that some metabolic effects in TS women may be attributed to the altered X chromosome dosage [57], [67]. It is important to recognize, however, that comparisons between TS and POI women remain confounded by the fact that TS individuals have aberrant development and impaired ovarian hormone secretion through their entire life, whereas POI women are normal during development and are only affected later in life.
Additional sex chromosome anomalies occurring in humans include Swyer syndrome and Triple X syndrome. Swyer syndrome, also known as XY gonadal dysgenesis is a rare disorder of sexual development in which 46,XY individuals have impaired gonadal tissue development [68]. These individuals present externally as females but fail to develop functional ovaries and do not undergo puberty unless treated with hormone therapy. This condition can be due to SRY gene mutations (thought to underlie 10–20% of cases) or mutations in other genes [68]. The Triple X syndrome (47, XXX) is characterized by variable presentation of phenotypes such as tall stature, cognitive and behavioral disorders and dental issues [69]. At present, no studies have been reported with adequate sample sizes of either Swyer or XXX syndromes to analyze effects of these conditions on adiposity or related metabolic traits. It should be noted that in XO, XX, and XXX cells, X chromosome inactivation silences most genes on all but a single X chromosome. The occurrence of abnormalities in both Turner (XO) and XXX syndromes therefore supports the view that X chromosome dosage is important, despite the process of inactivation of all but one X. Possible reasons for the importance of having exactly two X chromosomes is discussed in sections 5, 8.
5. A role for XX chromosome dosage in sex differences in adiposity
Conclusive studies of metabolic changes in XXY men and XO women have been hampered by the lack of suitable control groups with similar gonadal hormone levels, by limited statistical power due to small cohorts, and by heterogeneity among individuals in the genetic and environmental factors that influence obesity. The use of mouse models makes it possible to overcome some of these limitations and to evaluate the relative contributions of sex chromosomes and gonadal hormones to sex differences.
A traditional approach used in mouse models to identify the cause of sex differences is to assess the action of gonadal hormones after puberty [70]. Adult female and male mice are gonadectomized and assessed to find whether the original sex difference is eliminated. If so, replacement of hormones by an implanted pump or daily injection is performed and mice observed to determine if the original sex difference is restored. Follow-up studies may include identification of the hormone receptor that mediates the effect, which can be done through chemical or genetic inhibition of hormone receptor function. For sex differences that occur before puberty, or which are not altered by gonadectomy, the role of the surges in testosterone that occur pre- or –postnatally may be studied. For meaningful performance and interpretation of studies that manipulate gonadal hormones, many factors must be considered and are reviewed elsewhere [70]. If gonadal hormone differences cannot fully explain a sex difference, the potential effect of sex chromosome complement should be considered.
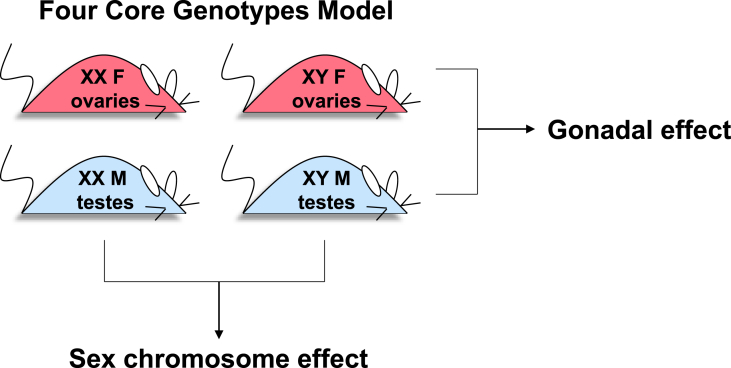
A mouse model that has been specifically designed to parse the effects of gonadal hormones as well as sex chromosomes is the Four Core Genotypes (FCG) mouse. The model involves the relocation of the Sry gene, which promotes the development of testes, from the Y chromosome to an autosome such that gonadal sex can segregate independently from sex chromosome complement [70], [71]. The position of the Sry gene on an autosome does not alter the prenatal or adult androgen levels [72]. Studies performed with FCG mice involve the comparison of four genotypes, which shuffle the chromosomal and gonadal components of sex to generate XX mice with either ovaries or testes and XY mice with either ovaries or testes (referred to, respectively, as XX F, XX M, XY F, and XY M). A two-by-two comparison of these four genotypes allows identification of effects that are dictated by either gonadal type or sex chromosome type (Figure 1). To eliminate the contribution of circulating gonadal hormones, the FCG mice are often studied after gonadectomy as adults.
Figure 1.
The Four Core Genotypes (FCG) mouse model. The FCG mouse model uncouples chromosomal sex from gonadal sex to reveal contributions of each to traits of interest. Traits that are influenced by gonadal hormones (or other effects conferred by ovaries or testes) appear similar between XX and XY mice with ovaries, and between XX and XY mice with testes, whereas the groups with ovaries and testes differ from one another. Conversely, traits that are influenced by sex chromosome complement appear similar between XX animals with ovaries or testes, and XY animals with ovaries or testes, and the XX and XY groups differ from one another. For information on the derivation of FCG mice, see Ref. [71].
