Abstract
Background
The sex of an individual affects glucose homeostasis and the pathophysiology, incidence, and prevalence of diabetes as well as the response to therapy.
Scope of the review
This review focuses on clinical and experimental sex differences in islet cell biology and dysfunction during development and in adulthood in human and animal models. We discuss sex differences in β-cell and α-cell function, heterogeneity, and dysfunction. We cover sex differences in communication between gonads and islets and islet-cell immune interactions. Finally, we discuss sex differences in β-cell programming by nutrition and other environmental factors during pregnancy.
Major conclusions
Important sex differences exist in islet cell function and susceptibility to failure. These differences represent sex-related biological factors that can be harnessed for gender-based prevention of and therapy for diabetes.
Keywords: Sex differences, Gender differences, Islet, β-cell, α-cell, Diabetes, Immune cells
1. Introduction
Sex differences in physiology begin early in development from the combination of genetic and hormonal cues and they continue after puberty [1]. These differences result from the combination of three major events: 1. The differences in the number and type of sex chromosomes; 2. The perinatal testosterone surges that masculinize the reproductive tract and the organization of neural circuits; and 3. The activity of gonadal hormones after puberty. The combination of these factors produces distinct male and female biological systems for islet cells in vivo.
Increasing evidence suggests that sex affects glucose homeostasis and the pathophysiology, incidence, and prevalence of diabetes, as well as response to therapy. Sex differences in glucose homeostasis and diabetes have been recently reviewed elsewhere [2], [3], [4]. Normoglycemic women have lower fasting plasma glucose concentrations than men and higher 2-h plasma glucose concentrations following an oral glucose tolerance test (OGTT) [5], slower gastric emptying [6], and higher insulin sensitivity than men [7]. In addition, in individuals with prediabetes, women tend to exhibit glucose intolerance, whereas men exhibit impaired fasting glucose [8]. This review will focus on clinical and experimental sex differences in islet biology and dysfunction during development and in adulthood. The study of sex differences in islet function and failure is of fundamental importance, because it will generate knowledge on sex-related biological factors that can be harnessed for better options for prevention of and therapy for diabetes.
2. Sex differences in β-cell function under normal and stress conditions
2.1. Clinical studies
Sex differences in β-cell function are apparent in clinical studies. Compared to healthy men and despite similar plasma glucose levels, healthy women exhibit enhanced postprandial plasma insulin and C-peptide concentrations after a meal [9], suggesting that women have increased insulin secretion for a given glucose load. In addition, the disposition index, which reflects insulin secretion for a given level of insulin action, is higher in women than in men, supporting greater insulin secretion in women [9]. In a recent study of 63 healthy Japanese men and women, the insulin responses to an oral glucose load were higher in females than males, with no differences in insulin sensitivity between the sexes [10]. Likewise, studies in older men and women suggest that for a given level of insulin action, women have higher levels of insulin secretion [11]. It has been hypothesized that the increased glucose-stimulated insulin secretion (GSIS) in females was due to sex differences in glucose-stimulated GLP-1 production. In the ADDITION-PRO study, a large study population of 1,462 Danish adults, normoglycemic women had a greater increase in serum GLP-1 concentrations following an OGTT, than normoglycemic men, even after adjusting for body weight, height or BMI [12]. This sex difference could be explained by the fact that the female hormone 17β-estradiol (E2) stimulates glucose-induced GLP-1 secretion as will be discussed below. However, the relationship between the increased serum GLP-1 following OGTT and the increased first phase insulin secretion was similar in men and women [12]. Therefore, sex differences in serum GLP-1 concentrations following OGTT do not seem to explain sex differences in β-cell function in humans. In the same cohort, individuals with prediabetes and type 2 diabetes (T2D) exhibited less of a GLP-1 increase than normoglycemic controls following an OGTT. This impaired serum GLP-1 increase in response to OGTT was most pronounced in women compared with men [12]. Thus, healthy women exhibit a greater increase in GLP-1 following an oral glucose challenge, but, as glucose tolerance deteriorates, this sex difference is no longer apparent. Interestingly, in individuals with prediabetes, women tend to be predisposed to impaired glucose tolerance, compared to men [8]. However, it is unknown if these phenotypic differences reflect differences in β-cell function.
Gender differences in β-cell failure are also observed in insulin-deficient forms of diabetes. For example, type 1 diabetes (T1D) is the only common autoimmune disease characterized by a male predominance [2], [3], [4], [13]. Girls presenting with T1D in pubertal years have higher residual β-cell function than boys at diagnosis [14]. Similarly, ketosis-prone diabetes (KPD) is a phenotypically-defined form of T2D characterized by a strong male predominance (75%) and rapid β-cell failure leading to severe insulin deficiency [15]. In subjects with KPD, the male gender is associated with a more pronounced decrease in β-cell insulin secretory reserve, assessed by fasting and glucagon-stimulated C-peptide [16]. The rare women developing KPD were in an anovulatory state. Finally, a mutation in the β-cell transcription factor V-Maf avian musculoaponeurotic fibrosarcoma oncogene homolog A (MAFA), causes autosomal dominant inheritance of diabetes or insulinomatosis (insulin-secreting tumors of the pancreas causing adult-onset hyperinsulinemic hypoglycemia) with a sex dimorphism. Women with the MAFA mutation are more likely to develop insulinomatosis while men are more likely to develop diabetes [17]. The reason for this divergent phenotype has not yet been determined, but, interestingly, the women who developed insulinomatosis had a prior pregnancy, suggesting that the hormonal milieu of pregnancy influences the effect of this mutation on the β-cell.
2.2. Studies using human islets and β-cells
The Integrated Islet Distribution Program (IIDP; https://iidp.coh.org/) has greatly facilitated the use of cadaver donor human islets in research. Thus, we can now more easily assess whether intrinsic differences exist between islets and β cells obtained from male and female individuals. In one study, 53 male and 34 female human pancreatic islet donors were compared [18]. There were no significant differences in age, body mass index (BMI), hemoglobin A1c (HbA1c), islet purity or β-cell content between males and females. However, ex vivo GSIS was slightly higher in islets from females compared with males.
Information generated from studies of pancreas development and endocrine differentiation from multiple organisms has resulted in multistep directed-differentiation protocols that can convert human embryonic stem cells (hESCs) into pancreatic progenitor cells, which, several months following implantation into immunocompromised mice, develop into mature glucose-responsive insulin-secreting cells that are capable of reversing experimentally-induced diabetes [19], [20]. To date, clinical trials assessing the utility of encapsulated hESC-derived pancreatic progenitors in patients with poorly controlled T1D have not been successful in regulating glycemia, likely due to fibrosis in the transplanted cells. Interestingly, recent studies suggest that the sex of the host into which hESC-derived pancreatic progenitors are transplanted may affect their ability to mature into optimally functional β-cells. Male and female mice were transplanted with two different stages of hESC-derived pancreatic cells: endocrine progenitors or insulin-positive cells. In vivo maturation of both cell populations into glucose-responsive insulin-secreting cells (as measured by circulating human C-peptide) was accelerated in female recipients (12 weeks compared to 16 weeks) compared with male hosts [21]. The authors concluded that E2 in female recipients promoted more rapid β-cell maturation. Indeed, a large body of evidence demonstrates that E2 protects rodent and human islets from multiple metabolic injuries [Reviewed in [22], [23]], and E2 promotes human islet engraftment and revascularization in diabetic mice [24]. Interestingly, long-term (35 weeks) graft function was higher in male hosts compared to females, potentially due to increased adipose tissue associated with the grafts in females [21].
