Abstract
Background
Over the past two decades, parallel recognition has grown of the importance of both sex steroids and immune activity in metabolic regulation. More recently, these discrete areas have been integrated in studies examining the metabolic effects of sex steroid immunomodulation. Implicit in these studies has been a traditional, endocrine model of sex steroid delivery from the gonads to target cells, including immune cells. Thus, research to date has focused on the metabolic effects of sex steroid receptor signaling in immune cells. This endocrine model, however, overlooks the extensive capacity of immune cells to generate and metabolize sex steroids, enabling the production of sex steroids for intracrine signaling – that is, sex steroid production for signaling within the cell of origin. Intracrine function allows highly cell-autonomous regulation of sex steroid exposure, and sex steroid secretion by immune cells could confer paracrine signaling effects in neighboring cells within metabolic tissues. In this review, immune cell intracrinology will denote sex steroid production within immune cells for either intracrine or paracrine signaling. This intracrine capacity of immune cells has been well established, and prior work has supported its importance in autoimmune disorders, trauma, and cancer. The potential relevance of immune cell intracrine function to the regulation of energy balance, body weight, body composition, and insulin sensitivity has yet to be explored.
Scope of review
The following review will detail findings to date regarding the steroidogenic and steroid metabolizing capacity of immune cells, the regulation of immune cell intracrine function, and the biological effects of immune-derived sex steroids, including the clinical relevance of immune cell intracrinology in fields other than metabolism. These findings will serve as the basis for a proposed model of immune cell intracrinology constituting a new frontier in metabolism research.
Major conclusions
The development of highly sensitive mass spectrometric methods for sex steroid measurement and quantitation of metabolic flux now allows unprecedented ability to interrogate sex steroid production, metabolism and secretion by immune cells. Immune cell intracrinology could reveal key mechanisms underlying immune cell-mediated metabolic regulation.
Keywords: Macrophages, Lymphocytes, Estrogens, Androgens, Intracrine, Metabolism
Highlights
-
•
Sex steroids exert immunomodulatory effects that may influence metabolic health.
-
•
Immune cells can synthesize, modify, and metabolize sex steroids.
-
•
Immune cell-derived sex steroids may play intracrine, autocrine, paracrine, and possibly even endocrine roles.
-
•
Immune cell steroidogenesis is a largely unexplored area of metabolism research.
1. Introduction
The term ‘intracrinology’ first was used almost 30 years ago to describe androgen synthesis in peripheral tissues in orchiectomized rats, denoting sex steroid production and signaling that occurred within the same cell [1]. Intracrinology, therefore, introduced unprecedented autonomy of peripheral cells and tissues to regulate sex steroid production locally, independent of gonadal sex steroid production. Tissue intracrinology long has been a central focus of research in rheumatology and oncology and has generated key insights into the pathogenesis of diseases including rheumatoid arthritis, breast cancer, and prostate cancer [2], [3], [4]. The steroidogenic or intracrine capacity of peripheral tissues now also has become an emergent area of interest in metabolism research [5], [6], as metabolic tissues including brain, liver, adipose tissue, and skeletal muscle all possess steroidogenic capacity. These metabolic tissues are enriched in sex steroids [5], and androgens and estrogens have well established roles in the regulation of energy metabolism, appetite, adipogenesis, and insulin sensitivity [7], [8], [9], [10], [11]. Importantly, clinical data consistently demonstrate a lack of uniform association between plasma and tissue-specific sex steroid concentrations, suggesting that altered sex steroid production within metabolic tissues may contribute to the evolution of obesity and associated metabolic disorders [5], [12], [13], [14]. Thus, identifying the mechanisms whereby local sex steroid production and metabolism are regulated within metabolic tissues could provide critical insight into the pathophysiology of metabolic disease.
Immune cell populations are present in all metabolic tissues, and steroidogenic capacity has been identified in immune cells, particularly in macrophages and T lymphocytes. Tissue immune cells, therefore, could be a key source of local sex steroid production, and immune cell-derived sex steroids may play important intracrine and paracrine roles, with signaling effects conferred both in the cell of origin and in surrounding cells. The metabolic significance of immune cell steroidogenesis remains almost wholly unexplored to date. Immune cell intracrinology is a novel facet of metabolism research that may prove essential for comprehensive elucidation of the mechanisms through which sex steroids and immunity regulate metabolic health.
2. Background: potential relevance of immune cell sex steroid production to metabolic regulation
2.1. A conceptual shift from endocrinology to intracrinology
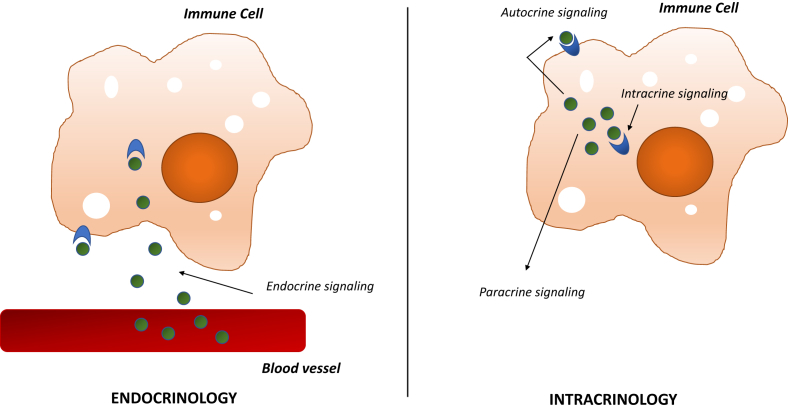
The classic, endocrine view of sex steroid biology is that sex steroids are produced in the gonads and secreted into circulation for conveyance to target tissues. Whereas an endocrine view assumes that circulating sex steroid concentrations determine sex steroid exposure for peripheral cells, an intracrine view requires attention to local, cell- or tissue-specific regulation of sex steroid production and metabolism. These locally produced sex steroids are primarily generated from circulating, adrenal-derived steroid precursors, and, strikingly, some data suggest that the majority of all androgens and estrogens may be produced in peripheral tissues rather than the gonads in both males and females [15]. In contrast to a traditional, endocrine model, intracrinology recognizes the extensive capacity of virtually all cell types to both generate and metabolize sex steroids and thereby tightly regulate sex steroid signaling in cell-autonomous fashion. In strict definition, intracrine signaling is specific to mediators that signal through cytosolic or nuclear receptors, as they can confer signaling effects without first being secreted from the cell of origin. Local mediators that are secreted into the extracellular space instead confer autocrine or paracrine effects, signaling through receptors on the plasma membrane of the cell of origin or on neighboring cells, respectively (Figure 1). In this review, immune cell intracrinology will refer to the capacity of immune cells to synthesize, modify, and metabolize sex steroids. These sex steroids and their derivatives - generated through intracrine pathways - may confer either intracrine, autocrine, or paracrine signaling effects. Thus, an intracrine view underscores the importance of interrogating local, tissue-specific regulation of sex steroid production and metabolism.
Figure 1.
Immune cell intracrinology. In an endocrine model of sex steroid biology, sex steroids are synthesized in classically steroidogenic tissues and disseminated to target cells through the circulation. In contrast, immune cell intracrinology denotes the capacity of immune cells to synthesize, modify, and metabolize sex steroids. Sex steroids signal through nuclear receptors, and a membrane receptor (GPR30) also has been identified for estrogen signaling. Therefore, intracrine function in immune cells can generate sex steroids and their derivatives that mediate intracrine, autocrine, and paracrine effects.
2.2. Sex steroid biosynthesis
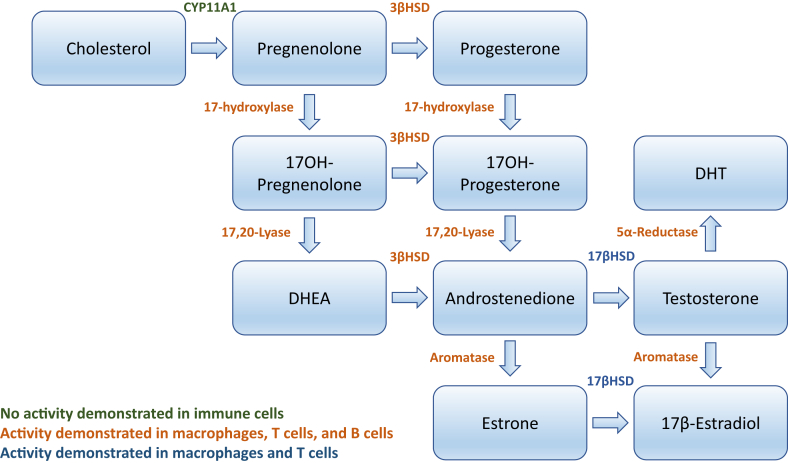
Sex steroids are synthesized from cholesterol through sequential enzymatic steps (Figure 2). The rate limiting step of de novo steroidogenesis from cholesterol is thought to be mediated by steroidogenic acute regulatory protein (StAR), which transports cholesterol to the inner mitochondrial membrane. Cholesterol is then converted to pregnenolone by CYP11A1 (side chain cleavage enzyme). Pregnenolone, in turn, can be converted to progesterone or to the weak androgen dehydroepiandrosterone (DHEA). DHEA subsequently can be converted to more potent androgens including testosterone. Testosterone can undergo conversion either to 17β-estradiol through aromatization or to dihydrotestosterone (DHT) through 5α-reductase activity.
Figure 2.
Steroidogenic enzymes involved in the de novo synthesis of sex steroids from cholesterol. Green font indicates that enzyme activity has yet to be demonstrated in immune cells. Orange font indicates that enzyme activity has been demonstrated in macrophages, T lymphocytes, and B lymphocytes. Blue font indicates that enzyme activity has been demonstrated in macrophages and T lymphocytes only.
