Abstract
Background and Aims:
Etomidate induced myoclonus (EM) is a common and hazardous sequel. Premedication with a number of opioids has been shown to effectively attenuate EM. However, there is no reported literature evaluating the effect of nalbuphine pretreatment on EM. The present study was designed to evaluate the efficacy of 0.2 mg/kg nalbuphine intravenous (IV) pretreatment for prevention of EM.
Material and Methods:
This prospective randomized double-blind and placebo controlled study was conducted in a medical college associated tertiary hospital. One hundred patients undergoing elective surgeries under general anesthesia were randomly allocated to one of two groups to receive: 10 ml of normal saline (Group I) or 0.2 mg/kg nalbuphine in 10 ml of normal saline (Group II) 150 s before injection etomidate 0.3 mg/kg administered IV over 20 s. The patients were assessed for the presence and severity of etomidate induced vascular pain (EP) and EM while injecting etomidate and for the next 2 min, respectively. The patients were monitored for sedation, nausea/vomiting, headache, dizziness, and respiratory depression for 24 h postoperatively. Student's t-test, Chi-square test, or Fisher exact test were used wherever appropriate and P < 0.05 was considered statistically significant.
Results and Conclusion:
Both the groups were comparable with respect to demographic characteristics. Nalbuphine pretreatment significantly reduced the incidence (20% vs. 72%; respiratory rate = 0.294, 95% confidence interval: 0.160–0.496, P < 0.01) and severity of EM without any significant increase in the incidence of adverse effects. Nalbuphine 0.2 mg/kg IV pretreatment significantly reduces the incidence and severity of EM with side-effect profile comparable to saline placebo.
Keywords: Anesthesia, etomidate, mixed agonist-antagonists, myoclonus, nalbuphine, opioid
Introduction
The hemodynamic stability associated with etomidate makes it the induction agent of choice in patients with compromised hemodynamic or cardiac reserves.[1,2] However, its use is associated with etomidate induced vascular pain (EP) and myoclonus jeopardizing its therapeutic use.[3] The etomidate induced myoclonus (EM) seen in up to 80% of un-premedicated patients is hazardous in patients with open globe injuries (prolapse of vitreous material), nonfasted patients (risk of regurgitation and aspiration), and patients with cardiac compromise.[3,4,5] EM may also lead to the loss of intravenous (IV) access, displacement of the electrocardiogram electrodes, and postoperative patient discomfort.[6] These potentially hazardous sequelae warrant devising effective strategies to prevent or limit the EM. The etiology of EM is unclear. It may represent a seizure-like activity.[5] Agonist modulation of kappa opioid receptors has been shown to limit seizure activitiy.[7] Based on this hypothesis opioid agonist-antagonists butorphanol and dezocine (μ antagonist, kappa agonist) have recently been investigated and found to be effective in reducing the EM.[3,8,9] The multiple advantages of prolonged analgesia, ceiling effect to the drug induced respiratory depression, low abuse potential and being outside the realm of controlled substances act makes them an attractive substitute to opioid agonists both for perioperative analgesia and for the prevention of EM.
Nalbuphine is an opioid belonging to the agonist-antagonist group and is recommended for the management of moderate to severe pain. There is no reported study evaluating the role of nalbuphine for prevention of EM. Therefore, this study was designed to evaluate the efficacy and safety of nalbuphine for prevention of EM. We hypothesized that nalbuphine in analgesic dose (0.2 mg/kg of body weight IV) will be effective in reducing the intensity and severity of EM.
Material and Methods
This prospective, placebo controlled, randomized and double-blind study was conducted after approval by the institutional research ethical committee (SGRR/IEC/30/15). Written informed consent was obtained preoperatively from all the participants. One hundred adult (20–60 years) participants belonging to the American Society of Anesthesiologists (ASA) physical Status I or II undergoing laparoscopic cholecystectomy under planned general anesthesia were included. Participants with history of allergy to any of the study drugs, anticipated or unanticipated difficult airway, cardiac disease, pregnant or lactating females, significant hepatic or renal insufficiency and those who received sedatives, analgesics or opioids in the 24 h preoperatively were excluded from the study. The principles (ethical) for medical research involving human subjects as specified in the Declaration of Helsinki were strictly adhered to while conducting this study.
