Abstract
We aimed to clarify the relationship between plasma-free amino acid (PFAA) profiles and the Crohn’s disease (CD) activity index (CDAI) in patients with CD.
Methods:
We measured fasting PFAA concentrations in 29 patients with CD and their correlation with disease activity.
Results:
In all patients, significant correlations were noted between CDAI and concentrations of valine, methionine, leucine, histidine, tryptophan, alanine, tyrosine, total amino acids (TAAs), nonessential amino acids (NEAAs), essential amino acids (EAAs), and branched-chain amino acids (BCAAs). In patients with the ileo-colonic type of CD, significant correlations were noted between CDAI and valine, histidine, tryptophan, glutamine, TAA, NEAA, EAA, and BCAA. In ileal type, significant correlations were observed between CDAI and threonine, valine, histidine, serine, and glycine. In colonic type, significant correlations were noted between CDAI and valine, histidine, tryptophan, TAA, NEAA, EAA, and BCAA.
Conclusions:
In patients with CD, plasma amino acids appear to be associated with disease activity.
Keywords: Amino acids, Crohn disease, disease activity, CDAI
Introduction
Crohn’s disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract characterized by recurrent inflammation at any location along its length. The disease typically manifests as bowel strictures, abscess formation, and fistulas. Malnutrition is one of the most significant problems in patients with CD, necessitating nutritional support for modulating intestinal inflammation.1,2
CD is characterized by damage of the epithelium and impaired the immune response of the bowel, function of the intestinal mucosal barrier, and absorption and/or wasting in the intestine of nutritional particles such as amino acids (AAs). Then, disturbances of metabolic homeostasis would be related to the pathogenesis of chronic inflammatory disorders such as CD.
The main role of AAs is to provide the building block for proteins, which are essential for life. Specific AAs belonging to the group of essential AAs (EAAs), such as glutamine (Gln) and arginine (Arg), have immunomodulatory effects during metabolic stress, especially when the gut is involved in systemic inflammation. Some AAs, such as glycine (Gly), histidine (His), cysteine (Cys), and taurine (Tau), are candidates for anti-inflammatory properties in intestinal epithelial cells.3 Recently, the anti-inflammatory activity of certain AAs has been reported,4,5 and antagonistic effects of AAs on intestinal inflammation have also been demonstrated.6–15
Gln is an immunomodulatory agent. It is the preferred energy source for intestinal epithelial cells, it protects the function of the intestinal mucosal barrier, and it serves as the basic nutritional element for cellular immune function.16 It has been shown that the combination of Gln and Arg suppresses the release of pro-inflammatory cytokines from colonic tissues of patients with CD.17 Gln supplementation increased plasma concentrations of threonine (Thr), citruline (Cit), and His; and Arg enhanced the ratio of AAs in rats with dextran sulfate sodium (DSS)-induced colitis.12 Gly prevents chemical-induced colitis by inhibiting the induction of inflammatory cytokines and chemokines.18 Plasma His and tryptophan (Trp) concentrations were significantly lower in patients with inflammatory bowel disease (IBD) than in healthy controls.19
These observations strongly suggest that plasma AA concentrations are closely associated with disease activity in CD. However, plasma-free AA (PFAA) profiles have not been well investigated in patients with CD. The goal of this study was to clarify the relationship between PFAAs and the CD activity index (CDAI)20,21 and to supplement patients with possibly beneficial AAs.
Patients and Methods
Patients
A total of 29 patients with CD were enrolled (6 women, 23 men; mean age, 41.0 years; 17 ileo-colonic type, 7 ileal type, 5 colonic type). The mean disease duration was 10.3 years (range, 3-30 years). The patients were evaluated with the CDAI at the time of blood sampling.20 The contributors to CDAI index consists of 8 factors, such as number of loose stools, abdominal pain, general well-being, presence of complications, taking antidiarrhea drug, presence of an abdominal mass, hematocrit, and percentage deviation from standard weight, each summed after adjustment with a weighting factor based on the past 7 days. Plasma samples for AA analysis were obtained in the morning before breakfast using EDTA as an anticoagulant. The study protocol was approved by the Institutional Review Board at Iwate Medical University and performed in accordance with the Declaration of Helsinki.