In addition to estrogens and androgens, steroid precursors derived principally from the adrenal glands circulate in high concentrations, the most abundant of which is DHEA sulfate (DHEA-S). These precursors can undergo conversion to more potent androgens or estrogens in peripheral tissues, enabling local concentrations of sex steroids to be determined in highly tissue-specific fashion. Thus, it has been estimated that nearly half of total androgens and the vast majority of estrogens in men are formed in peripheral tissues, with most androgens and ∼75% of total estrogens similarly attributed to peripheral formation in premenopausal women [15], [16], [17]. In postmenopausal women, essentially all estrogens and androgens are synthesized within peripheral tissues [15]. Some peripheral cells have the capacity not only for steroid conversion but also for the production of sex steroids de novo from cholesterol. Whereas de novo steroidogenesis was once believed to be exclusive to the gonads and adrenal glands, de novo sex steroid production now has been identified in numerous other tissues and cell types, including kidney, neurons, astrocytes and other glial cells, keratinocytes, adipocytes, and placental trophoblasts [18], [19], [20], [21], [22], [23]. Furthermore, sex steroid synthesis in brain and peripheral tissues involves steroidogenic pathways and enzymes that are not found in the gonads and adrenal glands; for example, peripheral tissues have been shown to generate 17β-estradiol and DHT through pathways that do not require testosterone as an intermediate [24]. These findings collectively underscore the intricacy of local sex steroid regulation and highlight the importance of understanding sex steroid production, signaling, and metabolism within a single cell or tissue [15].
2.3. Sex steroids may influence energy metabolism through immunomodulatory effects
The importance of sex steroids as key regulators of metabolic health has been well established in both men and women. In men, androgen deficiency promotes adverse changes in body composition and insulin resistance (IR) [25], [26], and men with rare syndromes of genetic estrogen deficiency similarly exhibit metabolic dysregulation characterized by increased visceral adiposity and IR [27]. Estradiol deficiency in women leads to redistribution of fat mass in association with increased risk of IR and metabolic syndrome [28], and androgen excess predisposes women to IR, impaired insulin secretion, and type 2 diabetes [29]. Sex steroids regulate metabolic health through signaling effects in both peripheral and central metabolic tissues, including adipose tissue, liver, skeletal muscle, and brain [30].
Within these metabolic tissues, sex steroids exert broad effects on diverse cell types. Immune cells are resident in all metabolic tissues, and sex steroids are key regulators of immune cell phenotype and function, with demonstrated roles in the regulation of both adaptive and innate immunity. The androgens testosterone and DHT signal through the androgen receptor (AR), whereas estrogens including estrone and 17β-estradiol signal through estrogen receptors alpha and beta (ERα and ERβ). In addition to these nuclear receptors, a membrane-bound, non-nuclear receptor (GPR30) has been identified for estrogens [11], [31], [32]. Both nuclear sex steroid receptors and GPR30 are expressed in immune cells, with expression patterns that vary as a function of cell type and stage of differentiation [33], [34], [35], [36]. Sex steroid receptor expression has been identified in bone marrow stromal cells, hematopoietic stem cells, and mature immune cells including macrophages, T and B lymphocytes, neutrophils, and dendritic cells [35], [36], [37]. Androgens and estrogens have been shown to regulate immune cell proliferation, differentiation, and apoptosis, as well as cytokine and immunoglobulin production [4], [35], [38], [39], [40]. The importance of sex steroid-mediated immunomodulation is underscored by sexual dimorphisms in vaccine and infection response, wound healing, and risk of autoimmune disease [41], [42].
Notably, immune cell populations in metabolic tissues contribute to the regulation of insulin sensitivity and energy metabolism in neighboring cells through the secretion of paracrine mediators [43], [44]. Consequently, the paracrine function of these tissue immune cells contributes to the regulation of both tissue-specific and systemic insulin sensitivity, energy balance, body weight, and body composition [43], [44], [45]. The observations that sex steroids exert immunomodulatory effects and that immune cells contribute to metabolic regulation have led to the premise that sex steroids could influence both tissue-specific and systemic energy metabolism in part through the regulation of immune cell phenotype and function.
Indeed, an emergent field of metabolism research now focuses on the significance of sex steroid-mediated immunomodulatory effects for metabolic regulation, and preclinical studies have demonstrated that sex steroid signaling specifically in immune cells helps maintain metabolic health in both males and females. Thus, loss of intact ERα signaling exclusively in myeloid cells led to increased adiposity and IR in female mice [46]. In parallel, AR deficiency in hematopoietic cells promoted visceral adiposity in male mice [47]. Critically, the effects of sex steroids on immune cell function are highly contingent on sex steroid concentration and specific biological context within the microenvironment [36], highlighting the importance of defining the mechanisms whereby local, tissue-specific sex steroid production and metabolism are regulated. Thus, aromatase expression has been identified in both central and peripheral metabolic tissues [48], allowing highly tissue- and cell-autonomous regulation of the conversion of androgens to estrogens.
3. Biological effects and clinical relevance of intracrine sex steroid production
3.1. Intracrine sex steroids: beyond 17β-estradiol and testosterone
Whereas 17β-estradiol and testosterone are the predominant circulating sex steroids, peripheral tissues have an extensive capacity to produce a broad array of sex steroids and their metabolites. Indeed, many peripheral cell types have steroidogenic and steroid modifying enzymes not found in classically steroidogenic tissues. These steroids and steroid metabolites may circulate at negligible concentrations but confer important biological effects within the tissue microenvironment.
Estrogens can undergo sulfonation, generating inactive, water-soluble metabolites that, importantly, may be converted back to the parent enzyme by steroid sulfatase. Alternatively, estrogens including estrone and 17β-estradiol can undergo hydroxylation and methylation, generating bioactive metabolites that mediate highly variable and even opposing effects from both each other and the parent estrogen. Both synovial and monocyte-derived macrophages produce 16-hydroxylated steroids [34], and 16-hydroxyestrone was shown to exert mitogenic and proliferative effects on neighboring synovial cells, suggestive of a key etiopathogenic role in rheumatoid arthritis [49]. In contrast, 2-hydroxy-17β-estradiol and 2-methoxy-17β-estradiol confer anti-proliferative and pro-apoptotic effects, suggesting that differential activity of the enzymes that generate these various estrogen metabolites could have profound effects on rheumatoid arthritis risk and progression. Similarly, estrone and estradiol metabolites exert discrete effects on cytokine production [49]; thus, 2- and 4-hydroxylated estrogens were shown to inhibit tumor necrosis factor alpha (TNFα) secretion from synovial cells, whereas no effect was found for 16-hydroxylated estrogens [40]. The potential biological roles of these estrogen metabolites in metabolic tissues are completely unknown.
Another estrogen that appears to play intracrine and paracrine rather than endocrine roles is 17α-estradiol. Supporting its intracrine rather than endocrine function, 17α-estradiol binds ERα but does not bind sex hormone binding globulin [50]. Once believed to be an inactive isomer of 17β-estradiol, 17α-estradiol now has well characterized biological roles. In male but not female mice, 17α-estradiol was shown to extend lifespan [51], and, in aged male mice, systemic treatment with 17α-estradiol led to diminished visceral fat mass through both inhibition of food intake and through selective activation of AMP-activated protein kinase alpha (AMPKα) in visceral adipose tissue [52]. Although local production of 17α-estradiol within brain has been demonstrated, the synthetic pathways and specific cell types implicated in its production remain incompletely understood [53]. Strikingly, we have found that 17α-estradiol is the most abundant estrogen produced in vitro by thioglycolate-elicited peritoneal immune cells (unpublished observation).
Similarly, locally produced androgens may play important intracrine, autocrine, and paracrine roles. DHT, converted from testosterone through 5α-reductase activity, was shown to suppress NFκB activity as well as downstream IL-6 production in fibroblasts [54]. DHT also increased cell motility and survival in human peripheral blood mononuclear cells (PBMCs) [55]. The weak androgen DHEA has been shown to have immunomodulatory effects including regulation of cytokine production [56]. Further, DHEA can be converted to 7α-hydroxy-DHEA by the enzyme steroid 7α-hydroxylase (CYP7B). 7α-Hydroxy-DHEA has been shown to counteract the immunosuppressive effects of glucocorticoids and augment antibody production and, moreover, may contribute to disease activity and progression in rheumatoid arthritis [57]. In all tissues except liver, including immune tissues and cells, CYP7B is the primary enzyme with 7α-hydroxylase activity, and its substrates include pregnenolone and androstenediol as well as DHEA [58]. CYP7B activity is stimulated by TNFα, interleukin 17 (IL-17) and IL-1β but inhibited by transforming growth factor beta (TGFβ) [57], suggesting potential autocrine signaling pathways through which macrophage- and lymphocyte-derived cytokines and growth factors regulate sex steroid modification within the same cell.
In addition to signaling through their cognate receptors, sex steroids also are key regulators of xenobiotic receptors including constitutive androstane receptor (CAR) and pregnane X receptor (PXR) [59]. CAR and PXR are most highly expressed in liver and play pivotal roles not only in drug metabolism but also in bile acid, lipid, and glucose metabolism, rendering their historical designation as xenobiotic receptors inadequate for capturing the scope of their biological roles [60]. Notably, a broad spectrum of estrogens and estrogen derivatives were found to activate PXR, with over half of all estrogenic compounds tested exhibiting capacity to regulate this receptor [61]. PXR expression has been identified in monocytes/macrophages and lymphocytes. In macrophages, PXR activation was found to inhibit NFκB signaling and TNFα secretion, and PXR also regulates B lymphocyte maturation as well as T lymphocyte proliferation and activation [62]. Importantly, endogenous ligands for PXR and CAR are thought only to activate these receptors at very high concentrations, as could be achieved intracellularly through intracrine production or within the tissue microenvironment if synthesized sex steroids and their derivatives were secreted into the extracellular space. Thus, through interaction with these noncanonical xenobiotic receptors, sex steroids produced through intracrine pathways could prove to be key regulators of immune cell energy metabolism. Further, immune cell-derived sex steroids could activate xenobiotic receptor signaling in neighboring cells, thereby contributing to the regulation of whole tissue energy metabolism as well as the catabolism of numerous signaling molecules [59].
