Abstract
So far, intravenous tissue-type plasminogen activator (tPA) and mechanical removal of arterial blood clot (thrombectomy) are the only available treatments for acute ischemic stroke. However, the short therapeutic window and the lack of specialized stroke unit care make the overall availability of both treatments limited. Additional agents to combine with tPA administration or thrombectomy to enhance efficacy and improve outcomes associated with stroke are needed. Stroke-induced inflammatory processes are a response to the tissue damage due to the absence of blood supply but have been proposed also as key contributors to all the stages of the ischemic stroke pathophysiology. Despite promising results in experimental studies, inflammation-modulating treatments have not yet been translated successfully into the clinical setting. This review will (a) describe the timing of the stroke immune pathophysiology; (b) detail the immune responses to stroke sift-through cell type; and (c) discuss the pitfalls on the translation from experimental studies to clinical trials testing the therapeutic pertinence of immune modulators.
Keywords: clinical trial, immunomodulatory drugs, inflammation, stroke models, translation
Introduction
Every year, 15 million people worldwide suffer a stroke. Of these, 5 million die and another 5 million are left permanently disabled, placing a burden on family and community (http://www.who.int/cardiovascular_diseases/en/cvd_atlas_15_burden_stroke.pdf?ua=1). In the United States a stroke event happens every 40 seconds, and every 4 minutes, someone dies of stroke (American Stroke Association). Ischemic stroke is provoked by an arterial occlusion in the brain that leads to the rapid death of the brain tissue irrigated by that particular artery. So far, intravenous tissue-type plasminogen activator (tPA) is the only available pharmacological agent for acute ischemic stroke, but this agent is frequently underutilized due to its limited therapeutic window (4.5 h) and increased risk of intracerebral hemorrhage. Recently, mechanical thrombectomy has demonstrated beneficial effects on ischemic stroke in selected patients1 and has become the standard of care for patients with large-vessel occlusion up to 24 h of stroke onset.2,3 Additional agents to combine with tPA administration or thrombectomy to improve efficacy and ameliorate outcomes associated with stroke are needed, in particular, for those patients not eligible for thrombolysis or thrombectomy, or with no access to specialized stroke unit care. Easily administrable agents reducing tissue damage even modestly would drastically decrease the burden of stroke on society and would ameliorate patient outcomes and quality of life.4
Stroke-induced inflammatory processes, which include mechanisms of innate and adaptive immunity, are a response to tissue damage due to the absence of blood supply but have also been proposed as key contributors to all the stages of the ischemic stroke pathophysiology.5 However, despite promising results in experimental studies, inflammation-modulating treatments have not yet been translated successfully into the clinical setting. This review will focus on the innate and adaptive immune responses participating in ischemic brain injury and their impact on tissue damage and repair in both experimental models of stroke and available clinical data. It has been structured in three parts: a first part describing the timing of the stroke pathophysiology from an immunological point of view (Figure 1); a second part detailing the immune responses to stroke sift-through cell type; and a third part discussing the pitfalls on the translation from preclinical to clinical stroke research of immunomodulating therapeutic agents.
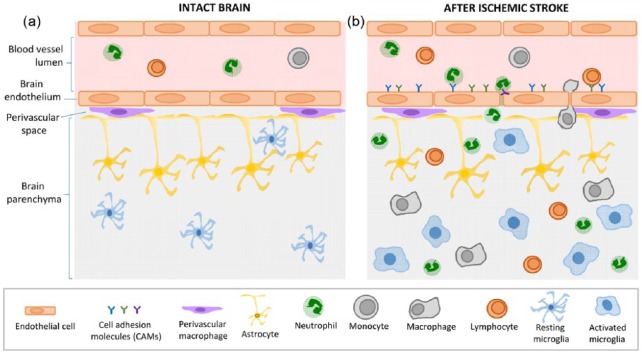
Figure 1.
Immune reactions after ischemic stroke.
(a) In the intact brain, the functional unit formed by tightly jointed ECs and astrocytic endfeet constitute the BBB, a strong protective blood–tissue barrier from pathogens. Immune cells circulate freely in the blood, and in the brain parenchyma, resting microglia survey the environment with their processes. (b) A few minutes after stroke onset, the BBB is disrupted and local ECs are activated. The tight junctions between ECs disappear and activated ECs express CAMs. This allows white cell rolling and adhesion at the luminal side of the blood vessel and then transmigration from the vascular compartment to the brain parenchyma. Once infiltrated in the tissue, neutrophils secrete pro-inflammatory factors that will recruit monocytes/macrophages, and later lymphocytes to the parenchyma. After stroke, microglia switches from a resting form to an activated state, adopting a phagocytic phenotype and secreting pro-inflammatory factors.
BBB, blood–brain barrier; CAM, cellular adhesion molecule; EC, endothelial cell.
Timeline of inflammatory events after stroke
Basically, inflammatory responses after stroke can be decomposed in three phases.5 The acute phase (first hours after stroke onset) corresponds to the clearing of dead cells mainly by resident phagocytic cells such as microglia/macrophages and a first entry of leukocytes, mainly neutrophils. The subacute phase (first days after stroke) corresponds to resolution of inflammation. The late phase (days and weeks after stroke) corresponds to tissue repair by astrocytes and microglia (glial scar).