Using the FCG model, effects of sex chromosome complement on several metabolic traits have been identified, including obesity, plasma lipid profile, food intake, and hypertension [73], [74], [75], [76], [77]. In C57BL/6 FCG mice that were gonadectomized as adults to remove acute effects of gonadal hormones, mice with XX versus XY chromosomes (regardless of male or female gonads present originally) gained weight more rapidly on a chow or high-fat diet, and accumulated greater body fat, particularly in the inguinal subcutaneous fat depot (Figure 2) [73]. Increased weight gain in XX compared to XY mice was promoted, at least in part, by enhanced food intake by XX mice, specifically during the light phase of the circadian cycle [74]. As nocturnal animals, mice consume approximately 70% of their calories during the dark phase, and they rest and snack (30% of total calories) during the light phase. The enhanced food intake by XX mice in the light phase was due to anticipatory eating beginning about 1.5 h before their XY counterparts [74]. In addition to increased adipose tissue accumulation, XX mice fed a high-fat diet for 16 weeks developed obesity-related conditions such as fatty liver, elevated total blood cholesterol levels, and elevated insulin levels [73]. It is likely that these metabolic disturbances are secondary to the enhanced fat accumulation in XX mice fed a high-fat diet since they are not seen in XX mice fed a chow diet.
Figure 2.
Summary of the influence of sex chromosome complement on adiposity and related traits in mice and humans. (A) Studies of the Four Core Genotypes mice revealed that the presence of XX chromosomes leads to increased body weight, fat mass and related morbidities compared to XY mice (all mice gonadectomized as adults to remove acute effects of gonadal hormones). (B) Studies of XY* mice determined that the presence of two X chromosomes promotes greater body weight and fat mass than those with one X chromosome, with no evident effect of the Y chromosome (all mice gonadectomized as adults). (C) XXY compared to XY men show greater abdominal fat, insulin resistance and related metabolic abnormalities. XXY men also have reduced gonadal hormone levels. (D) XO compared to XX women show increased body fat, but also suffer from developmental and hormonal abnormalities. Data are summarized from studies cited in the main text.
Data from the FCG mice clearly showed an effect of XX compared to XY chromosomes on adiposity and obesity-related morbidities. But these studies alone did not distinguish between an effect of X chromosome dosage (two versus one X) or an effect of the presence or absence of the Y chromosome. Analysis of mouse models with additional sex chromosome genotypes made it possible to identify the relevant sex chromosome effect. By making use of an unusual Y chromosome with sequence duplications in the pseudoautosomal region (known as the XY* model, [71]), mice with XX, XY, XO and XXY chromosomes were assessed for body weight and adiposity. The results showed that the presence of two X chromosomes (XX and XXY mice) led to higher body weight/fat than one X chromosome (XY and XO); the presence of the Y chromosome did not have an influence (Figure 2) [73].
6. Sex chromosome effects on lipid levels
Beyond adiposity, sex chromosome complement in mouse and humans influences plasma lipid levels. Elevated levels of total plasma cholesterol levels and of specific lipoprotein subclasses have well-known epidemiological associations with cardiovascular disease. At a general level, elevations in low-density lipoproteins (LDL) and reductions in high-density lipoprotein (HDL) levels are associated with increased cardiovascular disease risk [78]. Current results suggest that specific components of the lipid profile are influenced independently by X chromosome dosage, presence of the Y chromosome, and gonadal hormones; the sex differences observed are likely a composite of all of these contributions. For example, gonadal hormones appear to regulate lipoprotein metabolism, as pre-menopausal women tend to have higher HDL levels and lower LDL levels than men, and post-menopause, lipoprotein profiles become more similar to men (reviewed in Ref. [18]). Evidence for Y chromosome effects come from the demonstration that genetic variations in the Y chromosome are associated with lipoprotein profiles in men [79], [80] and mice [81]. In addition, studies with the FCG mouse model revealed effects of X chromosome dosage on HDL cholesterol levels, whereas gonadal hormones had effects on triglyceride and fatty acid levels [18], [77].
7. Sex differences in inflammation and immunometabolism
Obesity is an inflammatory disease, and it has been shown in mice that males and females differ in the inflammatory response that occurs in diet-induced obesity [82]. Sex differences in the inflammatory response that is elicited by high-fat diet in mice include increased adipose tissue infiltration with M1 (CD11c+) macrophages in males, but M2 (CD11c–) macrophages in females, and increased levels of inflammatory cytokines in males compared to females [83]. Bone marrow transplant studies demonstrated that the sex-dependent inflammatory response is related to a cell-intrinsic sexual dimorphism in the composition or activation of hematopoietic stem cell populations [83].
Autoimmune diseases are among the most strongly sex-biased of all diseases, and 80% of autoimmune patients are women [84]. Obesity is a risk factor for autoinflammatory and autoimmune diseases including systemic lupus erythematosus, type 1 diabetes, rheumatoid arthritis, and psoriasis [85]. Likely mechanistic links between obesity and autoimmune dysfunction include sex-dependent alterations in the levels of adipokines produced by adipocytes (e.g., leptin, resistin, adiponectin, visfatin), and production of inflammatory cytokines (e.g., tumor necrosis factor α, interleukin-6). Both gonadal hormones and X chromosome dosage have been implicated in the female bias in autoimmune disease [86]. Consistent with effects of gonadal hormones, sex bias in autoimmune disease is more evident after puberty. Estrogens and androgens exert distinct influences over cytokine production and T cell differentiation. Testosterone is suppressive of both the adaptive and innate immune response, whereas estrogen may promote inflammatory responses [87], [88]. An interaction between early life gut microbial composition and gonadal hormone levels has been identified in type 1 diabetes in the non-obese diabetic mouse [89]. In this model, the commensal bacteria in the gut elevated serum testosterone levels in males, which was protective against type 1 diabetes. The transfer of gut microbial populations from adult males to immature females resulted in elevated testosterone levels, reduced pancreatic islet inflammation, and protection against type 1 diabetes. These effects were dependent on androgen receptor activity.