2.3. Studies using animal models
Most rodent studies examining effects of gene and/or environmental manipulations on β-cell mass and function have traditionally focused only on male adults given the stronger diabetic phenotype compared to females. This biased approach already reflects differences between the two sexes and has led to a paucity of data on sex differences in islet gene expression or β-cell function or whether islets from males and females react differently to stressors. However, recent evidence suggests that GSIS is modulated in a sex-specific manner by gonadal hormones. For example, testosterone enhances GSIS in vivo in male mice via action on the androgen receptor (AR) in β cells [25]. In cultured male mouse and human islets, testosterone binding an extranuclear AR enhances cAMP production and the insulinotropic effect of GLP-1. In contrast, in females, E2 increases glucose-induced GLP-1 secretion in vivo and GLP-1 secretion from primary cultures of mouse and human α cells and intestinal explants through the activation of estrogen receptors (ERs) [26]. Thus, although male and female mammals exhibit the same overall mechanism of nutrient-induced insulin secretion, the fine-tuning of insulin secretion is regulated in a sex-specific manner by sex hormones. GSIS in rodents declines significantly with age in both sexes [27], [28]. However, isolated islets from female rats at 18 months of age still show higher glucose-stimulated insulin secretion (GSIS) ex vivo than those of males [27], confirming results obtained in humans [18]. Islets from elderly female rats showed elevated mitochondrial function (ATP content and oxygen consumption) compared with males when exposed to high glucose ex vivo. Mitochondrial biogenesis was also significantly higher in elderly female rats compared with males.
In multiple rodent models of diabetes in which β-cell failure is observed, there is also sex dimorphism. For instance, female animals are usually protected from development of the disease, with the exception of the NOD mouse as discussed in Section 4. Sexually dimorphic models including mice with streptozotocin-induced diabetes, the transgenic mouse overexpressing human islet amyloid polypeptide (hIAPP) in β cells, or the Zucker diabetic fatty (ZDF) rat have been reviewed elsewhere and will not be discussed in detail here [22], [23]. These models, however, have been instrumental in revealing the role of sex in islet pathophysiology and have contributed to identifying the main female ovarian hormone 17β-estradiol (E2) as a critical factor in protecting human islets from metabolic injuries including oxidative stress, gluco-lipotoxicity, and apoptosis.
3. Islet and β-cell heterogeneity
Islet endocrine cell composition is known to vary according to the anatomical location within the pancreas and among different species [29], [30], [31]. However, recent data from humans and animal models suggest that the proportion of different hormone-producing cells in the pancreas also differs between males and females of the same species. For example, in chickens, males have a greater proportion of β-cells within islets of the ventral pancreas, while females show a greater percentage of mixed islets in the splenic lobe of the pancreas [32]. In human pancreas biopsies, one study of 19 individuals found that females have an average of 6% more β-cells than males [33]. This is supported by an imaging study using radiolabeled exendin, which binds to the GLP-1 receptor on β-cells, where females showed increased absorbance compared to males [34]. Interestingly, islet transplants (14 in this study) performed with female donor islets showed improved clinical outcomes compared with islets obtained from male donors [33].
Over the past several years it has become apparent that individual β-cells from humans and animal models exhibit substantial heterogeneity in gene expression, electrical activity, secretory capacity, proliferation, and antigenicity [35], [36], [37]. These analyses have been greatly aided by the onset of single-cell ‘omics’ methodologies. Most animal studies have been undertaken with islets isolated from male mice, although in many publications, the sex from which the beta cells were obtained is not mentioned. In humans, four distinct subtypes of β-cells have been identified based on differential expression of cell surface markers [38]. In this study, islets from 16 non-diabetic donors (10M, 6F) and eight donors with T2D (2M, 6F) were assessed for the proportion of the four different β-cell subtypes. Although there is variability in the proportion of the four subtypes among individuals, and in general a decrease in the most commonly found β-cell subtype in individuals with T2D, there is no difference in subtype distribution between males and females. Studies using human cadaver donor islets also reveal differences in gene expression and epigenetic marks between males and females. For example, a study of 53 male and 34 female donors identified 2,853 sites on the X chromosome that were differentially methylated between males and females with >5% higher methylation in males [18]. These sites correspond to 757 individual genes out of a total of 890 genes on the X chromosome. There were no significant differences in age, body mass index (BMI), or hemoglobinA1c (HbA1c) between the two sexes. One of the genes that shows an increase in DNA methylation in male islets is DUSP9, a gene associated with insulin resistance and type 2 diabetes [39]. In addition, these analyses uncovered differential methylation in other diabetes and metabolism candidate genes located on autosomal chromosomes including BCL11A, HNF4a, CDKN2B, ATP11A, ADCY5, and IRS1. Several microRNAs were also differentially methylated.
In summary, in humans, female islets secrete more insulin in culture and in vivo, harbor more β-cells, and exhibit a lower degree of methylation of the X chromosome than male islets.
4. Islet and immune cell interactions
Studies in humans also show variable results with regards to sexual dimorphism in the presence of autoantigens associated with T1D progression. In one study of individuals at high risk for developing T1D, the frequency of autoantibodies against glutamic acid decarboxylase (GAD) was higher in females (88%) than males (71%), while insulin autoantibodies (IAA) were found at a higher prevalence in males (82% versus 60%) [40]. However, another study saw no difference in autoantibody prevalence in male versus female offspring of parents with T1D, although there was an increased prevalence of islet autoantibodies if the paternal parent had T1D versus the maternal parent [41].
Thus, T1D incidence is predominated by males and the onset of puberty is often associated with a decreased incidence of T1D in females suggesting that the female hormone E2 is protective [2], [3], [4], [13]. Surprisingly, this sex dimorphism is the opposite in the NOD mouse model of T1D, which is characterized by a female predominance as will be discussed below. Several possibilities can explain this relative female protection from T1D in humans. T1D progression results from immunological defects in central and peripheral immune tolerance that culminates in the destruction of β-cells [42], [43]. The immune system is one of the most sexually dimorphic. For example, females show heightened immunity to pathogens and are predisposed to autoimmune disorders compared to males [44], [45]. This female predominance, however, is not observed for T1D. One possibility is that E2 protects female islets from apoptosis in vivo but also promotes immune tolerance. Consistent with the first possibility, E2 is known to protect human islets from multiple pro-apoptotic stimuli in vitro and in vivo (Reviewed in [22], [23]). It is also plausible that in some women, decreased circulating levels of E2 contribute to defects in peripheral immune tolerance and progression to autoimmune responses in T1D. In support of this hypothesis, serum E2 levels and estrogenic activity are decreased in adolescents with T1D, and the potential protective effects of E2 are lost [46]. The immunological effects of E2 on the innate and adaptive immune arms of the immune system are being increasingly appreciated. The immunopathogenesis of T1D involves innate immune activation comprising of islet-resident macrophages and dendritic cells to engulf β cell autoantigens that can be ferried to the pancreatic lymph node to promote the activation of naïve autoreactive CD4 and CD8 T cells [47]. The activation of islet-resident macrophages and dendritic cells is a key initiating event since they can directly facilitate β cell destruction by generating pro-inflammatory cytokines, chemokines, and reactive oxygen species. Notably, E2 is protective against innate immune pro-inflammatory responses and prevents apoptosis of cultured human islets exposed to IFN-γ, IL-1β, and TNF-α [48]. Importantly, following transplantation in diabetic mice, islets treated with E2 ex vivo showed improved insulin secretion and were more efficient in restoring euglycemia than non-treated islets. Other beneficial effects of E2 on innate immunity were observed with Coxsackievirus-infected human islets in which E2 produced a profound decrease in the synthesis of the chemokine CXCL10 [49]. There is a wealth of evidence demonstrating an association of T1D onset with enteroviral infections [50], [51]. The ability of E2 to decrease CXCL10 synthesis is significant since this pro-inflammatory chemokine can recruit autoreactive T cells into the islets to propagate β cell destruction [52]. E2 can also suppress pro-inflammatory cytokine synthesis from neutrophils, macrophages, and dendritic cells including TNF-α, IL-1β, IL-12, and IL-23 [53]. Finally, E2 can decrease the synthesis of pro-inflammatory cytokines including IL-6 from stimulated macrophages. Interestingly, genetic polymorphisms within the IL-6 promoter element result in an inability of E2 to promote resistance and downregulate IL-6 synthesis and may confer an early risk of T1D onset in females [54]. Therefore, in females, E2 could delay islet destruction in T1D by suppressing the innate immune response.
