Abstract
Background
Estrogenic actions in the brain prevent obesity. Better understanding of the underlying mechanisms may facilitate development of new obesity therapies.
Scope of review
This review focuses on the critical brain regions that mediate effects of estrogens on food intake and/or energy expenditure, the molecular signals that are involved, and the functional interactions between brain estrogens and other signals modulating metabolism. Body weight regulation by estrogens in male brains will also be discussed.
Major conclusions
17β-estradiol acts in the brain to regulate energy homeostasis in both sexes. It can inhibit feeding and stimulate brown adipose tissue thermogenesis. A better understanding of the central actions of 17β-estradiol on energy balance would provide new insight for the development of therapies against obesity in both sexes.
Keywords: Estrogens, Energy balance, Obesity, Hypothalamus, Metabolism, Food intake, Brown adipose tissue
1. Introduction
Besides the regulation of the reproductive function, estrogens have a key role in the central regulation of the energy homeostasis including both modulation of feeding behavior and energy expenditure [1], [2], [3]. Increased life expectancy implies that many women will live an increasing number of years in a state of ovarian insufficiency. This leads to a steady surge in obesity incidence reaching a staggering figure of more than 70% in women older than 60 years [4]. Although, the interrelationship between estrogen deficiency and obesity was the subject of some discussion, pooled data derived from 107 trials showed that hormone-replacement therapy in menopausal patients led to reduced abdominal obesity, insulin resistance and new-onset diabetes [4], providing a cause–effect relationship between estrogen deficiency, obesity, and metabolic complications. However, this application has been hampered mainly due to side effects of 17β-estradiol, including venous thrombosis and endometrial and breast cancers [5], [6]. One solution is to better understand the mechanisms of estrogenic actions, which may facilitate development of novel therapies that provide anti-obesity benefits with fewer side effects.
Central action of 17β-estradiol in metabolic control has been a focus of many research groups and has been summarized by a number of recent review articles [7], [8], [9]. In this review, we will recap current literature regarding distinct brain regions that mediate 17β-estradiol's effects on feeding and energy expenditure. Importantly, we will discuss advances in our understanding about the molecular signals initiated by 17β-estradiol in neurons that are involved in body weight control. Further, the functional interactions between 17β-estradiol and other appetite-regulatory signals will also be discussed. 17β-estradiol also acts in the peripheral tissues to regulate energy homeostasis, and the audience is directed to read the other excellent reviews in this special issue of Molecular Metabolism.
2. Brain estrogens suppress feeding behavior
The anti-obesity actions of estrogens have been well documented. For instance, surgical depletion of endogenous estrogens by ovariectomy (OVX) causes increases in food intake, body weight, and body fat in female animals; 17β-estradiol administration in OVX animals can reduce feeding and prevent obesity [10], [11], [12], [13], [14], [15]. Bazedoxifene (a selective estrogen receptor modulator), combined with conjugated equine estrogens, has been used to provide estrogen-mediated benefits while reducing endometrial and breast cancer risk in post-menopausal women [16]. Interestingly, this regimen has been shown to reduce body weight but does not alter feeding in OVX mice [17]. The lack of effect on feeding by the conjugated equine estrogens is likely due to minimal penetration of estrogens to the brain [18], further highlighting the essential actions of brain estrogens in the regulation of feeding.
Anti-obesity effects of 17β-estradiol appear to be primarily mediated by estrogen receptor-α (ERα), one of the “classical” estrogen receptors. Mutations in the ERα (Esr1) gene cause obesity in mice and humans [19], [20]. Mice lacking ERα are not responsive to anti-obesity effects of 17β-estradiol [12]. In particular, injections of 17β-estradiol into various brain regions decrease food intake in animals [21], [22]. These early observations were further supported by findings from genetic mouse models. For instance, Clegg and colleagues generated mice lacking ERα only in the brain, which developed obesity [23]. Increased food intake, low energy expenditure, and low locomotion were observed in these mutant mice [23]. Interestingly, deletion of ERα in the brain also impairs negative feedback regulation by estrogens, resulting in higher 17β-estradiol in blood [23]; yet, the elevated 17β-estradiol level in the circulation fails to prevent obesity, suggesting that brain ERα plays a predominant role in the regulation of energy balance. Many brain regions express high levels of ERα, including the arcuate nucleus of hypothalamus (ARC), the nucleus of solitary tract (NTS), the dorsal raphe nuclei (DRN), and the medial preoptic area (MPOA) [24]. As discussed below, recent efforts using genetic mouse models have dissected out the physiological functions of ERα in some of these brain regions in the regulation of energy homeostasis.
2.1. ERα in POMC neurons
Pro-opiomelanocortin (POMC) neurons in the ARC are the first order neurons in the hypothalamus that sense and integrate nutritional and hormonal cues to regulate energy balance [25]. A portion (20–30%) of ARC POMC neurons co-express ERα [23], [26], [27]. 17β-estradiol was reported to enhance glutamatergic synapses onto POMC neurons, which results in stronger miniature excitatory postsynaptic currents [28]. Further, 17β-estradiol acutely activates firing of POMC neurons, which can be blocked by an inhibitor of the inwardly rectifying K+ channels [29]. Propylpyrazole triol (PPT, an agonist of ERα) depolarizes POMC neurons that express ERα, but not those without ERα [7]. Importantly, female mice with ERα selectively deleted in POMC neurons are hyperphagic and develop modest obesity [23]. 17β-estradiol-induced suppression in food intake is attenuated in these mutant mice [30]. Thus, 17β-estradiol's anorexigenic effects are at least partly mediated by POMC neurons that express ERα [23].
2.2. ERα in the NTS
The NTS, a brainstem center for satiety signals, also expresses high levels of ERα [24], [31], [32]. Increased NTS neural activities have been reported to be associated with 17β-estradiol-induced anorexia in female mice [12], [33]. Importantly, deletion of ERα blocks these responses [12], [33]. In addition, microinjections of 17β-estradiol into the NTS enhance feeding-suppressing effects of cholecystokinin (CCK), a well-known satiety hormone secreted from the gut [34]. Therefore, NTS ERα signals appear to also mediate inhibitory effects of 17β-estradiol on feeding.
2.3. ERα in the DRN
The DRN expresses ERα [24], and the majority of these ERα-positive neurons are shown to be serotonin (5-HT) neurons [35]. 17β-estradiol treatment enhances neural activities within the DRN [36], [37]; consistently, DRN 5-HT neurons are shown to be stimulated by PPT, an effect that can be blocked by genetic deletion of ERα [35]. Interestingly, female rats receiving 17β-estradiol injected into the DRN display anorexigenic responses [38]. Deletion of ERα selectively in 5-HT neurons blocks 17β-estradiol's effect of suppressing binge-like eating [35]. These observations indicate that 17β-estradiol can act upon DRN 5-HT neurons to inhibit food intake.
Roles of ERα in other brain regions remain elusive. For instance, 17β-estradiol injected directly into the MPOA suppresses feeding [38]. Similarly, suppression of food intake and body weight was observed when OVX female rats received 17β-estradiol implanted in the paraventricular nucleus of the hypothalamus (PVH) [21]. Supporting a role of the PVH, subcutaneous 17β-estradiol administration fails to reduce feeding in rats with the PVH being lesioned [39]. However, findings regarding the PVH were not duplicated by others [40]. Further, it is worth noting that only low levels of ERα are present in the PVH [24]. Importantly, 17β-estradiol is known to regulate food-associated reward [9], suggesting a role for ERs expressed by brain reward centers, including the nucleus accumbens (NAc) and the lateral hypothalamus (LH) [24]. Indeed, 17β-estradiol was shown to influence metabolism of several monoamines (e.g. dopamine, 5-HT, and norepinephrine) in the NAc [41], although effects of these estrogenic actions in the NAc on feeding behavior remain to be examined. Collectively, the current literature validated a few regions in the female brain (e.g. ARC, NTS and DRN) as important nodes that respond to 17β-estradiol and mediate its signals to suppress feeding; however, the roles of additional brain regions warrant further investigations.
3. Brain estrogens stimulate energy expenditure
3.1. BAT thermogenesis
In mammals, including humans, the main place for adaptive thermogenesis is the brown adipose tissue (BAT) [42], [43], [44], [45]. In small mammals living in sub-thermoneutral environment, large quantities of active BAT are encountered [42], [43]. In humans, BAT presence was shown to be inversely correlated with increasing age. However, recent evidences have demonstrated the presence of functional brown fat depots in healthy adults [44], [46], [47], [48], [49]. Histologically, brown adipocytes exhibit numerous small lipid droplets and a very high mitochondrial content. Through the movement of electron across the respiratory chain, the mitochondria produce energy temporarily stored as a proton gradient across the inner mitochondrial membrane. Later, the power derived from this proton gradient is used to generate ATP from ADP by the ATP synthase [50], [51], [52]. Alternative pathways, occurring in the internal membrane of BAT's mitochondria and involving the uncoupling protein 1 (UCP1), allow the retrograde transport of protons back into the mitochondrial matrix, sidestepping ATP synthase activity and releasing energy as heat [42], [43], [53].
