Abstract
Background and Aims:
Hyponatremia is one of the most common electrolyte abnormality encountered in postoperative patients especially in the elderly. We aimed to assess the efficacy of single-dose intravenous conivaptan vs. oral tolvaptan therapy for correction of hyponatremia in postoperative patients.
Material and Methods:
This prospective randomized study was conducted on 40 patients aged 20–70 years, who had undergone major head and neck surgeries with a serum sodium level of ≤130 mEq/L and were symptomatic. Patients were randomly allocated into two equal groups. Patients belonging to group C received single intravenous bolus dose of conivaptan 20mg, whereas group T received oral tolvaptan 15mg on the first day. At 24h, if sodium correction was <4mEq/L, dose of tolvaptan was increased to 30mg in group T or an infusion of conivaptan 20mg over next 24h was started in group C.
Results:
Chi-square test, independent sample t-test, and paired t-test were used as applicable. Though there was no significant difference in the baseline sodium values in both groups, at 12 and 24 h group C had significantly high values. At 48h sodium values in both the groups were comparable. Intra-group analysis had shown that there was a significant increase in sodium values from the baseline at 12, 24, and 48 h in both the groups.
Conclusion:
Single-dose intravenous conivaptan as well as oral tolvaptan were safe and effective in correcting hyponatremia in postoperative patients. Conivaptan could be considered superior as it resulted in faster sodium correction with effective aquaresis.
Keywords: Aquaresis, conivaptan, fluid balance, hyponatremia, tolvaptan
Introduction
Hyponatremia is the most common electrolyte abnormality encountered in clinical practice.[1] Syndrome of inappropriate secretion of antidiuretic hormone (SIADH) is common in postoperative patients, especially in the elderly and presents as hyponatremia. Traditionally, it is treated with infusions of hypertonic saline. Vaptans are a group of relatively new vasopressin receptor antagonists which directly combat elevated antidiuretic hormone (ADH) levels and are useful in managing hypervolemic or euvolemic hyponatremia.
The primary objective of our study was to assess the efficacy of single-dose intravenous conivaptan vs oral tolvaptan for correction of hyponatremia in postoperative patients. The secondary objectives were assessment of daily fluid balance, concomitant changes in serum potassium levels, and hemodynamic stability in these patients.
Material and Methods
The present study was a prospective, randomized one which was conducted after obtaining clearance from institutional ethical committee during the period January 2015 to January 2017. Forty patients aged 20 to 70 years, with American Society of Anesthesiologists (ASA) physical status 1 and 2 who had undergone major head and neck surgeries with a serum sodium level of ≤130 mEq/L and were symptomatic (headache, nausea, vomiting, lethargy, confusion, disorientation) were included in the study. Patients with hypovolemia, anuria, cirrhosis liver, and on CYP3A inhibitors such as ketoconazole, fluconazole, clarithromycin, or erythromycin were excluded. Hypovolemia was ruled out clinically with absence of reduced urine output along with signs such as reduced skin turgor and dry mucosa with or without hypotension.
Patients were randomly allocated into two equal groups, C and T, based on computer generated random sequence of numbers. Patients belonging to group C received single intravenous bolus dose of conivaptan 20 mg over 30 min, whereas those in group T received oral tolvaptan 15 mg on the first day. On the second day, if sodium correction in 24h was <4mEq/L, dose of tolvaptan was increased to 30 mg in group T or an infusion of conivaptan 20 mg over next 24h was started in group C. No diuretic was concurrently administered and there was no restriction of fluid intake.
Hemodynamic parameters, fluid intake, and urine output were documented hourly. Laboratory measurement of serum sodium was performed at the beginning of treatment, thereafter at 12, 24, and 48 h following intervention. Preoperative serum sodium level of all the patients was also collected. The fluid balance and serum potassium levels were recorded at 0, 24, and 48 h following initiation of treatment. Heart rate and mean arterial pressure (MAP) were documented 4 hourly. Reduction in MAP <20% from baseline was considered as hypotension and was managed with intravenous fluid bolus of 200 mL. Unresponsiveness to more than two fluid boluses warranted initiation of noradrenaline infusion to maintain blood pressure.
All normally distributed continuous variables were presented as mean with standard deviation (SD) and categorical variables as proportion. Chi-square test was used to compare distribution of gender and ASA physical status. Independent sample t-test was used to compare the continuous variables among Group C and Group T. Paired t-test was used to compare the change of sodium at different time points in each group. Statistical analyses were conducted using Statistical Package for the Social Sciences, version 20.0 for Windows (IBM Corporation ARMONK, NY, USA).