In addition to the effects of gonadal hormones on autoimmunity, sex chromosome complement likely plays a role. Using the FCG mouse model to study experimental autoimmune encephalomyelitis (relevant to multiple sclerosis) and spontaneous lupus, disease was more severe in XX compared to XY mice with female gonads [90], [91], [92]. Studies of DNA methylation suggested that maternal versus paternal imprinting of X chromosome genes could contribute to the sex differences in experimental immune encephalitis [93]. Y chromosome gene dosage has also been implicated in autoimmune disease. For example, natural genetic variation in the copy number of multicopy genes on the Y chromosome is associated with susceptibility to experimental allergic encephalomyelitis and experimental myocarditis in the mouse [94]. Studying consomic Y chromosome mouse strains (which possess the Y chromosome from one inbred mouse strain on the background of another inbred strain) revealed a link between copy number of two specific Y chromosome genes and gene expression in immune cells [94]. The role of Y chromosome sequences as a trans-acting regulator of gene expression may contribute to the sexual dimorphism in autoimmune disease. However, interpretation of such findings is complex. On one hand, they could indicate that the Y chromosome may be partly responsible for conferring the observed sex differences in susceptibility to autoimmune disease. On the other, it has been suggested that gene expression from the Y chromosome acts as a “balancer” to the effects of the X chromosome. In this view, expression of genes from the Y chromosome makes males more like females, such that altered Y chromosome gene dosage may tip the balance and influence sex differences in autoimmune diseases and other traits [95].
8. Potential mechanisms for sex chromosome effects on metabolism
There are multiple mechanisms that may underlie the effects of sex chromosome complement on obesity, lipid metabolism, autoimmunity, and other traits. The presence of XX or XY chromosomes profoundly influences the developmental path and sets up a lifelong difference between sexes in the levels of gonadal hormones. Both gonadal hormones and sex chromosomes influence gene expression, which likely underlies phenotypic differences observed between males and females. Early studies of gene expression in male and female mice from a genetic intercross of two inbred strains revealed male/female differences in gene expression in adipose tissue, liver, and skeletal muscle (thousands of genes) as well as brain (hundreds of genes) [96]. This study also provided evidence for the global regulation of subsets of the genes that were differentially expressed between the sexes. A subsequent study in the same mouse intercross population revealed correlations between body fat and lipid levels and sex-specific gene expression modules in adipose tissue and liver; many of the modules were shown to be regulated by gonadal hormones [97]. Additional studies have revealed gonadal hormone influences on transcriptional profiles of subcutaneous and visceral adipose tissue depots [31], [36].
Mouse models have also revealed effects of sex chromosomes on gene expression. Of note, sex chromosomes influence the expression of hundreds of autosomal genes and some of these effects are modulated by the presence of the testis-determining gene, Sry [98], [99], [100]. MicroRNAs (miRNAs), which modulate gene expression levels by interacting with specific mRNA species to increase their degradation or interfere with translation, are also influenced by sex. Using the FCG mouse model to parse effects of gonadal and chromosomal sex, the overall miRNA expression profile showed a bias toward higher expression in male compared to female gonadal adipose tissue [101]. This bias was normalized after gonadectomy, indicating a role for circulating gonadal hormones in modulating miRNA levels. In mice fed a high-fat diet, miRNA levels were higher in mice with XX versus XY chromosome complement. Thus, there is a compound effect of gonadal hormones and sex chromosomes on miRNA expression, and presumably, on the mRNAs regulated by these miRNAs.
Recent analysis of human transcriptome data from the GTEx project is consistent with mouse data in detecting thousands of genes that are differentially expressed in male and female tissues [102]. The GTEx project includes RNA-seq data from >50 tissues from hundreds of men and women [103]. Most genes that were found to have sex differences in expression levels exhibited these differences in one or a few tissues [102]. By contrast, 16 X-chromosome genes had sex differences across at least six tissues, suggesting widespread effects. Of tissues found in both males and females, the most sex-differentiated gene expression occurred in breast tissue, consistent with the sexually dimorphic physiology and structure of breast tissue [102].
It is straightforward to imagine how gonadal hormones could influence gene expression across the genome, but less intuitive to understand how differences in sex chromosome complement could alter expression of genes on autosomes. The presence of two X chromosomes in a cell confers unique properties that are not present in an XY cell. In XX cells, one X chromosome is transcriptionally silenced in cells outside of the germline to reduce the expression of most X chromosome genes so that levels are comparable between XX and XY cells. However, some X chromosome genes “escape” inactivation and remain transcriptionally active on both X chromosomes in XX cells. Importantly, genes that escape X inactivation are largely conserved between human and mouse [104], [105], [106], [107], [108].