The acute phase of stroke: tissue injury, microglial and endothelial activation
The arterial occlusion at the origin of ischemic stroke leads to deprivation of oxygen and nutrients that are essential for neuronal survival, leading to rapid neuronal death in the core of the lesion site only minutes after stroke onset. The absence of blood supply into the tissue is followed by a cascade of events starting with a reduction in cellular adenosine triphosphate (ATP) that is required to maintain ionic gradients. The disruption of ionic gradients leads to an influx of Na+ and Ca2+, cellular depolarization and release of neurotransmitters, including the excitatory neurotransmitter glutamate.6 As energy-dependent removal of glutamate is impaired, glutamate accumulation leads to overactivation and opening of monovalent ion channels followed by water influx, thus resulting in cellular swelling and death.7 Dying neurons at the core of the lesion express free radicals, damage-associated molecular patterns (DAMPs) and high-mobility group box 1 (HMGB1) protein that lead to inflammatory reactivity by microglia. Edaravone, a free radical scavenger, has shown beneficial properties in stroke patients with large-vessel occlusion, especially when combined with recombinant tissue-type plasminogen activator, but not in combination with thrombectomy.8 Activated microglia adopt a pro-inflammatory status, expressing pro-inflammatory cytokines [interleukin 1 (IL-1), IL-6] that are released into blood circulation.9
Pro-inflammatory cytokines exacerbate endothelial cell (EC) activation contributing to leukocyte rolling, adhesion and infiltration in brain tissue. Leukocytes start to roll on the vessel wall with the help of selectins expressed by activated EC. After rolling, leukocytes strongly adhere to the vessel wall by strong links with intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1).10,11 Into the brain, there is almost no way for leukocytes to pass through the blood–brain barrier (BBB) in nonpathological conditions.12 However, the BBB integrity is disrupted after stroke onset and tight junctions between EC disappear, allowing leukocyte infiltration into the injured brain tissue.13 Clinical data and experimental models of stroke have shown that neutrophils infiltrate within hours of stroke onset.14,15
The subacute phase: resolution of inflammation
In the first days after stroke, different leukocyte populations, including macrophages and lymphocytes, infiltrate the brain.14,15 The infiltration of leukocyte subpopulations differs among murine permanent and transient mechanical focal cerebral ischemia models16 and the inflammatory response seems to be more pronounced after permanent electrocoagulatory middle cerebral artery occlusion (MCAO) compared with 30-min and 90-min transient mechanical vascular occlusion (TMVO).16 Descriptive data on the timeline inflammatory responses in thrombus-induced experimental stroke models are missing.
Preclinical studies performed on TMVO models of ischemic stroke have shown a secondary injury after reperfusion (i.e. cerebral ischemia/reperfusion injury). While mechanical thrombectomy also promotes rapid reperfusion, it does not seem to provoke such a secondary injury in patients with proximal middle-cerebral-artery or internal-carotid-artery occlusion and a penumbral region of tissue (i.e. a brain region that is ischemic but not infarcted yet, which is therefore salvageable). This is probably due to a better collateral circulation and slower infarct growth in these patients and is associated to a higher proportion of good functional outcomes at 3 months after stroke, compared with patients with little or no penumbra, even when performed late (16–24 h) after stroke onset.2,3,17 In the DEFUSE 2 study, patients with little or no penumbra had a greater lesion growth despite reperfusion. This greater lesion growth could be the result of reperfusion-related edema based on the larger infarct core volumes in the no penumbra group.17
The late phase: tissue repair and glial scar
Neuroinflammation is also considered necessary for the reparation phase that persists after the initial brain insult.18–20 This phase aims at restoring tissue integrity and involves matrix remodeling, neurogenesis, axon sprouting, dendritogenesis and oligodendrogenesis.21 All these processes are common to acute brain injuries (including ischemia, hemorrhage, and trauma), neuroinflammatory (including multiple sclerosis) and neurodegenerative disorders,22 that initiate an extensive glial response known as reactive gliosis. Reactive gliosis involves an enhanced expression of specific markers, such as various extracellular matrix molecules (ECM) like chondroitin sulfate proteoglycans (CSPG) and glial fibrillary acidic protein (GFAP) for astrocytes. Glial scar formation is crucial for sealing the lesion site to remodel the injured tissue, in order to spatially and temporally control the local immune response and revascularization of blood capillaries. Nonetheless, the glial scar also acts as an obstacle to axon regeneration and thus avoids the recovery of central nervous system (CNS) function in the chronic phase after stroke. Experimental studies on the glial response to ischemic stroke have been performed in animal models using variations of permanent or focal transient MCAO and photothrombosis.23–25 The human brain after ischemic injury seems to share similar properties to these experimental models of stroke.26
Immunomodulating therapeutic strategies for stroke: sift-through cell type
Clinical trials on stroke immunology have targeted both innate and adaptive immune responses, by reducing microglial activation, inhibiting leukocyte or lymphocyte migration to the brain parenchyma, and blocking the IL-1 receptor. The increasing number of immunomodulatory treatments that are already established for other indications in humans have provided a good opportunity to fast-track innovative proof-of-concept trials in stroke. However, and despite promising preclinical results, immunomodulatory drugs have not shown clear benefits in large clinical trials on stroke. Another strategy has been the induction of hypothermia to reduce inflammatory responses and apoptosis triggered after stroke (among other mechanisms). Several trials have shown its safety and feasibility, alone or in combination with thrombolysis;27–30 however, its efficacy in the treatment of ischemic stroke is still debatable, especially because of increased pneumonia incidence and mortality in the hypothermia group.31 New clinical trials on the effects of the combination of thrombolysis/thrombectomy plus hypothermia are ongoing.32 Figure 2 summarizes the results of clinical trials targeting the immune responses triggered after ischemic stroke.
Figure 2.
Summary of clinical trials targeting the immune responses triggered after ischemic stroke.
CD11/CD18, LFA-1 for lymphocyte function-associated antigen 1; IL-1, interleukin 1; rhIL1-Ra, recombinant human interleukin 1 receptor antagonist; ICAM-1, intercellular adhesion molecule; VCAM-1, vascular cell adhesion molecule; VLA-4, leukocyte very late antigen-4.
