Abstract
Background
Atrioventricular nodal reentrant tachycardia (AVNRT) is one of the most common supraventricular tachycardia substrates. The aim of this study was to demonstrate the excellent outcomes of cryoablation without fluoroscopy in pediatric patients with AVNRT.
Methods
From September 2015 to October 2016, a transcatheter cryoablation procedure was performed in 109 patients using the EnSite® system. After electrophysiologic studies, a cryoablation catheter was advanced for the purpose of ablation of the slow pathway. Six to eight lesions were delivered in 240-300 seconds at -70 °C, with special effort being paid to obtain an eyeball formation around the first effective lesion.
Results
The mean weight and age of the patients were 24.6 ± 5.3 kg (15-68 kg) and 9.8 ± 2.6 years (5-18 years), respectively. The mean procedure time was 109.8 ± 46 minutes, and the acute procedural success rate was excellent (100%). Ablation procedures were performed during induced tachycardia in 67 patients and during sinus rhythm in 42. The mean follow-up period was 13.3 ± 5.8 months (4-17 months). Recurrence was noted in one patient during the follow-up period who received the ablation procedure with a 6-mm tip catheter. No recurrence was noted among the patients treated with an 8-mm cryocatheter. No permanent cryoablation-related complications occurred.
Conclusions
Cryoablation using an electroanatomic mapping system is safe and effective in pediatric patients with AVNRT, and has the advantage of avoiding ionizing radiation.
Keywords: Atrioventricular reentrant tachycardia, Cryoablation, Electroanatomic mapping, Zero-fluoroscopy
INTRODUCTION
Atrioventricular nodal reentrant tachycardia (AVNRT) is one of the most common supraventricular tachycardia (SVT) substrates in children, accounting for 13-24% of all pediatric cases. The incidence of AVNRT increases with age, and it has been reported to be responsible for up to 31% of SVTs in children older than 10 years of age. Even though AVNRT often occurs in patients with structurally normal hearts and is usually well tolerated, patients may present with signs and symptoms of hemodynamic compromise and syncope.1-8
Transcatheter radiofrequency (RF) and cryoablation are well established, safe and feasible techniques for the treatment of AVNRT in pediatric populations.9-14 Conventionally, X-rays have been used for the navigation of ablation catheters. Recently, however, these procedures have been shown to be safe and feasible via electroanatomic mapping systems without using radiation.14,15
Each hour of fluoroscopic imaging has been reported to increase the lifetime risk of a fatal malignancy by up to 1%.16 Radiation exposure should therefore be of particular concern in children due to their longer expected lifespan. Accordingly, the goal should be to keep ionizing radiation burden in children to a minimum to reduce long-term cancer risk.16-18 In this study, we report a series of pediatric patients with AVNRT who underwent successful catheter cryoablation procedures without the use of fluoroscopy. The aim of this study was to demonstrate the excellent outcomes of cryoablation techniques performed with an electroanatomic navigation system without fluoroscopy in a large patient cohort with AVNRT.
MATERIALS AND METHODS
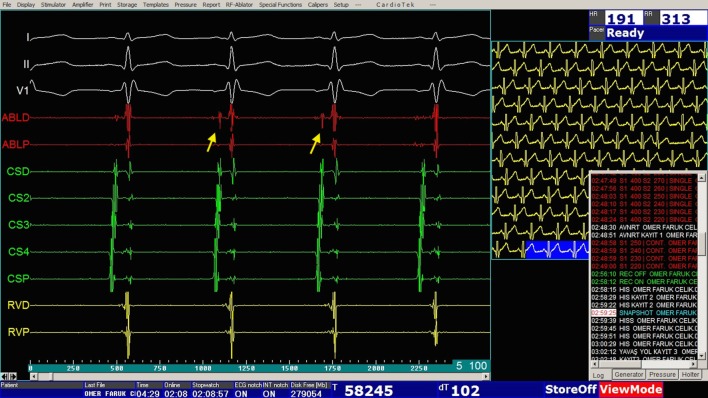
From September 2015 to August 2016, transcatheter cryoablation of slow pathways was performed in 109 patients. Informed consent was obtained from all patients or their legal guardians before the procedure. The study was approved by the local ethics committee. All procedures were performed under general anesthesia with endotracheal intubation. Antiarrhythmic drugs were stopped for at least five half-lives prior to the procedure. Two 5 or 6 French (Fr) standard sheaths were introduced into the left femoral vein, and the right femoral vein was cannulated with a 7 Fr sheath. Arterial cannulation was not required in any patient. A standard electrophysiological study was then performed with the guidance of an electroanatomic system (EnSite system, St. Jude Medical, St. Paul, MN, USA). The EnSite system allows for the reconstitution of 3-D geometry of cardiac chambers and visualization and navigation of ablation catheters using the signal characteristics of cardiac structures. For this purpose, a decapolar electrode catheter was advanced into the coronary sinus and then two quadripolar electrode catheters were placed at the right ventricle apex and high right atrium (HRA). We did not use a fourth catheter to avoid introducing an extra sheath into pediatric patients. Instead, we placed the HRA catheter in the His region when needed. Programmed stimulations were then performed to prove the presence of dual atrioventricular (AV) node physiology as described elsewhere in detail.19 A repeat programmed stimulation was undertaken during isoproterenol infusion, when a sustained tachycardia could not be induced. The area yielding prominent His signals (Figure 1) was marked on a 3-D electroanatomic map (Figure 2).