However, E2 can also protect islets from the adaptive immune response. One of the best examples demonstrating that E2 promotes peripheral immune tolerance is the immunological effects of E2 on regulatory T (Treg) cell and invariant natural killer T (iNKT) cell differentiation and effector responses. Treg cells provide self-tolerance and are essential for suppressing autoimmune responses in individuals with T1D [55], [56]. Previous studies have shown that E2 treatment promotes the expansion of immunosuppressive Treg cells including Foxp3 mRNA accumulation and protein expression to protect female mice from experimental autoimmune encephalomyelitis, an autoimmune mouse model of multiple sclerosis [57]. Recent molecular mechanistic studies demonstrated that E2 treatment of Treg cells facilitates FOXP3 expression via estrogen receptor-α (ERα) binding eight estrogen response elements in the FOXP3 promoter [58]. E2 can also influence the expression of inhibitory receptors and anti-inflammatory cytokines expressed by Treg cells to facilitate peripheral tolerance including PD-1 and IL-10, respectively [59], [60]. In addition to promoting the differentiation of immunosuppressive Treg cells, E2 also enhance the immunomodulatory function of iNKT cells. Although female NOD mice are predisposed to T1D, treatment of female NOD mice with E2 delays spontaneous autoimmune T1D partly due to the activation of iNKT cells [61]. Stimulation of iNKT cells with the glycolipid alpha-galactosylceramide (α-GalCer) was effective in delaying T1D in NOD mice by skewing T cell cytokine responses to a less inflammatory Th2 phenotype and also by recruiting tolerogenic dendritic cells to promote Treg cell differentiation [62], [63]. Notably, the immunomodulatory effects of iNKT cells to delay autoimmune diabetes could be enhanced with combinatorial E2 and α-GalCer treatment [61], [64].
Interestingly, the NOD mouse model of spontaneous autoimmune diabetes, when housed in specific pathogen free (SPF) conditions, displays a female sex bias in disease incidence (F:M, 2:1). Under germ-free (GF) conditions, this sex dimorphism is not observed suggesting that the microbiome is instrumental in the female bias [65]. Transfer of adult NOD male intestinal microbiota into female NOD mice increased serum testosterone levels and protected from T1D [65]. Another study also reported that colonization of GF NOD females with bacterial taxa from SPF males also increased serum testosterone levels [66]. Notably, the protection against T1D induced by male microbiome transfer was abolished in female recipients treated with an androgen receptor antagonist, again implicating testosterone in the transfer of T1D protection in NOD mice. Together these results suggest that sex and androgens contribute to microbiome composition, which uniquely affects immune responses and progression to autoimmunity in the NOD mouse.
5. α-cell function and dysfunction
5.1. Counter-regulatory responses to hypoglycemia
Insulin-induced hypoglycemia is counterregulated by glucagon, epinephrine (adrenaline), norepinephrine (noradrenaline), cortisol, and growth hormone [67]. Sex differences in counter regulation and especially α-cell function has been a matter of debate for years. Historically, premenopausal women were reported to exhibit lower blood glucose than men during a prolonged 72 h fasting providing the first indication of gender difference in the counterregulation to hypoglycemia [68]. However, glucagon concentrations were not assessed and the role of the α-cell vs catecholamines in counterregulation is unknown. Subsequent studies assessing the effect of gender on catecholamine responses to hypoglycemia during euglycemic-hypoglycemic clamps showed that catecholamines (epinephrine, norepinephrine) and/or cortisol/GH responses to hypoglycemia, but not to glucagon, were diminished in healthy women compared to men [69], [70].
Since the autonomic nervous system (ANS) contributes to increased glucagon secretion during insulin-induced hypoglycemia in rodents and primates [71], the diminished catecholamine responses to hypoglycemia would be predicted to reflect lower glucagon responses to hypoglycemia in women. Indeed, a study reported that the glucagon response to insulin-induced hypoglycemia was blunted in nondiabetic women in the presence of the ganglion nicotinic receptor trimethapan [72], suggesting that the ANS mediates the majority of the glucagon response to insulin-induced hypoglycemia. Further, Davis et al. performed glucose clamps on healthy human subjects and reported that females showed lower glucagon response to equivalent fixed hypoglycemia and hyperinsulinemia compared to age-and-BMI-matched males [73]. However, during low-dose insulin-induced hypoglycemia, suppression of endogenous glucose output was more prolonged in healthy women compared to men but catecholamine and glucagon concentrations did not differ between the two sexes during this challenge [69]. In mice, neuroglucopenia is associated with a stronger glucagon response in females [74]. In studies targeting healthy young Japanese adults, females exhibited a stronger suppression of glucagon secretion after an oral glucose load compared with males [10]. These data are consistent with female mice exhibiting an increased sensitivity of α-cells to cholinergic activation of glucagon secretion that in turn led to larger glucagon response to hypoglycemia [74].
In summary, it appears that women exhibit reductions in the counterregulatory hormones, glucagon, and epinephrine, together with blunted rates of endogenous glucose production compared to men. These differences could explain why blood glucose concentrations fall to lower levels during fasting in women. It is unclear, however, if this sex difference in the glucagon response to insulin-induced hypoglycemia reflects differences in autonomic activation or in direct pancreatic α-cell regulation.
5.2. Sex hormones and glucagon secretion
Early studies reported an increase in glucagon content in pancreases from female mice [75], [76]. The glucagon level in the portal vein was reduced relative to insulin in estrogen-treated ovariectomized rats [77]. In rodent models, ovariectomy increased circulating glucagon, and this effect was reversed by E2 administration [78]. Similarly, following islet transplantation, E2 suppresses hyperglucagonemia in hyperglycemic mice [24]. The effect of E2 is direct since the estrogen receptor-α (ERα) and the G protein-coupled ER (GPER) are expressed in islet cells [79], and E2 decreases glucagon secretion from islets isolated from males and females [80]. In addition, the GPER agonist G-1 inhibited glucagon secretion in cultured islets from female wild type mice to an extent similar to E2 [81]. However, it is unknown if these effects are mediated via a direct glucagonostatic action on ERs in α-cells or an indirect insulinotropic effect on ERs in β cells. Further, the above studies were undertaken in animal models, and the direct effects of estrogens on glucagon secretion in humans have not been studied.
Mechanistically, endocrine disruptors such as bisphenol A (BPA) and diethylstilbestrol (DES), which mimics E2, were shown to suppress low glucose-induced intracellular calcium ion ([Ca2+]i) oscillations, which triggers glucagon secretion from α-cells [82]. However, a detailed morphological analysis including single cell approaches in different genders has not been reported.
6. Cross-talk between organs and islet cells
Recent reviews have discussed the importance of organ cross-talk with regard to the regulation of islet biology [83]. Here we will focus on the effect of male and female gonadal hormones on islet cell biology.
6.1. Ovarian–islet axis in female
In women, the ovarian–islet axis influences β-cell biology during reproductive years and at menopause. As discussed earlier in Section 5, estrogens increase GLP-1 production in mice. Sex-specific effects of estrogens and progesterone on β-cell survival, function, and proliferation during pregnancy have been reviewed elsewhere [22]. Prolactin secreted from the pituitary and placental lactogen contribute to the expansion of β-cell mass during pregnancy [84]. Prolactin and lactogen mediate their actions on β-cell proliferation through HGF, Menin, serotonin, and/or osteoprotegerin pathways [85], [86], [87], [88], [89]. However, the factors that promote maternal β-cell adaptation during pregnancy in humans are still unclear and warrant additional studies. Studies of the effect of menopause on islet function in women suggest that it alters insulin secretion [90]. However, menopausal changes in insulin clearance and metabolism make this assessment particularly difficult to interpret.