BAT is regulated by both the central and peripheral nervous system. The sympathetic nervous system (SNS) has a critical role in BAT thermogenesis stimulation [42], [43], [45], [54], [55], [56]. Increased firing rate of the sympathetic nerves subserving BAT induces the secretion of norepinephrine (NE) at the nerve terminal, inducing the subsequent activation of G-protein coupled receptors, named β-adrenergic receptors (β-ARs), expressed in the brown adipocytes; mainly the β3 subtype (β3-AR). The associated G protein coupled to β3-AR activates adenylate cyclase (AC), inducing an increase of intracellular cAMP, which subsequently activates protein kinase A (PKA), inducing thermogenesis and downstream activation of p38 mitogen-activated protein kinase (MAPK) [42], [45], [54]. The acute response of PKA stimulates lipolysis leading to elevated cytosolic free fatty acid (FFA) levels. This FFA increase will occur following the sequential hydrolysis of triglycerides by the adipose triglyceride lipase (ATGL), the hormone-sensitive lipase (HSL; being pHSL the activated form), and the monoacylglycerol lipase (MGL). The FFAs-CoA - FFAs activated to acyl CoAs by acyl-CoA synthetase - are transferred into the mitochondria by the carnitine palmitoyltransferase 1a (CPT1a), where FA oxidation induces NADH and FADH production, which will be oxidized later in the electron transport chain [42], [43], [45], [54].
3.2. Direct effects of 17β-estradiol on BAT
For the first time, in the 70s, it was shown that 17β-estradiol could bind to both interscapular brown and white adipocytes [57]. Physiological evidence demonstrated that 17β-estradiol could lead to elevated energy expenditure in rodents - hamsters, rats, and mice - through an increase of BAT lipolysis and thermogenesis [58], [59], [60], [61], [62]. Notably, those effects were related to an increased NE turnover that was reduced by OVX [63], suggesting not only a direct action of 17β-estradiol on BAT but also an indirect regulatory action on the central control of the sympathetic firing on BAT. This modulatory mechanism would be comparable to the effects observed with thyroid hormones (THs), which, in addition to stimulating UCP1 expression, also induce NE-induced lipolysis [64], [65], [66], [67]. However, it has also been demonstrated that the direct effect of 17β-estradiol on BAT is more likely due to modulations in (i) adrenergic receptors (AR) and mitochondrial biogenesis-signaling factors, such as phosphatase and tensin homolog (PTEN), (ii) nuclear respiratory transcriptional factor 1 (NRF1), and (iii) probably peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), rather than to modifications in UCP1 expression [68], [69], [70].
Studies have provided strong evidences that ERs are expressed in a sex-dependent manner by the brown adipocytes, with higher ERα densities in male than female rats [71]. However, phenotype studies of ERα knockout mice have not provided conclusive data on any possible direct role of estrogens on BAT. Moreover, evidences were released demonstrating that ERα knockout mice display an age-dependent increased adiposity - associated with both hyperplasia and hypertrophy - of white fat depots. Conversely, despite impaired energy expenditure, no such differences were observed in the BAT (neither in male nor females) suggesting the possible existence of central effects [20], [72]. Nevertheless, the deletion of ERβ does not induce obesity or metabolic alterations in standard diet fed mice kept at 25 °C. However, animals fed with high-fat diet (HFD) exhibit an elevated body weight and an enlarged adiposity than their wild types kept at 25 °C [73]. Alternatively, aromatase knockout (ArKO) mice display larger BAT pads, reversed by peripheral 17β-estradiol treatment [74]. Concerning G protein-coupled estrogen receptor (GPER) implications in metabolism, it has been shown that mice - independently of the gender - lacking GPER show mild obesity associated with (i) decreased energy expenditure, (ii) enlarged BAT lipid content (suggesting a reduced thermogenic activity), and (iii) decreased expression of UCP1 and β3-AR when kept at 23-24 °C [75].
Until recently, the implication of ERs in human BAT had not been investigated deeply. As reported in 2014, both ERα and ERβ were found to be expressed in human fetal BAT, with higher ERα expression [76]. Although the role of ERs in adult human BAT remains undefined, the discovery of functional BAT in adulthood [44], [46], [47], [48], [49] suggests a possible implication of estrogens in human brown fat activity regulation. In line with those findings, recent studies showed that there is more functionally active brown adipose tissue present in women (greater 18F-fluorodeoxyglucose (18F-FDG) uptake) than in men, physiologically correlated with higher BAT activity [46]. This suggested sexual dimorphism indicates a possible direct modulation of human BAT by gonadal steroids, including estrogens.
3.3. Central effects of 17β-estradiol on BAT
The ventromedial nucleus of hypothalamus (VMH) was the first hypothalamic nucleus identified regulating energy expenditure, particularly in thermogenesis. Notably, the ERα is highly expressed in the VMH, suggesting estrogenic functional relevance [77], [78]. In addition, as demonstrated by diverse electrical, pharmacological, or hormonal stimulations of the VMH inducing an increase of BAT temperature, the VMH has an important role in energy expenditure modulation, mostly through the SNS [79], [80], [81], [82], [83], [84], [85] (for review [42], [45], [55], [86]). According to those findings, VMH neurons were also described to communicate with nuclei functionally linked to the modulation of BAT activity, such as the raphe pallidus (RPa) and inferior olive (IO), to increase the accuracy of SNS activity modulation [42], [45], [55] (Figure 1).
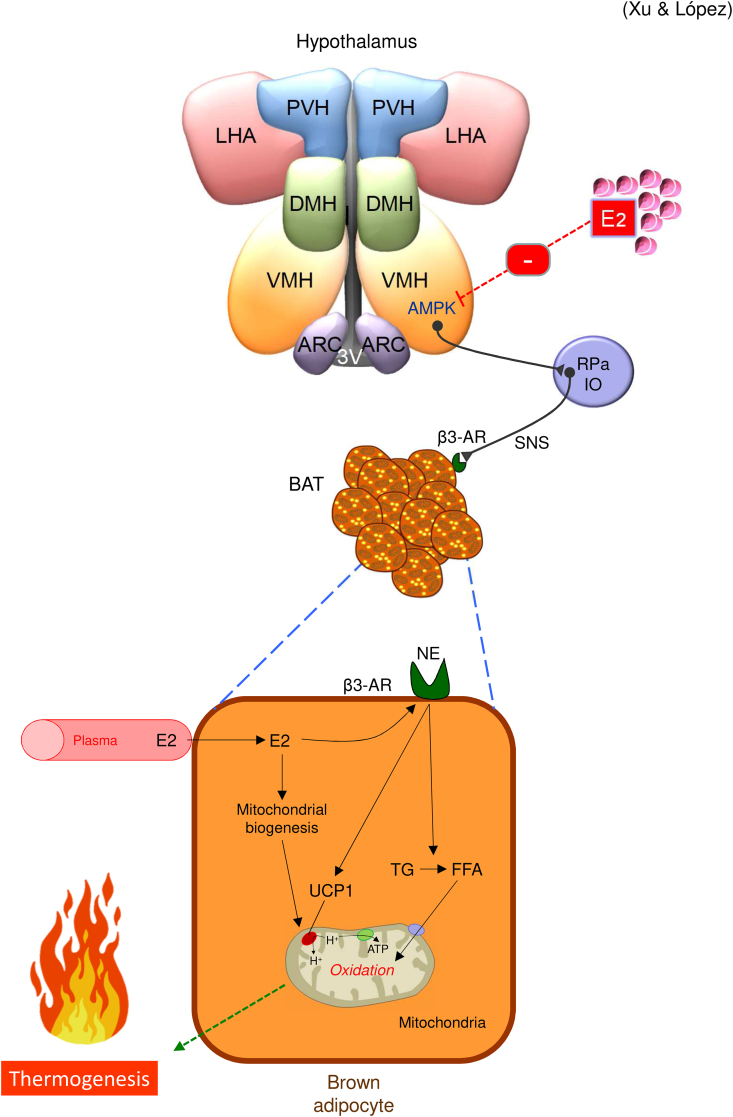
Figure 1.