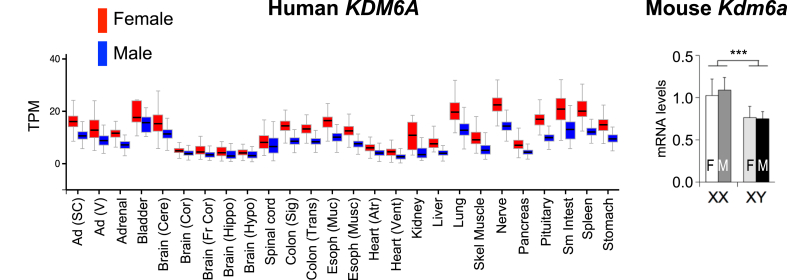
The X inactivation escapee genes exhibit increased expression in tissues from women compared to men, including in metabolic tissues such as adipose tissue and liver. An expression profile in men and women for a representative X chromosome escapee gene (KDM6A) is shown in Figure 3. The corresponding mouse gene (Kdm6a) exhibits increased expression levels in XX compared to XY FCG mice, showing that this effect is determined by sex chromosome complement rather than gonadal hormones (Figure 3). It is estimated that 3–6% of mouse X chromosome genes escape inactivation, and these same genes plus others (approximately 15% of X chromosome genes) escape inactivation in humans [104], [105], [106], [107], [108]. Some X escapee genes have partner genes that were retained on the Y chromosome, and escape from inactivation may help normalize expression of these genes between XX and XY cells. However, many more X chromosome genes escape inactivation than have partners on the Y chromosome [107]. The X escapee genes encode proteins that may influence gene expression broadly, including histone modifying enzymes, translation initiation proteins, and RNA helicases, to name a few. Differences also exist between XX and XY cells due to parent-of-origin imprinting. Genomic imprinting is the heritable DNA or histone methylation at specific regions of chromosomes that leads to transcriptional silencing. Imprints are established in the germline of the mother or father and passed to offspring such that the allele from only one parent will be expressed in the offspring. XX cells receive an X chromosome from both father and mother, whereas an XY cell receives an X exclusively from the mother. Thus, only XX cells undergo paternal X chromosome imprinting, which can lead to differences in gene expression in XX compared to XY cells. For more extensive discussion of these mechanisms, the reader is referred to excellent recent reviews [47], [71].
Figure 3.
Sex differences in expression of a representative gene that escapes X chromosome inactivation in humans and mice. Left, The X-linked KDM6A gene encodes a histone demethylase and escapes inactivation leading to increased gene expression levels across many human tissues. Data shown are transcripts per million (TPM) in RNA-seq data of the GTEx project [103] and downloaded from gtexportal.org. Ad (SC), subcutaneous adipose tissue; Ad (V), visceral adipose tissue; Cere, cerebellum; Cor, cortex; Fr Cor, frontal cortex, Hippo, hippocampus; Hypo, hypothalamus, Colon (Sig), sigmoidal colon; Colon (Trans), transcending colon; Esoph (Muc) mucosal esophagus, Esoph (musc), muscular esophagus; Atr, atrium; Vent, ventricle; Skel Muscle, skeletal muscle. Right, Hepatic mRNA levels for the mouse Kdm6a gene in Four Core Genotypes mice determined by qPCR (from Refs. [18], [77]). Expression levels in XX mice with ovaries (F) or testes (M) are higher than in XY mice with ovaries or testes, demonstrating a sex chromosome effect, and reflective of Kdm6a escape from X chromosome inactivation.
9. Future perspectives
We propose that we will not fully understand many aspects of metabolism until we understand sex differences in metabolism. This includes differences between women and men in the risk and development of obesity and in the manifestation of related morbidities. The identification of these differences may reveal how one sex is protected from specific disease outcomes compared to the other and suggest new prevention and treatment possibilities for both sexes. Research approaches that consider the components of sex-biasing effects—including differences dictated by gonad type and by sex chromosome complement—will provide the most complete understanding. The study of mouse models with unique sex chromosome combinations and humans with naturally occurring sex chromosome variations have already begun to hint at important effects of sex chromosomes on obesity, lipid metabolism, and inflammation. Further understanding will require a more complete elucidation of the molecular differences imparted by presence of XX or XY chromosome complements. The current recognition of sex as an important biological variable to be considered in research [109], [110], [111] will likely provide new (and welcome) motivation to push the field forward.
Funding
We are grateful for support from the Public Health Service (R01 DK083561, P01 HL28481).