Microglial cells
Pathophysiology
Microglial cells constitute 10–15% of the brain cells and are the resident mononuclear phagocytes of the brain parenchyma.33 As aforementioned, microglial response is one of the first steps of the innate immune responses triggered after stroke. Microglial cells are in close contact with neurons and constantly survey the environment with their processes.34 These cells can adopt a large spectrum of phenotypes ranging from pro-inflammatory to anti-inflammatory and neuroprotective. At the acute phase after stroke onset, microglial cells at the core of the lesion detect DAMPs and HMGB1 via their receptors mainly belonging to the Toll-like receptor (TLR) family.13,35–37 This ligand–receptor link leads to internalization of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor (IκB) kinase into the nucleus and the subsequent activation of the nuclear factor of kappa light chain enhancer of activated B-cells (NF-κB) pathway and thus to the activation of microglial cells. Also, loss of the constitutively expressed neuronal ligand CX3CL1 (fractalkine) after neuronal death results in enhanced microglial activation through their receptors CX3CR1.38 Experimental models of both transient and permanent ischemic stroke have shown an increase in the number of microglial cells on the ipsilateral cortex in the first 24 h after stroke onset.14,16 Once activated, microglial cells express high levels of CD11b, CD45 and CD68 corresponding to a phagocytic phenotype responsible for the clearance of cellular debris,9 as well as tumor necrosis factor and the pro-inflammatory interleukins IL-1 and IL-6 that are released into blood circulation.9 IL-1 has been targeted to reduce further cerebral injury mediated by inflammation. A meta-analysis showed that IL-1 receptor antagonist (IL-1Ra) administration was associated with a 38.2 % reduction in mean infarct volume across 16 published preclinical studies.39 In a phase II clinical study, recombinant human IL-1Ra (rhIL-1Ra; anakinra) administered at hospital arrival has shown beneficial effects in patients with cortical infarcts, with better clinical outcomes after 3 months in the treated group.40 However, the recently published results of a second phase II study on the effect of subcutaneous IL-1Ra administration (SCIL-STROKE) has shown that, in spite of a significant reduction in plasma inflammatory markers associated with a worse outcome after ischemic stroke, IL-1Ra treatment was not associated with a favorable outcome on modified Rankin Scale (mRS).41
Recently developed techniques of total microglial depletion have shown that microglia seem essential to the limitation of stroke damages, since microglia-depleted mice show larger infarct volumes than nondepleted mice.42
Therapeutic strategies targeting microglia
Minocycline, an anti-infective agent of the tetracycline family used for the treatment of infections caused by a wide range of organisms, has been tested for the treatment of stroke. It is highly lipophilic and crosses the blood–brain barrier. Minocycline, by acting through different pathways, is able to inhibit microglial activation, decrease migration of T cells, reduce neuronal apoptosis, block free radical production, decrease CNS expression of chemokines and their associated receptors, and inhibit matrix metalloproteinases, particulary matrix metalloproteinase-9.43–58 In preclinical stroke studies, minocycline ameliorated behavioral function and decreased lesion volume and hemorrhagic transformation.44–47 It did not alter the fibrinolytic effect of rtPA46 and could enlarge the time window for thrombolysis.49 Two phase II clinical trials have shown minocycline to be safe and potentially effective in acute ischemic stroke, alone or in combination with tPA, when administered in the first 24 h after stroke onset and for 5 days.59,60 However, a third pilot study performed in a small sample of acute stroke patients has shown that minocycline administration in the first 24 h was safe but not efficacious.43 The authors acknowledged that this third study was not powered to identify reliably or exclude a modest but clinically important treatment effect of minocycline.43 Larger clinical trials are thus needed to study the effect of minocycline in stroke patients.
Recently, preclinical studies have focused also on the switch of microglial cell phenotype from M1 to M2 as a potential target to improve stroke outcome, by administering IL-3361 or IL-4.62
Endothelial cells
Pathophysiology
In the brain, the functional unit formed by tightly jointed ECs and astrocytic endfeet constitute the BBB, a strong protective blood–tissue barrier against pathogens. A few minutes after stroke onset, the BBB is disrupted, and local ECs are activated. 63,64 When activated, ECs express cellular adhesion molecules (CAMs) which allow leukocyte rolling and adhesion to the luminal side of the EC and eventually leukocyte transmigration toward the brain parenchyma.5 After their activation, the first adhesion molecules expressed by ECs are the selectins (E- and P-selectins). They are constitutively produced by ECs and stored in Weibel-Palade bodies until EC activation. The initial adhesive interactions between leukocytes and selectins expressed on the luminal side of the venular endothelium are tethering (capture) and rolling. These low-affinity (weak) interactions are subsequently strengthened as a result of the sequential activation of different families of adhesion molecules that are located on the surface of leukocytes and ECs.65 A few hours after stroke onset, ECs start to express the VCAM-1 and the ICAM-1,63,66 allowing leukocytes to adhere and remain stationary on the vessel wall. Finally, ECs express the platelet endothelial cell adhesion molecule (PECAM-1), responsible for leukocyte transmigration into the brain parenchyma.11
Therapeutic strategies targeting selectins
Strategies for the blockade of CAMs through the intravenous injection of antibodies have failed in ameliorating stroke outcome both in clinical and preclinical trials.
Enlimomab, a murine ICAM-1 antibody, reduces leukocyte adhesion and infarct size in experimental stroke studies.67 However, the clinical trial performed in stroke patients showed that the administration of enlimomab within 6 h after stroke onset is not an effective treatment for ischemic stroke and, indeed, significantly worsened stroke outcome and increased adverse events.68 One of the possible explanations for this failure is the murine origin of the injected antibody to stroke patients.69
Induction of mucosal tolerance to E-selectin to minimize inflammation and risk of further cerebral insult has been tested in a phase I clinical trial, but results have not been disclosed.70
L-selectin, a CAM located in the surface of leukocytes, has also been targeted in an experimental stroke model in rabbits by using a humanized monoclonal antibody (HuDREG200). This strategy, alone or in combination with alteplase, failed to significantly ameliorate stroke outcome.71
Neutrophils
Pathophysiology
The principal role of neutrophils is to enhance leukocyte recruitment by degranulation of their content, rich in cytokines/chemokines, proteolytic enzymes and activated-complement system.72 The extent of neutrophil infiltration after stroke is still debated, being described either limited to the perivascular space or infiltrating the brain tissue in preclinical stroke models (permanent or mechanical transient occlusion of the MCA) and human postmortem samples.73–75
It was postulated that neutrophils could contribute to brain injury after ischemic stroke by obstructing microvessel circulation, damaging endothelial cells and ECM by hydrolytic enzymes and free radicals, promoting intravascular thrombus formation together with platelet activation, and releasing cytokines and chemotactic factors that could promote extension of the inflammatory response.76
Therapeutic strategies targeting neutrophils
Preclinical and clinical studies aiming at blocking neutrophil infiltration have targeted CD11/CD18 (LFA-1 for lymphocyte function-associated antigen 1), located at the surface of neutrophils, to prevent their adhesion to ICAM-1 (expressed at the luminal surface of ECs). Preclinical studies have shown that CD11/CD18 monoclonal antibodies are not beneficial in permanent stroke models,77,78 but are beneficial on a transient mechanical ischemic stroke model.79 In spite of this, two clinical trials have been performed to prevent neutrophil recruitment after stroke onset and have shown no beneficial effect. On the HALT phase III trial, the humanized antibody Hu23F2G, administered twice daily, appeared to produce an improvement in mRS score in a phase II study. However, the sponsor terminated a subsequent phase III study when a ‘futility analysis’ advised that no benefit of the treatment would occur if the study was completed. No public information regarding the database, outcomes, safety issues, or relative number of adverse events has appeared.76 On the ASTIN (Acute Stroke Therapy by Inhibition of Neutrophils) study, UK-279,276 (another CD18 antagonist) was administered within 6 h of stroke symptom onset, but, similar to the HALT study, the trial was stopped early due to futility.80
Lymphocytes
Pathophysiology
Adaptive immunity also plays an important role during stroke. Lymphocytes consist of distinct subpopulations with diverse functions and can be subdivided into two groups: pro- and anti-inflammatory lymphocytes. The consequences of the adaptive immune response on ischemic stroke are still debated, as both beneficial and deleterious results have been reported depending on the type of T-helper (Th) immune response set in motion after the activation of lymphocytes.81–83 Both transient (mechanical) and permanent experimental models of stroke have shown that lymphocyte infiltration into the ischemic tissue comes later after stroke onset (starting at 3 days).14,16 Postmortem human samples have shown that lymphocyte infiltration into the ischemic area occur from day 3 and can be present up to 53 years after stroke.15
The choroid plexuses (CP) have recently been proposed as the preferential route for lymphocyte infiltration into the lesion site after experimental stroke. Indeed, CP infarction reduces lymphocyte infiltration after stroke onset; however, the ischemic volume is not modified in these mice, even if lymphocyte infiltration is actually reduced.84 These intriguing results raise important questions about the role of lymphocytes on the development of the ischemic lesion.