Measurement of plasma AAs
Fasting plasma concentrations of the 20 AAs—Thr, valine (Val), methionine (Met), isoleucine (Iso), leucine (Leu), phenylalanine (Phe), His, Trp, lysine (Lys), aspartic acid (Asp), serine (Ser), asparagine (Asn), glutamic acid (Glu), Gln, proline (Pro), Gly, alanine (Ala), Cys, tyrosine (Tyr), arginine (Arg)—were measured by high-performance liquid chromatography. Subsequently, the concentrations of total AAs (TAAs), nonessential AAs (NEAAs), EAAs, and branched-chain AAs (BCAAs) were also calculated.
Statistical analyses
Patient characteristics data are presented as the mean ± SD. The AA levels are expressed as median (25th-75th percentiles). Within-group comparisons were analyzed by nonparametric comparisons with the Mann-Whitney U test. Multiple comparisons were performed using the Benjamini-Hochberg procedure. Correlations were evaluated with Pearson correlation coefficient test. A P value of <.05 was considered statistically significant.
Results
Patient characteristics
Table 1 summarizes the clinical characteristics of patients with the ileo-colonic, ileal, and colonic types of CD. A total of 17 patients had the ileo-colonic type (3 women, 14 men; mean age, 32.4 years), 7 patients had the ileal type (2 women, 5 men; mean age, 41.0 years), and 5 patients had the colonic type of CD (1 woman, 4 men; mean age, 29.0 years). All of the patients were treated with oral mesalamine (750-3000 mg/d). Two patients with the ileo-cecal type and patient with the colonic type also received oral prednisone (5-25 mg/d). Three patients with the ileo-colonic type, 2 patients with the ileal type and 1 patient with the colonic type were given oral azathioprine (50 mg/d). In addition, 13 patients with the ileo-colonic type, 3 patients with the ileal type, and 4 patients with the colonic type were treated with anti–TNF-α (tumor necrosis factor α) agents. No statistical differences in age, sex, disease duration, and CDAI were observed among the 3 groups.
Table 1.
Clinical characteristics of patients with the ileo-colonic, ileal, and colonic types of CD (n = 29).
| All |
Ileo-colonic type |
Ileal type |
Colonic type |
P valuea | |
|---|---|---|---|---|---|
| (n = 29) | (n = 17) | (n = 7) | (n = 5) | ||
| Age, y | 18-55 (41.0) | 18-55 (32.4) | 33-49 (41.0) | 27-32 (29.0) | .98 |
| Gender, female/male | 6/23 | 3/14 | 2/5 | 1/4 | .28 |
| Height, cm | 168.2 ± 8.9 | 167.5 ± 10.0 | 169.2 ± 4.8 | 169.0 ± 9.6 | .75 |
| Body weight, kg | 58.4 ± 9.9 | 56.2 ± 10.0 | 62.6 ± 6.2 | 60.0 ± 11.8 | .39 |
| Body mass index, kg/m2 | 20.6 ± 2.8 | 19.9 ± 2.4 | 22.0 ± 3.1 | 20.9 ± 3.0 | .43 |
| Disease duration, y | 1-19 (5.4) | 1-15 (6.1) | 2.3-19 (5.8) | 1-7 (2.8) | .19 |
| CDAI | 160.1 ± 74.6 | 160.2 ± 85.0 | 151.3 ± 51.1 | 151.7 ± 75.5 | .95 |
| Treatment | |||||
| 5-aminosalicylic acid, % | 29 (100) | 17 (100) | 7 (100) | 5 (100) | 1.00 |
| Prednisone, % | 3 (10.3) | 2 (11.2) | 1 (14.3) | 1 (25.0) | .28 |
| Azathioprine, % | 6 (20.7) | 3 (17.6) | 2 (28.5) | 1 (25.0) | .29 |
| Anti TNF-α agents, % | 20 (70.0) | 13 (76.5) | 3 (42.9) | 4 (80.0) | .28 |
Abbreviations: CDAI, Crohn’s disease activity index; TNF-α, tumor necrosis factor α.