3.2. Clinical relevance of immune cell intracrinology
The potential importance of immune cell intracrinology in metabolic disease is supported indirectly by evidence of its relevance to other disease states. The biological roles of immune cell steroidogenesis thus far have been most closely examined in research focused on sex steroid-dependent cancers and autoimmune disease; thus, local production particularly of estrogens by immune cells has been implicated in the pathogenesis of breast cancer and rheumatoid arthritis [40], [63], [64], [65].
In breast cancer research, intra-tumoral production of estrogens has received considerable attention. Estrogen production in post-menopausal women primarily occurs in the periphery through conversion of DHEA, DHEA-S, 4-androstene-3, 17-dione (4-dione), and estrone sulfate by the collective actions of aromatase, steroid sulfatase, and 17β-hydroxysteroid dehydrogenase (17βHSD) type 1 and type 7. These steroidogenic enzymes are present both in the malignant epithelial cells as well as in stromal cells, with estrogens promoting tumor cell proliferation through both intracrine and paracrine pathways [66]. In contrast, intra-tumoral production of DHT exerts anti-proliferative effects on tumor epithelial cells through AR signaling [66]. Tumor-associated macrophages produce cytokines including IL-6, IL-4, IL-13, and TNFα that may contribute to the induction of aromatase, 3β-hydroxysteroid dehydrogenase (3βHSD), and 17βHSD [67], [68], and this cytokine-mediated regulation of steroidogenesis has been proposed as a key pathogenic facet of breast cancer progression [69].
In addition to promoting estrogen generation in neighboring cells, tumor-associated immune cells also have been posited to be a critical source of intra-tumoral estrogens and therefore a critical component of disease pathogenesis [70]. This model is supported by findings that breast cancer severity correlated with the degree of aromatase expression in tumor-associated macrophages [71]. These same authors also showed that THP-1 macrophages were able to induce proliferation of breast cancer MCF-7 cells in vitro, an effect that was abrogated by macrophage treatment with an aromatase inhibitor [71]. As found in previous studies, differentiation into mature macrophages was required for estrogen production and, strikingly, macrophages produced estrogens at rates comparable to placenta. Therefore, macrophages not only may regulate steroidogenic enzyme activity in neighboring cells but also may be a key source of intra-tumoral estrogen production. A complex intracrine-paracrine model of steroidogenesis in breast cancer has thus emerged, but additional work is needed to identify the respective predominant steroidogenic pathways in tumor, stromal, and infiltrating immune cells and to elucidate fully the mechanisms through which steroidogenic enzyme activity is regulated.
Immune cell steroidogenesis also has been postulated to play an important role in autoimmune disorders including systemic lupus erythematosus and rheumatoid arthritis. Some autoimmune disorders disproportionately affect women, leading to the notion that differential sex steroid exposure may underlie this sexually dimorphic risk. The immunomodulatory roles of estrogens in particular have long been believed to contribute to the elevated risk of autoimmunity among women, but circulating estrogen concentrations do not differ between women with rheumatoid arthritis and healthy controls, suggesting that local rather than circulating estrogen production is a key determinant of risk [4]. Supporting the importance of local sex steroid production in rheumatoid arthritis, synovial fluid concentrations of free and total androgens were significantly lower among rheumatoid arthritis patients than controls, whereas synovial fluid from these patients exhibited higher concentrations of conjugated estrogens [65]. These findings, suggestive of increased local aromatase activity, were further supported by enhanced conversion of DHEA to estrone and 17β-estradiol in mixed synovial cell cultures from rheumatoid arthritis patients relative to healthy controls [65]. Synovial cells from rheumatoid arthritis patients also exhibited enhanced generation of 16α-hydroxyestrone and 4-hydroxyestradiol but not 2-hydroxyestrone. Though much of the intracrine research in rheumatoid arthritis has entailed mixed synovial cell cultures, macrophages and T lymphocytes in particular have been implicated as both sources and targets of local sex steroid signaling in this disease. Moreover, estrogen production in these immune cells has been proposed as a potential mechanistic link in the clear association between autoimmune disorders and cancer [72]. Notably, synovial fluid sex steroid concentrations did not differ between men and women with rheumatoid arthritis, suggesting that elevated risk for this autoimmune disorder in women does not derive from sexually dimorphic patterns of steroidogenesis in synovial immune cells.
In addition to risk of autoimmune disorders, sexual dimorphisms also have been well characterized for immune responses to infection, trauma, and vaccination. In contrast to findings in patients with rheumatoid arthritis, sexually dimorphic patterns of immune cell steroidogenesis appear to contribute to sex differences in the immune response to trauma. In a mouse model of trauma-hemorrhage, for example, splenic T lymphocytes were found to have altered patterns of steroid conversion, and, further, these patterns were sexually dimorphic. Thus, T lymphocytes from male mice exhibited augmented 5α-reductase activity in conjunction with decreased expression of 17βHSD type 4, leading to increased DHT availability [73]. Conversely, female mice in proestrous exhibited higher aromatase expression after trauma-hemorrhage in association with increased reductase activity of 17βHSD [73].
Immune activation by immunization also has been shown to influence immune cell steroidogenesis, as immunization challenge increased 3βHSD and 17βHSD enzymatic activity in mouse lymphoid tissues, although only male mice were studied [74]. Patterns of sex steroid conversion in immune cells may vary as a function of age, as PBMCs isolated from older men exhibited higher activity of 17βHSD type 5, with enhanced conversion of DHEA to androstenediol and testosterone relative to PBMCs from younger men [56]. Thus, extensive future work is necessary to define the influence of clinical variables including age and sex on patterns of immune cell steroidogenesis and, in addition, to identify potential mechanisms whereby altered patterns of immune cell sex steroid production may contribute to individual susceptibility to obesity and associated metabolic disorders.
3.3. Intracrinology and metabolic disease
In contrast to research in the fields of autoimmunity and cancer, metabolism research has yet to examine the possible relevance of immune cell steroidogenesis to the pathogenesis of metabolic disorders. However, the production of sex steroids within key metabolic tissues – tissue intracrinology – has been established as a critical facet of metabolic regulation with characterized roles in energy metabolism, adipogenesis, and insulin sensitivity. Androgens and estrogens play broad metabolic roles in these peripheral metabolic tissues and contribute to the regulation of adipogenesis, lipid and glucose metabolism, and mitochondrial function [6], [9], [10], [11]. However, the tissue-specific regulation of sex steroid synthesis and metabolism remains poorly understood, and the critical cell types responsible for local production, modification, and metabolism of sex steroids have yet to be clearly established.
Adipose tissue is highly enriched in sex steroids, with total concentrations estimated anywhere from 2 to 400 fold higher than plasma [5]. Highly variable sex steroid concentrations have been found both among individuals and in different fat depots within an individual, underscoring the potential for adipose tissue intracrinology to explain individual susceptibility to obesity and associated metabolic disorders, as well as sexual dimorphisms in fat distribution and total adiposity [5], [6]. Thus, differences in intra-adipose sex steroid production could help explain, for example, the higher risk of obesity in women but the disproportionate risk of obesity-related comorbidities in men [75]. Adipose tissue comprises numerous cell types, including adipocytes, pre-adipocytes, leukocytes, and other stromovascular cells including endothelial cells and fibroblasts. The widespread expression particularly of aromatase in these cell types suggests that all of these cell populations could contribute to whole tissue regulation of androgens and estrogens. Increased aromatase activity in adipose tissue has long been recognized as a feature of obesity, though whether this reflects an adaptive or pathogenic change remains uncertain. Recently, aromatase overexpression in white adipose tissue was shown to enhance adipogenesis and insulin sensitivity in male mice [76]. Of note, aromatase overexpression in this model was under control of the aP2 promoter, which is expressed in macrophages as well as adipocytes, raising the intriguing possibility that macrophage-specific aromatase activity contributed to the observed metabolic phenotype.
Extensive androgen metabolism also has been demonstrated within human adipose tissue in both men and women, and expression and activity of the steroidogenic enzymes 3βHSD, 3α-hydroxysteroid dehydrogenase (3αHSD), 17α-hydroxylase, 17βHSD, and 5α-reductase have been identified in adipose tissue [5]. Subcutaneous and visceral adipose tissue depots further exhibit activity of aldoketoreductase 1C (AKR1c) enzymes, which catalyze both the synthesis and inactivation of androgens [7]. Androgens exert myriad effects on lipid metabolism, insulin sensitivity, adipogenesis, and adipokine production within adipose tissue [77]; consequently, the local synthesis and metabolism of androgens are key determinants of tissue-specific energy metabolism as well as the endocrine function of adipose tissue. Notably, changes in the expression of steroid metabolism genes were among the most prominent changes in adipose tissue gene expression following a behavioral weight loss intervention in postmenopausal women [78], further supporting close associations among tissue intracrine function, energy balance, and adipose tissue remodeling. Importantly, steroidogenic activity in adipose tissue predominantly localizes to the stromovascular fraction rather than adipocytes [79]. This heterogeneous fraction constitutes vascular epithelial cells, fibroblasts, preadipocytes, and immune cells, and the respective contribution of each cell type to adipose tissue sex steroid concentrations has yet to be quantified.
