Abstract
Background and Aims:
Percutaneous radiofrequency ablation (PRFA) is a minimally invasive treatment for hepatic tumors. We assessed and compared the efficacy of right thoracic paravertebral block (TPVB) with that of local anesthetic infiltration for the anesthetic management of PRFA of liver tumors.
Material and Methods:
Sixty patients with hepatic tumors aged 50–80 years were randomly allocated into two groups. Group I received local anesthetic infiltration along the path of the ablation device with sedation. Group II received right TPVB at the level T7 and T9 with sedation. The pain was assessed using visual analog scale (VAS) at 1 min and then every 5 min during PRFA procedure, on admission, and discharge from the post-PRFA observation area. The total dose of rescue analgesia during PRFA procedure, number of patients requiring general anesthesia, patient and radiologist satisfaction were reported.
Results:
VAS was significantly lower in group II than group I during and after PRFA procedure (P < 0.05). General anesthesia was administered in 7 patients in group I, whereas no patient required general anesthesia in group II (P < 0.05). Patient and radiologist satisfaction were significantly higher in group II compared to group I (P < 0.05). There were no significant complications in group II compared to group I (P > 0.05).
Conclusions:
Right TPVB with sedation is an effective and safe anesthetic technique for the management of PRFA procedure of hepatic tumors. It is more effective than local anesthesia with sedation in relieving pain during PRFA procedure of hepatic tumors.
Keywords: Liver neoplasms, nerve block, paravertebral, radiofrequency ablation
Introduction
Percutaneous radiofrequency ablation (PRFA) is a minimally invasive and widely performed local treatment for patients with liver tumors.[1,2,3] Hepatic radiofrequency ablation is a painful procedure; patients often have pain during and/or after the procedure.[4]
Thoracic paravertebral block (TPVB), which anesthetizes the spinal nerve roots and sympathetic chain in the paravertebral space, has long been used to provide unilateral chest and abdominal wall analgesia.[5] The block has been used to control pain specifically of hepatic origin during percutaneous transhepatic biliary drainage[6,7] or after liver trauma.[8]
The present study was designed to assess and compare the efficacy of the right TPVB with sedation in relieving pain during PRFA procedure of liver tumors with that of local anesthetic infiltration with sedation as a primary goal and patient and interventional radiologist satisfaction as secondary goals.
Material and Methods
After obtaining approval from the Hospital Ethics Committee (30627/12/15) and informed written consent from the patients, a prospective double-blinded randomized study was carried out. Adult patients aged 50–80 years scheduled for PRFA of hepatic tumors were included in the study. Malignant liver tumors either primary or secondary were included in the study. The duration of the study was 9 months.
During the preoperative visit, every patient received an explanation of the study objectives, study protocol, and the visual analog scale (VAS) for pain (where 0= “no pain” and 10 = the “worst pain”). All patient data were used for the current study and securely maintained. Any untoward events were shared with the patients and the Hospital Ethical Committee. Patients with a Child–Pugh score more than B7, international normalized ratio value more than 1.5, platelets count <50,000/mm3, history of mental disorders or allergy to local anesthetics, chest wall deformity, or regular use of analgesics were excluded from the study.
Patients were randomized using computer-generated randomization numbers into two groups using sealed opaque envelopes, and each patient chose the envelope which determined the group in which the patient was allocated. The two studied groups were group I (local anesthetic infiltration group) and group II (TPVB group).
The patients did not receive premedication. After securing peripheral intravenous (IV) line and application of routine monitoring that included electrocardiography, pulse oximetry, and noninvasive arterial blood pressure, all patients received intravenous 20 μg/kg midazolam and 0.5 μg/kg fentanyl. Supplemental oxygen at a flow rate of 4 l/min was administered via nasal cannula. Normal saline solution infusion at a rate of 4 ml/kg/h was started.
Right TPVB was performed in group II patients and sham right TPVBin group I patients in a sitting position. Following this, local anesthetic infiltration along the path of the ablation device was given for group I patients, and sham local anesthetic infiltration was given for group II in a supine position. After adequate local anesthesia over the entry site was confirmed, propofol 0.5 mg/kg IV bolus dose was given to all patients followed by an infusion of propofol 25–50 μg/kg/min to provide moderate sedation during the PRFA procedure.
Under aseptic precautions, local anesthesia was done using 2% lidocaine (5–10 ml) infiltrated percutaneously along the path of the ablation device.