Data are presented as the mean ± SD. Age and disease duration are expressed as median (25th-75th percentiles).
Mann-Whitney U test.
Comparison of AA profiles among types of CD
The AA profiles were not significantly different among patients with the ileo-colonic, ileal, and colonic types of CD (Table 2).
Table 2.
Comparison of AA profiles in patients with the ileo-colonic, ileal, and colonic types of CD (n = 29).
| AAs, nmol/mL | All (n = 29) |
Ileo-colonic type (n = 17) |
Ileal type (n = 7) |
Colonic type (n = 5) |
P valuea | Normal range |
|---|---|---|---|---|---|---|
| EAAs | ||||||
| Thr | 103.4 (90.6-120.1) | 101.4 (92.1-120.1) | 118.7 (117.5-142.7) | 82.9 (82.5-110.1) | .81 | (66.5-188.9) |
| Val | 191.0 (156.2-232.2) | 189.4 (160.1-226.3) | 192.8 (173.6-243.3) | 146.8 (133.8-232.2) | .92 | (147.8-307.0) |
| Met | 23.8 (21.1-28.3) | 22.8 (21.1-28.3) | 24.6 (26.3-30.0) | 19.3 (18.4-23.8) | .50 | (18.9-40.5) |
| Iso | 63.4 (52.9-70.5) | 57.8 (51.2-69.2) | 69.1 (61.1-77.7) | 64.4 (59.3-70.8) | .21 | (43.0-112.8) |
| Leu | 99.1 (88.1-129.3) | 99.0 (88.1-127.4) | 111.7 (97.6-134.1) | 102.5 (80.9-133.9) | .57 | (76.6-171.3) |
| Phe | 51.4 (44.3-60.6) | 50.6 (43.9-61.1) | 54.1 (48.5-56.8) | 55.3 (44.3-68.8) | .24 | (42.6-75.7) |
| His | 73.7 (65.1-81.1) | 73.7 (65.1-79.7) | 74.4 (71.3-78.6) | 50.2 (47.0-88.9) | .81 | (59.0-92.0) |
| Trp | 42.4 (24.4-52.6) | 42.4 (29.0-48.9) | 49.1 (32.1-51.9) | 22.4 (19.3-53.6) | .73 | (37.0-74.9) |
| Lys | 157.9 (130.9-191.7) | 153.0 (118.4-181.1) | 189.5 (155.5-216.5) | 137.0 (132.5-162.0) | .70 | (108.7-242.2) |
| NEAAs | ||||||
| Asp | 3.1 (2.6-3.7) | 3.0 (2.6-3.4) | 3.2 (2.3-3.3) | 3.6 (3.0-4.5) | .95 | (<2.4) |
| Ser | 113.5 (102.5-125.0) | 115.6 (105.4-124.1) | 125.0 (107.7-129.6) | 105.5 (101.5-109.7) | .91 | (72.4-164.5) |
| Asn | 43.4 (37.5-47.3) | 42.6 (37.5-46.4) | 48.1 (39.2-52.7) | 38.9 (38.7-45.2) | .72 | (44.7-96.8) |
| Glu | 44.8 (37.5-54.6) | 41.5 (38.7-52.4) | 45.3 (22.1-62.2) | 44.8 (38.1-63.8) | .29 | (12.6-62.5) |