The intracrinology of other key peripheral metabolic tissues has also been demonstrated though has received less attention to date. Skeletal muscle expression and activity of 5α-reductase, 3βHSD, and 17βHSD have been identified, establishing the capacity for local production of testosterone and DHT from circulating DHEA. Similar to adipose tissue, sex steroid concentrations in skeletal muscle may be dissociated from circulating concentrations [80], and intramuscular concentrations of 17β-estradiol, testosterone, DHT, and DHEA proved independent predictors of muscle strength and power in women [81]. Estrogen generation through aromatase activity also has been demonstrated in skeletal muscle [82], and exercise training has been shown to induce dynamic changes in steroidogenic enzyme expression [83], [84]. Steroidogenic enzyme expression and activity have been identified in liver, as well, viewed principally through the lens of chronic liver disease rather than a perspective of energy metabolism [85], [86], [87]. As for adipose tissue, the relative contribution of resident immune cells in skeletal muscle and liver to tissue-specific sex steroid concentrations remains unknown.
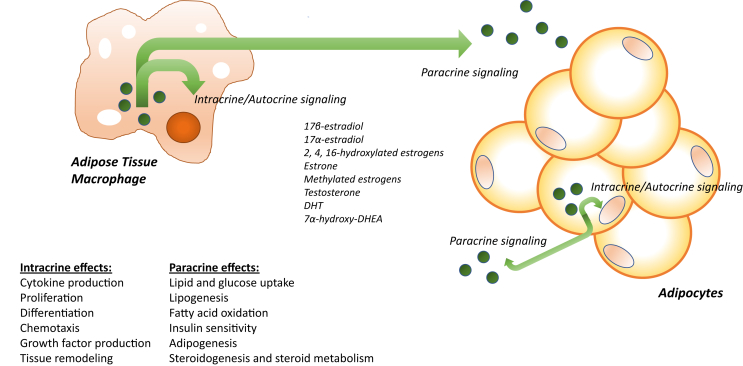
Extensive evidence now demonstrates that the phenotype and function of immune cells in peripheral metabolic tissues are critical determinants of energy balance, adiposity, and systemic insulin sensitivity [43], [44], [88]. Nonetheless, attention has focused predominantly on immune cell cytokine production with lesser focus on other nodes of immune cell function including energy metabolism, growth factor production, and tissue remodeling. Thus far, virtually no research efforts have examined the intracrine phenotype of immune cells in metabolic tissues nor possible changes in immune cell intracrine function that occur in the context of obesity, diabetes, and related metabolic disorders. The possible intracrine roles of immune cells in metabolic regulation may be viewed from two discrete perspectives. First, the generation of sex steroids for signaling within the same cell could exert effects on cellular differentiation, phenotype, and paracrine function. Through the regulation of immune cell paracrine function, intracrine sex steroid production therefore could influence energy metabolism, insulin sensitivity, as well as steroidogenesis in neighboring cells within metabolic tissues. Viewed from the second perspective, immune cells could secrete the synthesized sex steroids and thereby contribute to tissue intracrinology through direct regulation of tissue-specific sex steroid concentrations (Figure 3). These two perspectives therefore require simultaneous consideration of the intracrine/autocrine and paracrine effects of steroidogenesis in immune cells. When integrated, these perspectives also generate an integrated network of tissue-specific sex steroid regulation that may be independent of circulating sex steroid concentrations. Thus, immune cell intracrinology gives rise to a model wherein immune cells do not merely respond passively to ambient sex steroids but are critical, local determinants of the production and metabolism of these signals.
Figure 3.
Schematic of the proposed mechanisms by which sex steroid generation in immune cells could confer metabolic effects. Sex steroids could remain within the cell of origin and act as ligands for cognate nuclear receptors (intracrine signaling). Alternatively, sex steroids could be secreted from the cell and signal through membrane receptors on the same cell (autocrine effects). As sex steroids have demonstrated roles in regulating immune cell differentiation, proliferation, and production of other paracrine mediators, these intracrine and autocrine effects could be key regulators of cellular phenotype and function. Further, secreted sex steroids also could exert signaling effects in neighboring cells (paracrine effects). Estrogens and androgens are important regulators of insulin sensitivity and energy metabolism in key metabolic tissues; thus, tissue resident immune cells could prove an important source of tissue-specific sex steroid production. Adipocytes and other cell types in adipose tissue also express steroidogenic enzymes including aromatase, suggesting that sex steroids could be key signals for cellular crosstalk within metabolic tissues.
4. Steroidogenic capacity of immune cells
4.1. Immune cells: potential contributors to the intracrine function of metabolic tissues
Supporting the importance of tissue intracrinology in metabolic regulation, clinical data clearly demonstrate that sex steroid concentrations within metabolic tissues are not solely determined by circulating concentrations. Thus, in obese men and women, no correlation was found between 17β-estradiol concentrations in plasma and visceral fat, and disproportionately elevated estradiol concentrations were found in adipose tissue relative to plasma in obese men [89]. Dissociations also were found between plasma and breast fatty tissue estrogen concentrations in women as well as plasma and subcutaneous adipose tissue estrogen concentrations in healthy men subject to experimental sex steroid manipulation [12], [90]. Consequently, understanding the metabolic roles of sex steroid immunomodulation requires quantification of local, tissue-specific sex steroid concentrations, identification of the key cell types implicated in local sex steroid production, and delineation of the pathways that regulate sex steroid synthesis and metabolism within metabolic tissues.
4.2. Sex steroid production and metabolism in macrophages and lymphocytes
Research to date has examined the metabolic effects of sex steroid receptor signaling in immune cells; however, immune cells are not merely passive targets of sex steroid signaling; rather, they have the capacity to actively synthesize and metabolize sex steroids. The capacity for steroid conversion in immune cells first was demonstrated over 30 years ago. Conversion of androstenedione to other steroids by human alveolar macrophages led to the inference of 5α-reductase, 17βHSD, 3βHSD, and 3αHSD activity in these cells [91]. Through similar methods, activity of these steroidogenic enzymes also was shown in alveolar macrophages from both male and female guinea pigs [92]. Conversion of testosterone to DHT was demonstrated in primary human synovial macrophages, further corroborating 5α-reductase activity [93]. Another study showed that synovial macrophages could convert testosterone to both androstenedione and DHT, and, remarkably, rates of DHT formation were comparable to those in prostate cancer cells [94]. Steroidogenic enzyme activity also has been clearly demonstrated in human macrophages differentiated in vitro from monocytes, suggesting that sex steroid synthesis occurs in macrophages across a broad phenotypic spectrum, as would be found in diverse tissue types due to different microenvironmental contexts [95], [96]. However, patterns of steroid production clearly differ among these cell types, as monocyte-derived macrophages preferentially formed Δ4 and Δ5 steroids including testosterone, androstenedione, and androstenediol, whereas synovial macrophages produced more abundant estrogens, suggestive of higher aromatase activity [65], [95], [96]. Aromatase activity appears dependent on cellular differentiation as well as phenotype, as it has been demonstrated in mature macrophages but not monocytes [95], and aromatase expression was found in both THP-1 cells and primary monocytes only after differentiation into macrophages [71].
Macrophages also express steroid sulfatase and consequently can convert DHEA-S, the most abundant circulating adrenal androgen, to DHEA [97], [98], which, in turn, can be converted to androstenedione. Steroid sulfatase activity also renders macrophages capable of generating other biologically active sex steroids from their sulfated derivatives, including conversion of estrogen sulfates to estrone and 17β-estradiol. Therefore, based on evidence to date, macrophages have the requisite steroidogenic machinery to convert circulating DHEA, progesterone, 17-hydroxyprogesterone, and androstenedione to downstream androgens and estrogens, as well as the capacity to generate active sex steroids from their sulfated derivatives.
T and B lymphocytes also express steroidogenic enzymes. Murine splenic T lymphocytes exhibited 5α-reductase, aromatase, 3βHSD, and 17βHSD activity, whereas lower rates of steroid synthesis and no evidence of 17βHSD activity were found in B lymphocytes [73]. Aromatase-dependent conversion of androstenedione further has been shown in infiltrating lymphocytes in breast cancer [99]. Steroidogenic enzyme activity in macrophages and lymphocytes has not been compared directly, although aromatase expression was found to be substantially higher in macrophages than T lymphocytes [100].
To date, evidence of de novo synthesis of sex steroids from cholesterol has not been shown for any immune cell type. Interestingly, StAR expression has been identified in primary murine macrophages as well as immortalized macrophage cell lines [101]. However, CYP11A1 activity has yet to be identified in macrophages or other immune cells, leading to the current belief that immune cells are unable to perform the first enzymatic step of de novo steroidogenesis. In macrophages, StAR is believed to deliver cholesterol to the inner mitochondrial membrane for oxysterol rather than sex steroid synthesis [101]. Nonetheless, some evidence exists that StAR may influence steroid conversion within these cells through regulation of steroid sulfatase activity [102]. StAR expression and activity, in turn, have been implicated in extensive interactions with cytokines, growth factors, cholesterol metabolism and flux, and other signaling nodes in macrophages [103], [104], [105], [106], [107], [108]. The mechanisms underlying these StAR-mediated effects remain poorly understood. They may be conferred through oxysterol production, but the degree to which these effects may be mediated through regulation of steroid sulfatase activity and sex steroid conversion has not been determined. Thus, StAR may play a role in the regulation of macrophage sex steroid production distinct from its traditional role in steroidogenesis entailing cholesterol delivery to the inner mitochondrial membrane.
Importantly, numerous immune cell populations now have been implicated in metabolic regulation, particularly within adipose tissue. Thus, in addition to macrophages and lymphocytes, mast cells, natural killer cells, eosinophils, dendritic cells, and neutrophils all have been shown to influence energy metabolism, adipose tissue remodeling, and/or insulin sensitivity in metabolic tissues [109], [110], [111]. The intracrine capacity of these other immune cell types remains unexplored and constitutes a critical area of future research.