A detailed preanesthetic checkup was done. No premedication was given. The participants were kept fasting for 8 h preoperatively. In the operation theater baseline heart rate (HR), noninvasive systolic blood pressure (SBP), respiratory rate (RR), and peripheral oxygen saturation (SpO2) were recorded. A 20-gauge IV cannula was secured on the dorsum of left hand and connected to a Ringer lactate drip.
Randomization was achieved with the help of computer generated random number list. To ensure allocation concealment the randomization assignment was kept in sealed opaque envelopes opened at the time of the study drug preparation. The study solutions were prepared in identical 10 ml syringes outside the operation theatre and were labeled as the “study drug” by an independent anesthesiologist not involved further in the study. Depending upon the drug used as premedication the patients were allocated randomly into one of the two groups: Group I (placebo; n = 50): 10 ml of normal saline or Group II (nalbuphine; n = 50): 0.2 mg/kg of nalbuphine in 10 ml of normal saline. The patients as well as the anesthesiologist performing the induction were blinded to the group allocation.
One hundred and fifty seconds (time to onset of action of nalbuphine) after the pretreatment, anesthesia was induced with etomidate 0.3 mg/kg IV over 20 s while the patients were assessed for vascular pain using the following grading: 0: No pain, 1: Mild (pain reported only in response to questioning), 2: Moderate (pain reported spontaneously or pain reported in response to questioning with associated behavioral signs), 3: Severe (strong vocal response, arm withdrawal or tearing in the eyes). The patients were observed visually by an observer unaware of the group allocation for another 2 min for the presence of myoclonus defined as “involuntary short contractions of some muscle fibers or of a whole muscle or of different muscles of one group resulting in short observable movements in the body.”[3] The severity of myoclonus was graded as follows: 0: No myoclonus; 1: Mild (short movements of a body segment), 2: Moderate (mild movement of two different muscles) or 3: Severe myoclonus (clonic movements in two or more muscle groups or fast adduction of a limb).[3,10] If a patient had >1 episode of myoclonus during the 2 min observation period then the episode with the highest severity grading was recorded for statistical analysis. The time to first onset of myoclonus in seconds was recorded. During the 2 min observation period the ventilation was assisted with 100% oxygen. After the 2 min observation period, the group allocation was revealed to the concerned anesthesiologist and injection fentanyl 2 μg/kg IV was given to the placebo group and injection vecuronium bromide 0.1 mg/kg IV to both the groups. The patients were ventilated with 50% nitrous oxide and 1%–1.2% isoflurane in oxygen for 3 min. The patients were intubated with an appropriately sized endotracheal tube followed by a standardizedanesthesia and analgesia protocol. The HR, mean arterial pressure (MAP) and SpO2 (using Datex-Ohmeda, Cardiocap/5, GE Healthcare, Helsinki, Finland multichannel monitor) were monitored continuously and any episode of intraoperative bradycardia (HR <55/min) or hypotension (SBP <90 mmHg) was recorded and managed appropriately.
The primary outcome of our study was the incidence of EM. The secondary outcomes were the severity of EM, incidence of EP and nalbuphine related adverse effects (nausea, vomiting, dizziness, headache and respiratory depression).
The patients were assessed for sedation, nausea/vomiting, headache, dizziness, respiratory depression (RR < 10/min) at 2, 6, 12, and 24 h postoperatively by an anesthesiology resident unaware of the group allocation. The patients were assessed for sedation using the following sedation score: 1: Alert/oriented, 2: Sedated but responding to verbal commands, 3: Sedated but responsive to physical stimulation and 4: Sedated and unresponsive.
Assuming the incidence of EM as 70% in the placebo group with a clinically significant reduction rate as 0.35, a two-tailed alpha value of 0.05 and a power of 95% the sample size was calculated to be 49 patients per group. We decided to include fifty patients in each group. Statistical analyses were performed employing SPSS 19.0 (Statistical Package for Social Sciences, Chicago, IL, USA) for Windows. The continuous and categorical variables are presented as mean ± standard deviation and frequencies (percentages), respectively. The patient characteristics between the groups were compared using Student's t-test, Chi-square or Fisher exact test as appropriate. For all statistical tests, a P < 0.05 and 0.001 was taken to indicate a significant and highly significant difference, respectively.