6.2. Testicular-islet axis in males
In men, the testicular-islet axis also impacts islet biology via production of testosterone. Men with primary testosterone deficiency due to androgen-depletion therapy (ADT) for prostate cancer exhibit an increased diabetic risk. In two, large, population-based studies of men with prostate cancer, ADT with gonadotropin releasing hormone (GnRH) analogs was associated with a 28–44% increased risk of incident diabetes compared to controls [91], [92]. Thus, severe testosterone deficiency is instrumental in predisposing to hyperglycemia and diabetes in these patients. Obviously, the diabetogenic effect of androgen depletion in men involves the combination of insulin resistance and visceral adiposity [93]. However, evidence suggests that androgen action in β-cells enhances β-cell function in men [94]. First, testosterone increases GSIS via AR in human islets from male donors [25]. Second, in hypogonadal men with T2D, testosterone therapy improved β-cell function (measured by HOMA %B) [95]. In fact, women with ovarian hyperandrogenism also exhibit β-cell dysfunction. Some show higher basal insulin secretory rates and attenuated secretory responses to meals [96] or exhibit exaggerated acute insulin response to glucose [97]. These abnormalities are closely associated with testosterone levels [98] suggesting that excess testosterone leads to insulin hypersecretion in women [99]. Further, testosterone produces insulin hypersecretion from cultured islets of human female donors and chronic testosterone administration to female mice promotes insulin hypersecretion, islet oxidative injury and secondary β-cell failure as a result of AR activation in β-cells [100].
7. In utero β-cell programming
The Developmental Origins of Health and Disease (DOHaD) hypothesis states that the in utero environment impacts postnatal susceptibility to disease. Exposure to either under-nutrition or over-nutrition in utero has been shown to lead to increased susceptibility to obesity and diabetes in the offspring in humans and in animal models (reviewed in [101]). Taken together, the data reveal sexual dimorphism in susceptibility to environmental manipulations during critical periods of pancreas development that impact β-cell differentiation.
7.1. Effect of maternal obesity
Offspring born to overweight or obese mothers are more likely to be obese as children and into early adulthood [102]. Additionally, offspring of mothers who were obese before and during pregnancy are more likely to develop insulin resistance and impaired glucose tolerance as adults independent of birth weight [103]. Given the fact that nearly 50% of women of child-bearing years are currently overweight or obese [104], understanding how the in utero environment affects islet development, mature function, and adaptability to stress has become increasingly important. Molecular mechanisms underlying these phenomena are likely to include epigenetic modifications. Several different animal models have been used to study the effects of in utero over-nutrition on islet structure and function (reviewed in [101]). Three common threads emerge when considering the body of animal studies as a whole. First, overwhelmingly, maternal HFD results in significant changes in islet architecture and/or function, many of which would be predicted to increase susceptibility to Type 2 diabetes later in life. Second, although changes in α- or β-cell mass may vary, there is usually evidence of impaired islet function. Finally, in the minority of studies that focused on sex differences, there were observable differences in islet function between males and females exposed to HFD in utero, although which sex was more severely affected depended on the design of the study.
The non-human primate represents a model in which islet architecture, composition, and gene expression more closely resembles humans [31]. Studies in Japanese macaques reveal that in utero HFD exposure results in increased fasting blood glucose and decreased first phase insulin secretion in both males and females at 13 months, with males specifically showing an increased in total pancreas IL1-β (post-weaning) [105].
In one study using C57Bl/6J dams placed on a HFD during pregnancy and lactation, male and female offspring were compared after weaning onto a control chow diet (CD) or maintenance on the maternal HFD [106]. Adult female (but not male) HFD-exposed offspring that were maintained on the HFD post-weaning showed a significant increase in body weight, plasma insulin, and blood glucose compared with CD-exposed females weaned onto HFD. However, β-cell compensation and adaptability seem to be preserved in females exposed to HFD in utero, since islet size and insulin content were increased when either CD- or HFD-exposed females were placed on HFD post-weaning. In contrast, males exposed to HFD in utero showed higher blood glucose levels but lower plasma insulin and decreased islet insulin content as adults regardless of post-weaning diet. HFD-exposed males also showed reduced islet area and, unlike female offspring, were unable to expand islet mass when maintained on HFD post-weaning. Defects in β-cell compensation in response to post-weaning HFD were also observed in a rat model of maternal over-nutrition Expression of the β-cell transcription factor Pdx1 was decreased in islets from males exposed to maternal HFD regardless of post-weaning diet, while female offspring exposed to maternal HFD had increased Pdx1 expression regardless of post-weaning diet. Islets from HFD-exposed males showed increased expression of Nox4 and gp91phox, both involved in NAD(P)H oxidation and superoxide production. Other measures of oxidative stress were also higher specifically in HFD-exposed males regardless of the post-weaning diet. Thus, despite HFD-exposed female offspring having impaired glucose tolerance, they seem to be protected from oxidative stress and β-cell dysfunction. In rats, female offspring were more affected by maternal HFD than were male offspring [107]. Females had increased β-cell mass, but reduced expression of GLUT2 and decreased GSIS. In contrast, paternal high fat diet consumption and insulin resistance in rats is associated with impaired insulin secretion in female but not male offspring [108]. In this study, female offspring of obese fathers had reduced β-cell mass due to smaller islets, suggestive of decreased replication. Gene expression analyses of islets from female offspring revealed decreased expression of genes involved in cell proliferation, metabolism, and granule exocytosis.
7.2. Effect of maternal undernutrition
There is some evidence in humans that adverse in utero exposures have differential effects on male and female offspring that can increase risk for metabolic disease. For example, changes in DNA methylation at metabolic gene loci differed between male and female offspring born during the Dutch Hunger Winter in 1944–45 [109]. At age 50, women born during the Dutch Hunger Winter had increased BMI and waist circumference compared with men [110]. However, most of our current understanding of DOHaD comes from studies in animal models. In animal models of intrauterine growth restriction (IUGR), male offspring tend to show impaired insulin sensitivity as adults, while females tend to have reduced insulin secretion [111]. In rats, either IUGR or maternal under-nutrition results in decreased β-cell in both sexes [107], [112], [113]; however, males had a higher pancreatic insulin content than females [107] and females showed reduced expression of the critical β-cell transcription factor Pdx1 as well as insulin [113]. In mice, male offspring whose mothers were exposed to a low protein diet during gestation had decreased β-cell mass and a lower mitochondrial-nuclear DNA ratio compared with controls [112]. Expression levels of genes involved in the TCA cycle were reduced in islets of both males and females, while higher ROS production was observed specifically in islets from male offspring. The defective mitochondrial gene expression and increased ROS likely contributes to β-cell dysfunction and in the longer-term, β-cell demise, potentially leading to increased susceptibility to T2D later in life.
7.3. Effect of maternal hyperglycemia
There is evidence that human exposure to hyperglycemia in utero has differential effects on male and female offspring that can increase risk for diabetes. For example, human fetal exposure to maternal T1D is associated with altered GSIS in adult offspring of both sexes independent of adiposity and insulin resistance [114]. Notably, this β-cell defect is characterized by a sexual dimorphism [115]. In response to oral glucose stimulation, male and female offspring of diabetic mothers displayed reduced insulin secretion. In contrast, in response to I.V. glucose stimulation, only female offspring of diabetic mothers exhibited decreased insulin secretion. This suggests that males are protected against or can compensate for the deleterious effect of maternal hyperglycemia on fetal β-cells.
In a mouse model of hyperglycemia during pregnancy [116], both male and female offspring showed impaired glucose tolerance (although this was more pronounced in males). Interestingly, the F2 generation from these hyperglycemia-exposed offspring also showed impaired glucose tolerance regardless of whether it was the male or female parent who had been exposed to in utero hyperglycemia (again, male F2 offspring were more severely affected). Expression of two differentially imprinted genes, insulin-like growth factor 2 (Igf2) and the long non-coding RNA H19, was decreased in islets from both males and females, but again, the magnitude of the decrease was greater in male offspring than female offspring.