| Gln | 518.7 (453.7-561.7) | 517.6 (455.4-553.7) | 556.5 (493.8-617.6) | 510.8 (434.4-540.0) | .36 | (422.1-703.8) |
| Pro | 168.5 (123.7-204.3) | 144.8 (116.0-195.7) | 202.4 (168.6-214.2) | 123.7 (119.3-168.5) | .77 | (77.8-272.7) |
| Gly | 247.9 (210.3-321.2) | 247.9 (210.3-294.6) | 321.2 (249.3-373.1) | 201.5 (201.0-223.3) | .62 | (151.0-351.0) |
| Ala | 360.6 (285.4-463.0) | 319.0 (262.1-463.0) | 422.0 (367.7-533.2) | 311.6 (296.7-367.1) | .49 | (208.7-522.7) |
| Cys | 30.1 (21.7-36.7) | 30.5 (23.0-36.7) | 30.8 (21.7-37.5) | 29.4 (21.7-30.1) | .94 | (13.7-28.3) |
| Tyr | 52.1 (46.1-60.7) | 51.9 (46.2-59.7) | 54.0 (47.7-60.9) | 49.7 (33.4-61.9) | .69 | (40.4-90.3) |
| Arg | 68.2 (59.5-76.8) | 68.2 (59.5-76.1) | 79.8 (67.9-82.6) | 61.4 (47.8-61.7) | .64 | (53.6-133.6) |
| TAAs | 2781.7 (2242.0-3096.9) | 2553.1 (2244.6-2984.2) | 3086.0 (2890.5-3209.3) | 2242.0 (2135.5-2847.4) | .70 | (2068.2-3510.3) |
| NEAAs | 1844.7 (1600.2-2158.5) | 1757.3 (1590.2-2091.0) | 2137.9 (2046.9-2178.7) | 1614.4 (1572.9-1826.6) | .62 | (1381.6-2379.4) |
| EAAs | 851.4 (691.6-942.3) | 840.1 (758.0-923.8) | 884.8 (836.4-993.2) | 691.6 (627.6-943.9) | .75 | (660.0-1222.3) |
| BCAAs | 348.6 (297.2-433.2) | 346.8 (303.6-424.2) | 396.9 (322.9-450.6) | 308.6 (285.5-441.2) | .69 | (265.8-579.1) |
Abbreviations: AAs, amino acids; BCAAs, branched-chain amino acids; EAAs, essential amino acids; NEAAs, nonessential amino acids; NS, nonsignificant; TAAs, total amino acids.
Data are presented as median (25th-75th percentiles).
Multiple comparisons were performed using the Benjamini-Hochberg procedure.
Relationship between CDAI scores and concentrations of AAs
All patients with CD
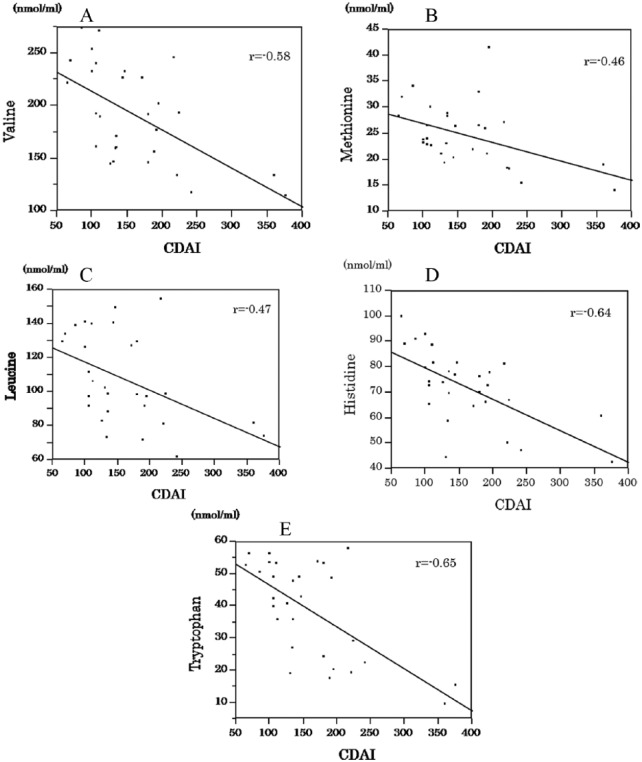
Significant correlations were noted between CDAI scores and concentrations of Val, Met, Leu, His, Trp, Ala, Tyr, TAA, NEAA, EAA, and BCAA (Table 3). Concentrations of 5 EAAs, Val, Met, Leu, His, and Trp were significantly correlated with CDAI scores (Figure 1A to E). No significant correlations were observed between CDAI scores and concentrations of the other AAs.