Therapeutic strategies targeting lymphocytes
Therapeutic strategies targeting lymphocytes would be desirable because they focus the delayed phase of the injury and thus could have a particularly wide therapeutic window. Several preclinical studies have shown that pro-inflammatory lymphocytes, such as TH1, TH17, and γδ T cells worsen stroke outcome, and that blocking of their brain invasion is neuroprotective.85–87
Contrary to pro-inflammatory lymphocytes, regulatory T cells (Treg) and B cells have been characterized as anti-inflammatory and as disease-limiting protective cells. Targeting Tregs as the endogenous orchestrators of the postischemic immune response has been proposed as a more effective therapy than blocking only a particular inflammatory pathway.5,13 However, because of the complex function of regulatory cells in immune homeostasis and disease, as well as partially divergent findings using different stroke models, the pathophysiologic function of regulatory lymphocytes in stroke remains uncertain. As an example, among nine studies using Treg-depletion paradigms, three of the studies revealed an increase in infarct volume88–90 whereas five studies did not detect any effect on stroke outcome88,89,91–93 and one study even observed a reduction of infarct size in Treg-deficient mice.94 To explain this, the experimental model used (permanent versus transient mechanical) and more particularly, the resulting volume of the ischemic lesion, has been proposed as a determinant on the overall effect of Treg depletion that seems to provide a benefit only on small lesion volumes provoked on the permanent stroke model.95
Another strategy for Treg modulation has been the administration of enhancers of Treg function by adoptive cell transfer of purified Treg to wild-type animals to increase circulating Treg numbers, or by the administration of a CD28 superagonist (CD28SA), which provokes in vivo expansion of Tregs and amplification of their suppressive function. However, the obtained results are again controversial, with some studies describing an improvement of stroke outcome96 and other studies describing an increase of ischemic volume.97 Once again, it has been proposed that stroke severity might predict the net biological effect of Tregs.95
It has been proposed that Tregs have a deleterious role on ischemic stroke not related to their established immunoregulatory characteristics but to a specific effect on microvascular thrombus formation in a model of transient mechanical occlusion.94 Secondary microthrombosis arises within minutes after reperfusion in transient mechanical models of stroke once the occluding filament has been removed. It provokes delayed cerebral blood flow (CBF) reduction, secondary ischemia and lesion growth, which is responsible for ~70% of the final ischemic lesion size.98 However, clinical studies have shown that secondary ischemia and lesion growth are modest or nonexistent after reperfusion in selected patients,2,17,99,100 and concerns have been raised about the clinical relevance of transient mechanical vascular occlusion stroke models.101 For these reasons, it would be interesting to test the role of Tregs in nonmechanical models of ischemic stroke, where secondary microthrombosis is absent.102
To increase the complexity of this question, fingolimod (FTY720), an immunomodulatory drug currently approved by the US Food and Drug Administration for the treatment of multiple sclerosis that inhibits lymphocyte circulation and brain immigration, has been tested in models of permanent and transient mechanical ischemia. As in the case of the aforementioned CP infarction,84 although a reduction of lymphocyte brain invasion was detected using fingolimod, no effect was observed on infarct volumes and behavioral dysfunction in any of the models. This lack of neuroprotection despite effective lymphopenia was attributed to a divergent impact of fingolimod on cytokine expression and possible activation of innate immune cells after brain ischemia.86 A phase II clinical trial using fingolimod (administered within 6 h after the onset of symptoms) combined with alteplase and mechanical thrombectomy on ischemic stroke (FAMTAIS) is ongoing.103
Two clinical pilot studies have tested fingolimod, alone and later than 4.5 h after stroke onset for 3 days,104 or in combination with tPA (thus before 4.5 h after stroke onset) for 3 days.105 These two studies have shown a beneficial effect of the oral administration of fingolimod within 72 h of stroke onset, especially when combined with tPA, where patients who received the combination exhibited smaller lesion volumes, less hemorrhage, and a better recovery at day 90.