Right TPVB was performed with the patient in the sitting position. The superior aspect of the spinous processes of T7 and T9 were identified and marked. Under aseptic precautions, skin anesthesia with 2 ml of 2% lidocaine was performed. A 22-gauge needle was inserted 2.5 cm lateral to the midline on the right side of the back at the selected two levels. The needle was advanced perpendicularly to the skin until the transverse process was contacted. Then, the needle was “walked off” above the transverse process and advanced until loss of resistance to the injection of air was felt. After negative aspiration of blood or cerebrospinal fluid, 25 ml of 0.5% bupivacaine was injected within the right paravertebral space at the levels of T7 and T9 (12.5 ml in each). After completion of the TPVB, the patient was returned to the supine position. Ten minutes later, loss of sensation to pinprick and cold sensations was evaluated over the targeted dermatomes (T6–11).
PRFA was done by a single radiologist blinded to the group assignment. PRFA was performed with ultrasound guidance, using expandable-tip electrode needles.
The degree of pain was assessed using VAS score; preprocedure (baseline), at 1 min, and then every 5 min during the procedure, on admission, and discharge from the post-PRFA observation area. The pain was considered mild if VAS range was 1–3, moderate if VAS range was 4–6, and severe if the VAS was ≥7. Rescue analgesia in the form of intravenous fentanyl 0.5 μg/kg was given if the VAS was ≥4 and repeated once if needed. The total dose of fentanyl administered as rescue analgesia during PRFA procedure was documented. If the patient still suffered from pain with VAS ≥4 after a repeated dose of fentanyl, total intravenous general anesthesia with propofol was administered. The number of patients requiring general anesthesia was recorded.
At the end of the PRFA procedure, propofol infusion was discontinued. The patients were transferred to the post-PRFA observation area where the vital signs were monitored for 30 min. Postprocedure analgesia in the form of paracetamol infusion (1g/8h) and tramadol (50 mg/12 h) was started if the VAS was ≥4. Before patient's discharge from the post-PRFA observation area, the patients and interventional radiologist were asked about the degree of their satisfaction with the anesthetic management using a four-point satisfaction scale (1 = very dissatisfied, 2 = dissatisfied, 3 = satisfied, and 4 = very satisfied).
Complications such as hypotension, bradycardia, pneumothorax, upper airway obstruction, contralateral segmental sensory blockade of TPVB, and respiratory depression (respiratory rate ≤10 breaths/min) were recorded. Hypotension was defined as a decrease of the mean arterial pressure more than 25% of the baseline value. Hypotension was treated with IV fluids (8 ml/kg) and ephedrine 10 mg, if needed. Bradycardia was defined as a decrease of the heart rate <50 beats/min and managed with IV atropine 0.5 mg.
Statistical analysis
Based on the results of the previous study,[9] the sample size estimation showed that at least 27 patients should be included for detecting a clinically meaningful difference of VAS during PRFA of 2.1 at an α error of 0.05 and study power of 80%. Sample size was calculated and assured using power and sample size calculation software program provided by the Department of Biostatistics, Vanderbilt University. Statistical Package for the Social Sciences (SPSS) 16 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Kolmogorov–Smirnov test was performed to verify the assumption of normality. Continuous data were presented as mean ± standard deviation (SD) or median with interquartile range, and were compared by using Student's t-test or the Mann–Whitney U-test as appropriate. Categorical data were expressed as number (n) or percentage (%) and compared using the chi-square test. The intragroup changes of VAS score were analyzed using the Wilcoxon signed-rank test. P < 0.05 was considered significant.
Results
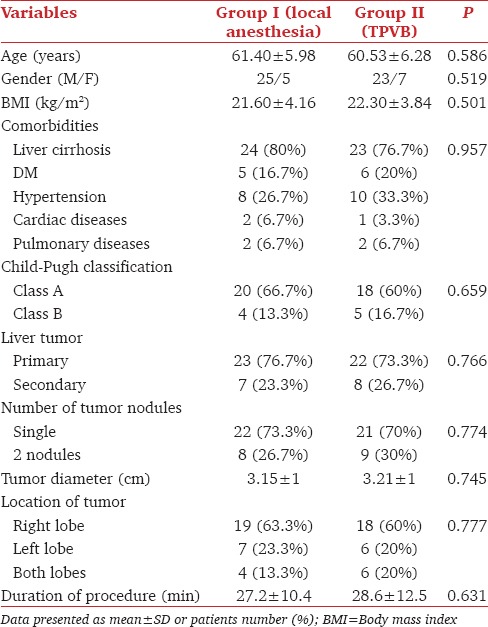
Sixty patients with liver tumors, either primary or secondary, scheduled for PRFA were enrolled in the study and randomly allocated into two groups. The demographic data including age, gender, body mass index (BMI), and the associated comorbidities were not statistically different between t groups (P > 0.05). The characteristics of liver tumors and the duration of PRFA procedure were comparable between the two studied groups (P > 0.05) [Table 1].