4.3. Regulation of steroidogenic enzymes in immune cells
The signals that regulate sex steroid synthesis and conversion in immune cells remain incompletely understood. Regulation of aromatase activity has received attention particularly given its modulatory roles in disease states including rheumatoid arthritis, atherosclerosis, obesity, and breast cancer. Macrophage aromatase activity is induced principally by glucocorticoids as well as cytokines including TNFα, IL-1, and IL-6 through activation of STAT3 [40], whereas 1,25-dihydroxy-vitamin D was shown to downregulate aromatase activity [112]. Macrophage steroid sulfatase activity was inhibited by lipopolysaccharide (LPS), interferon gamma (IFNϒ), and TNFα but not IL-1, IL-6, or TGFβ [97]. In other cell types, steroidogenic enzyme expression has been shown to be regulated by growth factors including insulin-like growth factor 1 (IGF-1) and insulin as well as lipoproteins, cyclooxygenase-2, and arachidonic acid [113], [114], [115], [116], [117], [118], though these regulatory pathways have yet to be demonstrated specifically in immune cells.
Sex steroids also have been implicated in the regulation of steroidogenic enzymes in immune cells. Thus, in male mice, castration inhibited 5α-reductase activity and enhanced 17βHSD oxidative activity in T lymphocytes, indicating decreased production and increased catabolism, respectively, of DHT [119]. These findings suggest that 5α-reductase and 17βHSD activity are regulated, at least in part, by the availability of circulating testosterone. Further, this reduction in DHT exposure associated with enhanced T lymphocyte production of IL-2 and IL-6 subsequent to trauma-hemorrhage. In mixed synovial cell cultures, treatment with testosterone was found to inhibit aromatization and favor formation of DHT, and testosterone treatment nearly completely abrogated aromatase activity [96]. Importantly, androgen inhibition of aromatase activity is concentration-dependent and may occur only at higher concentrations [120]. In contrast, treatment with DHEA promoted aromatase activity and formation of estrone, 17β-estradiol, and estriol as well as formation of 16α-hydroxy steroids. Notably, DHEA has been found to both inhibit [95] and promote [96] aromatase activity in different types of macrophages, underscoring the importance of cellular phenotype and biological context in influencing the regulatory effects of discrete signals on sex steroid production.
The regulation of intracrine function in microglia, resident macrophages of the central nervous system, has been studied extensively. Systemic administration of LPS to mice reduced microglial expression of 5α-reductase, 17βHSD, and StAR in vivo, whereas these steroidogenic proteins were upregulated in vitro by combined treatment with LPS and IFNϒ [121]. Aromatase expression was not identified in isolated microglia, again highlighting that intracrine capacity may vary as function of cellular differentiation and phenotype. The same authors also demonstrated that microglia do not express CYP11A1, suggesting that, unlike other types of glial cells, microglia are not able to synthesize sex steroids de novo from cholesterol.
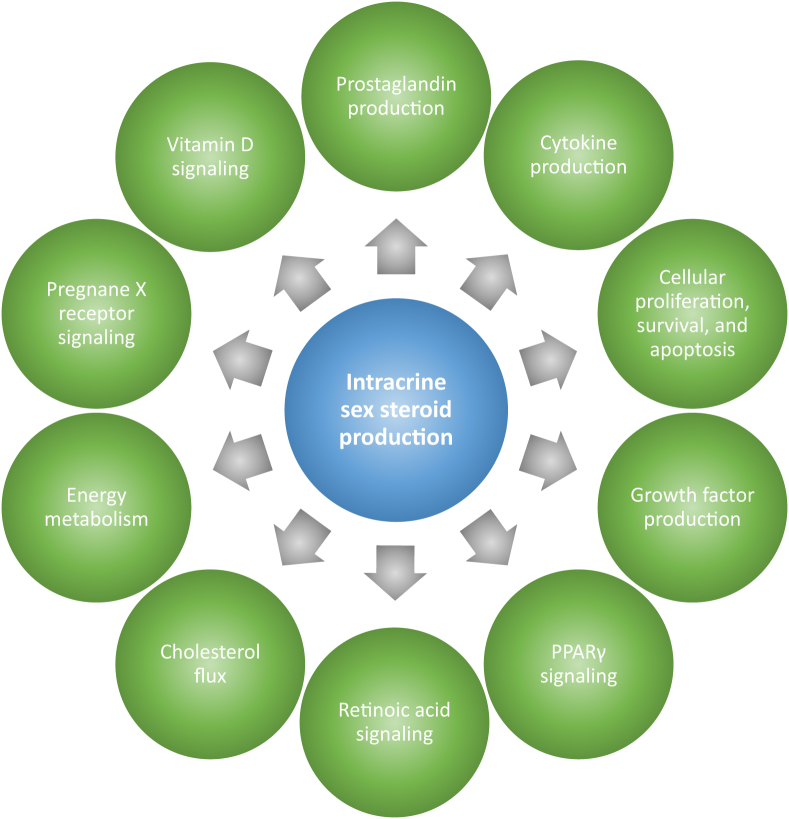
Collectively, these findings suggest a broad network of signaling nodes that interact with sex steroid production and metabolism, although additional work is needed to fully delineate the mechanisms that regulate intracrine function specifically in immune cells. In particular, steroidogenic pathways appear to intersect with cytokine and growth factor signaling as well as lipid and cholesterol metabolism, suggesting elaborate intracrine and autocrine signaling networks that could be critical for the exquisite regulation of immune cell phenotype and function (Figure 4).
Figure 4.
Reciprocal regulation of steroidogenic enzymes and other signaling nodes. Cytokines, prostanoids, and growth factors regulate the expression and/or activity of steroidogenic enzymes, and sex steroids, in turn, have been shown to regulate the production of these autocrine and paracrine mediators. Similarly, many nuclear receptors act as both regulators of steroidogenic enzymes and targets of sex steroid signaling. Thus, sex steroid generation may be a central facet of immune cell phenotype and function.
5. Future directions and conclusions
A vast spectrum of sex steroid mediators can be produced through immune cell intracrinology, and these mediators can influence the phenotype and function not only of the cell of origin but also of surrounding cells through paracrine actions. Extensive future work is required to delineate the kinetics and regulation of the production, secretion, and metabolism of these sex steroids and their derivatives to understand the full spectrum of biological roles of each respective mediator. After a period of prolonged quiescence, this area of investigation is reemerging in a new era in which mass spectrometric approaches are available for quantification of low abundance steroids and kinetic modeling of steroidogenic enzyme activity, finally offering the methodologic means of reinvigorating this field of immunobiology [122]. These state-of-the-art quantitative methods allow performance of metabolic flux studies using physiologically based pharmacokinetic modeling that has been applied in other fields to elucidate complex metabolic pathways including retinoic acid metabolism [123], [124], [125]. Thus, a critical future direction in immune cell intracrinology will be the novel application of these methods to better understand how patterns of sex steroid production, metabolism, and secretion vary as a function of cellular phenotype, as well as how immune cells contribute to whole tissue and systemic sex steroid regulation. This strategy allows a systems biology approach to understanding tissue- and cell type-specific intracrinology, as opposed to historical focus on the activity of a single steroidogenic enzyme in isolation.
Peripheral tissues exhibit multiple pathways for the production of sex steroids that extend beyond those present in classically steroidogenic tissues. An intracrine perspective mandates attention to an expanded scope of sex steroids as well as their metabolites and modified forms, rather than restricted focus on the predominant circulating species. Thus, steroids including pregnenolone, modified estrogens, and 17α-estradiol have no described endocrine function but nonetheless could perform critical intracrine and paracrine roles in metabolic regulation. Accordingly, the entire repertoire of steroidogenic enzymes and pathways requires delineation in each respective immune cell type, including careful assessment of the capacity for de novo steroidogenesis in immune cells. To date, sex steroid production has not been demonstrated in immune cell types other than monocytes/macrophages and lymphocytes. Notably, however, intracrine capacity in neutrophils is supported by recent evidence that these cells highly express 11β-hydroxysteroid dehydrogenase type 1 and therefore are able to synthesize corticosterone and cortisol [126].
Another critical area of future research will be determination of possible sexual dimorphisms that may in part explain sex differences in total adiposity, body fat mass distribution, and risk of obesity-associated complications. Sexual dimorphisms may exist in whole tissue endocrinology, in the metabolic roles of immune cells, as well as specifically in immune cell intracrine function. Immune cell intracrinology underscores the importance of recognizing marked discord between circulating and local, tissue-specific sex steroid concentrations [48], [127] and therefore cautions against the measurement of plasma sex steroid concentrations as an index of sex steroid signaling in metabolic tissues. Further, the relative contribution of immune cells to total tissue concentrations of all of these sex steroid species remains to be determined. Finally, interfaces between immune cell intracrinology and myriad other facets of immune cellular function must be clearly defined in order to fully delineate the respective intracrine/autocrine and paracrine roles of immune cell intracrine function in metabolic regulation.
Despite the many challenges that historically have impeded progress in understanding the physiologic and pathologic implications of immune cell intracrinology, current efforts now benefit from mass spectrometric methods that offer unprecedented ability to address these unanswered questions. Immune cell intracrinology may prove a completely novel and integral facet of metabolism research.
Conflict of interest
The author has no competing interests to disclose.
Acknowledgements
The author is supported in part by an American Heart Association (16GRNT30700006) Grant-in-Aid Award; this funding source had no input into the writing of this review. The author would like to thank David Rubinow, MD, for his gracious review and thoughtful critique of this work.