Therapeutic strategies targeting global leukocyte infiltration
The transendothelial migration of leukocytes through the interaction between the molecules VLA-4 (leukocyte very late antigen-4) (present at the surface of neutrophils, monocytes, and T and B lymphocytes among other blood cell types) and VCAM-1 (vascular cell adhesion molecule-1) has been targeted in several studies. Antibodies against the alpha chain of VLA-4 (anti-CD49d antibodies) have shown efficacy in several models of autoimmune diseases, and the humanized antibody natalizumab is currently one of the most effective therapies for patients with multiple sclerosis.106 However, preclinical data have shown conflicting results from four positive and one inconclusive studies on the use of anti-CD49d antibodies on ischemic stroke.86,107–110 Recently, a preclinical randomized controlled multicenter trial highlighted the importance of testing new therapies in different experimental stroke models (discussed further on), since the administration of CD49d-antibodies reduced both leukocyte invasion and infarct volume after the permanent distal occlusion of the MCA (which causes a small cortical infarction), whereas it did not reduce leukocyte invasion or infarct volume after transient proximal mechanical occlusion of the MCA (which induces large lesions).111 These results could suggest that the benefits of immune-targeted therapies may be dependent on infarct localization and severity.
The recently published results of the administration of one dose of natalizumab in patients with acute ischemic stroke (ACTION trial) have shown that natalizumab administered up to 9 h after stroke onset did not reduce infarct growth (primary endpoint of the study), but more patients in the natalizumab group than in the placebo group had mRS scores of 0 or 1 at day 30, although this beneficial effect disappeared 90 days after stroke.112 These results reinforce the need of new studies to understand (a) how natalizumab exerts a positive effect on functional outcome without reducing the infarct volume at 30 days, and (b) why this beneficial effect on functional outcome is lost at 90 days. Additionally, the ACTION II trial has been recently completed. Its primary objective was to assess the effects of natalizumab on functional independence and activities of daily living.113 Results have not been published yet.
Immunomodulating therapies in stroke: a gap between our expectations and reality
The reasons for the failure of immunomodulatory therapies in clinical trials are multifactorial. As discussed, one of the main issues of the bench-to-bed translation of immunomodulatory therapies for stroke is the huge gap between human pathophysiology and the stroke models that have mainly been used to study stroke immunology. In experimental stroke models, preclinical stroke research nowadays disposes of a wide range of experimental models including permanent and transient arterial occlusions. In addition to this, the origin of the occlusion may also vary from a transient mechanical occlusion to real blood clots located in the lumen of the artery. In the case of clot-induced stroke models, researchers can even adopt different strategies to determine the nature of clots (fibrin-rich or platelet-rich) and, consequently, clot susceptibility to thrombolytic agents114–116 to mimic the heterogeneity of human thrombi subtypes. It is important to note that despite the variety of available stroke models, the vast majority of preclinical data on transient models of ischemic stroke has been obtained on mechanical occlusion models that provoke thromboinflammation and secondary microthrombosis. This secondary microthrombosis is a major pathophysiologic mechanism leading to brain damage which does not occur in other models based on clot-induced ischemic stroke like the thrombin-induced stroke model.102,116 As in the aforementioned case of Treg-targeted drugs, immunomodulatory agents that have clearly shown beneficial effects on mechanical occlusion models of stroke could actually be targeting thromboinflammation and secondary microthrombosis. Since it is unclear whether thromboinflammation is a universal pathophysiological mechanism of human stroke, immunomodulatory drugs deserve to be evaluated or re-evaluated in experimental stroke models not inducing secondary thromboinflammation before trying to apply them in clinical practice.
Other reasons for the failure in the translation of immunomodulatory therapies from bench to bedside may include poorly designed preclinical and clinical studies, and underpowered clinical trials with overambitious and pathophysiologically irrelevant therapeutic windows.117,118 In addition, a biased selection of substances for clinical testing may partially explain the slow progress in developing treatments.118
It is also possible that the positive results seen with immunomodulatory drugs in experimental studies are not transposable to the complexity and the variability of the immunologic response in human stroke, which may depend on different parameters including prestroke status of the patient and the natural history of the disease.63,93 In most animal studies, complete occlusion is induced and is either maintained or followed by complete reperfusion. In human stroke, however, only a few patients are likely to experience both complete occlusion and complete reperfusion (excepting thrombectomized patients). The unlimited variety of outcomes seen in stroke patients suggests that stroke itself, as well as the body’s response to ischemic stroke represents a seamless continuum of exigencies rather than the definitive scenario employed in animal studies.68 In addition, stroke risk factors such as diet, smoking, aging, atherosclerosis, hypertension, alcohol consumption and stress cannot be replicated well in the laboratory; actually, most of the preclinical studies are performed in healthy mice. Moreover, although stroke remains the third cause of death in women,119 most of the preclinical studies are performed on male animals. Inflammatory cells such as microglia, dendritic cells, neutrophils and lymphocytes show variations between sex, and so may play different roles in the pathophysiology of stroke.120–123
The failure of immunomodulatory drugs clinically tested on stroke to date may also indicate that the inflammatory response after stroke is an important aspect of the regenerative process triggered after stroke and only becomes detrimental when exaggerated, perhaps in relation to the severity of ischemic injury (as shown in preclinical stroke models) or to concomitant disease states or risk factors. However, many of the preclinical studies examining immunomodulation were targeted at the acute phase, not during the phases where recovery is expected to occur. Thus, this hypothesis needs to be addressed in future studies.
Another important element to be considered when testing immunomodulatory drugs for stroke is that postischemic systemic immunomodulation and infectious complications are some of the main comorbidities after stroke. In experimental models, the systemic immune responses differ substantially among stroke models and infarct volume.89,124 Translational studies of immunomodulatory therapies for stroke must account for this heterogeneity, especially in the case of drugs with long-term effects such as natalizumab, that blocks the α-4 integrin for at least 4 weeks.125
Conclusion
Factors contributing to the failure in the translation of therapies targeting stroke-induced immune responses to clinical practice include nonmodifiable factors (differences in the cerebrovascular system in humans and rodents, variability in the immune response in patients depending on their prestroke inflammatory status, which is extremely difficult to mimic in animal models of stroke…) that need to be considered when interpreting results. However, most of these factors are modifiable and include, among others, the use of clinically relevant stroke models and therapeutic time windows, the comparison of a same molecule in different stroke models, the improvement in the experimental designs and the inclusion of females and comorbidities in preclinical studies.