Results
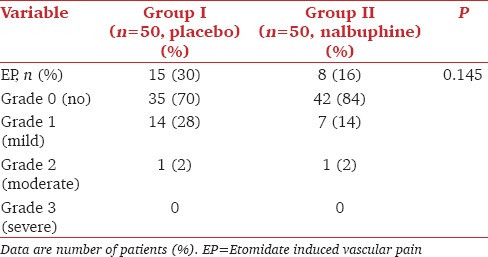
Mean age, weight, HR, MAP, distribution of sex, and ASA status were comparable between the groups [Table 1]. There were 15 (30%) and 8 (16%) patients who had EP in the control and nalbuphine group respectively [Table 2]. No statistically significant difference was observed with respect to the severity of EP, with one patient having moderate EP in each group. None of the patients had severe EP.
Table 1.
Demographics
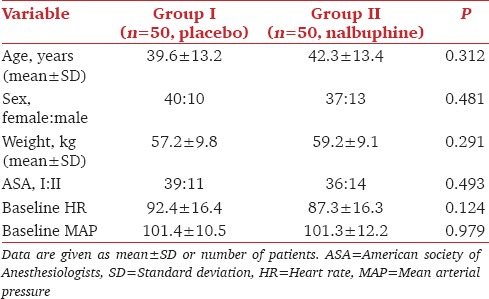
Table 2.
Incidence and severity of etomidate induced vascular pain (EP)
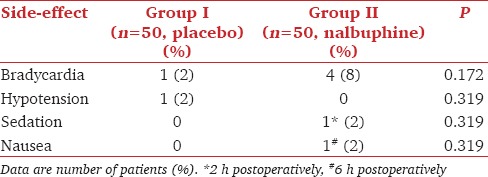
A statistically significant lower incidence of myoclonus both during the 1 and 2 min observation periods was observed with nalbuphine pretreatment compared with the control group (RR = 0.294, 95% confidence interval: 0.160–0.496, P < 0.001) [Table 3]. Nalbuphine pretreatment significantly reduced the severity of myoclonus with the mean intensity of EM during the 2 min observation period being 1.8 versus 0.3 in Group N versus Group P, respectively. Only 40% of those having myoclonus in the nalbuphine group had in the 1st min following etomidate administration compared to 70% of those in the control group. No statistically significant difference was observed among the groups with respect to the incidence of hemodynamic or any other side-effect [Table 4]. Mild sedation (Grade 2) was observed at 2 h postoperatively in only one patient in the nalbuphine group compared to 0 in group P. None of the patients in any of the group had postoperative respiratory depression.
Table 3.
Incidence and severity of etomidate induced myoclonus (EM)
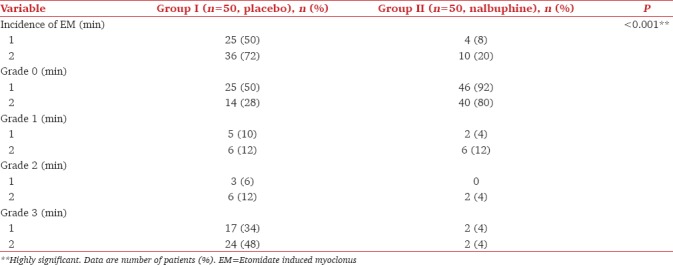
Table 4.
Side-effects
Discussion
Our study results indicate that 0.2 mg/kg nalbuphine pretreatment 150 s before etomidate effectively reduces the intensity and severity of EM. We chose 0.2 mg/kg as the study dose of nalbuphine as its equi-analgesic dose of butorphanol (2 mg) and fentanyl (100 μg) have been shown to effectively reduce the incidence as well as severity of EM.[3] The EM incidence of 20% found in the nalbuphine group lies in between that reported with butorphanol 2 mg (4%) and fentanyl 100 μg (24%). The varying affinity and intrinsic activity on κ-opioid receptors might be responsible for this observed variation in the inhibitory effect of equi-analgesic doses of different opioids on the EM.[3] Furthermore, 0.2 mg nalbuphine has been shown by other investigators to provide adequate postoperative analgesia, and prevent stress response to laryngoscopy without any significant side-effects.[11] Although a lower dose of butorphanol (0.015 mg/kg) and higher doses of fentanyl (up to 500 μg) have been found to be effective in reducing EM, they are either sub-analgesic doses, partially effective for EM or associated with higher incidence of apnea respectively.[12,13]
Nalbuphine is a synthetic strong opioid (μ antagonist, kappa agonist), effective in the dose range of 0.2–0.4 mg/kg.[14,15] Its faster onset, longer duration of action (3–6 h), reduced incidence of postoperative nausea, and vomiting and the absence of cardiovascular or respiratory depression makes it an attractive intraoperative analgesic.[11] The pretreatment in our study was administered 150 s before etomidate to justify its time to onset of action of 2–3 min.[11] The incidence of EM has been shown to vary with the speed and the rate of etomidate injection.[10,16,17] The incidence of EM in un-premedicated patients has been found to be 55%, 77%, and 84% depending on the observation period (1, 2, and 3 min, respectively).[16,18] Therefore, a standardized technique consisting of the IV injection of etomidate over 20 s followed by 2 min observation period was selected to avoid bias due to the above mentioned clinical factors. The incidence of myoclonus in the placebo group of 50% (at 1 min) and 72% (at 2 min) in our study is consistent with that observed by the previous authors with a similar speed of injection and the observation period.[16,18]