Actions of 17β-estradiol on BAT. 17β-estradiol modulates the brown adipose tissue (BAT) thermogenesis via AMP-activated protein kinase (AMPK) in the ventromedial nucleus of the hypothalamus (VMH) and the sympathetic nervous system (SNS). 17β-estradiol also regulates brown adipocytes directly by affecting adrenergic receptors (AR) and mitochondrial biogenesis. The overall effect of these actions is a catabolic response, linked to increased temperature, energy expenditure, and weight loss. 3V: third ventricle; β3-AR: beta 3 adrenergic receptor; DMH: dorsomedial nucleus of the hypothalamus; FFA: free fatty acid; IO: inferior olive; LHA: lateral hypothalamic area; NE: norepinephrine; PVH: paraventricular nucleus of the hypothalamus; RPa: raphe pallidus; UCP1: uncoupling protein 1; TG: triglyceride.
Electrophysiological studies showed that 17β-estradiol could also modulate the excitability of VMH neurons through a mechanism specifically dependent of cAMP [87]. Later, Clegg and colleagues provided strong evidence showing that the VMH is as major hypothalamic center mediating estrogen's effect on energy expenditure. They reported that VMH-specific silencing of ERα elicited obesity, hyperglycemia, and decreased energy expenditure either in mice and rats [88]. Further studies showed that the action of 17β-estradiol on thermogenesis depends on steroidogenic factor-1 (SF1) neurons of the VMH. As reported, female (not male) ERα/SF1 null mice fed with a normal diet exhibited a food intake-independent increase of body weight associated to visceral adiposity, which was increased when animals were fed with HFD [23]. Of note, that phenotype was linked to a lower metabolic rate, impaired BAT thermogenesis and subsequently diminished heat production in mice maintained at 22 °C. In keeping with this, the specific depletion of ERα in SF1 neurons in mice induced a reduced sympathetic outflow on BAT and lower expression of UCP1, peroxisome proliferator-activated receptor gamma (PPARγ), PGC-1α, and β3-AR in BAT [23]. Accordingly, the simultaneous deletion of ERα in SF1 and POMC neurons has been shown to induce hypometabolism, hyperphagia, and severe obesity [23]. However, as demonstrated by the treatment with an ERβ-selective agonist in female mice under HFD inducing an increased expression of UCP1 in BAT and reduced obesity, it is likely that some of 17β-estradiol effects on BAT thermoregulation could be mediated by ERβ [89]. Current data have also described a new subset of ERα positive neurons in the ventrolateral region of the ventromedial hypothalamus (VMHvl) that promotes hormone-dependent female locomotor activity [90]. The impaired development of those neurons elicits inactivity and obesity, without changes in BAT thermogenesis below thermoneutrality [90]; overall, this evidence suggests that 17β-estradiol likely induces specific effects on energy homeostasis depending on the targeted VMH neuronal populations.
Recently, AMP-activated protein kinase (AMPK), a cellular energy sensor [91], has been described to be the principal molecular mediator of 17β-estradiol's actions within the VMH. Stereotaxic microinjections of 17β-estradiol in the VMH at non-thermoneutral conditions induce multiple effects: (i) a catabolic response associated with increased neuronal firing in the RPa and IO, (ii) an increased sympathetic tone, (iii) higher BAT and body temperatures, and (iv) increased energy expenditure and lower respiratory quotient (RQ), all of which lead to body weight decrease. Interestingly, these 17β-estradiol-mediated effects were correlated with an inhibition of hypothalamic AMPK function, specifically in the VMH, but not in the ARC [85]. Remarkably, the 17β-estradiol-induced activation of BAT thermogenesis associated with body weight loss could be prevented by the genetic re-activation of AMPK in the VMH [85]. Notably, it has been reported recently that other molecules implicated in the modulation of BAT activity, such as leptin, THs, bone morphogenetic protein 8b (BMP8b), and glucagon-like peptide 1 (GLP1) [81], [82], [86], [92], [93], [94], [95], [96], [97] are also sharing this AMPK(VMH)-SNS-BAT axis to mediate their effects, suggesting that this could represent a canonical mechanism modulating energy balance [45], [91]. This indicates that the axis might be a suitable target for the treatment of obesity, such as after ovariectomy and/or during menopausal states [2], [91], [98], [99], [100]. However, it is worth mentioning that although the central effects of 17β-estradiol on BAT thermogenesis are preserved in diet-induced obese models feeding a HFD at 24 °C [101], they disappear during pregnancy. Actually, despite the fact that hypothalamic AMPK has been reported to be inhibited by 17β-estradiol during gestation, pregnant rats exhibit lower temperature and BAT function, as well as hyperphagia [78]. The mechanistic details of these effects remain unclear, but they are associated with reduced POMC expression (which would be under the increased appetite) and decreased UCP1 and β3-AR expression in BAT at non thermoneutral housing temperatures [78]. These data indicate that pregnancy elicits a state of resistance to the central actions of 17β-estradiol on BAT thermogenesis, which is likely required to maintain a positive energy balance essential to successfully endure the energetic demands of embryonic development.
Besides BAT thermogenesis, AMPK may be important for understanding estrogen actions on glucose homeostasis. Hypothalamic AMPK, particularly in the VMH, has been demonstrated to have a key role in hypoglycemia sensing and the regulation of counterregulatory responses, such as increased levels of corticosterone, glucagon, and catecholamines [102], [103], [104], [105]. Current evidence has shown that estradiol controls hypothalamic astrocyte AMPK and hindbrain catecholamine-dependent activation of this cell-specific sensor by hypoglycemia [106]. In keeping with this evidence, recent data have also proposed that 17β-estradiol acting on AMPK in the hindbrain dorsal vagal complex (DVC) might be also involved in the induction of counterregulatory responses [107]. Although these data are of relevance, further work involving genetic models will be necessary to address the physiological relevance of estrogen on AMPK-induced effects on glucose homeostasis.
4. Estrogen-coupled intracellular signals
One major question in the field is what estrogen-initiated molecular signals mediate its anti-obesity functions. ER-initiated signals are known to include several distinct pathways. As classic nuclear receptors, ERs can directly bind to estrogen-responsive element (ERE) on DNA to regulate expression of its target genes. Interestingly, a mutation in the DNA binding domain of ERα does not affect energy homeostasis in female mice [108], despite the fact that this mutation diminishes ERα′s binding to the ERE motifs on the chromosome [109]. Thus, the ERE-dependent ERα functions do not appear to be essential for energy balance. However, Handgraaf et al. developed mice with mutations in the activation function motif-2 (AF-2), which is required for transcriptional activity of ERα; these mutation causes obesity and diabetes in mice [110], suggesting that transcriptional activity of ERα, perhaps through ERE-independent mechanisms, is important for body weight control.
In parallel, it has been increasingly appreciated that a portion of ER molecules exist on the cytomembrane and in the cytosol, and these ERs can trigger rapid signaling cascades, e.g. PI3K/Akt. Indeed, ARC POMC neurons are shown to acutely (in the time scale of milliseconds) respond to 17β-estradiol with increased firing and depolarization, and these responses are mediated by an ERα-PI3K-mediated mechanism [29], [30]. Further, female mice lacking the catalytic PI3K subunit selectively in ARC POMC neurons are less responsive to anti-obesity effects of 17β-estradiol [30], [111]. However, when the full length ERα protein is replaced by a ERα domain that exists on the cytomembrane and retains capacity of initiating rapid signals (e.g. PI3K), mice display a similar obesity as seen in ERα knockout mice [112]. Thus, the ERα-initiated rapid signaling may not be sufficient to prevent obesity. Nevertheless, anti-obesity effects of estrogens appear to require not only ERα-initiated rapid signaling pathways but also ERα′s transcriptional activity. The detailed intracellular ERα functions that regulate energy balance remain to be fully revealed.
5. 17β-estradiol and other body weight-regulatory signals
5.1. Leptin
Leptin is an adipocyte-derived hormone that reflects energy storage [113]. Leptin prevents body weight gain through suppressing feeding [114], [115] and increasing energy expenditure [116], [117], [118]. The long form leptin receptor (also known as LEPR-B) mediates effects of leptin on body weight [119], and the majority of leptin's actions are mediated by LEPR-B in the brain [120]. Many hypothalamic nuclei, including the ARC, express abundant LEPR-B. In particular, some ARC neurons expressing LEPR-B also co-express ERα [121], and 17β-estradiol can alter LEPR-B mRNA levels in the ARC [122]. Thus, 17β-estradiol and leptin may interact within ARC neurons. Indeed, enhanced leptin sensitivity is correlated with increased 17β-estradiol levels; on the other hand, surgical depletion of ovarian hormones reduces leptin sensitivity, which can be rescued by 17β-estradiol treatment [123].