Table 3.
Correlation between the CDAI score and amino acid concentrations in patients with the ileo-colonic, ileal, and colonic types of CD (n = 29).
| AAs | All (n = 29) |
Ileo-colonic
type (n = 17) |
Ileal
type (n = 7) |
Colonic
type (n = 5) |
||||
|---|---|---|---|---|---|---|---|---|
| r | P valuea | r | P valuea | r | P valuea | r | P valuea | |
| EAAs | ||||||||
| Thr | −.36 | .054 | −.34 | .17 | −.44 | .32 | −.83 | .07 |
| Val | −.58 | .0009 | −.57 | .0174 | −.47 | .29 | −.90 | .0349 |
| Met | −.46 | .0124 | −.44 | .07 | −.17 | .71 | −.87 | .053 |
| Iso | −.27 | .15 | −.29 | .25 | .17 | .71 | −.50 | .39 |
| Leu | −.47 | .0105 | −.47 | .055 | .03 | .94 | −.95 | .0124 |
| Phe | −.37 | .0483 | −.29 | .25 | .30 | .50 | −.95 | .0115 |
| His | −.64 | .0002 | −.73 | .0008 | −.39 | .38 | −.77 | .12 |
| Trp | −.65 | .0001 | −.77 | .0003 | −.18 | .70 | −.80 | .10 |
| Lys | −.39 | .03 | −.46 | .07 | .02 | .59 | −.68 | .33 |
| NEAAs | ||||||||
| Asp | .36 | .06 | .31 | .23 | −.01 | .98 | .69 | .19 |
| Ser | −.38 | .0444 | −.35 | .17 | −.85 | .0164 | −.29 | .64 |
| Asn | −.06 | .76 | −.0017 | .99 | .00 | .99 | −.53 | .35 |
| Glu | −.24 | .21 | −.22 | .39 | .07 | .88 | −.77 | .13 |
| Gln | −.34 | .06 | −.57 | .0164 | .23 | .62 | −.24 | .69 |
| Pro | −.28 | .13 | −.34 | .18 | −.01 | .98 | −.23 | .70 |
| Gly | −.23 | .23 | −.19 | .45 | −.57 | .18 | −.13 | .83 |
| Ala | −.52 | .0042 | −.62 | .0073 | −.07 | .88 | −.85 | .06 |
| Cys | −.24 | .20 | −.35 | .17 | .03 | .94 | −.50 | .39 |
| Tyr | −.51 | .0049 | −.42 | .09 | −.42 | .34 | −.99 | .0017 |
| Arg | −.22 | .24 | −.44 | .07 | −.26 | .59 | .56 | .33 |
| TAAs | −.56 | .0015 | −.63 | .0063 | −.26 | .58 | −.68 | .19 |
| NEAAs | −.48 | .0082 | −.56 | .02 | −.23 | .62 | −.47 | .42 |
| EAAs | −.61 | .0004 | −.66 | .0037 | −.24 | .59 | −.92 | .0230 |
| BCAAs | −.53 | .0031 | −.52 | .0327 | −.24 | .61 | −.90 | .0328 |
Abbreviations: AAs, amino acids; BCAAs, branched-chain amino acids; EAAs, essential amino acids; NEAAs, nonessential amino acids; NS, nonsignificant; TAAs, total amino acids.
Pearson correlation coefficient test.
Figure 1.
Correlation between CDAI and valine, methionine, leucine, histidine and tryptophan. (A) The concentration of valine was significantly correlated with the CDAI score in patients with CD. (B) The concentration of methionine was significantly correlated with the CDAI score in patients with CD. (C) The concentration of leucine was significantly correlated with the CDAI score in patients with CD. (D) The concentration of histidine was significantly correlated with the CDAI score in patients with CD. (E) The concentration of tryptophan was significantly correlated with the CDAI score in patients with CD.