Among the neurophysiological hypotheses proposed behind the mechanism of EM, the ones proposed by the Kugler et al. and Doenicke et al. are most popular.[10,19] Although the excitatory effect of myoclonus has been hypothesized not to be generated by an epileptic focus, a number of drugs such as dexmedetomidine (alpha-2 agonism mediated reduction in the severity of convulsions), thiopentone sodium (N-methyl-D-aspartate receptor blockade and reduction of excitatory output from pyramidal cells), dezocine and butorphanol have been found to effective in alleviating EM based on the above mechanism.[3,9,12,14,20] A number of randomized controlled trials have demonstrated multitude of opioids such as fentanyl, sufentanil, alfentanil, remifentanil, butorphanol, and dezocine to be effective in reducing the incidence and severity of EM with the site of their inhibitory action still being obscure.[4,9,12,14,15,16,21,22,23] Although pretreatment with pure agonists like fentanyl and remifentanil effectively reduces EM, their use is associated with higher incidence of apnea, nausea, vomiting, and bradycardia compared to the placebo.[4] The low incidence of side-effects like bradycardia (4%) sedation (2%) and nausea (2%) observed with nalbuphine was comparable to the placebo group and in accordance with findings by previous authors.[10]
The main limitation of our study is that we used only a single dose (0.2 mg/kg) of nalbuphine. A dose-response decrease in the incidence of EM has been established with increasing doses of fentanyl and dexmedetomidine by previous authors.[13,24] Nalbuphine 0.2 mg/kg provides satisfactory postoperative analgesia as well as effectively blunts the sympathetic response to laryngoscopy and intubation. Therefore, through our study we sought to establish the effect of the most commonly employed dose, that is, 0.2 mg/kg of nalbuphine on EM. Establishing a dose-response relationship between EM and varying doses of nalbuphine after establishing a drug-response relationship as demonstrated by our study is a way-forward.
Conclusion
Pretreatment with nalbuphine 0.2 mg/kg IV 150 s before etomidate injection significantly reduces the incidence and severity of EM without any significant increase in adverse-effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Erdoes G, Basciani RM, Eberle B. Etomidate – A review of robust evidence for its use in various clinical scenarios. Acta Anaesthesiol Scand. 2014;58:380–9. doi: 10.1111/aas.12289. [DOI] [PubMed] [Google Scholar]
- 2.Patkar CS, Baldwa N, Dave S, Gujjar P. Perioperative anaesthetic management of phaeochromocytoma associated with uncorrected tetralogy of Fallot. Indian J Anaesth. 2015;59:816–8. doi: 10.4103/0019-5049.171591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Bao R, Zhu J, Liu Z, Meng Y, Fan X, et al. Pretreatment with butorphanol reduces myoclonus after etomidate. J Anesthesiol Clin Sci. 2013;2:2. [Google Scholar]
- 4.Berry JM, Merin RG. Etomidate myoclonus and the open globe. Anesth Analg. 1989;69:256–9. [PubMed] [Google Scholar]
- 5.Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: Seizures and general anesthetic drugs. Anesth Analg. 2008;107:1689–703. doi: 10.1213/ane.0b013e3181852595. [DOI] [PubMed] [Google Scholar]