Results
Mean age, height, weight, preoperative serum sodium levels (137.4 ± 2.2 vs 137.0 ± 2.7, P = 0.548) as well as distribution of gender and ASA physical status were comparable between groups C and T [Table 1]. Though there was no significant difference in the baseline sodium values in the two groups, group C had significantly higher values than group T (P < 0.05) at 12 and 24 hours. At 48h sodium values in both the groups were comparable [Table 2]. Intra-group analysis had shown that there was a significant increase in sodium values from the baseline at 12, 24, and 48 h in both the groups [Table 2](P < 0.001).
Table 1.
Comparison of demographics, gender, and ASA status
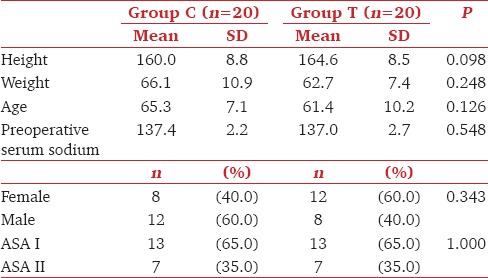
Table 2.
Comparison of serum sodium and potassium levels
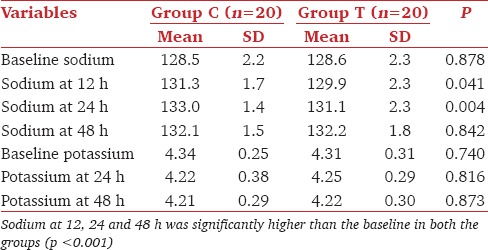
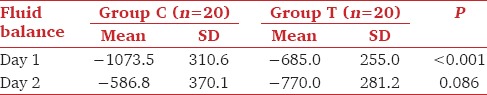
Baseline potassium values as well as that at 24 and 48h were comparable in both the groups [Table 2]. Fluid balance was significantly more negative in group C at day 1(P < 0.001). But the difference between groups was statistically insignificant on day 2, though fluid balance became more negative in group T by that time [Table 3]. Majority of patients in group C had sodium correction of >4mEq/L at 24h as compared to group T (95% vs 10%). No patient in either of the groups developed hypotension (fall in MAP >20% from baseline) requiring fluid bolus or vasoconstrictors to maintain blood pressures. Ten percentage of patients in group C developed thrombophlebitis. The average time elapsed from surgical procedure to development of hyponatremia was 5.2 days. The onset of symptoms varied from third to 16th postoperative day.
Table 3.
Comparison of daily fluid balance
Discussion
Hyponatremia (serum sodium levels <135mEq/L) usually reflects a state of hypotonicity of blood with a relative excess of body water rather than an actual decrease in total sodium content. It is common in elderly postoperative patients and the outcome could be catastrophic if there is delay in diagnosis and proper management.[2,3] Traditionally, hyponatremia is managed with fluid restriction and intravenous administration of 3% hypertonic saline.[3]
Acute hyponatremia results in life-threatening cerebral edema and 4–6 mEq/L increase in serum sodium concentration is sufficient for symptomatic relief. In chronic (duration more than 48 h) as well as severe (serum sodium levels <120 mEq/L) hyponatremia, correction by >8 to 10 mEq/L/day carries risk of development of iatrogenic osmotic demyelination syndrome. So it is recommended to aim daily sodium correction to 4–6 mEq/L in most patients.[4]
Vaptans are nonpeptide vasopressin antagonists that act by inhibiting ADH, also known as arginine vasopressin (AVP). They competitively and reversibly bind to selected AVP receptors and inhibit actions of ADH. There are three subtypes of vasopressin antagonist (VPA) receptors: V1a, V1b, and V2. V1a receptors are mainly distributed in the blood vessels and myocardium, whereasV1b receptors are found in the anterior pituitary gland. V2 receptors are present in the renal collecting tubular cells. Vaptans result in “aquaresis” which is characterized by free water elimination without electrolyte excretion. They are effective in management of hypervolemic hyponatremia seen in congestive heart failure and hepatic cirrhosis as well as in normovolemic hyponatremia of SIADH.[5]
Conivaptan is a dual V1 and V2 antagonist and is commonly administered intravenously. It is a nonselective vasopressin receptor antagonist.[6] Though it has high affinities for both V1a and V2 receptors, affinity for V2 is tenfold higher and the aquaretic effect is predominantly V2-associated.[6,7] Conivaptan is usually started at a dose of 20 mg intravenously over 30 min, followed by an infusion up to 20 mg over the next 24 h and may be given to a maximum of 4 days. The maximum daily dose should not exceed 40 mg.[8]
A single intravenous bolus of 20 or 40 mg conivaptan was found to be effective for the correction of acute hyponatremia and the effect of intermittent bolus dosing lasts up to 72 h.[9,10] Bolus dose of conivaptan 20 mg followed by an infusion of 40 mg over 24 h for the next 72 h was found to be superior than hypertonic saline in correcting hyponatremia.[11] Oral conivaptan 40 or 80 mg/day for 5 days had also shown similar effects.[12] The side effects include postural hypotension,[13,14] hypokalemia and osmotic demyelination syndrome on rapid overcorrection. Other possible side effects are rebound hyponatremia and renal damage due to significant hypovolemia leading to hypotension and acute tubular necrosis. Increased frequency of adverse cardiac events such as atrial dysrhythmias and sepsis in the presence of congestive cardiac failure had also been reported.[7]