Ileo-colonic type
Significant correlations were noted between CDAI scores and concentrations of Val, His, Trp, Gln, Ala, TAA, EAA, and BCAA in patients with the ileo-colonic type of CD (Table 3). No significant correlations were observed between CDAI scores and concentrations of the other AAs.
Ileal type
Significant correlations were observed between CDAI scores and the concentration of Ser in patients with the ileal type of CD (Table 3). No significant correlations were observed between CDAI scores and concentrations of the other AAs.
Colonic type
In patients with the colonic type of CD, significant correlations were noted between CDAI scores and concentrations of Val, Leu, Phe, Tyr, EAA, and BCAA (Table 3). No significant correlations were observed between CDAI scores and concentrations of the other AAs.
Discussion
In this study, we analyzed the PFAAs profiles of patients with CD, with an emphasis on correlations with disease activity. In particular, concentrations of 5 EAAs—Val, Met, Leu, His, and Trp—were significantly correlated with CDAI scores in patients with CD, which would reflect the degree of inflammation (Figure 1A to E). These AAs belong to the EAAs, which are supplied in the diet. Furthermore, significant correlations were shown between CDAI scores and a certain proportion of EAAs and NEAAs in patients with CD. Nutritional deficiencies in patients with active CD would be the result of insufficient intake, malabsorption, and protein-losing enteropathy, as well as the metabolic disturbances induced by chronic disease.
In this study, Gln concentrations were correlated with CDAI in ileo-colonic CD. Gln plays a role in a variety of biochemical functions. Gln-enriched diets have been linked with the maintenance of gut barrier function and cell differentiation, suggesting that Gln may help protect the lining of the gastrointestinal mucosa.22 The mucosal content of Gln has been shown to be decreased in inflamed ileum and colon from patients with CD.23 It has also been shown that Gln reduces the production of pro-inflammatory cytokines (IL-8 and IL-6) and enhances the production of the anti-inflammatory cytokine IL-10 in patients with CD.17 In addition, it has been demonstrated that Gln supplementation improved outcomes in in vitro and in vivo experimental colitis models.24 Gln administration failed to produce obvious biochemical or clinical benefit in patients with active IBD,25 and according to a Cochrane Database System review, there is insufficient evidence to allow firm conclusions regarding the efficacy and safety of Gln for induction of remission in CD.26
In this study, the concentration of Trp was correlated with the CDAI score in patients with CD (Figure 1), especially those with the ileo-colonic type. Supplementation of L-Trp not only ameliorated clinical symptoms and increased weight gain but also improved histological scores and decreased the expression of pro-inflammatory cytokines in a porcine model of DSS colitis.27
In a rat model of DSS colitis, the administration of L-Tre, L-Pro, L-Cys, and L-Ser enhanced colonic protection and mucosal healing by increasing mucin synthesis and promoting gut microflora reequilibration.28 Concentrations of AAs changed depending on the degree of colitis in DSS-treated mice, and serum levels of Gln, Trp, Tyr, Asn, and Gly were significantly lower than in control mice.29 In patients with CD, the levels of Ala, Asp, Gly, Met, and Pro were significantly decreased in comparison with those of healthy volunteers.30 Active CD is associated with depression of serum Trp.31 However, AAs were not significantly different between ileo-colonic, ileal, and colonic type of CD in this study under the nutrition guidance of the dietician.