Author contributions
KR conceptualized the manuscript; TZ, MP, and KR wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Voskuhl R. Sex differences in autoimmune diseases. Biology of Sex Differences. 2011;2(1):1. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nature Reviews Immunology. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 3.Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clinical Reviews in Allergy and Immunology. 2017 doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 4.Alswat K.A. Gender disparities in osteoporosis. Journal of Clinical Medicine Research. 2017;9(5):382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laws K.R., Irvine K., Gale T.M. Sex differences in Alzheimer's disease. Current Opinion in Psychiatry. 2018;31(2):133–139. doi: 10.1097/YCO.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 6.Rainville J.R., Tsyglakova M., Hodes G.E. Deciphering sex differences in the immune system and depression. Frontiers in Neuroendocrinology. 2017 doi: 10.1016/j.yfrne.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Werling D.M., Geschwind D.H. Sex differences in autism spectrum disorders. Current Opinion in Neurology. 2013;26(2):146–153. doi: 10.1097/WCO.0b013e32835ee548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurado-Coronel J.C., Cabezas R., Ávila Rodríguez M.F., Echeverria V., García-Segura L.M., Barreto G.E. Sex differences in Parkinson's disease: features on clinical symptoms, treatment outcome, sexual hormones and genetics. Frontiers in Neuroendocrinology. 2017 doi: 10.1016/j.yfrne.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Li R., Ma X., Wang G., Yang J., Wang C. Why sex differences in schizophrenia? Journal of Translational Neuroscience. 2016;1(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Regitz-Zagrosek V. Sex and gender differences in health. EMBO Reports. 2012;13(7):596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobyns W. The pattern of inheritance of X-linked traits is not dominant or recessive, just X-linked. Acta Paediatrica. 2006;95(0):11–15. doi: 10.1111/j.1651-2227.2006.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 12.Shah R.R., Bird A.P. MeCP2 mutations: progress towards understanding and treating Rett syndrome. Genome Medicine. 2017;9(1):17. doi: 10.1186/s13073-017-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34(3):309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karastergiou K., Smith S.R., Greenberg A.S., Fried S.K. Sex differences in human adipose tissues – the biology of pear shape. Biology of Sex Differences. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpe F., Pinnick K.E. Biology of upper-body and lower-body adipose tissue—link to whole-body phenotypes. Nature Reviews Endocrinology. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 16.Karastergiou K., Fried S.K. Cellular mechanisms driving sex differences in adipose tissue biology and body shape in humans and mouse models. Advances in Experimental Medicine and Biology. 2017;1043:29–51. doi: 10.1007/978-3-319-70178-3_3. [DOI] [PubMed] [Google Scholar]
- 17.Mundi M.S., Koutsari C., Jensen M.D. Effects of increased free fatty acid availability on adipose tissue fatty acid storage in men. The Journal of Clinical Endocrinology & Metabolism. 2014;99(12):E2635–E2642. doi: 10.1210/jc.2014-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link J.C., Reue K. Genetic basis for sex differences in obesity and lipid metabolism. Annual Review of Nutrition. 2017;37(1):225–245. doi: 10.1146/annurev-nutr-071816-064827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulit S.L., Karaderi T., Lindgren C.M. Sexual dimorphisms in genetic loci linked to body fat distribution. Bioscience Reports. 2017;37(1) doi: 10.1042/BSR20160184. BSR20160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchard C., Pérusse L., Leblanc C., Tremblay A., Thériault G. Inheritance of the amount and distribution of human body fat. International Journal of Obesity. 1988;12(3):205–215. [PubMed] [Google Scholar]
- 21.Selby J.V., Newman B., Quesenberry C.P., Fabsitz R.R., Carmelli D., Meaney F.J. Genetic and behavioral influences on body fat distribution. International Journal of Obesity. 1990;14(7):593–602. [PubMed] [Google Scholar]
- 22.van Dongen J., Willemsen G., Chen W.-M., de Geus E.J.C., Boomsma D.I. Heritability of metabolic syndrome traits in a large population-based sample. Journal of Lipid Research. 2013;54(10):2914–2923. doi: 10.1194/jlr.P041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lear S.A., Humphries K.H., Kohli S., Chockalingam A., Frohlich J.J., Birmingham C.L. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT) The American Journal of Clinical Nutrition. 2007;86(2):353–359. doi: 10.1093/ajcn/86.2.353. [DOI] [PubMed] [Google Scholar]
- 24.Demerath E.W., Sun S.S., Rogers N., Lee M., Reed D., Choh A.C. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity. 2007;15(12):2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaderi T., Drong A.W., Lindgren C.M. Insights into the genetic susceptibility to type 2 diabetes from genome-wide association studies of obesity-related traits. Current Diabetes Reports. 2015;15(10):83. doi: 10.1007/s11892-015-0648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes M.V., Pulit S.L., Lindgren C.M. Genetic and epigenetic studies of adiposity and cardiometabolic disease. Genome Medicine. 2017;9(1):82. doi: 10.1186/s13073-017-0474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodarzi M.O. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. The Lancet Diabetes & Endocrinology. 2018;6(3):223–236. doi: 10.1016/S2213-8587(17)30200-0. [DOI] [PubMed] [Google Scholar]
- 28.Shungin D., Winkler T.W., Croteau-Chonka D.C., Ferreira T., Locke A.E., Mägi R. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson P.R., Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. Journal of Lipid Research. 1972;13(1):2–11. [PubMed] [Google Scholar]
- 30.Medrikova D., Jilkova Z.M., Bardova K., Janovska P., Rossmeisl M., Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. International Journal of Obesity. 2012;36(2):262–272. doi: 10.1038/ijo.2011.87. [DOI] [PubMed] [Google Scholar]