5.2. Cholecystokinin
CCK is synthesized and released by the proximal intestine. CCK acts upon CCK-A receptors to trigger satiety signal and suppress feeding [124]. Increased 17β-estradiol can potentiate CCK's effects to suppress feeding [33], [125], [126], [127], and this effect is shown to be mediated through enhanced sensitivity of vagal CCK-A receptors [126], [128], [129]. CCK decreases meal size, which is associated with increased c-fos expression (a marker for neuronal activation) after feeding [130], [131], [132], [133] or CCK treatment [134], [135]. Interestingly, 17β-estradiol potentiates c-fos expression in the NTS, the PVH, and the central nucleus of the amygdala evoked by meals or by CCK treatment [131], [136]. Thus, 17β-estradiol decreases meal size partly through potentiating satiety signals evoked by CCK. However, it needs to be pointed out that the phasic meal size reduction during the estrus in gonad-intact female rats can only be partially attenuated but not be fully blocked by a CCK antagonist [127], indicating that other mechanisms/signals are involved in the phasic regulation of feeding across the estrous cycle.
5.3. Ghrelin
Ghrelin is a stomach-derived hormone, which stimulates feeding via its growth hormone secretagogue receptors (GHSRs) [137], [138], [139], [140], [141], [142]. Interestingly, effectiveness of ghrelin to promote feeding is less robust in gonad-intact female rats than in male rats or in female rats depleted of ovarian hormones [143]. In gonad-intact female rats, ghrelin significantly increases food intake during the diestrus when 17β-estradiol is low, but this effect is not observed during the proestrus or estrus when 17β-estradiol is high [143]. In addition, male rats receiving 17β-estradiol also show decreased orexigenic responses to ghrelin treatment [143]. Interestingly, while depletion of ovarian hormones increases feeding and body weight in wild type female mice, these effects are blunted in mice lacking GHSR [143]. Thus, effects of 17β-estradiol to suppress feeding appear to require intact ghrelin signaling [143], although the detailed mechanisms for this functional interaction remains unclear.
5.4. Central melanocortin system
Neurons that produce endogenous melanocortins and downstream neurons that express melanocortin receptors are known to play essential roles in body weight control [144], [145], [146], [147], [148]. The melanocortin neurons include POMC neurons and agouti-related peptide (AgRP) neurons, and both these populations are located within the ARC. POMC neurons secret α-melanocyte-stimulating hormone (α-MSH) to stimulate melanocortin receptors and suppress body weight gain; on the other hand, AgRP functions as an endogenous antagonist of melanocortin receptors to promote weight gain [144], [145], [146]. 17β-estradiol has been shown to regulate POMC expression levels. For instance, depletion of ovarian hormones reduces POMC mRNA levels in female animals, but this response can be reversed by 17β-estradiol [85], [149]. Decreased POMC levels are also observed in mice lacking ERα [150]. In addition, the number of excitatory synaptic inputs to ARC POMC neurons is highest during the proestrus [28]. Further, it has been shown that 17β-estradiol acutely stimulates firing of POMC neurons [29], [30], [151]. Thus, 17β-estradiol appears to regulate both POMC expression and excitability of POMC neurons, two essential functions of these neurons to regulate body weight balance.
5.5. NPY
ARC AgRP neurons also co-release neuropeptide Y (NPY), which is a potent orexigenic signal that promotes feeding [152], [153]. Expression of NPY and AgRP in the hypothalamus is at the lowest level during estrus in female mice [154]. 17β-estradiol decreases activity of NPY/AgRP neurons and reduces hunger-driven feeding [154]. Importantly, ablation of NPY/AgRP neurons attenuates the estrus-dependent feeding fluctuations and anorexia induced by 17β-estradiol [154]. Thus, NPY/AgRP neurons are functionally involved in 17β-estradiol's actions to suppress feeding.
5.6. Serotonin
Brain 5-HT, primarily synthesized by DRN neurons [155], plays important roles in feeding control. Meals enhance brain 5-HT content, whereas hunger suppresses it [156]. Pharmacological agents that increase brain 5-HT content, e.g. d-fenfluramine (d-Fen) [157], decrease food intake in rodents and humans [158], [159], [160]. On the other hand, blockade of 5-HT signals in the brain leads to increased food intake and weight gain [161], [162], [163], [164]. 17β-estradiol increases the expression of the serotonin transporter, which may result in enhanced brain 5-HT bioavailability [165]. Indeed, 5-HT-induced anorexia is potentiated by 17β-estradiol treatment [166]. As mentioned above, loss of ERα selectively in 5-HT neurons blunts effects of 17β-estradiol to inhibit binge-like eating in mice [35]. Thus, 17β-estradiol and 5-HT interact within the brain to provide synergistic effects to inhibit feeding.
6. Estrogenic actions in male brains
Male animals also need estrogens to maintain normal body weight. For instance, deletion of ERα causes obesity in male mice [20], [167]; ERα mutation also leads to obesity in men [168], [169]. Treatment with 17β-estradiol or its analogs decreases body weight in male animals [28], [98]. Testosterone, as a male sex hormone, can be converted into 17β-estradiol by aromatase. Thus, aromatase knockout mice represent a good model to examine functions of endogenous estrogens not only in female but also in male animals. Female aromatase knockout mice show increased body weight from 3 months of age, while male mutant mice show late onset obesity one year later [170]. Both male and female aromatase knockout mice show increased gonadal and infrarenal fat pads compared to control littermates. This increased adiposity is associated with reduced spontaneous physical activity levels, reduced glucose oxidation, and a decrease in lean body mass [170]. These findings indicate that endogenous estrogens prevent obesity in both sexes, but the underlying mechanisms may not be identical.
Tissues that express aromatase include not only the gonads but also the breast, brain, muscle, bone, and adipose tissue [171], [172], [173]. Notably, abundant aromatase is expressed in a few brain regions, including the medial amygdala (MeA), the bed nucleus of stria terminalis, the septum, and the pre-optic area [174]. Thus, despite the lack of circulating estrogens, these brain regions in males could be exposed to high levels of 17β-estradiol. In fact, deletion of ERα selectively in the MeA leads to obesity in both female mice and male mice [175]. Thus, it is conceivable that ER populations in brain regions or peripheral tissues with aromatase activity may play important roles in the regulation of energy balance in both sexes. This possibility needs further investigation.
7. Estrogenic actions in developing brains
The critical brain regions regulating energy balance (e.g. the hypothalamus) are structurally and functionally immature at birth [176]. In rodents, the basic anatomy of the hypothalamus is formed between embryonic day 12–15, and neurochemical identities of most of hypothalamic neurons are established before birth [176]. However, the majority of neural innervations to or from the hypothalamus do not fully develop till about postnatal day 21 [177], [178], [179]. After weaning, hypothalamic neurons still undergo substantial remodeling [180]. Events (e.g. nutrition and hormone milieu) during this critical window have profound effects on the development of neural circuits controlling body weight and program energy balance later in life [181]. Despite the well-known anti-obesity effects of estrogens in adults, their roles in the metabolic programming are not fully understood. Emerging evidence suggests that ERα in developing brains may program energy balance. For example, rodent brains start to express ERα at E21 [182]. The number of ERα neurons increases postnatally (P0-P35) in key hypothalamic regions that are relevant to energy balance, including the ARC and VMH [183]. Further, rodents experience a transient estrogen surge in the brain immediately after birth [184], [185]. Similar estrogen surges have been reported in infant boys and girls (1–6 months) [186]. Another estrogen surge occurs prior to the onset of puberty [187], [188]. Both these P0 and pre-pubertal, endogenous, estrogen surges could exert profound effects on brain development. Indeed, manipulations of testosterone in male and female rat pups, which presumably alter estrogens originating from aromatization, lead to permanent changes in synaptological morphology and neural excitability of ARC neurons [189]. Further, postnatal manipulations of gonadal steroids permanently alter feeding behavior and body weight in adults [190], [191]. In addition, it has been reported that genetic effects on eating disorders are significantly stronger in post-pubertal girls than in pre-pubertal girls [192], [193], suggesting a potential effect of sex hormones (e.g. estrogens) on feeding behavior specifically during the puberty development. Collectively, the current literature suggests robust effects of estrogens on developmental programming of energy balance, but the detailed mechanisms for such effects require further investigations.
8. Conclusions
17β-estradiol's actions in the brain contribute to the regulation of energy homeostasis in both sexes, although its actions in females are more extensively explored. Multiple brain regions that express high levels of ERα mediate the effects of 17β-estradiol to inhibit feeding; 17β-estradiol stimulates BAT thermogenesis primarily via its actions in the VMH. Both rapid signals and “classic” transcriptional activities of 17β-estradiol are involved in body weight control; however, the detailed molecular mechanisms remain unclear. 17β-estradiol has broad interactions with many other body weight-regulatory signals, and these interactions provide a coordinated control of feeding behavior and body weight. Further, 17β-estradiol can be locally produced in male brain regions with aromatase activity and exerts physiologically relevant functions to maintain body weight balance, and these estrogenic functions in male brains remain to be further explored.