References
- 1.Labrie F. Intracrinology. Molecular and Cellular Endocrinology. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 2.McNamara K.M., Sasano H. The intracrinology of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2015;145:172–178. doi: 10.1016/j.jsbmb.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Mostaghel E.A., Page S.T., Lin D.W., Fazli L., Coleman I.M., True L.D. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Research. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 4.Cutolo M., Capellino S., Montagna P., Villaggio B., Sulli A., Seriolo B. New roles for estrogens in rheumatoid arthritis. Clinical and Experimental Rheumatology. 2003;21:687–690. [PubMed] [Google Scholar]
- 5.Bélanger C., Luu-The V., Dupont P., Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Hormone and Metabolic Research. 2002;34:737–745. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- 6.Waraich R.S., Mauvais-Jarvis F. Paracrine and intracrine contributions of androgens and estrogens to adipose tissue biology: physiopathological aspects. Hormone Molecular Biology and Clinical Investigation. 2013;14:49–55. doi: 10.1515/hmbci-2013-0022. [DOI] [PubMed] [Google Scholar]
- 7.Blouin K., Veilleux A., Luu-The V., Tchernof A. Androgen metabolism in adipose tissue: recent advances. Molecular and Cellular Endocrinology. 2009;301:97–103. doi: 10.1016/j.mce.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Dubois V., Laurent M., Boonen S., Vanderschueren D., Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cellular and Molecular Life Sciences. 2012;69:1651–1667. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro G., Allard C., Xu W., Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23:713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foryst-Ludwig A., Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. The Journal of Steroid Biochemistry and Molecular Biology. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankenstein M.A., Szymczak J., Daroszewski J., Milewicz A., Thijssen J.H. Estrogens in plasma and fatty tissue from breast cancer patients and women undergoing surgery for non-oncological reasons. Gynecological Endocrinology. 1992;6:13–17. doi: 10.3109/09513599209081001. [DOI] [PubMed] [Google Scholar]
- 13.Deslypere J.P., Verdonck L., Vermeulen A. Fat tissue: a steroid reservoir and site of steroid metabolism. Journal of Clinical Endocrinology & Metabolism. 1985;61:564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 14.Szymczak J., Milewicz A., Thijssen J.H., Blankenstein M.A., Daroszewski J. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–321. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 15.Labrie F. Extragonadal synthesis of sex steroids: intracrinology. Annales d'Endocrinologie (Paris) 2003;64:95–107. [PubMed] [Google Scholar]
- 16.Hemsell D.L., Grodin J.M., Brenner P.F., Siiteri P.K., MacDonald P.C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. Journal of Clinical Endocrinology & Metabolism. 1974;38:476–479. doi: 10.1210/jcem-38-3-476. [DOI] [PubMed] [Google Scholar]
- 17.Labrie F., Bélanger A., Luu-The V., Labrie C., Simard J., Cusan L. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Kuo J., Wong A., Micevych P. Estradiol modulates translocator protein (TSPO) and steroid acute regulatory protein (StAR) via protein kinase A (PKA) signaling in hypothalamic astrocytes. Endocrinology. 2014;155:2976–2985. doi: 10.1210/en.2013-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannen R.F., Michael A.E., Jaulim A., Bhogal R., Burrin J.M., Philpott M.P. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochemical and Biophysical Research Communications. 2011;404:62–67. doi: 10.1016/j.bbrc.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 20.Escobar J.C., Patel S.S., Beshay V.E., Suzuki T., Carr B.R. The human placenta expresses CYP17 and generates androgens de novo. Journal of Clinical Endocrinology & Metabolism. 2011;96:1385–1392. doi: 10.1210/jc.2010-2504. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Daly E., Campioli E., Wabitsch M., Papadopoulos V. De novo synthesis of steroids and oxysterols in adipocytes. Journal of Biological Chemistry. 2014;289:747–764. doi: 10.1074/jbc.M113.534172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagotto M.A., Roldán M.L., Pagotto R.M., Lugano M.C., Pisani G.B., Rogic G. Localization and functional activity of cytochrome P450 side chain cleavage enzyme (CYP11A1) in the adult rat kidney. Molecular and Cellular Endocrinology. 2011;332:253–260. doi: 10.1016/j.mce.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Robel P., Baulieu E.E. Neurosteroids: biosynthesis and function. Critical Reviews in Neurobiology. 1995;9:383–394. [PubMed] [Google Scholar]
- 24.Luu-The V., Labrie F. The intracrine sex steroid biosynthesis pathways. Progress in Brain Research. 2010;181:177–192. doi: 10.1016/S0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton E.J., Gianatti E., Strauss B.J., Wentworth J., Lim-Joon D., Bolton D. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clinical Endocrinology (Oxf) 2011;74:377–383. doi: 10.1111/j.1365-2265.2010.03942.x. [DOI] [PubMed] [Google Scholar]
- 26.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nature Reviews Endocrinology. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 27.Simpson E.R. Genetic mutations resulting in estrogen insufficiency in the male. Molecular and Cellular Endocrinology. 1998;145:55–59. doi: 10.1016/s0303-7207(98)00169-5. [DOI] [PubMed] [Google Scholar]
- 28.Pu D., Tan R., Yu Q., Wu J. Metabolic syndrome in menopause and associated factors: a meta-analysis. Climacteric. 2017;20:583–591. doi: 10.1080/13697137.2017.1386649. [DOI] [PubMed] [Google Scholar]
- 29.Condorelli R.A., Calogero A.E., Di Mauro M., Mongioi' L.M., Cannarella R., Rosta G. Androgen excess and metabolic disorders in women with PCOS: beyond the body mass index. Journal of Endocrinology Investigation. 2017 doi: 10.1007/s40618-017-0762-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Morselli E., Santos R.S., Criollo A., Nelson M.D., Palmer B.F., Clegg D.J. The effects of oestrogens and their receptors on cardiometabolic health. Nature Reviews Endocrinology. 2017;13:352–364. doi: 10.1038/nrendo.2017.12. [DOI] [PubMed] [Google Scholar]
- 31.Feldman R.D., Limbird L.E. GPER (GPR30): a nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annual Review of Pharmacology and Toxicology. 2017;57:567–584. doi: 10.1146/annurev-pharmtox-010716-104651. [DOI] [PubMed] [Google Scholar]
- 32.Lucas-Herald A.K., Alves-Lopes R., Montezano A.C., Ahmed S.F., Touyz R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clinical Science (Lond) 2017;131:1405–1418. doi: 10.1042/CS20170090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider A.E., Kárpáti E., Schuszter K., Tóth E.A., Kiss E., Kulcsár M. A dynamic network of estrogen receptors in murine lymphocytes: fine-tuning the immune response. Journal of Leukocyte Biology. 2014;96:857–872. doi: 10.1189/jlb.2A0214-080RR. [DOI] [PubMed] [Google Scholar]
- 34.Cutolo M., Sulli A., Capellino S., Villaggio B., Montagna P., Seriolo B. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- 35.Trigunaite A., Dimo J., Jørgensen T.N. Suppressive effects of androgens on the immune system. Cellular Immunology. 2015;294:87–94. doi: 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cellular Immunology. 2015;294:63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakiani S., Olsen N.J., Kovacs W.J. Gonadal steroids and humoral immunity. Nature Reviews Endocrinology. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 38.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmunity Reviews. 2012;11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 39.Gilliver S.C. Sex steroids as inflammatory regulators. The Journal of Steroid Biochemistry and Molecular Biology. 2010;120:105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Capellino S., Straub R.H., Cutolo M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Annals of the New York Academy of Sciences. 2014;1317:24–31. doi: 10.1111/nyas.12398. [DOI] [PubMed] [Google Scholar]
- 41.Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Hormones and Behavior. 2012;62:254–262. doi: 10.1016/j.yhbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilliver S.C., Ashworth J.J., Mills S.J., Hardman M.J., Ashcroft G.S. Androgens modulate the inflammatory response during acute wound healing. Journal of Cell Science. 2006;119:722–732. doi: 10.1242/jcs.02786. [DOI] [PubMed] [Google Scholar]
- 43.DiSpirito J.R., Mathis D. Immunological contributions to adipose tissue homeostasis. Seminars in Immunology. 2015;27:315–321. doi: 10.1016/j.smim.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Federico A., D'Aiuto E., Borriello F., Barra G., Gravina A.G., Romano M. Fat: a matter of disturbance for the immune system. World Journal of Gastroenterology. 2010;16:4762–4772. doi: 10.3748/wjg.v16.i38.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribas V., Drew B.G., Le J.A., Soleymani T., Daraei P., Sitz D. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubinow K.B., Wang S., den Hartigh L.J., Subramanian S., Morton G.J., Buaas F.W. Hematopoietic androgen receptor deficiency promotes visceral fat deposition in male mice without impairing glucose homeostasis. Andrology. 2015;3:787–796. doi: 10.1111/andr.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simpson E.R. Aromatase: biologic relevance of tissue-specific expression. Seminars in Reproductive Medicine. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt M., Hartung R., Capellino S., Cutolo M., Pfeifer-Leeg A., Straub R.H. Estrone/17beta-estradiol conversion to, and tumor necrosis factor inhibition by, estrogen metabolites in synovial cells of patients with rheumatoid arthritis and patients with osteoarthritis. Arthritis & Rheumatism. 2009;60:2913–2922. doi: 10.1002/art.24859. [DOI] [PubMed] [Google Scholar]