- 6.Van Keulen SG, Burton JH. Myoclonus associated with etomidate for ED procedural sedation and analgesia. Am J Emerg Med. 2003;21:556–8. doi: 10.1016/j.ajem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: Anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130(Pt 4):1017–28. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Li QB, Wu YY, Wang BN, Kang JL, Xu XW. Efficacy and safty of opioids for the prevention of etomidate-induced myoclonus: A meta-analysis. Am J Ther. 2016 doi: 10.1097/MJT.0000000000000404. DOI:10.1097/MJT.0000000000000404 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.He L, Ding Y, Chen H, Qian Y, Li Z. Dezocine pretreatment prevents myoclonus induced by etomidate: A randomized, double-blinded controlled trial. J Anesth. 2015;29:143–5. doi: 10.1007/s00540-014-1854-2. [DOI] [PubMed] [Google Scholar]
- 10.Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. Reducing myoclonus after etomidate. Anesthesiology. 1999;90:113–9. doi: 10.1097/00000542-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Chawda PM, Pareek MK, Mehta KD. Effect of nalbuphine on haemodynamic response to orotracheal intubation. J Anaesthesiol Clin Pharmacol. 2010;26:458–60. [PMC free article] [PubMed] [Google Scholar]
- 12.He L, Ding Y, Chen H, Qian Y, Li Z. Butorphanol pre-treatment prevents myoclonus induced by etomidate: A randomised, double-blind, controlled clinical trial. Swiss Med Wkly. 2014;144:w14042. doi: 10.4414/smw.2014.14042. [DOI] [PubMed] [Google Scholar]
- 13.Stockham RJ, Stanley TH, Pace NL, Gillmor S, Groen F, Hilkens P. Fentanyl pretreatment modifies anaesthetic induction with etomidate. Anaesth Intensive Care. 1988;16:171–6. doi: 10.1177/0310057X8801600207. [DOI] [PubMed] [Google Scholar]
- 14.Lv Z, Fang J, Zhu J, Liang B, Li F, Jiang S, et al. Intravenous dezocine pretreatment reduces the incidence and intensity of myoclonus induced by etomidate. J Anesth. 2014;28:944–7. doi: 10.1007/s00540-014-1842-6. [DOI] [PubMed] [Google Scholar]
- 15.Zeng Z, Lu J, Shu C, Chen Y, Guo T, Wu QP, et al. A comparision of nalbuphine with morphine for analgesic effects and safety: Meta-analysis of randomized controlled trials. Sci Rep. 2015;5:10927. doi: 10.1038/srep10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do SH, Han SH, Park SH, Kim JH, Hwang JY, Son IL, et al. The effect of injection rate on etomidate-induced myoclonus. Korean J Anesthesiol. 2008;55:305–7. [Google Scholar]
- 17.Isitemiz I, Uzman S, Toptas M, Vahapoglu A, Gül YG, Inal FY, et al. Prevention of etomidate-induced myoclonus: Which is superior: Fentanyl, midazolam, or a combination? A Retrospective comparative study. Med Sci Monit. 2014;20:262–7. doi: 10.12659/MSM.889833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satılmıs T, Guler A, Pamuk AG. The comparison of different doses of magnesium sulfate to prevent myoclonus after etomidate induction. J Turk Anaesth Int Care. 2010;38:184–9. [Google Scholar]
- 19.Suttmann H, Doenicke A, Kugler J, Laub M. A new formulation of etomidate in lipid emulsion – Bioavailability and venous provocation. Anaesthesist. 1989;38:421–3. [PubMed] [Google Scholar]
- 20.Mizrak A, Koruk S, Bilgi M, Kocamer B, Erkutlu I, Ganidagli S, et al. Pretreatment with dexmedetomidine or thiopentone decreases myoclonus after etomidate: A randomized double blind controlled trial. J Surg Res. 2010;159:e11–6. doi: 10.1016/j.jss.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 21.Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47:482–4. doi: 10.1034/j.1399-6576.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 22.Khalil SN, Lawson KS, Hanis CL, Lemak NA, Ruiz RS. Alfentanil decreases myoclonus caused by etomidate. Middle East J Anaesthesiol. 1999;15:185–92. [PubMed] [Google Scholar]
- 23.Kelsaka E, Karakaya D, Sarihasan B, Baris S. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth. 2006;18:83–6. doi: 10.1016/j.jclinane.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Luan HF, Zhao ZB, Feng JY, Cui JZ, Zhang XB, Zhu P, et al. Prevention of etomidate-induced myoclonus during anesthetic induction by pretreatment with dexmedetomidine. Braz J Med Biol Res. 2015;48:186–90. doi: 10.1590/1414-431X20144100. [DOI] [PMC free article] [PubMed] [Google Scholar]