7.4. Effect of in utero environmental exposures
Studies in mice also suggest that male and female offspring respond differently to in utero environmental exposures. For example, exposure of dams to bisphenol A (BPA), a xenoestrogen used in the manufacturing of some plastics, causes a reduction in β-cell mass and impairs GSIS in male offspring at the F1 and F2 generation, but has no effect on these parameters in female offspring [117]. BPA influences on pancreatic islet mitochondrial function could play a role since abnormal mitochondrial number and function generates reactive oxygen species (ROS) and affects GSIS. Indeed, in rodent islets, BPA exposure is known to alter expression of key mitochondrial genes such as oxoglutarate dehydrogenase (Ogdh) and uncoupling protein 2 (Ucp2) [118], [119]. Thus, insulin secretory defects in males exposed to BPA may be due to greater susceptibility of mitochondria in males to environmental insults. Although there were no differences in total oxygen consumption in islets isolated from males exposed in utero to BPA, there were reductions in basal and maximal oxygen consumption. Ogdh expression was mildly increased and Ucp2 levels were significantly increased in BPA-exposed males compared with control males consistent with mitochondrial impairment. In BPA-exposed mouse dams, metabolic phenotypes in the F1 and F2 male offspring were linked to fetal over-expression of Igf2 [120]. Both IGF2 and H19 are involved in growth, proliferation and weight gain.
8. Conclusion and future perspectives
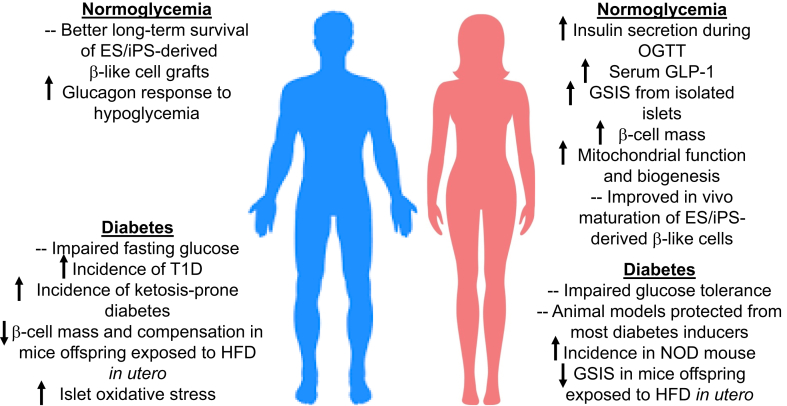
Evidence presented in this review underscores the influence of sex and gender that contribute to differences in islet cell function and susceptibility to failure (Figure 1). These differences represent sex-related biological factors that can be harnessed for developing better approaches to prevent and/or treat diabetes. However, major obstacles that currently prevent scientific progress in sex differences in islet biology should be addressed. For one, there are no suitable male and female islet cell lines available to study sex differences in culture. Establishing genetically suitable immortalized human islet lines of both sexes seems unrealistic. The utility of primary islet cells from inbred rodents of the same genetic background is limited due to their short lifespan in culture and the inability to expand and passage these cells. The development of clonal, immortalized cell lines from littermate inbred animals seems a logical first step in creating suitable male and female islet cell lines. While the comparison of cultured male and female islet cells oversimplifies the biology of sex because of the limitations of the in vitro environment, their availability as in vitro ‘tools’ will be useful for certain experiments that are difficult to undertake using primary islet tissue. Because of the sex differences described above, the in vivo environment of male and female islets differs in multiple factors including hormones, metabolites, and neural inputs. These defined phenotypic sex differences cannot be fully reproduced in culture. Finally, studies on sex differences will inform islet transplant procedures in male and female recipients and for generation of embryonic or adult stem cell derived islet cells for research and therapy that continues to be an important aim in diabetes research. In conclusion, the current research tools are not fully adapted to the study of sex differences in islet cell biology and dysfunction. The recent advances in technology should allow the development of modern research tools for experimental purposes as well as to contribute to precision and personalized medicine in the context of gender-specific therapies for diabetes.
Figure 1.
Summary of sex differences in islet biology and failure.
Funding
MG was supported by grants from the National Institutes of Health (NIH) (R01 DK105689 and 2R24 DK090964-06) and a Department of Veterans Affairs Merit Review Award (BX003744). RNK was supported by grants from the NIH (R01 DK67536 and R01 DK103215). HT was supported by grants from the NIH (R01 DK099550) and JDRF (2-SRA-2016-270-S-B, 2-PNF-2016-322-S-B). FMJ was supported by grants from the NIH (R01 DK074970 and R01 DK107444) and a Department of Veterans Affairs Merit Review Award (BX003725).
Authors contribution
MG edited the review and contributed to sections on β-cell function, heterogeneity, and in utero programming. RNK edited the review and contributed to sections on organ crosstalk and alpha cells and acknowledges assistance from J. Shirakawa MD PhD (Joslin Diabetes Center, Boston and Yokohoma University, Japan) and K. Shibue MD PhD (Joslin Diabetes Center). HT contributed to section on islet cell and immune interactions. FMJ contributed to all sections and edited the manuscript.
Conflict of interest
None declared.
References
- 1.Mauvais-Jarvis F., Arnold A.P., Reue K. A Guide for the Design of pre-clinical studies on sex differences in metabolism. Cell Metabolism. 2017;25(6):1216–1230. doi: 10.1016/j.cmet.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varlamov O., Bethea C.L., Roberts C.T., Jr. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne) 2014;5:241. doi: 10.3389/fendo.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences. 2015;6:14. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiology & Behavior. 2018;187:20–23. doi: 10.1016/j.physbeh.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicree R.A., Zimmet P.Z., Dunstan D.W., Cameron A.J., Welborn T.A., Shaw J.E. Differences in height explain gender differences in the response to the oral glucose tolerance test- the AusDiab study. Diabetic Medicine. 2008;25(3):296–302. doi: 10.1111/j.1464-5491.2007.02362.x. [DOI] [PubMed] [Google Scholar]
- 6.Hutson W.R., Roehrkasse R.L., Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96(1):11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 7.Nuutila P., Knuuti M.J., Maki M., Laine H., Ruotsalainen U., Teras M. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44(1):31–36. doi: 10.2337/diab.44.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Kautzky-Willer A., Kosi L., Lin J., Mihaljevic R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes, Obesity and Metabolism. 2015;17(6):533–540. doi: 10.1111/dom.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu R., Dalla Man C., Campioni M., Basu A., Klee G., Toffolo G. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 10.Horie I., Abiru N., Eto M., Sako A., Akeshima J., Nakao T. Sex differences in insulin and glucagon responses for glucose homeostasis in young healthy Japanese adults. Journal of Diabetes Investigation. 2018 doi: 10.1111/jdi.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu A., Dube S., Basu R. Men are from Mars, women are from venus: sex differences in insulin action and secretion. Advances in Experimental Medicine & Biology. 2017;1043:53–64. doi: 10.1007/978-3-319-70178-3_4. [DOI] [PubMed] [Google Scholar]
- 12.Faerch K., Torekov S.S., Vistisen D., Johansen N.B., Witte D.R., Jonsson A. GLP-1 Response to oral glucose is Reduced in prediabetes, screen-detected type 2 diabetes, and Obesity and Influenced by sex: the addition-pro study. Diabetes. 2015;64(7):2513–2525. doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F. Are estrogens promoting immune modulation and islet protection in type 1 diabetes? Journal of Diabetic Complications. 2017;31(11):1563–1564. doi: 10.1016/j.jdiacomp.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Samuelsson U., Lindblad B., Carlsson A., Forsander G., Ivarsson S., Kockum I. Residual beta cell function at diagnosis of type 1 diabetes in children and adolescents varies with gender and season. Diabetes Metab Res Rev. 2013;29(1):85–89. doi: 10.1002/dmrr.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauvais-Jarvis F., Sobngwi E., Porcher R., Riveline J.P., Kevorkian J.P., Vaisse C. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645–653. doi: 10.2337/diabetes.53.3.645. [DOI] [PubMed] [Google Scholar]