- 50.Toran-Allerand C.D., Tinnikov A.A., Singh R.J., Nethrapalli I.S. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 51.Harrison D.E., Strong R., Allison D.B., Ames B.N., Astle C.M., Atamna H. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stout M.B., Steyn F.J., Jurczak M.J., Camporez J.G., Zhu Y., Hawse J.R. 17α-estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. Journals of Gerontology Series A: Biological and Medical Sciences. 2017;72:3–15. doi: 10.1093/gerona/glv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda T., Makino Y., Yamada M.K. 17α-estradiol is generated locally in the male rat brain and can regulate GAD65 expression and anxiety. Neuropharmacology. 2015;90:9–14. doi: 10.1016/j.neuropharm.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Keller E.T., Chang C., Ershler W.B. Inhibition of NFkappaB activity through maintenance of IkappaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. Journal of Biological Chemistry. 1996;271:26267–26275. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- 55.Imperlini E., Mancini A., Spaziani S., Martone D., Alfieri A., Gemei M. Androgen receptor signaling induced by supraphysiological doses of dihydrotestosterone in human peripheral blood lymphocytes. Proteomics. 2010;10:3165–3175. doi: 10.1002/pmic.201000079. [DOI] [PubMed] [Google Scholar]
- 56.Hammer F., Drescher D.G., Schneider S.B., Quinkler M., Stewart P.M., Allolio B. Sex steroid metabolism in human peripheral blood mononuclear cells changes with aging. Journal of Clinical Endocrinology & Metabolism. 2005;90:6283–6289. doi: 10.1210/jc.2005-0915. [DOI] [PubMed] [Google Scholar]
- 57.Dulos J., van der Vleuten M.A., Kavelaars A., Heijnen C.J., Boots A.M. CYP7B expression and activity in fibroblast-like synoviocytes from patients with rheumatoid arthritis: regulation by proinflammatory cytokines. Arthritis & Rheumatism. 2005;52:770–778. doi: 10.1002/art.20950. [DOI] [PubMed] [Google Scholar]
- 58.Lathe R. Steroid and sterol 7-hydroxylation: ancient pathways. Steroids. 2002;67:967–977. doi: 10.1016/s0039-128x(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 59.Banerjee M., Robbins D., Chen T. Modulation of xenobiotic receptors by steroids. Molecules. 2013;18:7389–7406. doi: 10.3390/molecules18077389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yan J., Xie W. A brief history of the discovery of PXR and CAR as xenobiotic receptors. Acta Pharmaceutica Sinica B. 2016;6:450–452. doi: 10.1016/j.apsb.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mnif W., Pascussi J.M., Pillon A., Escande A., Bartegi A., Nicolas J.C. Estrogens and antiestrogens activate hPXR. Toxicology Letters. 2007;170:19–29. doi: 10.1016/j.toxlet.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Gao J., Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends in Pharmacological Sciences. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown K.A. Impact of obesity on mammary gland inflammation and local estrogen production. Journal of Mammary Gland Biology and Neoplasia. 2014;19:183–189. doi: 10.1007/s10911-014-9321-0. [DOI] [PubMed] [Google Scholar]
- 64.de Jong P.C., Blankenstein M.A., van de Ven J., Nortier J.W., Blijham G.H., Thijssen J.H. Importance of local aromatase activity in hormone-dependent breast cancer: a review. Breast. 2001;10:91–99. doi: 10.1054/brst.2000.0209. [DOI] [PubMed] [Google Scholar]
- 65.Castagnetta L.A., Carruba G., Granata O.M., Stefano R., Miele M., Schmidt M. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. Journal of Rheumatology. 2003;30:2597–2605. [PubMed] [Google Scholar]
- 66.Wang X., Sang X., Diorio C., Lin S.X., Doillon C.J. In vitro interactions between mammary fibroblasts (Hs 578Bst) and cancer epithelial cells (MCF-7) modulate aromatase, steroid sulfatase and 17β-hydroxysteroid dehydrogenases. Molecular and Cellular Endocrinology. 2015;412:339–348. doi: 10.1016/j.mce.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 67.Turgeon C., Gingras S., Carrière M.C., Blais Y., Labrie F., Simard J. Regulation of sex steroid formation by interleukin-4 and interleukin-6 in breast cancer cells. The Journal of Steroid Biochemistry and Molecular Biology. 1998;65:151–162. doi: 10.1016/s0960-0760(98)00031-4. [DOI] [PubMed] [Google Scholar]
- 68.Gingras S., Moriggl R., Groner B., Simard J. Induction of 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase type 1 gene transcription in human breast cancer cell lines and in normal mammary epithelial cells by interleukin-4 and interleukin-13. Molecular Endocrinology. 1999;13:66–81. doi: 10.1210/mend.13.1.0221. [DOI] [PubMed] [Google Scholar]
- 69.Reed M.J., Purohit A. Breast cancer and the role of cytokines in regulating estrogen synthesis: an emerging hypothesis. Endocrine Reviews. 1997;18:701–715. doi: 10.1210/edrv.18.5.0314. [DOI] [PubMed] [Google Scholar]
- 70.Berstein L.M., Santen R.J., Santner S.J. Three-component model of oestrogen formation and regulation of intratumoural oestrogen pool in breast neoplasms. Medical Hypotheses. 1995;45:588–590. doi: 10.1016/0306-9877(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 71.Mor G., Yue W., Santen R.J., Gutierrez L., Eliza M., Berstein L.M. Macrophages, estrogen and the microenvironment of breast cancer. The Journal of Steroid Biochemistry and Molecular Biology. 1998;67:403–411. doi: 10.1016/s0960-0760(98)00143-5. [DOI] [PubMed] [Google Scholar]
- 72.Castagnetta L., Granata O.M., Traina A., Cocciadiferro L., Saetta A., Stefano R. A role for sex steroids in autoimmune diseases: a working hypothesis and supporting data. Annals of the New York Academy of Sciences. 2002;966:193–203. doi: 10.1111/j.1749-6632.2002.tb04215.x. [DOI] [PubMed] [Google Scholar]
- 73.Samy T.S., Knöferl M.W., Zheng R., Schwacha M.G., Bland K.I., Chaudry I.H. Divergent immune responses in male and female mice after trauma-hemorrhage: dimorphic alterations in T lymphocyte steroidogenic enzyme activities. Endocrinology. 2001;142:3519–3529. doi: 10.1210/endo.142.8.8322. [DOI] [PubMed] [Google Scholar]
- 74.Mukhopadhyay R., Mishra M.K., Basu A., Bishayi B. Modulation of steroidogenic enzymes in murine lymphoid organs after immune activation. Immunological Investigations. 2009;38:14–30. doi: 10.1080/08820130802480570. [DOI] [PubMed] [Google Scholar]
- 75.Kautzky-Willer A., Handisurya A. Metabolic diseases and associated complications: sex and gender matter! European Journal of Clinical Investigation. 2009;39:631–648. doi: 10.1111/j.1365-2362.2009.02161.x. [DOI] [PubMed] [Google Scholar]
- 76.Ohlsson C., Hammarstedt A., Vandenput L., Saarinen N., Ryberg H., Windahl S. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. American Journal of Physiology. Endocrinology and Metabolism. 2017;313:E450–E462. doi: 10.1152/ajpendo.00093.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Reilly M.W., House P.J., Tomlinson J.W. Understanding androgen action in adipose tissue. The Journal of Steroid Biochemistry and Molecular Biology. 2014;143:277–284. doi: 10.1016/j.jsbmb.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Campbell K.L., Makar K.W., Kratz M., Foster-Schubert K.E., McTiernan A., Ulrich C.M. A pilot study of sampling subcutaneous adipose tissue to examine biomarkers of cancer risk. Cancer Prevention Research (Phila) 2009;2:37–42. doi: 10.1158/1940-6207.CAPR-08-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simpson E.R., Merrill J.C., Hollub A.J., Graham-Lorence S., Mendelson C.R. Regulation of estrogen biosynthesis by human adipose cells. Endocrine Reviews. 1989;10:136–148. doi: 10.1210/edrv-10-2-136. [DOI] [PubMed] [Google Scholar]
- 80.Pöllänen E., Sipilä S., Alen M., Ronkainen P.H., Ankarberg-Lindgren C., Puolakka J. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell. 2011;10:650–660. doi: 10.1111/j.1474-9726.2011.00701.x. [DOI] [PubMed] [Google Scholar]
- 81.Pöllänen E., Kangas R., Horttanainen M., Niskala P., Kaprio J., Butler-Browne G. Intramuscular sex steroid hormones are associated with skeletal muscle strength and power in women with different hormonal status. Aging Cell. 2015;14:236–248. doi: 10.1111/acel.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longcope C., Pratt J.H., Schneider S.H., Fineberg S.E. Aromatization of androgens by muscle and adipose tissue in vivo. Journal of Clinical Endocrinology & Metabolism. 1978;46:146–152. doi: 10.1210/jcem-46-1-146. [DOI] [PubMed] [Google Scholar]
- 83.Vingren J.L., Kraemer W.J., Hatfield D.L., Anderson J.M., Volek J.S., Ratamess N.A. Effect of resistance exercise on muscle steroidogenesis. Journal of Applied Physiology (1985) 2008;105:1754–1760. doi: 10.1152/japplphysiol.91235.2008. [DOI] [PubMed] [Google Scholar]
- 84.Yarrow J.F., McCoy S.C., Borst S.E. Intracrine and myotrophic roles of 5α-reductase and androgens: a review. Medicine & Science in Sports & Exercise. 2012;44(5):818–826. doi: 10.1249/MSS.0b013e31823bfcbf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimizu I. Impact of oestrogens on the progression of liver disease. Liver International. 2007;23:63–69. doi: 10.1034/j.1600-0676.2003.00811.x. [DOI] [PubMed] [Google Scholar]
- 86.Shimizu I., Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatology Research. 2007;37:239–247. doi: 10.1111/j.1872-034X.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- 87.Yamaguchi M., Yu L., Nazmy El-Assal O., Satoh T., Kumar Dhar D., Yamanoi A. Androgen metabolism in regenerating liver of male rats: evidence for active uptake and utilization of testosterone. Hepatology Research. 2001;20:114–127. doi: 10.1016/s1386-6346(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 88.Rakhshandehroo M., Kalkhoven E., Boes M. Invariant natural killer T cells in adipose tissue: novel regulators of immune-mediated metabolic disease. Cellular and Molecular Life Sciences. 2013;70:4711–4727. doi: 10.1007/s00018-013-1414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang F., Vihma V., Soronen J., Turpeinen U., Hämäläinen E., Savolainen-Peltonen H. 17â-estradiol and estradiol fatty acyl esters and estrogen-converting enzyme expression in adipose tissue in obese men and women. Journal of Clinical Endocrinology & Metabolism. 2013;98:4923–4931. doi: 10.1210/jc.2013-2605. [DOI] [PubMed] [Google Scholar]