Table 1.
Demographic data and patient characteristics in both groups
The VAS data are presented in Table 2. In Group I 25 patients experienced mild-to-severe pain. Twenty-one patients reported visceral and 4 patients reported referred shoulder pain. Twelve patients received a single dose of the rescue analgesia (fentanyl 0.5 μg/kg) during the PRFA procedure. Nine patients received two doses of the rescue analgesia (fentanyl 0.5 μg/kg) during the PRFA procedure. General anesthesia was conducted in 7 patients.
Table 2.
Visual analog scale in both groups
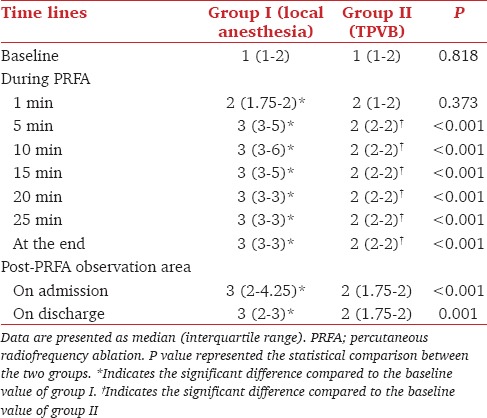
In group II (TPVB) no patient reported severe pain, 5 reported moderate pain, and 13 mild pain. Fourteen patients reported visceral and 4 referred shoulder pain. Five patients received a single dose of rescue analgesia (fentanyl 0.5 μg/kg) during the PRFA procedure due to moderate pain. General anesthesia was not required.
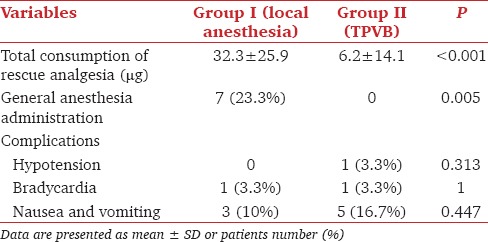
Total consumption of the rescue analgesia during the PRFA procedure was significantly lower in group II (TPVB) than group I (P < 0.05). There were no significant complications in group II (TPVB) compared to group I (P > 0.05) [Table 3]. Four patients in group II (TPVB) had contralateral segmental sensory blockade from T6 to T11.
Table 3.
Consumption of rescue analgesia and complications
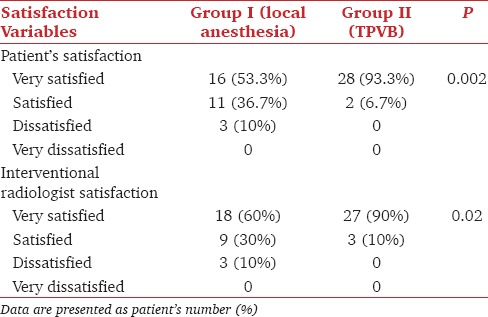
Patient and interventional radiologist satisfaction were significantly higher in group II (TPVB) compared to group I (P < 0.05) [Table 4].
Table 4.
Patient and radiologist satisfaction in both groups
Discussion
The results of the present study showed that the right TPVB with sedation effectively reduced the pain experienced during and after PRFA procedure, decreased the consumption of rescue analgesia during PRFA procedure, with better patient, and interventional radiologist satisfaction. The decreased requirement for opioids during the PRFA procedure is considered as an important advantage of TPVB that can improve patient safety, maintain an adequate conscious level, and preserve the respiratory drive.