Tolvaptan is a selective V2 antagonist and is administered through the oral route. It is usually started at 15 mg/day, and the dose may be increased at daily intervals to 30 mg/day, then to a maximum of 60 mg/day depending on the response. On initiation of treatment fluid restriction should be avoided during the first 24 h, and patients should be advised to take fluids as per need in response to thirst. The common side effects of tolvaptan are thirst, dry mouth, lethargy, constipation, polyuria, and hyperglycemia.[15] It is commonly used to treat hypervolemic or euvolemic hyponatremia associated with congestive heart failure, hepatic cirrhosis, SIADH, and autosomal-dominant polycystic kidney disease.[16,17,18,19]
The advantage of using vaptans over traditional management with hypertonic saline for correcting hyponatremia is avoidance of a prolonged intravenous infusion. Tolvaptan is administered orally and conivaptan, even if given as bolus without a subsequent infusion, is equally effective. Though less expensive, hypertonic saline has to be given as an infusion and the duration of drug administration is usually prolonged over many hours to days depending on the adequacy of sodium correction. Intravenous infusions require careful monitoring by nursing staff to avoid risks of accidental administration of a bolus dose. Though it is always safer to administer the drug using an infusion pump, it adds to the cost of treatment.
In hyponatremia patients with concentrated urine, with the exception of hypovolemic hyponatremia, vaptans are considered as the primary agents of choice as they result in aquaresis. But those patients presenting with dilute urine or those who develop urinary dilution after saline infusion, desmopressin is indicated as it leads to urinary concentration.[20]
Effectiveness of conivaptan for management of hyponatremia in postsurgical patients had been investigated by Rajan et al.,[10] Potts et al.,[21] Breshears et al.,[22] Buckley et al.,[23] and Marik and Rivera.[24] They have concluded that such an approach is safe and will result in a controlled and predictable increase in the serum sodium concentration. Though overcorrection of sodium could be a concern with the use of conivaptan as with any other traditional agents in common use, none of these studies showed an added risk. Use of tolvaptan for correction of postoperative hyponatremia has also shown promising results as observed by Kimura et al.,[25] Nishi et al.,[26] Ichimura et al.,[27] and Geka.[28] However, to reduce risk of overcorrection, we omitted infusion of conivaptan following the initial bolus, and tolvaptan was started with a low dose and increased subsequently based on the response as recommended.
We used only a single dose of conivaptan in group C, whereas dose of oral tolvaptan was hiked on second day in group T. So comparisons were made between one group (C) where plasma concentration of conivaptan was decreasing and another group (T) where plasma concentration tolvaptan was increasing. This was done to find out the duration of efficacy of a single bolus of conivaptan as against an escalating dose of tolvaptan. The initial dose recommended for tolvaptan is 15 mg/day to be increased by 15 mg on a daily basis depending on response to a maximum of 60 mg/day, whereas a single bolus of conivaptan 20 mg was shown to have effect lasting upto 72 h.
We had also observed that in 18 patients (90%) in group T, the sodium correction in first 24 h was <4 mEq/L, which necessitated the dose of tolvaptan to be increased to 30 mg/day. But only one patient (5%) in group C needed infusion of conivaptan due to inadequate correction at 24 h. Hence it was inferred that following single-dose conivaptan and oral tolvaptan administration though there was a significant increase in sodium values from baseline in both the groups, conivaptan was better for faster correction within 24 h. By 48 h sodium levels in both groups reached almost similar levels. Though hypovolemia was ruled out clinically before initiating interventions, we did not administer diuretics and fluid intake was not restricted to avoid excessive diuresis and development of hypernatremia.