It has been shown that patients with ulcerative colitis displayed increased concentrations of 3-hydroxybutyrate, glucose, and Phe and decreased concentrations of lipids in serum.32 In an IL-10 (−/−) cell transfer model of colitis recent studies, His prevented IL-8 secretion by intestinal epithelial cells33 and reduced colitis lesions.6 Trp metabolism has recently been highlighted as an immunological regulator,34–36 and supplementation of His and Trp has been suggested as a therapeutic strategy for IBD.21 The AA-based elemental diets would be useful by virtue of their low antigenicity,37 resulting in the reduction in mucosal cytokine production such as IL-1, IL-6, IL-8, and TNF-α.38 Then, disease activity and nutritional status were improved, particularly effective for poorly nourished with CD after administration of AAs, increases in the concentrations of His, Trp, and other plasma AAs in the remission group, whereas no increase in the level of AAs in the nonremission group.39 Beneficial action of mucosal immunity from AAs in CD would be the ability to stimulate the integrity of the intestinal mucosal barrier and promote the restoration of the inflammatory reaction.40
There are several limitations in this study. First, we could not determine AA profiles specific to patients with CD because we did not enroll healthy subjects as controls. However, we believe that our data would aid in provisional supplementation for patients with active CD. Future research should evaluate the efficacy of the supplementation of the AA specifically depleted in active CD. Second, the low number of participants per study group was enrolled in this study; a threat to statistical validity would be worried. Third, most of the patients were treated with anti TNF-α agents in this study, a future study should investigate the AA profiles before and after administration of anti–TNF-α agents in patients with CD.
In conclusion, plasma concentrations of some AAs such as Val, Met, Leu, His, and Trp are closely associated with disease activity in patients with CD.
Acknowledgments
A portion of this manuscript was presented at the Annual Meeting of the Japanese Society of Gastroenterology, 2012 and JDDW 2014.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: TC and KS designed the study, TC collected samples and analyzed the data. All authors were involved in drafting and revision of the manuscript and have approved the final manuscript for submission.
References
- 1. Hanauer SB, Feagan BG, Lichtenstein GR, et al. ; ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. [DOI] [PubMed] [Google Scholar]
- 2. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroentrol Hepatol. 2006;4:621–630. [DOI] [PubMed] [Google Scholar]
- 3. Coeffier M, Marion-Letellier R, Dechelotte P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:518–524. [DOI] [PubMed] [Google Scholar]
- 4. Coeffier M, Dechelotte P. The role of glutamine in intensive care unit patients: mechanisms of action and clinical outcome. Nutr Rev. 2005;63:65–69. [DOI] [PubMed] [Google Scholar]
- 5. Wu G, Morris SM., Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andou A, Hisamatsu T, Okamoto S, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–574. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease—epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112. [DOI] [PubMed] [Google Scholar]
- 8. Matsui T, Sakurai T, Yao T. Nutritional therapy for Crohn’s disease in Japan. J Gastroenterol. 2005;40:25–31. [DOI] [PubMed] [Google Scholar]
- 9. Ameho CK, Adjei AA, Harrison EK, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Israeli E, Berenshtein E, Wengrower D, et al. Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci. 2004;49:1705–1712. [DOI] [PubMed] [Google Scholar]
- 11. Giriş M, Erbil Y, Dogru-Abbasoglu S, et al. The effect of heme oxygenase-1 induction by glutamine on TNBS-induced colitis. The effect of glutamine on TNBS colitis. Int J Colorectal Dis. 2007;22:591–599. [DOI] [PubMed] [Google Scholar]
- 12. Vicario M, Amat C, Rivero M, et al. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr. 2007;137:1931–1937. [DOI] [PubMed] [Google Scholar]
- 13. Arndt H, Kullmann F, Reuss F, et al. Glutamine attenuates leukocyte-endothelial cell adhesion in indomethacin-induced intestinal inflammation in the rat. J Parenteral Enteral Nutr. 1999;23:12–18. [DOI] [PubMed] [Google Scholar]