- 31.Grove K.L., Fried S.K., Greenberg A.S., Xiao X.Q., Clegg D.J. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. International Journal of Obesity (2005) 2010;34(6):989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong J., Stubbins R.E., Smith R.R., Harvey A.E., Núñez N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutrition Journal. 2009;8(1):11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira Potter V.J., Strissel K.J., Xie C., Chang E., Bennett G., Defuria J. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology. 2012;153(9):4266–4277. doi: 10.1210/en.2011-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salinero A.E., Anderson B.M., Zuloaga K.L. Sex differences in the metabolic effects of diet-induced obesity vary by age of onset. International Journal of Obesity. 2018;(2005) doi: 10.1038/s41366-018-0023-3. [DOI] [PubMed] [Google Scholar]
- 35.Joe A.W.B., Yi L., Even Y., Vogl A.W., Rossi F.M.V. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells. 2009;27(10):2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y., Lee M.-J., Ido Y., Fried S.K. High-fat diet-induced obesity regulates MMP3 to modulate depot- and sex-dependent adipose expansion in C57BL/6J mice. American Journal of Physiology-Endocrinology and Metabolism. 2017;312(1):E58–E71. doi: 10.1152/ajpendo.00128.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery E., Wing A., Holtrup B., Sebo Z., Kaplan J.L., Saavedra-Peña R. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metabolism. 2016;24(1):142–150. doi: 10.1016/j.cmet.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature Medicine. 2013;19(10):1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeffery E., Church C.D., Holtrup B., Colman L., Rodeheffer M.S. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nature Cell Biology. 2015;17(4):376–385. doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garaulet M., Pérez-Llamas F., Baraza J.C., Garcia-Prieto M.D., Fardy P.S., Tébar F.J. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables. The Journal of Nutrition, Health & Aging. 2002;6(2):123–126. [PubMed] [Google Scholar]
- 41.Lima R., Wofford M., Reckelhoff J.F. Hypertension in postmenopausal women. Current Hypertension Reports. 2012;14(3):254–260. doi: 10.1007/s11906-012-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas A.H.E.M., Appelman Y.E.A. Gender differences in coronary heart disease. Netherlands Heart Journal. 2010;18(12):598–602. doi: 10.1007/s12471-010-0841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamboni M., Armellini F., Milani M.P., De Marchi M., Todesco T., Robbi R. Body fat distribution in pre- and post-menopausal women: metabolic and anthropometric variables and their inter-relationships. International Journal of Obesity and Related Metabolic Disorders. 1992;16(7):495–504. [PubMed] [Google Scholar]
- 44.Santosa S., Bonnes S.L., Jensen M.D. Acute female hypogonadism alters adipose tissue fatty acid storage factors and chylomicronemia. The Journal of Clinical Endocrinology & Metabolism. 2016;101(5):2089–2098. doi: 10.1210/jc.2015-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santosa S., Bush N.C., Jensen M.D. Acute testosterone deficiency alters adipose tissue fatty acid storage. The Journal of Clinical Endocrinology & Metabolism. 2017;102(8):3056–3064. doi: 10.1210/jc.2017-00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuente-Martín E., Argente-Arizón P., Ros P., Argente J., Chowen J.A. Sex differences in adipose tissue. Adipocyte. 2013;2(3):128–134. doi: 10.4161/adip.24075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold A.P. A general theory of sexual differentiation. Journal of Neuroscience Research. 2017;95(1–2):291–300. doi: 10.1002/jnr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bukowski R., Smith G.C.S., Malone F.D., Ball R.H., Nyberg D.A., Comstock C.H. Human sexual size dimorphism in early pregnancy. American Journal of Epidemiology. 2007;165(10):1216–1218. doi: 10.1093/aje/kwm024. [DOI] [PubMed] [Google Scholar]
- 49.Burgoyne P.S., Thornhill A.R., Boudrean S.K., Darling S.M., Bishop C.E., Evans E.P. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philosophical Transactions of the Royal Society B: Biological Sciences. 1995;350(1333):253–261. doi: 10.1098/rstb.1995.0159. [DOI] [PubMed] [Google Scholar]
- 50.Wells J.C.K. Sexual dimorphism of body composition. Best Practice & Research. Clinical Endocrinology & Metabolism. 2007;21(3):415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Taylor R.W., Grant A.M., Williams S.M., Goulding A. Sex differences in regional body fat distribution from pre- to postpuberty. Obesity. 2010;18(7):1410–1416. doi: 10.1038/oby.2009.399. [DOI] [PubMed] [Google Scholar]
- 52.Klinefelter H.F. Background of the recognition of Klinefelter's syndrome as a distinct pathologic entity. American Journal of Obstetrics and Gynecology. 1973;116(3):436–437. doi: 10.1016/s0002-9378(15)31307-7. [DOI] [PubMed] [Google Scholar]
- 53.Smyth C.M., Bremner W.J. Klinefelter syndrome. Archives of Internal Medicine. 1998;158(12):1309–1314. doi: 10.1001/archinte.158.12.1309. [DOI] [PubMed] [Google Scholar]
- 54.Bojesen A., Kristensen K., Birkebaek N.H., Fedder J., Mosekilde L., Bennett P. The metabolic syndrome is frequent in Klinefelter's Syndrome and is associated with abdominal obesity and hypogonadism. Diabetes Care. 2006;29(7):1591–1598. doi: 10.2337/dc06-0145. [DOI] [PubMed] [Google Scholar]
- 55.Lusis A.J., Attie A.D., Reue K. Metabolic syndrome: from epidemiology to systems biology. Nature Reviews. Genetics. 2008;9(11):819–830. doi: 10.1038/nrg2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bojesen A., Host C., Gravholt C.H. Klinefelter's syndrome, type 2 diabetes and the metabolic syndrome: the impact of body composition. Molecular Human Reproduction. 2010;16(6):396–401. doi: 10.1093/molehr/gaq016. [DOI] [PubMed] [Google Scholar]