- 16.Louet J.F., Smith S.B., Gautier J.F., Molokhia M., Virally M.L., Kevorkian J.P. Gender and neurogenin3 influence the pathogenesis of ketosis-prone diabetes. Diabetes, Obesity and Metabolism. 2008;10(10):912–920. doi: 10.1111/j.1463-1326.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 17.Iacovazzo D., Flanagan S.E., Walker E., Quezado R., de Sousa Barros F.A., Caswell R. MAFA missense mutation causes familial insulinomatosis and diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(5):1027–1032. doi: 10.1073/pnas.1712262115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall E., Volkov P., Dayeh T., Esguerra J.L., Salo S., Eliasson L. Sex differences in the genome-wide DNA methylation pattern and impact on gene expression, microRNA levels and insulin secretion in human pancreatic islets. Genome Biology. 2014;15(12):522. doi: 10.1186/s13059-014-0522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 20.Robert T., De Mesmaeker I., Stange G.M., Suenens K.G., Ling Z., Kroon E.J. Functional Beta Cell Mass from Device-Encapsulated hESC-Derived Pancreatic Endoderm Achieving Metabolic Control. Stem Cell Reports. 2018;10(3):739–750. doi: 10.1016/j.stemcr.2018.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saber N., Bruin J.E., O'Dwyer S., Schuster H., Rezania A., Kieffer T.J. Sex Differences in Maturation of Human Embryonic Stem Cell-Derived beta Cells in Mice. Endocrinology. 2018;159(4):1827–1841. doi: 10.1210/en.2018-00048. [DOI] [PubMed] [Google Scholar]
- 22.Mauvais-Jarvis F. Role of Sex Steroids in beta Cell Function, Growth, and Survival. Trends in Endocrinology and Metabolism. 2016 doi: 10.1016/j.tem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiano J.P., Mauvais-Jarvis F. Importance of oestrogen receptors to preserve functional beta-cell mass in diabetes. Nature Reviews Endocrinology. 2012;8(6):342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 24.Liu S., Kilic G., Meyers M.S., Navarro G., Wang Y., Oberholzer J. Oestrogens improve human pancreatic islet transplantation in a mouse model of insulin deficient diabetes. Diabetologia. 2013;56(2):370–381. doi: 10.1007/s00125-012-2764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro G., Xu W., Jacobson D.A., Wicksteed B., Allard C., Zhang G. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metabolism. 2016;23(5):837–851. doi: 10.1016/j.cmet.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handgraaf S., Dusaulcy R., Visentin F., Philippe J., Gosmain Y. 17-beta Estradiol regulates proglucagon-derived peptide secretion in mouse and human alpha- and L cells. JCI Insight. 2018;3(7) doi: 10.1172/jci.insight.98569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li T., Jiao W., Li W., Li H. Sex effect on insulin secretion and mitochondrial function in pancreatic beta cells of elderly Wistar rats. Endocrine Research. 2016;41(3):167–179. doi: 10.3109/07435800.2015.1124437. [DOI] [PubMed] [Google Scholar]
- 28.Helman A., Avrahami D., Klochendler A., Glaser B., Kaestner K.H., Ben-Porath I. Effects of ageing and senescence on pancreatic beta-cell function. Diabetes, Obesity and Metabolism. 2016;18(Suppl. 1):58–62. doi: 10.1111/dom.12719. [DOI] [PubMed] [Google Scholar]
- 29.Steiner D.J., Kim A., Miller K., Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2(3):135–145. doi: 10.4161/isl.2.3.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poudel A., Fowler J.L., Zielinski M.C., Kilimnik G., Hara M. Stereological analyses of the whole human pancreas. Scientific Reports. 2016;6:34049. doi: 10.1038/srep34049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conrad E., Dai C., Spaeth J., Guo M., Cyphert H.A., Scoville D. The MAFB transcription factor impacts islet alpha-cell function in rodents and represents a unique signature of primate islet beta-cells. American Journal of Physiology Endocrinology Metabolism. 2016;310(1):E91–E102. doi: 10.1152/ajpendo.00285.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parchami A., Kusha S. Effect of sex on histomorphometric properties of Langerhans islets in native chickens. Veterinary Research Forum. 2015;6(4):327–330. [PMC free article] [PubMed] [Google Scholar]
- 33.Marchese E., Rodeghier C., Monson R.S., McCracken B., Shi T., Schrock W. Enumerating beta-cells in whole human islets: sex differences and associations with clinical outcomes after islet transplantation. Diabetes Care. 2015;38(11) doi: 10.2337/dc15-0723. e176–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Kroon I., Woliner-van der Weg W., Brom M., Joosten L., Frielink C., Konijnenberg M.W. Whole organ and islet of Langerhans dosimetry for calculation of absorbed doses resulting from imaging with radiolabeled exendin. Scientific Reports. 2017;7:39800. doi: 10.1038/srep39800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roscioni S.S., Migliorini A., Gegg M., Lickert H. Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nature Reviews Endocrinology. 2016;12(12):695–709. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- 36.Avrahami D., Wang Y.J., Klochendler A., Dor Y., Glaser B., Kaestner K.H. Beta-cells are not uniform after all-novel insights into molecular heterogeneity of insulin-secreting cells. Diabetes Obes Metab. 2017;19(Suppl. 1):147–152. doi: 10.1111/dom.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benninger R.K.P., Hodson D.J. New Understanding of beta-Cell Heterogeneity and in situ islet function. Diabetes. 2018;67(4):537–547. doi: 10.2337/dbi17-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T. Human islets contain four distinct subtypes of beta cells. Nature Communications. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emanuelli B., Eberle D., Suzuki R., Kahn C.R. Overexpression of the dual-specificity phosphatase MKP-4/DUSP-9 protects against stress-induced insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3545–3550. doi: 10.1073/pnas.0712275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmet P.Z., Elliott R.B., Mackay I.R., Tuomi T., Rowley M.J., Pilcher C.C. Autoantibodies to glutamic acid decarboxylase and insulin in islet cell antibody positive presymptomatic type 1 diabetes mellitus: frequency and segregation by age and gender. Diabetic Medicine. 1994;11(9):866–871. doi: 10.1111/j.1464-5491.1994.tb00370.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu L., Chase H.P., Falorni A., Rewers M., Lernmark A., Eisenbarth G.S. Sexual dimorphism in transmission of expression of islet autoantibodies to offspring. Diabetologia. 1995;38(11):1353–1357. doi: 10.1007/BF00401769. [DOI] [PubMed] [Google Scholar]
- 42.Bettini M.L., Bettini M. Understanding Autoimmune Diabetes through the Prism of the Tri-Molecular Complex. Frontiers in Endocrinology (Lausanne) 2017;8:351. doi: 10.3389/fendo.2017.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bluestone J.A., Herold K., Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein S.L., Roberts C.W., editors. Sex hormones and immunity to infection. Springer Verlag; Berlin: 2010. [Google Scholar]
- 45.Jacobson D.L., Gange S.J., Rose N.R., Graham N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clinical Immunology and Immunopathology. 1997;84(3):223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- 46.Salonia A., Lanzi R., Scavini M., Pontillo M., Gatti E., Petrella G. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care. 2006;29(2):312–316. doi: 10.2337/diacare.29.02.06.dc05-1067. [DOI] [PubMed] [Google Scholar]
- 47.Padgett L.E., Broniowska K.A., Hansen P.A., Corbett J.A., Tse H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Annals of the New York Academy of Sciences. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contreras J.L., Smyth C.A., Bilbao G., Young C.J., Thompson J.A., Eckhoff D.E. 17beta-Estradiol protects isolated human pancreatic islets against proinflammatory cytokine-induced cell death: molecular mechanisms and islet functionality. Transplantation. 2002;74(9):1252–1259. doi: 10.1097/00007890-200211150-00010. [DOI] [PubMed] [Google Scholar]
- 49.Skog O., Korsgren O., Frisk G. Modulation of innate immunity in human pancreatic islets infected with enterovirus in vitro. Journal of Medical Virology. 2011;83(4):658–664. doi: 10.1002/jmv.21924. [DOI] [PubMed] [Google Scholar]