Acknowledgements
The authors thank Dr. Ismael González-García (University of Santiago de Compostela; Spain) for his comments. The research leading to these results has received funding from, National Institutes of Health (YX: R01DK093587 and R01DK101379), Xunta de Galicia (ML: 2015-CP079), MINECO co-funded by the FEDER Program of EU (ML: SAF2015-71026-R and BFU2015-70454-REDT/Adipoplast). CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Yong Xu, Email: yongx@bcm.edu.
Miguel López, Email: m.lopez@usc.es.
Conflict of interest
There are no known conflicts of interest.
References
- 1.Mauvais-Jarvis F., Clegg D.J., Hevener A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez M., Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in Endocrinology and Metabolism. 2015;26:411–421. doi: 10.1016/j.tem.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Palmer B.F., Clegg D.J. The sexual dimorphism of obesity. Molecular and Cellular Endocrinology. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 5.Billeci A.M., Paciaroni M., Caso V., Agnelli G. Hormone replacement therapy and stroke. Current Vascular Pharmacology. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 6.Canonico M., Oger E., Plu-Bureau G., Conard J., Meyer G., Levesque H. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 7.Saito K., Cao X., He Y., Xu Y. Progress in the molecular understanding of central regulation of body weight by estrogens. Obesity (Silver Spring) 2015;23:919–926. doi: 10.1002/oby.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez M., Tena-Sempere M. Estradiol and brown fat. Best Practice and Research Clinical Endocrinology and Metabolism. 2016;30:527–536. doi: 10.1016/j.beem.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Asarian L., Geary N. Sex differences in the physiology of eating. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaustein J.D., Wade G.N. Ovarian influences on the meal patterns of female rats. Physiology and Behavior. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 11.Drewett R.F. Sexual behaviour and sexual motivation in the female rat. Nature. 1973;242:476–477. doi: 10.1038/242476a0. [DOI] [PubMed] [Google Scholar]
- 12.Geary N., Asarian L., Korach K.S., Pfaff D.W., Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 13.Wallen W.J., Belanger M.P., Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. Journal of Nutrition. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]
- 14.Roesch D.M. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology and Behavior. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Rogers N.H., Perfield J.W., 2nd, Strissel K.J., Obin M.S., Greenberg A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009 doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komm B.S., Mirkin S. Incorporating bazedoxifene/conjugated estrogens into the current paradigm of menopausal therapy. International Journal of Womens Health. 2012;4:129–140. doi: 10.2147/IJWH.S29346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.H., Meyers M.S., Khuder S.S., Abdallah S.L., Muturi H.T., Russo L. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Molecular Metabolism. 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cedars M.I., Judd H.L. Nonoral routes of estrogen administration. Obstetrics and Gynecology Clinics of North America. 1987;14:269–298. [PubMed] [Google Scholar]
- 19.Okura T., Koda M., Ando F., Niino N., Ohta S., Shimokata H. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. International Journal of Obesity and Related Metabolic Disorders. 2003;27:1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- 20.Heine P.A., Taylor J.A., Iwamoto G.A., Lubahn D.B., Cooke P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butera P.C., Beikirch R.J. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Research. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 22.Palmer K., Gray J.M. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiology and Behavior. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Nedungadi T.P., Zhu L., Sobhani N., Irani B.G., Davis K.E. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchenthaler I., Lane M.V., Numan S., Dellovade T.L. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. Journal of Comparative Neurology. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 25.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 26.Miller M.M., Tousignant P., Yang U., Pedvis S., Billiar R.B. Effects of age and long-term ovariectomy on the estrogen-receptor containing subpopulations of beta-endorphin-immunoreactive neurons in the arcuate nucleus of female C57BL/6J mice. Neuroendocrinology. 1995;61:542–551. doi: 10.1159/000126878. [DOI] [PubMed] [Google Scholar]
- 27.de Souza F.S., Nasif S., Lopez-Leal R., Levi D.H., Low M.J., Rubinsten M. The estrogen receptor alpha colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. European Journal of Pharmacology. 2011;660:181–187. doi: 10.1016/j.ejphar.2010.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao Q., Mezei G., Nie Y., Rao Y., Choi C.S., Bechmann I. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 29.Malyala A., Zhang C., Bryant D.N., Kelly M.J., Ronnekleiv O.K. PI3K signaling effects in hypothalamic neurons mediated by estrogen. Journal of Comparative Neurology. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 30.Zhu L., Xu P., Cao X., Yang Y., Hinton A.O., Jr., Xia Y. The eralpha-PI3K cascade in proopiomelanocortin Progenitor neurons regulates feeding and glucose balance in female mice. Endocrinology. 2015;156:4474–4491. doi: 10.1210/en.2015-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osterlund M., Kuiper G.G., Gustafsson J.A., Hurd Y.L. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Research Molecular Brain Research. 1998;54:175–180. doi: 10.1016/s0169-328x(97)00351-3. [DOI] [PubMed] [Google Scholar]
- 32.Schlenker E.H., Hansen S.N. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Research. 2006;1123:89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 33.Asarian L., Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- 34.Thammacharoen S., Lutz T.A., Geary N., Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X., Xu P., Oyola M.G., Xia Y., Yan X., Saito K. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. Journal of Clinical Investigation. 2014;124:4351–4362. doi: 10.1172/JCI74726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalmasso C., Amigone J.L., Vivas L. Serotonergic system involvement in the inhibitory action of estrogen on induced sodium appetite in female rats. Physiology and Behavior. 2011;104:398–407. doi: 10.1016/j.physbeh.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Robichaud M., Debonnel G. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. Journal of Neuroendocrinology. 2005;17:179–185. doi: 10.1111/j.1365-2826.2005.01292.x. [DOI] [PubMed] [Google Scholar]
- 38.Santollo J., Torregrossa A.M., Eckel L.A. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Hormones and Behavior. 2011;60:86–93. doi: 10.1016/j.yhbeh.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butera P.C., Willard D.M., Raymond S.A. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Research. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. [DOI] [PubMed] [Google Scholar]
- 40.Hrupka B.J., Smith G.P., Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiology and Behavior. 2002;77:233–241. doi: 10.1016/s0031-9384(02)00857-0. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu H., Bray G.A. Effects of castration, estrogen replacement and estrus cycle on monoamine metabolism in the nucleus accumbens, measured by microdialysis. Brain Research. 1993;621:200–206. doi: 10.1016/0006-8993(93)90107-x. [DOI] [PubMed] [Google Scholar]
- 42.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 43.Silva J.E. Thermogenic mechanisms and their hormonal regulation. Physiological Reviews. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 44.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology Endocrinology and Metabolism. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 45.Contreras C., Gonzalez F., Ferno J., Dieguez C., Rahmouni K., Nogueiras R. The brain and brown fat. Annals of Medicine. 2015;47:150–168. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 48.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 49.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabolism. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Futai M., Noumi T., Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annual Review of Biochemistry. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- 51.Boyer P.D. The ATP synthase–a splendid molecular machine. Annual Review of Biochemistry. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 52.von Ballmoos C., Wiedenmann A., Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annual Review of Biochemistry. 2009;78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- 53.Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiological Reviews. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Whittle A.J., Lopez M., Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends in Molecular Medicine. 2011;17:405–411. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Morrison S.F., Madden C.J., Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in Endocrinology. 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison S.F., Madden C.J., Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metabolism. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wade G.N., Gray J.M. Cytoplasmic 17 beta-[3H]estradiol binding in rat adipose tissues. Endocrinology. 1978;103:1695–1701. doi: 10.1210/endo-103-5-1695. [DOI] [PubMed] [Google Scholar]