Now that we are in the age of acute revascularization, one might wonder if we can do better than thrombectomy for patients with penumbra. If the answer is ‘no’ then there is probably no need for immunomodulatory treatments for these patients. If the answer is ‘yes,’ and also for all the other patients, immunomodulation will retain therapeutic interest. Preclinical studies should be conceived according to the targeted stroke subpopulation. In any case, a strictly linear bench-to-bedside paradigm is probably not optimal for translating basic scientific findings into clinically effective stroke therapies, and a bedside-back-to-bench paradigm will help in this translation.
In spite of the limitations of experimental stroke models, our knowledge about the inflammatory responses triggered after stroke onset exponentially increases thanks to preclinical and clinical studies, and gives rise to novel therapeutic targets and improved strategies, with the hope of ameliorating stroke outcome in patients.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Marina Rubio  https://orcid.org/0000-0002-6120-4053
https://orcid.org/0000-0002-6120-4053
Contributor Information
Antoine Drieu, Pathophysiology and Imaging of Neurological Disorders, Normandy University, Caen, France.
Damien Levard, Pathophysiology and Imaging of Neurological Disorders, Normandy University, Caen, France.
Denis Vivien, Pathophysiology and Imaging of Neurological Disorders, Normandy University, Caen, France Pathophysiology and Imaging of Neurological Disorders, Centre Hospitalier Universitaire de Caen, Caen, France.
Marina Rubio, Pathophysiology and Imaging of Neurological Disorders, Normandy University, Boulevard Henri Becquerel BP 5229, Caen Cedex, 14000, France.
References
- 1. Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 2. Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 3. Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fagan SC, Cronic LE, Hess DC. Minocycline development for acute ischemic stroke. Transl Stroke Res. 2011; 2: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 1985; 58: 293–297. [DOI] [PubMed] [Google Scholar]
- 7. Koistinaho M, Koistinaho J. Interactions between Alzheimer’s disease and cerebral ischemia—focus on inflammation. Brain Res Brain Res Rev 2005; 48: 240–250. [DOI] [PubMed] [Google Scholar]
- 8. Miyaji Y, Yoshimura S, Sakai N, et al. Effect of edaravone on favorable outcome in patients with acute cerebral large vessel occlusion: subanalysis of RESCUE-Japan Registry. Neurol Med Chir (Tokyo) 2015; 55: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perego C, Fumagalli S, De Simoni M-G. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation 2011; 8: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol 2006; 66: 232–245. [DOI] [PubMed] [Google Scholar]
- 11. Muller WA. Leukocyte-endothelial cell interactions in the inflammatory response. Lab Investig J Tech Methods Pathol 2002; 82: 521–533. [DOI] [PubMed] [Google Scholar]
- 12. Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 2005; 26: 485–495. [DOI] [PubMed] [Google Scholar]
- 13. Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med 2011; 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009; 40: 1849–1857. [DOI] [PubMed] [Google Scholar]
- 15. Mena H, Cadavid D, Rushing EJ. Human cerebral infarct: a proposed histopathologic classification based on 137 cases. Acta Neuropathol (Berl) 2004; 108: 524–530. [DOI] [PubMed] [Google Scholar]
- 16. Zhou W, Liesz A, Bauer H, et al. Postischemic brain infiltration of leukocyte subpopulations differs among murine permanent and transient focal cerebral ischemia models: infiltration differs among cerebral ischemia. Brain Pathol 2013; 23: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol 2012; 11: 860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kyritsis N, Kizil C, Zocher S, et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012; 338: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 19. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10: 471–480. [DOI] [PubMed] [Google Scholar]
- 20. Tobin MK, Bonds JA, Minshall RD, et al. Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2014; 34: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peruzzotti-Jametti L, Donegá M, Giusto E, et al. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience 2014; 283: 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz M, Kipnis J, Rivest S, et al. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci Off J Soc Neurosci 2013; 33: 17587–17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nowicka D, Rogozinska K, Aleksy M, et al. Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp (Warsz) 2008; 68: 155–168. [DOI] [PubMed] [Google Scholar]
- 24. Lively S, Moxon-Emre I, Schlichter LC. SC1/hevin and reactive gliosis after transient ischemic stroke in young and aged rats. J Neuropathol Exp Neurol 2011; 70: 913–929. [DOI] [PubMed] [Google Scholar]
- 25. Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol 1993; 327: 123–132. [DOI] [PubMed] [Google Scholar]
- 26. Huang L, Wu Z-B, Zhuge Q, et al. Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci 2014; 11: 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krieger DW, Georgia MAD, Abou-Chebl A, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke 2001; 32: 1847–1854. [DOI] [PubMed] [Google Scholar]
- 28. De Georgia MA, Krieger DW, Abou-Chebl A, et al. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology 2004; 63: 312–317. [DOI] [PubMed] [Google Scholar]
- 29. Lyden PD, Allgren RL, Ng K, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2005; 14: 107–114. [DOI] [PubMed] [Google Scholar]
- 30. Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke 2010; 41: 2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyden P, Hemmen T, Grotta J, et al. Results of the ICTuS 2 trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke 2016; 47: 2888–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Worp HB1, Macleod MR, Bath PM, et al. Trial of therapeutic cooling in patients with acute ischaemic stroke, Int J Stroke 2014; 9(5): 642–645. doi: 10.1111/ijs.12294. https://www.eurohyp1.eu/ (accessed 15 May 2018). [DOI] [PubMed] [Google Scholar]