- 50.Richer M.J., Horwitz M.S. Coxsackievirus infection as an environmental factor in the etiology of type 1 diabetes. Autoimmunity Reviews. 2009;8(7):611–615. doi: 10.1016/j.autrev.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 51.Morse Z.J., Horwitz M.S. Innate viral receptor signaling determines type 1 diabetes onset. Frontiers Endocrinology (Lausanne) 2017;8:249. doi: 10.3389/fendo.2017.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppieters K.T., Amirian N., Pagni P.P., Baca Jones C., Wiberg A., Lasch S. Functional redundancy of CXCR3/CXCL10 signaling in the recruitment of diabetogenic cytotoxic T lymphocytes to pancreatic islets in a virally induced autoimmune diabetes model. Diabetes. 2013;62(7):2492–2499. doi: 10.2337/db12-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan D., Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Frontiers in Immunology. 2015;6:635. doi: 10.3389/fimmu.2015.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristiansen O.P., Nolsoe R.L., Larsen L., Gjesing A.M., Johannesen J., Larsen Z.M. Association of a functional 17beta-estradiol sensitive IL6-174G/C promoter polymorphism with early-onset type 1 diabetes in females. Human Molecular Genetics. 2003;12(10):1101–1110. doi: 10.1093/hmg/ddg132. [DOI] [PubMed] [Google Scholar]
- 55.Tang Q., Bluestone J.A. Regulatory T-cell physiology and application to treat autoimmunity. Immunological Reviews. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 56.Bluestone J.A., Tang Q., Sedwick C.E. T regulatory cells in autoimmune diabetes: past challenges, future prospects. Journal of Clinical Immunology. 2008;28(6):677–684. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 57.Polanczyk M.J., Carson B.D., Subramanian S., Afentoulis M., Vandenbark A.A., Ziegler S.F. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. Journal of Clinical Immunology. 2004;173(4):2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 58.Adurthi S., Kumar M.M., Vinodkumar H.S., Mukherjee G., Krishnamurthy H., Acharya K.K. Oestrogen Receptor-alpha binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer. Scientific Reports. 2017;7(1):17289. doi: 10.1038/s41598-017-17102-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polanczyk M.J., Hopke C., Vandenbark A.A., Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) International Immunology. 2007;19(3):337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 60.Tai P., Wang J., Jin H., Song X., Yan J., Kang Y. Induction of regulatory T cells by physiological level estrogen. Journal of Cellular Physiology. 2008;214(2):456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 61.Gourdy P., Bourgeois E.A., Levescot A., Pham L., Riant E., Ahui M.L. Estrogen therapy delays autoimmune diabetes and promotes the protective efficiency of natural killer T-cell activation in female nonobese diabetic mice. Endocrinology. 2016;157(1):258–267. doi: 10.1210/en.2015-1313. [DOI] [PubMed] [Google Scholar]
- 62.Sharif S., Arreaza G.A., Zucker P., Mi Q.S., Sondhi J., Naidenko O.V. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nature Medicine. 2001;7(9):1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 63.Hong S., Wilson M.T., Serizawa I., Wu L., Singh N., Naidenko O.V. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nature Medicine. 2001;7(9):1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 64.Gourdy P., Araujo L.M., Zhu R., Garmy-Susini B., Diem S., Laurell H. Relevance of sexual dimorphism to regulatory T cells: estradiol promotes IFN-gamma production by invariant natural killer T cells. Blood. 2005;105(6):2415–2420. doi: 10.1182/blood-2004-07-2819. [DOI] [PubMed] [Google Scholar]
- 65.Markle J.G., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle Kampczyk U. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 66.Yurkovetskiy L., Burrows M., Khan A.A., Graham L., Volchkov P., Becker L. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beall C., Ashford M.L., McCrimmon R.J. The physiology and pathophysiology of the neural control of the counterregulatory response. American Journal Physiology Regulatory Integrative Comparative Physiology. 2012;302(2):R215–R223. doi: 10.1152/ajpregu.00531.2011. [DOI] [PubMed] [Google Scholar]
- 68.Merimee T.J., Tyson J.E. Stabilization of plasma glucose during fasting; Normal variations in two separate studies. New England Journal of Medicine. 1974;291(24):1275–1278. doi: 10.1056/NEJM197412122912404. [DOI] [PubMed] [Google Scholar]
- 69.Amiel S.A., Maran A., Powrie J.K., Umpleby A.M., Macdonald I.A. Gender differences in counterregulation to hypoglycaemia. Diabetologia. 1993;36(5):460–464. doi: 10.1007/BF00402284. [DOI] [PubMed] [Google Scholar]
- 70.Diamond M.P., Jones T., Caprio S., Hallarman L., Diamond M.C., Addabbo M. Gender influences counterregulatory hormone responses to hypoglycemia. Metabolism. 1993;42(12):1568–1572. doi: 10.1016/0026-0495(93)90152-e. [DOI] [PubMed] [Google Scholar]
- 71.Havel P.J., Parry S.J., Curry D.L., Stern J.S., Akpan J.O., Gingerich R.L. Autonomic nervous system mediation of the pancreatic polypeptide response to insulin-induced hypoglycemia in conscious rats. Endocrinology. 1992;130(4):2225–2229. doi: 10.1210/endo.130.4.1347741. [DOI] [PubMed] [Google Scholar]
- 72.Havel P.J., Ahren B. Activation of autonomic nerves and the adrenal medulla contributes to increased glucagon secretion during moderate insulin-induced gypoglycemia in women. Diabetes. 1997;46(5):801–807. doi: 10.2337/diab.46.5.801. [DOI] [PubMed] [Google Scholar]
- 73.Davis S.N., Cherrington A.D., Goldstein R.E., Jacobs J., Price L. Effects of insulin on the counterregulatory response to equivalent hypoglycemia in normal females. American Journal of Physiology. 1993;265(5 Pt 1) doi: 10.1152/ajpendo.1993.265.5.E680. E680-9. [DOI] [PubMed] [Google Scholar]
- 74.Karlsson S., Scheurink A.J., Ahren B. Gender difference in the glucagon response to glucopenic stress in mice. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2002;282(1) doi: 10.1152/ajpregu.2002.282.1.R281. R281-8. [DOI] [PubMed] [Google Scholar]
- 75.Bonnevie-Nielsen V. Experimental diets affect pancreatic insulin and glucagon differently in male and female mice. Metabolism. 1980;29(4):386–391. doi: 10.1016/0026-0495(80)90014-1. [DOI] [PubMed] [Google Scholar]
- 76.Bonnevie-Nielsen V. Different effects of high glucose and high fat diet on pancreatic insulin and glucagon in female and male mice. Diabete & Metabolisme. 1982;8(4):271–277. [PubMed] [Google Scholar]
- 77.Mandour T., Kissebah A.H., Wynn V. Mechanism of oestrogen and progesterone effects on lipid and carbohydrate metabolism: alteration in the insulin: glucagon molar ratio and hepatic enzyme activity. European Journal of Clinical Investigation. 1977;7(3):181–187. doi: 10.1111/j.1365-2362.1977.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 78.Faure A., Billaudel B., Sutter B.C. Adrenal interference of insulin secretion after 14-days oestradiol treatment in female rats. Diabetologia. 1984;26(1):76–80. doi: 10.1007/BF00252268. [DOI] [PubMed] [Google Scholar]
- 79.Liu S., Le May C., Wong W.P., Ward R.D., Clegg D.J., Marcelli M. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes. 2009;58(10):2292–2302. doi: 10.2337/db09-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martensson U.E., Salehi S.A., Windahl S., Gomez M.F., Sward K., Daszkiewicz Nilsson J. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 81.Balhuizen A., Kumar R., Amisten S., Lundquist I., Salehi A. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Molecular and Cellular Endocrinology. 2010;320(1–2):16–24. doi: 10.1016/j.mce.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 82.Alonso-Magdalena P., Laribi O., Ropero A.B., Fuentes E., Ripoll C., Soria B. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environmental Health Perspectives. 2005;113(8):969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirakawa J., De Jesus D.F., Kulkarni R.N. Exploring inter-organ crosstalk to uncover mechanisms that regulate beta-cell function and mass. European Journal of Clinical Nutrition. 2017;71(7):896–903. doi: 10.1038/ejcn.2017.13. [DOI] [PubMed] [Google Scholar]