- 90.Rubinow K.B., Chao J., Hagman D., Kratz M., Van Yserloo B., Gaikwad N.W. Circulating sex steroids co-regulate adipose tissue immune cell populations in healthy men. American Journal of Physiology. Endocrinology and Metabolism. 2017;313:E528–E539. doi: 10.1152/ajpendo.00075.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Milewich L., Kaimal V., Toews G.B. Androstenedione metabolism in human alveolar macrophages. Journal of Clinical Endocrinology & Metabolism. 1983;56:920–924. doi: 10.1210/jcem-56-5-920. [DOI] [PubMed] [Google Scholar]
- 92.Milewich L., Lipscomb M.F., Whisenant M.G., MacDonald P.C. Conversion of androstenedione to testosterone and other androgens in Guinea-pig alveolar macrophages. Journal of Steroid Biochemistry. 1983;19:1611–1615. doi: 10.1016/0022-4731(83)90378-3. [DOI] [PubMed] [Google Scholar]
- 93.Cutolo M., Accardo S., Villaggio B., Barone A., Sulli A., Balleari E. Androgen metabolism and inhibition of interleukin-1 synthesis in primary cultured human synovial macrophages. Mediators of Inflammation. 1995;4:138–143. doi: 10.1155/S096293519500024X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cutolo M., Villaggio B., Barone A., Sulli A., Accardo S., Granata O.M. Primary cultures of human synovial macrophages metabolize androgens. Annals of the New York Academy of Sciences. 1996;784:534–541. doi: 10.1111/j.1749-6632.1996.tb16277.x. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt M., Kreutz M., Löffler G., Schölmerich J., Straub R.H. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. Journal of Endocrinology. 2000;164:161–169. doi: 10.1677/joe.0.1640161. [DOI] [PubMed] [Google Scholar]
- 96.Schmidt M., Weidler C., Naumann H., Anders S., Schölmerich J., Straub R.H. Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes–androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5alpha-reduced androgens. Arthritis Research and Therapy. 2005;7:R938–R948. doi: 10.1186/ar1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hennebold J.D., Daynes R.A. Regulation of macrophage dehydroepiandrosterone sulfate metabolism by inflammatory cytokines. Endocrinology. 1994;135:67–75. doi: 10.1210/endo.135.1.8013393. [DOI] [PubMed] [Google Scholar]
- 98.Weidler C., Struharova S., Schmidt M., Ugele B., Schölmerich J., Straub R.H. Tumor necrosis factor inhibits conversion of dehydroepiandrosterone sulfate (DHEAS) to DHEA in rheumatoid arthritis synovial cells: a prerequisite for local androgen deficiency. Arthritis & Rheumatism. 2005;52:1721–1729. doi: 10.1002/art.21112. [DOI] [PubMed] [Google Scholar]
- 99.Berstein L.M., Poroshina T.E., Zimarina T.S., Larionov A.A., Kovalenko I.G., Uporov A.V. Ability of lymphocytes infiltrating breast-cancer tissue to convert androstenedione. International Journal of Cancer. 1998;77:485–487. doi: 10.1002/(sici)1097-0215(19980812)77:4<485::aid-ijc1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 100.Berstein L.M., Larionov A.A., Poroshina T.E., Zimarina T.S., Leenman E.E. Aromatase (CYP19) expression in tumor-infiltrating lymphocytes and blood mononuclears. Journal of Cancer Research and Clinical Oncology. 2002;128:173–176. doi: 10.1007/s00432-002-0322-9. [DOI] [PubMed] [Google Scholar]
- 101.Anuka E., Gal M., Stocco D.M., Orly J. Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Molecular and Cellular Endocrinology. 2013;371:47–61. doi: 10.1016/j.mce.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 102.Sugawara T., Fujimoto S. The potential function of steroid sulphatase activity in steroid production and steroidogenic acute regulatory protein expression. Biochemical Journal. 2004;380:153–160. doi: 10.1042/BJ20031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Manna P.R., Stetson C.L., Slominski A.T., Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016;51:7–21. doi: 10.1007/s12020-015-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bai Q., Li X., Ning Y., Zhao F., Yin L. Mitochondrial cholesterol transporter, StAR, inhibits human THP-1 monocyte-derived macrophage apoptosis. Lipids. 2010;45:29–36. doi: 10.1007/s11745-009-3375-6. [DOI] [PubMed] [Google Scholar]
- 105.Ning Y., Bai Q., Lu H., Li X., Pandak W.M., Zhao F. Overexpression of mitochondrial cholesterol delivery protein, StAR, decreases intracellular lipids and inflammatory factors secretion in macrophages. Atherosclerosis. 2009;204:114–120. doi: 10.1016/j.atherosclerosis.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borthwick F., Taylor J.M., Bartholomew C., Graham A. Differential regulation of the STARD1 subfamily of START lipid trafficking proteins in human macrophages. FEBS Letters. 2009;583:1147–1153. doi: 10.1016/j.febslet.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 107.Ma Y., Ren S., Pandak W.M., Li X., Ning Y., Lu C. The effects of inflammatory cytokines on steroidogenic acute regulatory protein expression in macrophages. Inflammation Research. 2007;56:495–501. doi: 10.1007/s00011-007-6133-3. [DOI] [PubMed] [Google Scholar]
- 108.Manna P.R., Sennoune S.R., Martinez-Zaguilan R., Slominski A.T., Pruitt K. Regulation of retinoid mediated cholesterol efflux involves liver X receptor activation in mouse macrophages. Biochemical and Biophysical Research Communications. 2015;464:312–317. doi: 10.1016/j.bbrc.2015.06.150. [DOI] [PubMed] [Google Scholar]
- 109.Żelechowska P., Agier J., Kozłowska E., Brzezińska-Błaszczyk E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obesity Reviews. 2018 doi: 10.1111/obr.12670. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 110.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metabolism. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonamichi B.D.S.F., Lee J. Unusual suspects in the development of obesity-induced inflammation and insulin resistance: NK cells, iNKT cells, and ILCs. Diabetes & Metabolism Journal. 2017;41:229–250. doi: 10.4093/dmj.2017.41.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Villaggio B., Soldano S., Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clinical & Experimental Rheumatology. 2012;30:934–938. [PubMed] [Google Scholar]
- 113.Eimerl S., Orly J. Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: differential responses of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, and 3beta-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biology of Reproduction. 2002;67:900–910. doi: 10.1095/biolreprod.101.002170. [DOI] [PubMed] [Google Scholar]
- 114.Wang X., Shen C.L., Dyson M.T., Eimerl S., Orly J., Hutson J.C. Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology. 2005;146:4202–4208. doi: 10.1210/en.2005-0298. [DOI] [PubMed] [Google Scholar]
- 115.Zhang J.Y., Wu Y., Zhao S., Liu Z.X., Zeng S.M., Zhang G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (StAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology. 2015;84:811–817. doi: 10.1016/j.theriogenology.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 116.Locke J.A., Guns E.S., Lehman M.L., Ettinger S., Zoubeidi A., Lubik A. Arachidonic acid activation of intratumoral steroid synthesis during prostate cancer progression to castration resistance. The Prostate. 2010;70:239–251. doi: 10.1002/pros.21057. [DOI] [PubMed] [Google Scholar]
- 117.Lubik A.A., Gunter J.H., Hendy S.C., Locke J.A., Adomat H.H., Thompson V. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Research. 2011;71:5754–5764. doi: 10.1158/0008-5472.CAN-10-2470. [DOI] [PubMed] [Google Scholar]
- 118.Lubik A.A., Gunter J.H., Hollier B.G., Ettinger S., Fazli L., Stylianou N. IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocrine-Related Cancer. 2013;20:173–186. doi: 10.1530/ERC-12-0250. [DOI] [PubMed] [Google Scholar]
- 119.Zheng R., Samy T.S., Schneider C.P., Rue L.W., Bland K.I., Chaudry I.H. Decreased 5alpha-dihydrotestosterone catabolism suppresses T lymphocyte functions in males after trauma-hemorrhage. American Journal of Physiology, Cell Physiology. 2002;282:C1332–C1338. doi: 10.1152/ajpcell.00560.2001. [DOI] [PubMed] [Google Scholar]
- 120.Schmidt M., Naumann H., Weidler C., Schellenberg M., Anders S., Straub R.H. Inflammation and sex hormone metabolism. Annals of the New York Academy of Sciences. 2006;1069:236–246. doi: 10.1196/annals.1351.021. [DOI] [PubMed] [Google Scholar]
- 121.Gottfried-Blackmore A., Sierra A., Jellinck P.H., McEwen B.S., Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. The Journal of Steroid Biochemistry and Molecular Biology. 2008;109:96–107. doi: 10.1016/j.jsbmb.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalhorn T.F., Page S.T., Howald W.N., Mostaghel E.A., Nelson P.S. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- 123.Arnold S.L., Amory J.K., Walsh T.J., Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. The Journal of Lipid Research. 2012;53:587–598. doi: 10.1194/jlr.D019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Foti R.S., Isoherranen N., Zelter A., Dickmann L.J., Buttrick B.R., Diaz P. Identification of tazarotenic acid as the first xenobiotic substrate of human retinoic acid hydroxylase CYP26A1 and CYP26B1. Journal of Pharmacology and Experimental Therapeutics. 2016;357:281–292. doi: 10.1124/jpet.116.232637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jing J., Nelson C., Paik J., Shirasaka Y., Amory J.K., Isoherranen N. Physiologically based pharmacokinetic model of all-trans-retinoic acid with application to cancer populations and drug interactions. Journal of Pharmacology and Experimental Therapeutics. 2017;361:246–258. doi: 10.1124/jpet.117.240523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coutinho A.E., Kipari T.M., Zhang Z., Esteves C.L., Lucas C.D., Gilmour J.S. 11β-hydroxysteroid dehydrogenase type 1 is expressed in neutrophils and restrains an inflammatory response in male mice. Endocrinology. 2016;157:2928–2936. doi: 10.1210/en.2016-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simpson E.R. Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]