- 57.Salgin B., Amin R., Yuen K., Williams R.M., Murgatroyd P., Dunger D.B. Insulin resistance is an intrinsic defect independent of fat mass in women with Turner's syndrome. Hormone Research. 2006;65(2):69–75. doi: 10.1159/000090907. [DOI] [PubMed] [Google Scholar]
- 58.Han S.J., Kim K.S., Kim W., Kim J.H., Lee Y.H., Nam J.S. Obesity and hyperglycemia in Korean men with Klinefelter syndrome: the Korean Endocrine Society Registry. Endocrinology and Metabolism. 2016;31(4):598–603. doi: 10.3803/EnM.2016.31.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aksglaede L., Molgaard C., Skakkebaek N.E., Juul A. Normal bone mineral content but unfavourable muscle/fat ratio in Klinefelter syndrome. Archives of Disease in Childhood. 2008;93(1):30–34. doi: 10.1136/adc.2007.120675. [DOI] [PubMed] [Google Scholar]
- 60.Bardsley M.Z., Falkner B., Kowal K., Ross J.L. Insulin resistance and metabolic syndrome in prepubertal boys with Klinefelter syndrome. Acta Paediatrica. 2011;100(6):866–870. doi: 10.1111/j.1651-2227.2011.02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhong Q., Layman L.C. Genetic considerations in the patient with Turner syndrome – 45,X with or without mosaicism. Fertility and Sterility. 2012;98(4):775–779. doi: 10.1016/j.fertnstert.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McDonough P.G., Byrd J.R. Gonadal dysgenesis. Clinical Obstetrics and Gynecology. 1977;20(3):565–579. doi: 10.1097/00003081-197709000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Gravholt C.H. Turner syndrome and the heart: cardiovascular complications and treatment strategies. American Journal of Cardiovascular Drugs: Drugs, Devices, and Other Interventions. 2002;2(6):401–413. doi: 10.2165/00129784-200202060-00005. [DOI] [PubMed] [Google Scholar]
- 64.Gravholt C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. European Journal of Endocrinology. 2004;151(6):657–687. doi: 10.1530/eje.0.1510657. [DOI] [PubMed] [Google Scholar]
- 65.Gravholt C.H., Jensen A.S., Høst C., Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatrica. 2011;100(6):871–877. doi: 10.1111/j.1651-2227.2011.02233.x. [DOI] [PubMed] [Google Scholar]
- 66.Baldin A.D., Siviero-Miachon A.A., Fabbri T., de Lemos-Marini S.H.V., Spinola-Castro A.M., Baptista M.T.M. Turner syndrome and metabolic derangements: another example of fetal programming. Early Human Development. 2012;88(2):99–102. doi: 10.1016/j.earlhumdev.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 67.Van P.L., Bakalov V.K., Bondy C.A. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. The Journal of Clinical Endocrinology & Metabolism. 2006;91(8):2867–2870. doi: 10.1210/jc.2006-0503. [DOI] [PubMed] [Google Scholar]
- 68.King T.F.J., Conway G.S. Swyer syndrome. Current Opinion in Endocrinology, Diabetes, and Obesity. 2014;21(6):504–510. doi: 10.1097/MED.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 69.Wigby K., D'Epagnier C., Howell S., Reicks A., Wilson R., Cordeiro L. Expanding the phenotype of Triple X syndrome: a comparison of prenatal versus postnatal diagnosis. American Journal of Medical Genetics. Part A. 2016;170(11):2870–2881. doi: 10.1002/ajmg.a.37688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mauvais-Jarvis F., Arnold A.P., Reue K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metabolism. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgoyne P.S., Arnold A.P. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biology of Sex Differences. 2016;7(1):68. doi: 10.1186/s13293-016-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Itoh Y., Mackie R., Kampf K., Domadia S., Brown J.D., O'Neill R. Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Research Notes. 2015;8(1):69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen X., McClusky R., Chen J., Beaven S.W., Tontonoz P., Arnold A.P. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genetics. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X., Wang L., Loh D.H., Colwell C.S., Taché Y., Reue K. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Hormones and Behavior. 2015;75:55–63. doi: 10.1016/j.yhbeh.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonthuis P.J., Rissman E.F. Neural growth hormone implicated in body weight sex differences. Endocrinology. 2013;154(10):3826–3835. doi: 10.1210/en.2013-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji H., Zheng W., Wu X., Liu J., Ecelbarger C.M., Watkins R. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55(5):1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Link J.C., Chen X., Prien C., Borja M.S., Hammerson B., Oda M.N. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(8):1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castelli W.P., Anderson K., Wilson P.W., Levy D. Lipids and risk of coronary heart disease. The Framingham Study. Annals of Epidemiology. 1992;2(1–2):23–28. doi: 10.1016/1047-2797(92)90033-m. [DOI] [PubMed] [Google Scholar]
- 79.Charchar F.J., Tomaszewski M., Lacka B., Zakrzewski J., Zukowska-Szczechowska E., Grzeszczak W. Association of the human Y chromosome with cholesterol levels in the general population. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(2):308–312. doi: 10.1161/01.ATV.0000113291.39267.0a. [DOI] [PubMed] [Google Scholar]
- 80.Russo P., Siani A., Miller M.A., Karanam S., Esposito T., Gianfrancesco F. Genetic variants of Y chromosome are associated with a protective lipid profile in black men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(8):1569–1574. doi: 10.1161/ATVBAHA.108.168641. [DOI] [PubMed] [Google Scholar]