Figure 1.
An electrogram demonstrating His bundle signals (arrows).
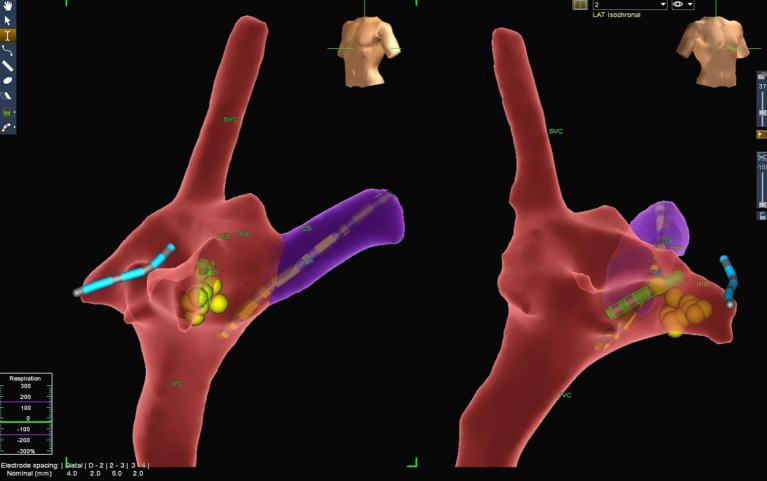
Figure 2.
3D anatomy of the right atrium (RA), superior vena cava (SVC), inferior vena cava (IVC), coronary sinus (CS) and his bundle region (HIS) are demonstrated in left anterior oblique and right anterior oblique views. The coronary sinus catheter (yellow), cryoablation catheter (green), right ventricle catheter (blue) and cryolesions given (yellow dots) are shown on the 3D electroanatomic mapping system.
The diagnosis of typical AVNRT was established when a septal ventriculo-atrial interval was determined to be < 70 ms. The diagnosis of atypical AVNRT was made when the postpacing interval minus the tachycardia cycle length was > 115 ms and via parahisian pacing and differential pacing (RV apical-basal pacing) maneuvers. The diagnosis of atrial tachycardia was made when an atrial-atrial-ventricular response after cessation of ventricular overdrive pacing was observed. Patients in whom the underlying mechanism of SVT was determined to be AVNRT were included into the study.