- 84.Huang C., Snider F., Cross J.C. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009;150(4):1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- 85.Karnik S.K., Chen H., McLean G.W., Heit J.J., Gu X., Zhang A.Y. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318(5851):806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 86.Kim H., Toyofuku Y., Lynn F.C., Chak E., Uchida T., Mizukami H. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demirci C., Ernst S., Alvarez-Perez J.C., Rosa T., Valle S., Shridhar V. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61(5):1143–1152. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kondegowda N.G., Fenutria R., Pollack I.R., Orthofer M., Garcia-Ocana A., Penninger J.M. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-kappab ligand pathway. Cell Metabolism. 2015;22(1):77–85. doi: 10.1016/j.cmet.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Jesus D.F., Kulkarni R.N. Epigenetic modifiers of islet function and mass. Trends in Endocrinology and Metabolism. 2014;25(12):628–636. doi: 10.1016/j.tem.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 90.Mauvais-Jarvis F., Manson J.E., Stevenson J.C., Fonseca V.A. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms and clinical implications. Endocrine Reviews. 2017 doi: 10.1210/er.2016-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keating N.L., O'Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 92.Keating N.L., O'Malley A.J., Freedland S.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. Journal of the National Cancer Institute. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Navarro G., Allard C., Xu W., Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring) 2015;23(4):713–719. doi: 10.1002/oby.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mauvais-Jarvis F. Androgen-deprivation therapy and pancreatic beta-cell dysfunction in men. Journal of Diabetic Complications. 2016 doi: 10.1016/j.jdiacomp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Dimitriadis G.K., Randeva H.S., Aftab S., Ali A., Hattersley J.G., Pandey S. Metabolic phenotype of male obesity-related secondary hypogonadism pre-replacement and post-replacement therapy with intra-muscular testosterone undecanoate therapy. Endocrine. 2018;60(1):175–184. doi: 10.1007/s12020-017-1516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O'Meara N.M., Blackman J.D., Ehrmann D.A., Barnes R.B., Jaspan J.B., Rosenfield R.L. Defects in beta-cell function in functional ovarian hyperandrogenism. Journal of Clinical Endocrinology & Metabolism. 1993;76(5):1241–1247. doi: 10.1210/jcem.76.5.8496316. [DOI] [PubMed] [Google Scholar]
- 97.Dunaif A., Finegood D.T. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 1996;81(3):942–947. doi: 10.1210/jcem.81.3.8772555. [DOI] [PubMed] [Google Scholar]
- 98.Holte J., Bergh T., Berne C., Berglund L., Lithell H. Enhanced early insulin response to glucose in relation to insulin resistance in women with polycystic ovary syndrome and normal glucose tolerance. Journal of Clinical Endocrinology & Metabolism. 1994;78(5):1052–1058. doi: 10.1210/jcem.78.5.8175959. [DOI] [PubMed] [Google Scholar]
- 99.Goodarzi M.O., Erickson S., Port S.C., Jennrich R.I., Korenman S.G. Beta-cell function: a key pathological determinant in polycystic ovary syndrome. The Journal of Clinical Endocrinology and Metabolism. 2005;90(1):310–315. doi: 10.1210/jc.2004-1006. [DOI] [PubMed] [Google Scholar]
- 100.Navarro G.N., Allard C., Morford J.J., Xu W., Liu S., Molinas A.J. Androgen excess in pancreatic β-cells and neurons predisposes to type 2 diabetes in female mice. JCI Insight. 2018 doi: 10.1172/jci.insight.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elsakr J.M., Gannon M. Developmental programming of the pancreatic islet by in utero overnutrition. Trends in Developmental Biology. 2017;10:79–95. [PMC free article] [PubMed] [Google Scholar]
- 102.Drake A.J., Reynolds R.M. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387–398. doi: 10.1530/REP-10-0077. [DOI] [PubMed] [Google Scholar]
- 103.Mingrone G., Manco M., Mora M.E., Guidone C., Iaconelli A., Gniuli D. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. 2008;31(9):1872–1876. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Branum A.M., Kirmeyer S.E., Gregory E.C. Prepregnancy body mass index by maternal characteristics and state: data from the birth certificate, 2014. National Vital Statistics Reports. 2016;65(6):1–11. [PubMed] [Google Scholar]
- 105.Nicol L.E., Grant W.F., Comstock S.M., Nguyen M.L., Smith M.S., Grove K.L. Pancreatic inflammation and increased islet macrophages in insulin-resistant juvenile primates. Journal of Endocrinology. 2013;217(2):207–213. doi: 10.1530/JOE-12-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yokomizo H., Inoguchi T., Sonoda N., Sakaki Y., Maeda Y., Inoue T. Maternal high-fat diet induces insulin resistance and deterioration of pancreatic beta-cell function in adult offspring with sex differences in mice. American Journal of Physiology Endocrinology and Metabolism. 2014;306(10):E1163–E1175. doi: 10.1152/ajpendo.00688.2013. [DOI] [PubMed] [Google Scholar]
- 107.Theys N., Ahn M.T., Bouckenooghe T., Reusens B., Remacle C. Maternal malnutrition programs pancreatic islet mitochondrial dysfunction in the adult offspring. The Journal of Nutritional Biochemistry. 2011;22(10):985–994. doi: 10.1016/j.jnutbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 108.Ng S.F., Lin R.C., Laybutt D.R., Barres R., Owens J.A., Morris M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467(7318):963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 109.Tobi E.W., Lumey L.H., Talens R.P., Kremer D., Putter H., Stein A.D. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics. 2009;18(21):4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein A.D., Ravelli A.C., Lumey L.H. Famine, third-trimester pregnancy weight gain, and intrauterine growth: the Dutch Famine Birth Cohort Study. Human Biology. 1995;67(1):135–150. [PubMed] [Google Scholar]
- 111.Fowden A.L., Giussani D.A., Forhead A.J. Endocrine and metabolic programming during intrauterine development. Early Human Development. 2005;81(9):723–734. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 112.Theys N., Bouckenooghe T., Ahn M.T., Remacle C., Reusens B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. American Journal of Physiology Regulatory Integrative Comparative Physiology. 2009;297(5):R1516–R1525. doi: 10.1152/ajpregu.00280.2009. [DOI] [PubMed] [Google Scholar]
- 113.Chamson-Reig A., Thyssen S.M., Arany E., Hill D.J. Altered pancreatic morphology in the offspring of pregnant rats given reduced dietary protein is time and gender specific. Journal of Endocrinology. 2006;191(1):83–92. doi: 10.1677/joe.1.06754. [DOI] [PubMed] [Google Scholar]
- 114.Sobngwi E., Boudou P., Mauvais-Jarvis F., Leblanc H., Velho G., Vexiau P. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet. 2003;361(9372):1861–1865. doi: 10.1016/S0140-6736(03)13505-2. [DOI] [PubMed] [Google Scholar]
- 115.Gautier J.F., Fetita L.S., Riveline J.P., Ibrahim F., Porcher R., Khalil C.A. Sex Difference In the Effect of Fetal Exposure to Maternal Diabetes on Insulin Secretion. Journal of the Endocrine Society. 2018;2(5):391–397. doi: 10.1210/js.2017-00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ding G.L., Wang F.F., Shu J., Tian S., Jiang Y., Zhang D. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bansal A., Rashid C., Xin F., Li C., Polyak E., Duemler A. Sex- and dose-specific effects of maternal bisphenol a exposure on pancreatic islets of first- and second-generation adult mice offspring. Environmental Health Perspectives. 2017;125(9):097022. doi: 10.1289/EHP1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wei J., Sun X., Chen Y., Li Y., Song L., Zhou Z. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. Journal of Endocrinology. 2014;222(3):313–325. doi: 10.1530/JOE-14-0356. [DOI] [PubMed] [Google Scholar]
- 119.Song L., Xia W., Zhou Z., Li Y., Lin Y., Wei J. Low-level phenolic estrogen pollutants impair islet morphology and beta-cell function in isolated rat islets. Journal of Endocrinology. 2012;215(2):303–311. doi: 10.1530/JOE-12-0219. [DOI] [PubMed] [Google Scholar]
- 120.Susiarjo M., Xin F., Bansal A., Stefaniak M., Li C., Simmons R.A. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015;156(6):2049–2058. doi: 10.1210/en.2014-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]