- 33. Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992; 48: 405–415. [DOI] [PubMed] [Google Scholar]
- 34. Kettenmann H, Hanisch U-K, Noda M, et al. Physiology of microglia. Physiol Rev 2011; 91: 461–553. [DOI] [PubMed] [Google Scholar]
- 35. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10: 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernandez-Fernandez S, Almeida A, Bolaños JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J 2012; 443: 3–11. [DOI] [PubMed] [Google Scholar]
- 37. Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 2009; 183: 4733–4744. [DOI] [PubMed] [Google Scholar]
- 38. Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 2006; 9: 917–924. [DOI] [PubMed] [Google Scholar]
- 39. Banwell V, Sena ES, Macleod MR. Systematic review and stratified meta-analysis of the efficacy of interleukin-1 receptor antagonist in animal models of stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 2009; 18: 269–276. [DOI] [PubMed] [Google Scholar]
- 40. Emsley HCA, Smith CJ, Georgiou RF, et al. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry 2005; 76: 1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith CJ, Hulme S, Vail A, et al. SCIL-STROKE (Subcutaneous Interleukin-1 Receptor Antagonist in Ischemic Stroke): a randomized controlled phase 2 trial. Stroke 2018; 49: 1210–1216. [DOI] [PubMed] [Google Scholar]
- 42. Szalay G, Martinecz B, Lénárt N, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun 2016; 7: 11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohler E, Prentice DA, Bates TR, et al. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke 2013; 44: 2493–2499. [DOI] [PubMed] [Google Scholar]
- 44. Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 2000; 31: 3034–3040. [DOI] [PubMed] [Google Scholar]
- 45. Lee CZ, Xue Z, Zhu Y, et al. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 2007; 38: 2563–2568. [DOI] [PubMed] [Google Scholar]
- 46. Machado LS, Sazonova IY, Kozak A, et al. Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke 2009; 40: 3028–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsukawa N, Yasuhara T, Hara K, et al. Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci 2009; 10: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morimoto N, Shimazawa M, Yamashima T, et al. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res 2005; 1044: 8–15. [DOI] [PubMed] [Google Scholar]
- 49. Murata Y, Rosell A, Scannevin RH, et al. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 2008; 39: 3372–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Plane JM, Shen Y, Pleasure DE, et al. Prospects for minocycline neuroprotection. Arch Neurol 2010; 67: 1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power C, Henry S, Del Bigio MR, et al. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann Neurol 2003; 53: 731–742. [DOI] [PubMed] [Google Scholar]
- 52. Sanchez Mejia RO, Ona VO, Li M, et al. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 2001; 48: 1393–1399; discussion 1399–1401. [DOI] [PubMed] [Google Scholar]
- 53. Stirling DP, Khodarahmi K, Liu J, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci Off J Soc Neurosci 2004; 24: 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Teng YD, Choi H, Onario RC, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A 2004; 101: 3071–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang CX, Yang T, Noor R, et al. Delayed minocycline but not delayed mild hypothermia protects against embolic stroke. BMC Neurol 2002; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wells JEA, Hurlbert RJ, Fehlings MG, et al. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain J Neurol 2003; 126(Pt 7): 1628–1637. [DOI] [PubMed] [Google Scholar]
- 57. Xu L, Fagan SC, Waller JL, et al. Low dose intravenous minocycline is neuroprotective after middle cerebral artery occlusion-reperfusion in rats. BMC Neurol 2004; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yong VW, Wells J, Giuliani F, et al. The promise of minocycline in neurology. Lancet Neurol 2004; 3: 744–751. [DOI] [PubMed] [Google Scholar]
- 59. Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 2007; 69: 1404–1410. [DOI] [PubMed] [Google Scholar]
- 60. Padma Srivastava MV, Bhasin A, Bhatia R, et al. Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India 2012; 60: 23–28. [DOI] [PubMed] [Google Scholar]
- 61. Yang Y, Liu H, Zhang H, et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci Off J Soc Neurosci 2017; 37: 4692–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu X, Liu J, Zhao S, et al. Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke 2016; 47: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gauberti M, Montagne A, Marcos-Contreras OA, et al. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke 2013; 44: 1988–1996. [DOI] [PubMed] [Google Scholar]
- 64. Montagne A, Gauberti M, Macrez R, et al. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. NeuroImage 2012; 63: 760–770. [DOI] [PubMed] [Google Scholar]
- 65. Granger DN, Senchenkova E. Inflammation and the microcirculation. San Rafael (CA): Morgan & Claypool Life Sciences, http://www.ncbi.nlm.nih.gov/books/NBK53373/ (2010, accessed 12 March 2018). [PubMed] [Google Scholar]
- 66. Gauberti M, Fournier AP, Docagne F, et al. Molecular magnetic resonance imaging of endothelial activation in the central nervous system. Theranostics 2018; 8: 1195–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bowes MP, Rothlein R, Fagan SC, et al. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology 1995; 45: 815–819. [DOI] [PubMed] [Google Scholar]
- 68. Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology 2001; 57: 1428–1434. [DOI] [PubMed] [Google Scholar]
- 69. DeGraba TJ. The role of inflammation after acute stroke: utility of pursuing anti-adhesion molecule therapy. Neurology 1998; 51(3 Suppl 3): S62–S68. [DOI] [PubMed] [Google Scholar]
- 70. Cayce Onks. E-selectin nasal spray to prevent stroke recurrence. https://clinicaltrials.gov/ct2/show/NCT00012454 (accessed 22 March 2018).