- 81.Suto J., Satou K. Effect of the Y chromosome on plasma high-density lipoprotein-cholesterol levels in Y-chromosome-consomic mouse strains. BMC Research Notes. 2014;7(1):393. doi: 10.1186/1756-0500-7-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffin C., Lanzetta N., Eter L., Singer K. Sexually dimorphic myeloid inflammatory and metabolic responses to diet-induced obesity. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2016;311(2):R211–R216. doi: 10.1152/ajpregu.00136.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singer K., Maley N., Mergian T., DelProposto J., Cho K.W., Zamarron B.F. Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet-induced obesity. The Journal of Biological Chemistry. 2015;290(21):13250–13262. doi: 10.1074/jbc.M114.634568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rubtsova K., Marrack P., Rubtsov A.V. Sexual dimorphism in autoimmunity. Journal of Clinical Investigation. 2015;125(6):2187–2193. doi: 10.1172/JCI78082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Versini M., Jeandel P.-Y., Rosenthal E., Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmunity Reviews. 2014;13(9):981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 86.Theofilopoulos A.N., Kono D.H., Baccala R. The multiple pathways to autoimmunity. Nature Immunology. 2017;18(7):716–724. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trigunaite A., Dimo J., Jørgensen T.N. Suppressive effects of androgens on the immune system. Cellular Immunology. 2015;294(2):87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 88.Maret A., Coudert J.D., Garidou L., Foucras G., Gourdy P., Krust A. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. European Journal of Immunology. 2003;33(2):512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 89.Markle J.G.M., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 90.Palaszynski K.M., Smith D.L., Kamrava S., Burgoyne P.S., Arnold A.P., Voskuhl R.R. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146(8):3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 91.Smith-Bouvier D.L., Divekar A.A., Sasidhar M., Du S., Tiwari-Woodruff S.K., King J.K. A role for sex chromosome complement in the female bias in autoimmune disease. The Journal of Experimental Medicine. 2008;205(5):1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sasidhar M.V., Itoh N., Gold S.M., Lawson G.W., Voskuhl R.R. The XX sex chromosome complement in mice is associated with increased spontaneous lupus compared with XY. Annals of the Rheumatic Diseases. 2012;71(8):1418–1422. doi: 10.1136/annrheumdis-2011-201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Voskuhl R.R., Sawalha A.H., Itoh Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Multiple Sclerosis Journal. 2018;24(1):22–31. doi: 10.1177/1352458517737394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Case L.K., Wall E.H., Dragon J.A., Saligrama N., Krementsov D.N., Moussawi M. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Research. 2013;23(9):1474–1485. doi: 10.1101/gr.156703.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arnold A.P. Y chromosome's roles in sex differences in disease. Proceedings of the National Academy of Sciences. 2017;114(15):3787–3789. doi: 10.1073/pnas.1702161114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X., Schadt E.E., Wang S., Wang H., Arnold A.P., Ingram-Drake L. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Research. 2006;16(8):995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Nas A., GuhaThakurta D., Wang S.S., Yehya N., Horvath S., Zhang B. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. 2009;150(3):1235–1249. doi: 10.1210/en.2008-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wijchers P.J., Yandim C., Panousopoulou E., Ahmad M., Harker N., Saveliev A. Sexual dimorphism in mammalian autosomal gene regulation is determined not only by Sry but by sex chromosome complement as well. Developmental Cell. 2010;19(3):477–484. doi: 10.1016/j.devcel.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Dewing P., Chiang C.W.K., Sinchak K., Sim H., Fernagut P.-O., Kelly S. Direct regulation of adult brain function by the male-specific factor SRY. Current Biology. 2006;16(4):415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 100.Prokop J.W., Deschepper C.F. Chromosome Y genetic variants: impact in animal models and on human disease. Physiological Genomics. 2015;47(11):525–537. doi: 10.1152/physiolgenomics.00074.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Link J.C., Hasin-Brumshtein Y., Cantor R.M., Chen X., Arnold A.P., Lusis A.J. Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression. BMC Genomics. 2017;18(1) doi: 10.1186/s12864-017-3484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gershoni M., Pietrokovski S. The landscape of sex-differential transcriptome and its consequent selection in human adults. BMC Biology. 2017;15(1):7. doi: 10.1186/s12915-017-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ardlie K.G., Deluca D.S., Segre A.V., Sullivan T.J., Young T.R., Gelfand E.T. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carrel L., Willard H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7031):400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 105.Brown C.J., Greally J.M. A stain upon the silence: genes escaping X inactivation. Trends in Genetics. 2003;19(8):432–438. doi: 10.1016/S0168-9525(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 106.Berletch J.B., Ma W., Yang F., Shendure J., Noble W.S., Disteche C.M. Escape from X inactivation varies in mouse tissues. PLoS Genetics. 2015;11(3):e1005079. doi: 10.1371/journal.pgen.1005079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berletch J.B., Yang F., Disteche C.M. Escape from X inactivation in mice and humans. Genome Biology. 2010;11(6):213. doi: 10.1186/gb-2010-11-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prothero K.E., Stahl J.M., Carrel L. Dosage compensation and gene expression on the mammalian X chromosome: one plus one does not always equal two. Chromosome Research. 2009;17(5):637–648. doi: 10.1007/s10577-009-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clayton J.A., Collins F.S. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miller L.R., Marks C., Becker J.B., Hurn P.D., Chen W.-J., Woodruff T. Considering sex as a biological variable in preclinical research. The FASEB Journal. 2016;3(1):29–34. doi: 10.1096/fj.201600781R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCullough L.D., de Vries G.J., Miller V.M., Becker J.B., Sandberg K., McCarthy M.M. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biology of Sex Differences. 2014;5(1):15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]