- 58.Edens N.K., Wade G.N. Effects of estradiol on tissue distribution of newly-synthesized fatty acids in rats and hamsters. Physiology and Behavior. 1983;31:703–709. doi: 10.1016/s0031-9384(83)80007-9. [DOI] [PubMed] [Google Scholar]
- 59.Bartness T.J., Wade G.N. Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behavioral Neuroscience. 1984;98:674–685. doi: 10.1037//0735-7044.98.4.674. [DOI] [PubMed] [Google Scholar]
- 60.Schneider J.E., Palmer L.A., Wade G.N. Effects of estrous cycles and ovarian steroids on body weight and energy expenditure in Syrian hamsters. Physiology and Behavior. 1986;38:119–126. doi: 10.1016/0031-9384(86)90141-1. [DOI] [PubMed] [Google Scholar]
- 61.Richard D. Effects of ovarian hormones on energy balance and brown adipose tissue thermogenesis. American Journal of Physiology. 1986;250:R245–R249. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- 62.Kamei Y., Suzuki M., Miyazaki H., Tsuboyama-Kasaoka N., Wu J., Ishimi Y. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. Journal of Nutritional Science and Vitaminology. 2005;51:110–117. doi: 10.3177/jnsv.51.110. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida T., Nishioka H., Yoshioka K., Kondo M. Reduced norepinephrine turnover in interscapular brown adipose tissue of obese rats after ovariectomy. Metabolism. 1987;36:1–6. doi: 10.1016/0026-0495(87)90054-0. [DOI] [PubMed] [Google Scholar]
- 64.Lopez M., Alvarez C.V., Nogueiras R., Dieguez C. Energy balance regulation by thyroid hormones at central level. Trends in Molecular Medicine. 2013;19:418–427. doi: 10.1016/j.molmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro M.O., Lebrun F.L., Christoffolete M.A., Branco M., Crescenzi A., Carvalho S.D. Evidence of UCP1-independent regulation of norepinephrine-induced thermogenesis in brown fat. American Journal of Physiology Endocrinology and Metabolism. 2000;279:E314–E322. doi: 10.1152/ajpendo.2000.279.2.E314. [DOI] [PubMed] [Google Scholar]
- 66.de Jesus L.A., Carvalho S.D., Ribeiro M.O., Schneider M., Kim S.W., Harney J.W. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. Journal of Clinical Investigation. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinez-Sanchez N., Alvarez C.V., Ferno J., Nogueiras R., Dieguez C., Lopez M. Hypothalamic effects of thyroid hormones on metabolism. Best Practice and Research Clinical Endocrinology and Metabolism. 2014;28:703–712. doi: 10.1016/j.beem.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez A.M., Monjo M., Roca P., Palou A. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cellular and Molecular Life Sciences. 2002;59:1714–1723. doi: 10.1007/PL00012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monjo M., Rodriguez A.M., Palou A., Roca P. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144:4923–4930. doi: 10.1210/en.2003-0537. [DOI] [PubMed] [Google Scholar]
- 70.Rodriguez-Cuenca S., Monjo M., Gianotti M., Proenza A.M., Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. American Journal of Physiology Endocrinology and Metabolism. 2007;292:E340–E346. doi: 10.1152/ajpendo.00175.2006. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Cuenca S., Monjo M., Frontera M., Gianotti M., Proenza A.M., Roca P. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiology Biochemistry. 2007;20:877–886. doi: 10.1159/000110448. [DOI] [PubMed] [Google Scholar]
- 72.Cooke P.S., Heine P.A., Taylor J.A., Lubahn D.B. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Molecular and Cellular Endocrinology. 2001;178:147–154. doi: 10.1016/s0303-7207(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 73.Foryst-Ludwig A., Clemenz M., Hohmann S., Hartge M., Sprang C., Frost N. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genetics. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hewitt K.N., Pratis K., Jones M.E., Simpson E.R. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–1848. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- 75.Davis K.E., Carstens E.J., Irani B.G., Gent L.M., Hahner L.M., Clegg D.J. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Hormones and Behavior. 2014;66:196–207. doi: 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Velickovic K., Cvoro A., Srdic B., Stokic E., Markelic M., Golic I. Expression and subcellular localization of estrogen receptors alpha and beta in human fetal brown adipose tissue. The Journal of Cinical Endocrinology and Metabolism. 2014;99:151–159. doi: 10.1210/jc.2013-2017. [DOI] [PubMed] [Google Scholar]
- 77.Shughrue P.J., Lane M.V., Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 78.Martinez de Morentin P.B., Lage R., Gonzalez-Garcia I., Ruiz-Pino F., Martins L., Fernandez-Mallo D. Pregnancy induces resistance to the anorectic effect of hypothalamic malonyl-CoA and the thermogenic effect of hypothalamic AMPK inhibition in female rats. Endocrinology. 2015;156:947–960. doi: 10.1210/en.2014-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perkins M.N., Rothwell N.J., Stock M.J., Stone T.W. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- 80.Yoshimatsu H., Egawa M., Bray G.A. Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate. Brain Research. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- 81.Lopez M., Varela L., Vazquez M.J., Rodriguez-Cuenca S., Gonzalez C.R., Velagapudi V.R. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nature Medicine. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whittle A.J., Carobbio S., Martins L., Slawik M., Hondares E., Vazquez M.J. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez de Morentin P.B., Whittle A.J., Ferno J., Nogueiras R., Dieguez C., Vidal-Puig A. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes. 2012;61:807–817. doi: 10.2337/db11-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seoane-Collazo P., Martinez de Morentin P.B., Ferno J., Dieguez C., Nogueiras R., Lopez M. Nicotine improves obesity and hepatic steatosis and ER stress in diet-induced obese male rats. Endocrinology. 2014;155:1679–1689. doi: 10.1210/en.2013-1839. [DOI] [PubMed] [Google Scholar]
- 85.Martinez de Morentin P.B., Gonzalez-Garcia I., Martins L., Lage R., Fernandez-Mallo D., Martinez-Sanchez N. Estradiol regulates Brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contreras C., Gonzalez-Garcia I., Seoane-Collazo P., Martinez-Sanchez N., Linares-Pose L., Rial-Pensado E. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes. 2017;66:87–99. doi: 10.2337/db15-1547. [DOI] [PubMed] [Google Scholar]
- 87.Minami T., Oomura Y., Nabekura J., Fukuda A. 17 beta-estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Research. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- 88.Musatov S., Chen W., Pfaff D.W., Mobbs C.V., Yang X.J., Clegg D.J. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yepuru M., Eswaraka J., Kearbey J.D., Barrett C.M., Raghow S., Veverka K.A. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. Journal of Biological Chemistry. 2010;285:31292–31303. doi: 10.1074/jbc.M110.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Correa S.M., Newstrom D.W., Warne J.P., Flandin P., Cheung C.C., Lin-Moore A.T. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Reports. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez M., Nogueiras R., Tena-Sempere M., Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nature Reviews Endocrinology. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 92.Tanida M., Yamamoto N. Central AMP-activated protein kinase affects sympathetic nerve activity in rats. Neuroscience Letters. 2011;503:167–170. doi: 10.1016/j.neulet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Tanida M., Yamamoto N., Shibamoto T., Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8:e56660. doi: 10.1371/journal.pone.0056660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beiroa D., Imbernon M., Gallego R., Senra A., Herranz D., Villarroya F. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 95.Alvarez-Crespo M., Csikasz R.I., Martinez-Sanchez N., Dieguez C., Cannon B., Nedergaard J. Essential role of UCP1 modulating the central effects of thyroid hormones on energy balance. Molecular Metabolism. 2016;5:271–282. doi: 10.1016/j.molmet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martins L., Seoane-Collazo P., Contreras C., Gonzalez-Garcia I., Martinez-Sanchez N., Gonzalez F. A functional link between AMPK and orexin mediates the effect of BMP8B on energy balance. Cell Reports. 2016;16:2231–2242. doi: 10.1016/j.celrep.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martinez-Sanchez N., Moreno-Navarrete J.M., Contreras C., Rial-Pensado E., Ferno J., Nogueiras R. Thyroid hormones induce browning of white fat. Journal of Endocrinology. 2017;232:351–362. doi: 10.1530/JOE-16-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Finan B., Yang B., Ottaway N., Stemmer K., Muller T.D., Yi C.X. Targeted estrogen delivery reverses the metabolic syndrome. Nature Medicine. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lopez M., Tena-Sempere M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: a gateway for obesity treatment? Pharmacology and Therapeutics. 2017;178:109–122. doi: 10.1016/j.pharmthera.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 100.Lopez M., EJE P.R.I.Z.E. 2017: hypothalamic AMPK: a golden target against obesity? European Journal of Endocrinology. 2017;176:R235–R246. doi: 10.1530/EJE-16-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yonezawa R., Wada T., Matsumoto N., Morita M., Sawakawa K., Ishii Y. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. American Journal of Physiology Endocrinology and Metabolism. 2012;303:E445–E456. doi: 10.1152/ajpendo.00638.2011. [DOI] [PubMed] [Google Scholar]