From an anatomical viewpoint, the liver is innervated by the hepatic nerve plexus containing both sympathetic (T6–11) and parasympathetic (vagus) innervations.[9] PRFA is associated with intense pain during and after the procedure,[10,11] especially during PRFA for superficial tumors or tumors located in close proximity to large hepatic vessels.[4,12] Pain during hepatic procedures is mediated from afferent somatic and autonomic innervations, sympathetic nerve (T6–11), and parasympathetic fiber.[13]
Local anesthetic infiltration with sedation is a widely used anesthetic technique for PRFA.[14] However, this technique is often associated with pain during and after PRFA procedure.[15,16,17,18] In addition, the IV sedative agents must be titrated meticulously during PRFA procedure to maintain a balance between maximum patient comfort and patient cooperation (to perform Valsalva maneuvers at the request of the radiologist).[14]
TPVB is an anesthetic technique of administering local anesthetic agents close to where the spinal nerves emerge from the thoracic intervertebral foramina.[5] TPVB causes sympathetic and spinal nerve fibers block in the paravertebral space without affecting the parasympathetic fibers (vagus), which is considered the main drawback of this technique.[19]
TPVB has been reported as effective in controlling hepatic pain in cases of blunt abdominal trauma[8] or percutaneous transhepatic biliary drainage.[6,7] Previous studies have reported that TPVB is an effective anesthetic technique for management of PRFA for hepatic tumors.[9,19,20,21]
Ning et al.[9] used the right TPVB for anesthesia during PRFA of hepatic tumors in 20 patients and concluded that TPVB is a safe and effective technique for the anesthetic management of PRFA of hepatic tumors. Piccioni et al.[20] evaluated the efficacy and safety of TPVB in 12 patients with hepatic tumors undergoing PRFA and concluded that TPVB produced satisfactory unilateral anesthesia for PRFA of liver tumors without significant side effects. Gazzera et al.[21] evaluated the efficacy of ultrasound-guided right TPVB for anesthetic management of PRFA in 30 patients with liver tumors. Ten patients (33.3%) experienced moderate-to-severe pain that required IV sedation.
In our study, visceral pain reported in the TPVB group may be due to the inability of TPVB to block the parasympathetic nerve fibers (vagus) as well as the contralateral sympathetic fibers.[9] Referred shoulder pain experienced during PRFA of liver tumors could be due to the thermal ablation of peripheral hepatic tumors that are located adjacent to the diaphragm. Phrenic nerve is not blocked by TPVB nor local anesthesia infiltration.
There were no major complications reported in either group. The safety of TPVB was reported in previous studies.[9,20,21,22] In group II, the contralateral segmental sensory blockade was reported in 4 patients (13.3%). The previously reported rate was 35%,[9]20%,[23] and 1.1%.[24] The mechanism of the contralateral segmental sensory block may be due to epidural[25] or prevertebral[26,27] spread of local anesthetic.
There are some limitations of our study; first is the limited number of patients included in our study. Second, the documentation of pain assessment was limited to during PRFA procedure till the discharge from the post-PRFA observation area.
In conclusion, right TPVB with sedation is an effective and safe anesthetic technique for management of patients with hepatic tumors undergoing PRFA. Right TPVB with sedation is more effective than local anesthetic infiltration with sedation in relieving pain during PRFA of hepatic tumors. Further studies are recommended to validate our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kudo M. Local ablation therapy for hepatocellular carcinoma: Current status and future perspectives. J Gastroenterol. 2004;39:205–14. doi: 10.1007/s00535-003-1280-y. [DOI] [PubMed] [Google Scholar]
- 2.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–8. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 3.Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, et al. Radiofrequency ablation of hepatocellular carcinoma: Value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol. 2008;190:W335–41. doi: 10.2214/AJR.07.3092. [DOI] [PubMed] [Google Scholar]
- 4.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–18. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 5.Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95:771–80. doi: 10.1097/00000542-200109000-00033. [DOI] [PubMed] [Google Scholar]
- 6.Culp Jr WC, Culp WC. Thoracic paravertebral block for percutaneous transhepatic biliary drainage. J Vasc Interv Radiol. 2005;16:1397–400. doi: 10.1097/01.RVI.0000174285.84995.7F. [DOI] [PubMed] [Google Scholar]