- 71. Bednar M, Gross C, Russell S, et al. Humanized anti-L-selectin monoclonal antibody DREC200 therapy in acute thromboembolic stroke. Neurol Res 1998; 20: 403–408. [DOI] [PubMed] [Google Scholar]
- 72. Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 2006; 6: 173–182. [DOI] [PubMed] [Google Scholar]
- 73. Enzmann G, Mysiorek C, Gorina R, et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol (Berl) 2013; 125: 395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chu HX, Kim HA, Lee S, et al. Immune cell infiltration in malignant middle cerebral artery infarction: comparison with transient cerebral ischemia. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2014; 34: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol (Berl) 2015; 129: 239–257. [DOI] [PubMed] [Google Scholar]
- 76. Del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci 2010; 1207: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Garcia JH, Liu KF, Bree MP. Effects of CD11b/18 monoclonal antibody on rats with permanent middle cerebral artery occlusion. Am J Pathol 1996; 148: 241–248. [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang N, Chopp M, Chahwala S. Neutrophil inhibitory factor treatment of focal cerebral ischemia in the rat. Brain Res 1998; 788: 25–34. [DOI] [PubMed] [Google Scholar]
- 79. Prestigiacomo CJ, Kim SC, Connolly ES, et al. CD18-mediated neutrophil recruitment contributes to the pathogenesis of reperfused but not nonreperfused stroke. Stroke 1999; 30: 1110–1117. [DOI] [PubMed] [Google Scholar]
- 80. Krams M, Lees KR, Hacke W, et al. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke 2003; 34: 2543–2548. [DOI] [PubMed] [Google Scholar]
- 81. Gee JM, Kalil A, Shea C, et al. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke 2007; 38(2 Suppl): 783–788. [DOI] [PubMed] [Google Scholar]
- 82. Hallenbeck J, Del Zoppo G, Jacobs T, et al. Immunomodulation strategies for preventing vascular disease of the brain and heart: workshop summary. Stroke 2006; 37: 3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schwartz M, Kipnis J. Autoimmunity on alert: naturally occurring regulatory CD4(+)CD25(+) T cells as part of the evolutionary compromise between a « need » and a « risk ». Trends Immunol 2002; 23: 530–534. [DOI] [PubMed] [Google Scholar]
- 84. Llovera G, Benakis C, Enzmann G, et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol (Berl) 2017; 134: 851–868. [DOI] [PubMed] [Google Scholar]
- 85. Gelderblom M, Weymar A, Bernreuther C, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood 2012; 120: 3793–3802. [DOI] [PubMed] [Google Scholar]
- 86. Liesz A, Zhou W, Mracskó É, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain J Neurol 2011; 134(Pt 3): 704–720. [DOI] [PubMed] [Google Scholar]
- 87. Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med 2009; 15: 946–950. [DOI] [PubMed] [Google Scholar]
- 88. Liesz A, Zhou W, Na S-Y, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci Off J Soc Neurosci 2013; 33: 17350–17362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Liesz A, Suri-Payer E, Veltkamp C, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15: 192–199. [DOI] [PubMed] [Google Scholar]
- 90. Xie L, Sun F, Wang J, et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol Baltim Md 1950 2014; 192: 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li P, Gan Y, Sun B-L, et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol 2013; 74: 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ren X, Akiyoshi K, Vandenbark AA, et al. CD4+FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis 2011; 26: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stubbe T, Ebner F, Richter D, et al. Regulatory T cells accumulate and proliferate in the ischemic hemisphere for up to 30 days after MCAO. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2013; 33: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kleinschnitz C, Kraft P, Dreykluft A, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013; 121: 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liesz A, Hu X, Kleinschnitz C, et al. Functional role of regulatory lymphocytes in stroke: facts and controversies. Stroke 2015; 46: 1422–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Na S-Y, Mracsko E, Liesz A, et al. Amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice. Stroke 2015; 46: 212–220. [DOI] [PubMed] [Google Scholar]
- 97. Schuhmann MK, Kraft P, Stoll G, et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2015; 35: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pham M, Kleinschnitz C, Helluy X, et al. Enhanced cortical reperfusion protects coagulation factor XII-deficient mice from ischemic stroke as revealed by high-field MRI. NeuroImage 2010; 49: 2907–2914. [DOI] [PubMed] [Google Scholar]
- 99. Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008; 7: 299–309. [DOI] [PubMed] [Google Scholar]
- 100. Delgado-Mederos R, Rovira A, Alvarez-Sabín J, et al. Speed of tPA-induced clot lysis predicts DWI lesion evolution in acute stroke. Stroke 2007; 38: 955–960. [DOI] [PubMed] [Google Scholar]
- 101. Gauberti M, Vivien D. Letter by Gauberti and Vivien regarding article, « amplification of regulatory T cells using a CD28 superagonist reduces brain damage after ischemic stroke in mice ». Stroke 2015; 46: e50–e51. [DOI] [PubMed] [Google Scholar]
- 102. Gauberti M, Montagne A, Quenault A, et al. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci 2014; 8: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Min Lou. Combinating fingolimod with alteplase bridging with mechanical thrombectomy in acute ischemic stroke, https://clinicaltrials.gov/ct2/show/NCT02956200 (2016, accessed 15 May 2018). [DOI] [PubMed]
- 104. Fu Y, Zhang N, Ren L, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014; 111: 18315–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation 2015; 132: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rudick R, Polman C, Clifford D, et al. Natalizumab: bench to bedside and beyond. JAMA Neurol 2013; 70: 172–182. [DOI] [PubMed] [Google Scholar]
- 107. Becker K, Kindrick D, Relton J, et al. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke 2001; 32: 206–211. [DOI] [PubMed] [Google Scholar]
- 108. Langhauser F, Kraft P, Göb E, et al. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke 2014; 45: 1799–1806. [DOI] [PubMed] [Google Scholar]
- 109. Neumann J, Riek-Burchardt M, Herz J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol (Berl) 2015; 129: 259–277. [DOI] [PubMed] [Google Scholar]
- 110. Relton JK, Sloan KE, Frew EM, et al. Inhibition of alpha4 integrin protects against transient focal cerebral ischemia in normotensive and hypertensive rats. Stroke 2001; 32: 199–205. [DOI] [PubMed] [Google Scholar]
- 111. Llovera G, Hofmann K, Roth S, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci Transl Med 2015; 7: 299ra121. [DOI] [PubMed] [Google Scholar]
- 112. Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol 2017; 16: 217–226. [DOI] [PubMed] [Google Scholar]
- 113. Jake Elkins. Safety and efficacy of intravenous natalizumab in acute ischemic stroke, https://clinicaltrials.gov/ct2/show/NCT02730455 (accessed 31 May 2018.)
- 114. Martinez de Lizarrondo S, Gakuba C, Herbig BA, et al. Potent thrombolytic effect of n-acetylcysteine on arterial thrombi. Circulation 2017; 136: 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Orset C, Haelewyn B, Allan SM, et al. Efficacy of alteplase in a mouse model of acute ischemic stroke: a retrospective pooled analysis. Stroke 2016; 47: 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Orset C, Macrez R, Young AR, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 2007; 38: 2771–2778. [DOI] [PubMed] [Google Scholar]
- 117. Hossmann K-A. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 2012; 32: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. O’Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 119. Spychala MS, Honarpisheh P, McCullough LD. Sex differences in neuroinflammation and neuroprotection in ischemic stroke. J Neurosci Res 2017; 95(1–2): 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuro Molecular Med 2010; 12: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Felger JC, Abe T, Kaunzner UW, et al. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain Behav Immun 2010; 24: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol 2011; 2011: 464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hug A, Dalpke A, Wieczorek N, et al. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke 2009; 40: 3226–3232. [DOI] [PubMed] [Google Scholar]
- 125. Veltkamp R, Gill D. Clinical trials of immunomodulation in ischemic stroke. Neurother J Am Soc Exp Neurother 2016; 13: 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]