The same trend was observed in the negative fluid balance also. Throughout the study period, serum potassium levels remained well within normal limits presumably because the vaptans act through aquaresis preserving the electrolytes. The diminishing aquaretic effect observed in conivaptan group on second day could be because the drug was not administered on that day, whereas the progressive effect seen in tolvaptan group could be due to administration of a higher dose on the second day. The same could be the reason for the effect on sodium levels also. Though 10% of patients in group C developed thrombophlebitis, we were not able to attribute it solely to conivaptan as the same intravenous line was used for administration of other drugs as well.
The major drawbacks of our study were that it was not blinded and urine osmolality was not analyzed. We have compared efficacy of two drugs belonging to a same group with similar modes of action. However, as routes of administration were different, the bioavailability could also have been different. Fixed doses of conivaptan and tolvaptan were administered without taking into account the body weight of the patients. As tolvaptan was available as tablets, titration of the dose could not have been possible.
There was a lack of standardization of postoperative fluid management as some patients were exclusively on intravenous fluids, whereas some were receiving oral fluids as well depending on which postoperative day they became symptomatic and got recruited into the study. The study period was only 48 h in our study. An extended study period could have revealed the actual effective period of single intravenous bolus of conivaptan. We were unable to assess the effect of vaptan treatment on Intensive Care Unit (ICU) stay as many patients continued to remain in the ICU due to reasons other than symptomatic hyponatremia.
Conclusion
Single-dose intravenous conivaptan as well as oral tolvaptan were safe and effective in correcting hyponatremia in postoperative patients. Conivaptan could be considered superior as it resulted in faster sodium correction with effective aquaresis. Both vaptans maintained serum potassium at normal levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Upadhyay A, Jaber BL, Madias NE. Epidemiology of hyponatremia. Semin Nephrol. 2009;29:227–38. doi: 10.1016/j.semnephrol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Eaton J. Detection of hyponatremia in the PACU. J Perianesth Nurs. 2003;18:392–7. doi: 10.1016/j.jopan.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Mujtaba B, Sarmast AH, Shah NF, Showkat HI, Gupta RP. Hyponatremia in postoperative patients. Gen Med (Los Angel) 2016;4:224. [Google Scholar]
- 4.Sterns RH, Hix JK, Silver SM. Management of hyponatremia in the ICU. Chest. 2013;144:672–9. doi: 10.1378/chest.12-2600. [DOI] [PubMed] [Google Scholar]
- 5.Villabona C. Vasopressin receptor antagonists: The vaptans. Endocrinol Nutr. 2010;57:41–52. doi: 10.1016/S1575-0922(10)70021-8. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez M, Perez JA, Patel CB. Efficacy of 3% saline vs. conivaptan in achieving hyponatremia treatment goals. MDCVJ. 2013;IX:49–53. doi: 10.14797/mdcj-9-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hline SS, Pham PT, Pham PT, Aung MH, Pham PM, Pham PC. Conivaptan: A step forward in the treatment of hyponatremia? Ther Clin Risk Manage. 2008;4:315–26. doi: 10.2147/tcrm.s340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willett K. Conivaptan (Vaprisol) for the Treatment of Hyponatremia. Am Fam Physician. 2008;78:984–9. [Google Scholar]
- 9.Murphy T, Dhar R, Diringer M. Conivaptan bolus dosing for the correction of hyponatremia in the neurointensive care unit. Neurocrit Care. 2009;11:14. doi: 10.1007/s12028-008-9179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajan S, Srikumar S, Paul J, Kumar L. Effectiveness of single dose conivaptan for correction of hyponatraemia in post-operative patients following major head and neck surgeries. Indian J Anaesth. 2015;59:416–20. doi: 10.4103/0019-5049.160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy SN, Rangappa P, Jacob I, Janakiraman R, Rao K. Efficacy of conivaptan and hypertonic (3%) saline in treating hyponatremia due to syndrome of inappropriate antidiuretic hormone in a tertiary Intensive Care Unit. Indian J Crit Care Med. 2016;20:714–8. doi: 10.4103/0972-5229.195708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annane D, Decaux G, Smith N. Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am J Med Sci. 2009;337:28–36. doi: 10.1097/MAJ.0b013e31817b8148. [DOI] [PubMed] [Google Scholar]