- 7.Culp WC, McCowan TC, DeValdenebro M, Wright LB, Workman JL, Culp WC., Jr Paravertebral block: An improved method of pain control in percutaneous transhepatic biliary drainage. Cardiovasc Intervent Radiol. 2006;29:1015–21. doi: 10.1007/s00270-005-0273-z. [DOI] [PubMed] [Google Scholar]
- 8.Hall H, Leach A. Paravertebral block in the management of liver capsule pain after blunt trauma. Br J Anaesth. 1999;83:819–21. doi: 10.1093/bja/83.5.819. [DOI] [PubMed] [Google Scholar]
- 9.Cheung Ning M, Karmakar MK. Right thoracic paravertebral anaesthesia for percutaneous radiofrequency ablation of liver tumours. Br J Radiol. 2011;84:785–9. doi: 10.1259/bjr/28983063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callstrom MR, Charboneau JW. Technologies for ablation of hepatocellular carcinoma. Gastroenterology. 2008;134:1831–5. doi: 10.1053/j.gastro.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Cheung TT, Ng KK, Chok KS, Chan SC, Poon RT, Lo CM, et al. Combined resection and radiofrequency ablation for multifocal hepatocellular carcinoma: Prognosis and outcomes. World J Gastroenterol. 2010;16:3056–62. doi: 10.3748/wjg.v16.i24.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann H, Beckh K. Oxford Textbook of Clinical Hepatology. In: McIntyre N, Benhamou JP, Bircher J, editors. Nerve supply and nervous control of liver function. Vol. 1. Oxford, UK: Oxford University Press; 1992. pp. 38–9. Sections 1.13. [Google Scholar]
- 13.Kopacz DJ, Thompson GE. Neural blockade in clinical anesthesia and management of pain. In: Cousins MJ, Bridenbaugh PO, editors. Celiac and hypogastric plexus, intercostals, interpleural, and peripheral neural blockade of the thorax and abdomen. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998. pp. 451–85. [Google Scholar]
- 14.Sabo B, Dodd GD, 3rd, Halff GA, Naples JJ. Anesthetic considerations in patients undergoing percutaneous radiofrequency interstitial tissue ablation. AANA J. 1999;67:467–8. [PubMed] [Google Scholar]
- 15.Goldberg SN, Charboneau JW, Dodd III GD, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–45. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 16.Chakravorty N, Jaiswal S, Chakravarty D, Jain RK, Agarwal RC. Anaesthetic management of radiofrequency tumor ablation: Our experience. Indian J Anaesth. 2005;50:123–7. [Google Scholar]
- 17.Giorgio A, Tarantino L, Stefano G, Coppola C, Ferraioli G. Complications after percutaneous saline-enhanced radiofrequency ablation of liver tumors: 3-year experience with 336 patients at a single center. AJR Am J Roentgenol. 2005;184:207–11. doi: 10.2214/ajr.184.1.01840207. [DOI] [PubMed] [Google Scholar]
- 18.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Nahum GS. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology. 2003;226:441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 19.Culp WC, Jr, Payne MN, Montgomery ML. Thoracic paravertebral block for analgesia following liver mass radiofrequency ablation. Br J Radiol. 2008;81:e23–5. doi: 10.1259/bjr/61546726. [DOI] [PubMed] [Google Scholar]
- 20.Piccioni F, Fumagalli L, Garbagnati F, Di Tolla G, Mazzaferro V, Langer M. Thoracic paravertebral anesthesia for percutaneous radiofrequency ablation of hepatic tumors. J Clin Anesth. 2014;26:271–5. doi: 10.1016/j.jclinane.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Gazzera C, Fonio P, Faletti R, Dotto MC, Gobbi F, Donadio P, et al. Role of paravertebral block anaesthesia during percutaneous transhepatic thermoablation. Radiol Med. 2014;119:549–57. doi: 10.1007/s11547-013-0372-x. [DOI] [PubMed] [Google Scholar]
- 22.Richardson J, Sabanathan S. Thoracic paravertebral analgesia. Acta Anaesthesiol Scand. 1995;39:1005–15. doi: 10.1111/j.1399-6576.1995.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 23.Karmakar MK, Critchley LA, Ho AM, Gin T, Lee TW, Yim AP. Continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with multiple fractured ribs. Chest. 2003;123:424–31. doi: 10.1378/chest.123.2.424. [DOI] [PubMed] [Google Scholar]
- 24.Lo¨nnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade: Failure rate and complications. Anaesthesia. 1995;50:813–15. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 25.Purcell-Jones G, Pither CE, Justins DM. Paravertebral somatic nerve block: A clinical, radiographic, and computed tomographic study in chronic pain patients. Anesth Analg. 1989;68:32–9. [PubMed] [Google Scholar]
- 26.Karmakar MK, Kwok WH, Kew J. Thoracic paravertebral block: Radiological evidence of contralateral spread anterior to the vertebral bodies. Br J Anaesth. 2000;84:263–5. doi: 10.1093/oxfordjournals.bja.a013417. [DOI] [PubMed] [Google Scholar]
- 27.Karmakar MK, Chui PT, Joynt GM, Ho AM. Thoracic paravertebral block for management of pain associated with multiple fractured ribs in patients with concomitant lumbar spinal trauma. Reg Anesth Pain Med. 2001;26:169–73. doi: 10.1053/rapm.2001.21086. [DOI] [PubMed] [Google Scholar]