- 102.McCrimmon R.J., Fan X., Ding Y., Zhu W., Jacob R.J., Sherwin R.S. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53:1953–1958. doi: 10.2337/diabetes.53.8.1953. [DOI] [PubMed] [Google Scholar]
- 103.Han S.M., Namkoong C., Jang P.G., Park I.S., Hong S.W., Katakami H. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- 104.McCrimmon R.J., Fan X., Cheng H., McNay E., Chan O., Shaw M. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes. 2006;55:1755–1760. doi: 10.2337/db05-1359. [DOI] [PubMed] [Google Scholar]
- 105.McCrimmon R.J., Shaw M., Fan X., Cheng H., Ding Y., Vella M.C. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 106.Tamrakar P., Briski K.P. Estradiol regulation of hypothalamic astrocyte adenosine 5'-monophosphate-activated protein kinase activity: role of hindbrain catecholamine signaling. Brain Research Bulletin. 2015;110:47–53. doi: 10.1016/j.brainresbull.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Briski K.P., Ibrahim B.A., Tamrakar P. Energy metabolism and hindbrain AMPK: regulation by estradiol. Hormone Molecular Biology and Clinical Investigation. 2014;17:129–136. doi: 10.1515/hmbci-2013-0067. [DOI] [PubMed] [Google Scholar]
- 108.Park C.J., Zhao Z., Glidewell-Kenney C., Lazic M., Chambon P., Krust A. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese Eralpha-null mutant mice. Journal of Clinical Investigation. 2011;121:604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jakacka M., Ito M., Martinson F., Ishikawa T., Lee E.J., Jameson J.L. An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Molecular Endocrinology. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 110.Handgraaf S., Riant E., Fabre A., Waget A., Burcelin R., Liere P. Prevention of obesity and insulin resistance by estrogens requires ERalpha activation function-2 (ERalphaAF-2), whereas ERalphaAF-1 is dispensable. Diabetes. 2013;62:4098–4108. doi: 10.2337/db13-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saito K., He Y., Yang Y., Zhu L., Wang C., Xu P. PI3K in the ventromedial hypothalamic nucleus mediates estrogenic actions on energy expenditure in female mice. Scientific Reports. 2016;6:23459. doi: 10.1038/srep23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pedram A., Razandi M., Kim J.K., O'Mahony F., Lee E.Y., Luderer U. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. Journal of Biological Chemistry. 2008 doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leibel R.L., Chung W.K., Chua S.C., Jr. The molecular genetics of rodent single gene obesities. Journal of Biological Chemistry. 1997;272:31937–31940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 114.Alingh Prins A., de Jong-Nagelsmit A., Keijser J., Strubbe J.H. Daily rhythms of feeding in the genetically obese and lean Zucker rats. Physiology and Behavior. 1986;38:423–426. doi: 10.1016/0031-9384(86)90115-0. [DOI] [PubMed] [Google Scholar]
- 115.McLaughlin C.L., Baile C.A. Ontogeny of feeding behavior in the Zucker obese rat. Physiology and Behavior. 1981;26:607–612. doi: 10.1016/0031-9384(81)90132-3. [DOI] [PubMed] [Google Scholar]
- 116.Trayhurn P., Thurlby P.L., James W.P. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 117.Dauncey M.J. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia. 1986;42:547–549. doi: 10.1007/BF01946696. [DOI] [PubMed] [Google Scholar]
- 118.Dauncey M.J., Brown D. Role of activity-induced thermogenesis in twenty-four hour energy expenditure of lean and genetically obese (ob/ob) mice. Quarterly Journal of Experimental Physiology. 1987;72:549–559. doi: 10.1113/expphysiol.1987.sp003096. [DOI] [PubMed] [Google Scholar]
- 119.Tartaglia L.A. The leptin receptor. Journal of Biological Chemistry. 1997;272:6093–6096. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 120.Halaas J.L., Boozer C., Blair-West J., Fidahusein N., Denton D.A., Friedman J.M. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diano S., Kalra S.P., Sakamoto H., Horvath T.L. Leptin receptors in estrogen receptor-containing neurons of the female rat hypothalamus. Brain Research. 1998;812:256–259. doi: 10.1016/s0006-8993(98)00936-6. [DOI] [PubMed] [Google Scholar]
- 122.Lindell K., Bennett P.A., Itoh Y., Robinson I.C., Carlsson L.M., Carlsson B. Leptin receptor 5'untranslated regions in the rat: relative abundance, genomic organization and relation to putative response elements. Molecular and Cellular Endocrinology. 2001;172:37–45. doi: 10.1016/s0303-7207(00)00382-8. [DOI] [PubMed] [Google Scholar]
- 123.Clegg D.J., Brown L.M., Woods S.C., Benoit S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 124.Raybould H.E. Mechanisms of CCK signaling from gut to brain. Current Opinion in Pharmacology. 2007;7:570–574. doi: 10.1016/j.coph.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang Y.S., Doi R., Chowdhury P., Pasley J.N., Nishikawa M., Huang T.J. Effect of cholecystokinin on food intake at different stages of the estrous cycle in female rats. Journal of the Association for Academic Minority Physicians. 1993;4:56–58. [PubMed] [Google Scholar]
- 126.Asarian L., Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides. 1999;20:445–450. doi: 10.1016/s0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 127.Eckel L.A., Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 128.Geary N., Smith G.P., Corp E.S. The increased satiating potency of CCK-8 by estradiol is not mediated by upregulation of NTS CCK receptors. Brain Research. 1996;719:179–186. doi: 10.1016/0006-8993(96)00099-6. [DOI] [PubMed] [Google Scholar]
- 129.Butera P.C., Bradway D.M., Cataldo N.J. Modulation of the satiety effect of cholecystokinin by estradiol. Physiology and Behavior. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- 130.DiNardo L.A., Travers J.B. Distribution of fos-like immunoreactivity in the medullary reticular formation of the rat after gustatory elicited ingestion and rejection behaviors. Journal of Neuroscience. 1997;17:3826–3839. doi: 10.1523/JNEUROSCI.17-10-03826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eckel L.A., Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2001;281:R738–R746. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- 132.Rinaman L., Baker E.A., Hoffman G.E., Stricker E.M., Verbalis J.G. Medullary c-Fos activation in rats after ingestion of a satiating meal. American Journal of Physiology. 1998;275:R262–R268. doi: 10.1152/ajpregu.1998.275.1.R262. [DOI] [PubMed] [Google Scholar]
- 133.Park T.H., Carr K.D. Neuroanatomical patterns of fos-like immunoreactivity induced by a palatable meal and meal-paired environment in saline- and naltrexone-treated rats. Brain Research. 1998;805:169–180. doi: 10.1016/s0006-8993(98)00719-7. [DOI] [PubMed] [Google Scholar]
- 134.Rinaman L., Hoffman G.E., Dohanics J., Le W.W., Stricker E.M., Verbalis J.G. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. Journal of Comparative Neurology. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- 135.Li B.H., Rowland N.E. Cholecystokinin- and dexfenfluramine-induced anorexia compared using devazepide and c-fos expression in the rat brain. Regulatory Peptides. 1994;50:223–233. doi: 10.1016/0167-0115(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 136.Eckel L.A., Houpt T.A., Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2002;283:R1378–R1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- 137.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 138.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 139.Wren A.M., Small C.J., Abbott C.R., Dhillo W.S., Seal L.J., Cohen M.A. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 140.Wren A.M., Seal L.J., Cohen M.A., Brynes A.E., Frost G.S., Murphy K.G. Ghrelin enhances appetite and increases food intake in humans. The Journal of Cinical Endocrinology and Metabolism. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 141.Arnold M., Mura A., Langhans W., Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. Journal of Neuroscience. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Davidson T.L., Kanoski S.E., Tracy A.L., Walls E.K., Clegg D., Benoit S.C. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 143.Clegg D.J., Brown L.M., Zigman J.M., Kemp C.J., Strader A.D., Benoit S.C. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 144.Elmquist J.K., Elias C.F., Saper C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 145.Cone R.D. Anatomy and regulation of the central melanocortin system. Nature Neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 146.Williams D.L., Schwartz M.W. The melanocortin system as a central integrator of direct and indirect controls of food intake. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2005;289:R2–R3. doi: 10.1152/ajpregu.00226.2005. [DOI] [PubMed] [Google Scholar]