- 13.Udelson JE, Smith WB, Hendrix GH, Painchaud CA, Ghazzi M, Thomas I, et al. Acute hemodynamic effects of conivaptan, a dual V1a and V2 vasopressin receptor antagonist, in patients with advanced heart failure. Circulation. 2001;104:2417–23. doi: 10.1161/hc4501.099313. [DOI] [PubMed] [Google Scholar]
- 14.Ghali JK, Koren MJ, Taylor JR, Brooks-Asplund E, Fan K, Long WA, et al. Efficacy and safety of oral conivaptan: A V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab. 2006;91:2145–52. doi: 10.1210/jc.2005-2287. [DOI] [PubMed] [Google Scholar]
- 15.Zmily HD, Daifallah S, Ghali JK. Tolvaptan, hyponatremia, and heart failure. Int J Nephrol Renovasc Dis. 2011;4:57–71. doi: 10.2147/IJNRD.S7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon MB, Lien YH. Tolvaptan and its potential in the treatment of hyponatremia. Ther Clin Risk Manag. 2008;4:1149–55. doi: 10.2147/tcrm.s3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly T, Chavez B. Tolvaptan (Samsca) for hyponatremia: Is it worth its salt? P T. 2009;34:543–7. [Google Scholar]
- 18.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 19.Horie S. Will introduction of tolvaptan change clinical practice in autosomal dominant polycystic kidney disease? Kidney Int. 2015;88:14–6. doi: 10.1038/ki.2015.143. [DOI] [PubMed] [Google Scholar]
- 20.Tzamaloukas AH, Shapiro JI, Raj DS, Murata GH, Glew RH, Malhotra D. Management of severe hyponatremia: Infusion of hypertonic saline and desmopressin or infusion of vasopressin inhibitors? Am J Med Sci. 2014;348:432–9. doi: 10.1097/MAJ.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potts MB, DeGiacomo AF, Deragopian L, Blevins LS., Jr Use of intravenous conivaptan in neurosurgical patients with hyponatremia from syndrome of inappropriate antidiuretic hormone secretion. Neurosurgery. 2011;69:268–73. doi: 10.1227/NEU.0b013e318218c78f. [DOI] [PubMed] [Google Scholar]
- 22.Breshears JD, Jiang B, Rowland NC, Kunwar S, Blevins LS. Use of conivaptan for management of hyponatremia following surgery for Cushing's disease. Clin Neurol Neurosurg. 2013;115:2358–61. doi: 10.1016/j.clineuro.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Buckley MS, Patel SA, Hattrup AE, Kazem NH, Jacobs SC, Culver MA. Conivaptan for treatment of hyponatremia in neurologic and neurosurgical adults. Ann Pharmacother. 2013;47:1194–200. doi: 10.1177/1060028013503126. [DOI] [PubMed] [Google Scholar]
- 24.Marik PE, Rivera R. Therapeutic effect of conivaptan bolus dosing in hyponatremic neurosurgical patients. Pharmacotherapy. 2013;33:51–5. doi: 10.1002/phar.1169. [DOI] [PubMed] [Google Scholar]
- 25.Kimura M, Nawata K, Kinoshita O, Hatano M, Imamura T, Kinugawa K, et al. Successful treatment of intractable fluid retention using tolvaptan after treatment for postoperative mediastinitis in a patient with a left ventricular assist device. Int Heart J. 2015;56:574–7. doi: 10.1536/ihj.14-412. [DOI] [PubMed] [Google Scholar]
- 26.Nishi H, Toda K, Miyagawa S, Yoshikawa Y, Fukushima S, Kawamura M, et al. Effects of tolvaptan in the early postoperative stage after heart valve surgery: Results of the STAR (Study of Tolvaptan for fluid retention AfteR valve surgery) trial. Surg Today. 2015;45:1542–51. doi: 10.1007/s00595-015-1251-y. [DOI] [PubMed] [Google Scholar]
- 27.Ichimura S, Fahlbusch R, Lüdemann W. Treatment of hyponatremia with tolvaptan in a patient after neurosurgical treatment of a pituitary tumor: Case report and review of literature. J Neurol Surg Rep. 2015;76:e279–81. doi: 10.1055/s-0035-1564605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geka K. Effectiveness of tolvaptan for postoperative heart failure in a patient with combined valvular disease and pulmonary hypertension. Japanese J Thorac Surg. 2014;67:117–20. [PubMed] [Google Scholar]