- 14. Basivireddy J, Jakob M, Balasubramanian KA. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci. 2004;107:281–289. [DOI] [PubMed] [Google Scholar]
- 15. Fillmann H, Kretzmann NA, San-Miguel B, et al. Glutamine inhibits over-expression of pro-inflammatory genes and down-regulates the nuclear factor kappaB pathway in an experimental model of colitis in the rat. Toxicology. 2007;236:217–226. [DOI] [PubMed] [Google Scholar]
- 16. Grimble RF. Nutritional modulation of immune function. Froc Nutr Soc. 2001;60:389–397. [DOI] [PubMed] [Google Scholar]
- 17. Lecleire S, Hassan A, Marion-Letellier R, et al. Combined glutamine and arginine decrease proinflammatory cytokine production by biopsies from Crohn’s patients in association with changes in nuclear factor-kappaB and p38 mitogen-activated protein kinase pathways. J Nutr. 2008;138:2481–2486. [DOI] [PubMed] [Google Scholar]
- 18. Tsune I, Ikejima K, Hirose M, et al. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775–785. [DOI] [PubMed] [Google Scholar]
- 19. Hisamatsu T, Okamoto S, Hashimoto M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE. 2012;7:e31131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National cooperative Crohn’s disease study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 21. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–530. [DOI] [PubMed] [Google Scholar]
- 22. Jiang ZM, Cao JD, Zhu XG, et al. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double-blind, controlled study of 120 patients. J Parenteral Enteral Nutr. 1999;23:S62–S66. [DOI] [PubMed] [Google Scholar]
- 23. Sido B, Seel C, Hochlehnert A, et al. Low intestinal glutamine level and low glutaminase activity in Crohn’s disease: a rational for glutamine supplementation? Dig Dis Sci. 2006;51:2170–2179. [DOI] [PubMed] [Google Scholar]
- 24. Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. J Parenteral Enteral Nutr. 2011;35:188–197. [DOI] [PubMed] [Google Scholar]
- 25. Ockenga J, Borchert K, Stüber E, et al. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur J Clin Nutr. 2005;59:1302–1309. [DOI] [PubMed] [Google Scholar]
- 26. Akobeng AK1, Elawad M, Gordon M. Glutamine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;2:CD007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim CJ, Kovacs-Nolan JA, Yang C, et al. l-Tryptophan exhibits therapeutic function in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J Nutr Biochem. 2010;21:468–475. [DOI] [PubMed] [Google Scholar]
- 28. Faure M, Mettraux C, Moennoz D, et al. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr. 2006;136:1558–1564. [DOI] [PubMed] [Google Scholar]
- 29. Shiomi Y, Nishiumi S, Ooi M, et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis. 2011;17:2261–2274. [DOI] [PubMed] [Google Scholar]
- 30. Ooi M, Nishiumi S, Yoshie T, et al. GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm Res. 2011;60:831–840. [DOI] [PubMed] [Google Scholar]
- 31. Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Y, Lin L, Xu Y, et al. 1H NMR-based spectroscopy detects metabolic alterations in serum of patients with early-stage ulcerative colitis. Biochem Biophys Res Commun. 2013;433:547–551. [DOI] [PubMed] [Google Scholar]
- 33. Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005;579:4671–4677. [DOI] [PubMed] [Google Scholar]
- 34. Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. [DOI] [PubMed] [Google Scholar]
- 35. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. [DOI] [PubMed] [Google Scholar]
- 36. Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. [DOI] [PubMed] [Google Scholar]
- 37. Fernández-Banares F, Cabré E, Esteve-Comas M, et al. How effective is enteral nutrition in inducing clinical remission in active Crohn’s disease? A meta-analysis of the randomized clinical trials. J Parenteral Enteral Nutr. 1995;19:356–364. [DOI] [PubMed] [Google Scholar]
- 38. Yamamoto T, Nakahigashi M, Umegae S, et al. Impact of elemental diet on mucosal inflammation in patients with active Crohn’s disease: cytokine production and endoscopic and histological findings. Inflamm Bowel Dis. 2005;11:580–588. [DOI] [PubMed] [Google Scholar]
- 39. Nakano M, Tominaga K, Hoshino A, et al. Therapeutic efficacy of an elemental diet for patients with Crohn’s disease and its association with amino acid metabolism. Saudi J Gastroenterol. 2017;23:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rowlands BJ, Gardiner KR. Nutritional modulation of gut inflammation. Proc Nutr Soc. 1998;57:395–401. [DOI] [PubMed] [Google Scholar]