- 147.Cone R.D. The central melanocortin system and energy homeostasis. Trends in Endocrinology and Metabolism. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 148.Huszar D., Lynch C.A., Fairchild-Huntress V., Dunmore J.H., Fang Q., Berkemeier L.R. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 149.Pelletier G., Li S., Luu-The V., Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. Journal of Neuroendocrinology. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 150.Hirosawa M., Minata M., Harada K.H., Hitomi T., Krust A., Koizumi A. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochemical and Biophysical Research Communications. 2008;371:320–323. doi: 10.1016/j.bbrc.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 151.Qiu J., Bosch M.A., Tobias S.C., Grandy D.K., Scanlan T.S., Ronnekleiv O.K. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. Journal of Neuroscience. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paez X., Myers R.D. Insatiable feeding evoked in rats by recurrent perfusion of neuropeptide Y in the hypothalamus. Peptides. 1991;12:609–616. doi: 10.1016/0196-9781(91)90109-3. [DOI] [PubMed] [Google Scholar]
- 153.Pierroz D.D., Catzeflis C., Aebi A.C., Rivier J.E., Aubert M.L. Chronic administration of neuropeptide Y into the lateral ventricle inhibits both the pituitary-testicular axis and growth hormone and insulin-like growth factor I secretion in intact adult male rats. Endocrinology. 1996;137:3–12. doi: 10.1210/endo.137.1.8536627. [DOI] [PubMed] [Google Scholar]
- 154.Olofsson L.E., Pierce A.A., Xu A.W. Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15932–15937. doi: 10.1073/pnas.0904747106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lechin F., van der Dijs B., Hernandez-Adrian G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Progress In Neuro-Psychopharmacology and Biological Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 156.De Fanti B.A., Hamilton J.S., Horwitz B.A. Meal-induced changes in extracellular 5-HT in medial hypothalamus of lean (Fa/Fa) and obese (fa/fa) Zucker rats. Brain Research. 2001;902:164–170. doi: 10.1016/s0006-8993(01)02371-x. [DOI] [PubMed] [Google Scholar]
- 157.Rowland N.E., Carlton J. Neurobiology of an anorectic drug: fenfluramine. Progress in Neurobiology. 1986;27:13–62. doi: 10.1016/0301-0082(86)90011-0. [DOI] [PubMed] [Google Scholar]
- 158.Foltin R.W., Moran T.H. Food intake in baboons: effects of a long-acting cholecystokinin analog. Appetite. 1989;12:145–152. doi: 10.1016/0195-6663(89)90103-7. [DOI] [PubMed] [Google Scholar]
- 159.McGuirk J., Goodall E., Silverstone T., Willner P. Differential effects of d-fenfluramine, l-fenfluramine and d-amphetamine on the microstructure of human eating behaviour. Behavioural Pharmacology. 1991;2:113–119. [PubMed] [Google Scholar]
- 160.Rogers P.J., Blundell J.E. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology (Berl) 1979;66:159–165. doi: 10.1007/BF00427624. [DOI] [PubMed] [Google Scholar]
- 161.Blundell J.E., Leshem M.B. Central action of anorexic agents: effects of amphetamine and fenfluramine in rats with lateral hypothalamic lesions. European Journal of Pharmacology. 1974;28:81–88. doi: 10.1016/0014-2999(74)90115-0. [DOI] [PubMed] [Google Scholar]
- 162.Geyer M.A., Puerto A., Menkes D.B., Segal D.S., Mandell A.J. Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Research. 1976;106:257–269. doi: 10.1016/0006-8993(76)91024-6. [DOI] [PubMed] [Google Scholar]
- 163.Ghosh M.N., Parvathy S. The effect of cyproheptadine on water and food intake and on body weight in the fasted adult and weanling rats. British Journal of Pharmacology. 1973;48 328P-329P. [PMC free article] [PubMed] [Google Scholar]
- 164.Saller C.F., Stricker E.M. Hyperphagia and increased growth in rats after intraventricular injection of 5,7-dihydroxytryptamine. Science. 1976;192:385–387. doi: 10.1126/science.1257774. [DOI] [PubMed] [Google Scholar]
- 165.Rivera H.M., Oberbeck D.R., Kwon B., Houpt T.A., Eckel L.A. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Research. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rivera H.M., Santollo J., Nikonova L.V., Eckel L.A. Estradiol increases the anorexia associated with increased 5-HT(2C) receptor activation in ovariectomized rats. Physiology and Behavior. 2012;105:188–194. doi: 10.1016/j.physbeh.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Callewaert F., Venken K., Ophoff J., De Gendt K., Torcasio A., van Lenthe G.H. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. The FASEB Journal. 2009;23:232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 168.Grumbach M.M., Auchus R.J. Estrogen: consequences and implications of human mutations in synthesis and action. Journal of Clinics in Endocrinology and Metabolism. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 169.Smith E.P., Boyd J., Frank G.R., Takahashi H., Cohen R.M., Specker B. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. New England Journal of Medicine. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 170.Jones M.E., Thorburn A.W., Britt K.L., Hewitt K.N., Wreford N.G., Proietto J. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Simpson E.R. Sources of estrogen and their importance. The Journal of Steroid Biochemistry and Molecular Biology. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 172.Nelson L.R., Bulun S.E. Estrogen production and action. Journal of the American Academy of Dermatology. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 173.Simpson E.R., Misso M., Hewitt K.N., Hill R.A., Boon W.C., Jones M.E. Estrogen–the good, the bad, and the unexpected. Endocrine Reviews. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 174.Wu M.V., Manoli D.S., Fraser E.J., Coats J.K., Tollkuhn J., Honda S. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu P., Cao X., He Y., Zhu L., Yang Y., Saito K. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. Journal of Clinical Investigation. 2015;125:2861–2876. doi: 10.1172/JCI80941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Markakis E.A. Development of the neuroendocrine hypothalamus. Frontiers in Neuroendocrinology. 2002;23:257–291. doi: 10.1016/s0091-3022(02)00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grove K.L., Smith M.S. Ontogeny of the hypothalamic neuropeptide Y system. Physiology and Behavior. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 178.Rinaman L. Ontogeny of hypothalamic-hindbrain feeding control circuits. Developmental Psychobiology. 2006;48:389–396. doi: 10.1002/dev.20146. [DOI] [PubMed] [Google Scholar]
- 179.Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Frontiers in Neuroendocrinology. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McNay D.E., Briancon N., Kokoeva M.V., Maratos-Flier E., Flier J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. Journal of Clinical Investigation. 2011 doi: 10.1172/JCI43134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Remmers F., Delemarre-van de Waal H.A. Developmental programming of energy balance and its hypothalamic regulation. Endocrine Reviews. 2011;32:272–311. doi: 10.1210/er.2009-0028. [DOI] [PubMed] [Google Scholar]
- 182.MacLusky N.J., Lieberburg I., McEwen B.S. The development of estrogen receptor systems in the rat brain: perinatal development. Brain Research. 1979;178:129–142. doi: 10.1016/0006-8993(79)90093-3. [DOI] [PubMed] [Google Scholar]
- 183.Yokosuka M., Okamura H., Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. Journal of Comparative Neurology. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 184.Rhoda J., Corbier P., Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 beta-estradiol. Endocrinology. 1984;114:1754–1760. doi: 10.1210/endo-114-5-1754. [DOI] [PubMed] [Google Scholar]
- 185.Amateau S.K., Alt J.J., Stamps C.L., McCarthy M.M. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 186.Bidlingmaier F., Strom T.M., Dorr H.G., Eisenmenger W., Knorr D. Estrone and estradiol concentrations in human ovaries, testes, and adrenals during the first two years of life. The Journal of Cinical Endocrinology and Metabolism. 1987;65:862–867. doi: 10.1210/jcem-65-5-862. [DOI] [PubMed] [Google Scholar]
- 187.Corpechot C., Baulieu E.E., Robel P. Testosterone, dihydrotestosterone and androstanediols in plasma, testes and prostates of rats during development. Acta Endocrinologica (Copenh) 1981;96:127–135. doi: 10.1530/acta.0.0960127. [DOI] [PubMed] [Google Scholar]
- 188.Seiki K., Sakabe K., Fujii-Hanamoto H., Kawashima I., Ogawa H. Hormonal events surrounding spontaneous onset of puberty in female rats. Tokai Journal of Experimental and Clinical Medicine. 1989;14:45–54. [PubMed] [Google Scholar]
- 189.Matsumoto A., Arai Y. Sexual dimorphism in 'wiring pattern' in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Research. 1980;190:238–242. doi: 10.1016/0006-8993(80)91173-7. [DOI] [PubMed] [Google Scholar]
- 190.Nohara K., Zhang Y., Waraich R.S., Laque A., Tiano J.P., Tong J. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology. 2011;152:1661–1669. doi: 10.1210/en.2010-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cederroth C.R., Nef S. Fetal programming of adult glucose homeostasis in mice. PLoS One. 2009;4:e7281. doi: 10.1371/journal.pone.0007281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klump K.L., Culbert K.M., O'Connor S., Fowler N., Burt S.A. The significant effects of puberty on the genetic diathesis of binge eating in girls. International Journal of Eating Disorders. 2017;50:984–989. doi: 10.1002/eat.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Klump K.L., Culbert K.M., Slane J.D., Burt S.A., Sisk C.L., Nigg J.T. The effects of puberty on genetic risk for disordered eating: evidence for a sex difference. Psychological Medicine. 2012;42:627–637. doi: 10.1017/S0033291711001541. [DOI] [PMC free article] [PubMed] [Google Scholar]