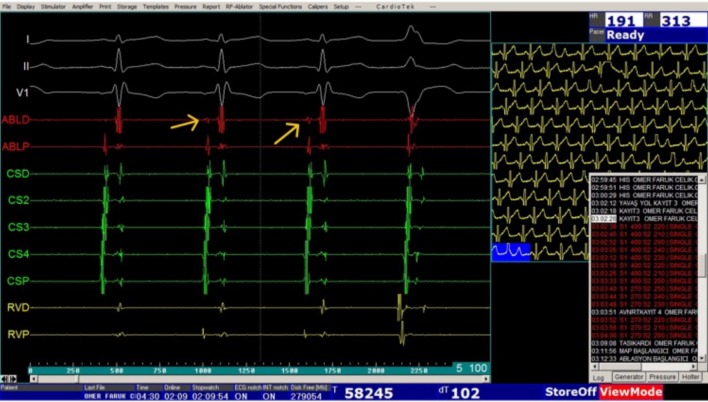
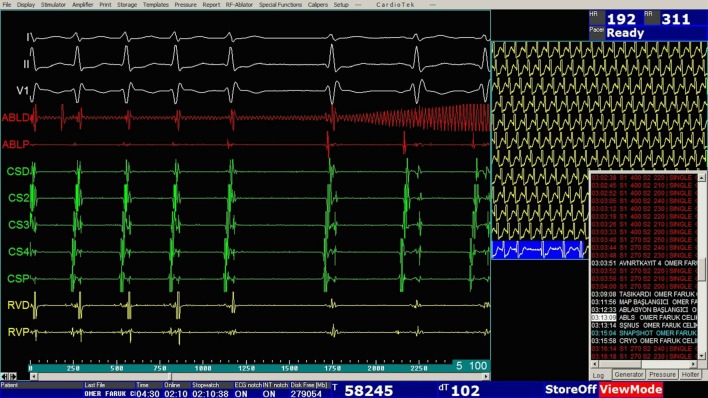
After electrophysiologic studies, a standard cryoablation catheter (Freezor Xtra, Medtronic, Minneapolis, MN, USA) with a 6- or 8-mm tip was advanced to the right atrium for the purpose of ablation of the slow pathway. An 8-mm tip catheter was employed in patients over 35 kg in weight. Cryomapping was performed using a cryoablation catheter at -30 °C for safe arrhythmia ablation whenever applicable. During sinus rhythm, the tip of the cryoablation catheter was placed at the posteroseptal tricuspid annulus. The area yielding an electrogram with an A:V ratio of 1:3 to 1:4 and/or slow pathway signals was considered to be the target area for cryoablation (Figure 3). During induced sustained tachycardia, the target area was identified by the ability of the cryoablation catheter to cease the induced SVT at -30 °C in less than 30 seconds (Figure 4). Six to eight lesions were delivered in 240-300 seconds at -70 °C, with the utmost attention being given to the development of AV conduction delay, and with special effort being paid to obtain an eyeball formation around the first effective lesion (Figure 2). The activation intervals between atria and ventricles were monitored via the EnSite system. Ablation was ceased immediately when any sign of sudden AV conduction delay was noted.
Figure 3.
An electrogram showing the slow-pathway signals (arrows).
Figure 4.
An electrogram demonstrating the cessation of tachycardia during cryomapping. Cryomapping was then switched to cryoablation at -70 °C.
We considered the procedure to be successful when no more than one echo beat could be induced on programmed stimulation or a complete conduction block through the slow pathway was noted 30 minutes after the final ablation lesion. All patients were observed in the hospital for at least 24 hours, especially for the development of complete AV block. Electrocardiography (ECG) and echocardiographic examinations were performed in the morning after the procedure day. Clinical examinations, ECG, echocardiography and Holter ECG were scheduled at 1 month and then at 6 and 12 months after the procedure. Complaints including fatigue, palpitation and syncope were recorded at each visit. Patients complaining of palpitation received ECG, additional 24-hour Holter ECG and event recorder monitoring for 7 days. Recurrence was defined as the presence of clinical symptoms consistent with those prior to the ablation procedure or documented tachycardia episodes on ECG, 24-hour Holter monitoring or event recorder.
Statistical analysis
SPSS 22.0 (IBM Corporation, Armonk, New York, United States) was used to analyze all data. Quantitative data were expressed as mean ± SD (standard deviation) and median range (maximum-minimum) values. Categorical values were stated as n (number) and % (percentage).
RESULTS
One-hundred and nine pediatric patients with AVNRT underwent transcatheter cryoablation during the study period, of whom 52 were male and 57 were female. The mean weight of the patients was 24.6 ± 5.3 kg (15-68 kg), and the mean age was 9.8 ± 2.6 years (5-18 years). The mean procedure time was 109.8 ± 46 minutes (Table 1). A mitral valve prolapse was present in two patients and an atrial septal defect was detected in one. No fluoroscopy was needed in any patient.
Table 1. Demographic, procedural characteristics and follow-up data.
| Age, years (min, max) | 9.8 ± 2.6 (5-18) |
| Male | 52 (48%) |
| Weight, kg (min, max) | 24.6 ± 5.3 (15-68) |
| Procedure time, minute | 109.8 ± 46 |
| Follow up, month (min, max) | 13.3 ± 5.8 (4-17) |
| Acute procedural success | 109/109 (100%) |
| Typical AVNRT (slow-fast) | 94 (86%) |
| Fast-slow AVNRT | 13 (12%) |
| Slow-slow AVNRT | 2 (2%) |
| 6 mm cryoablation catheter | 93 (85%) |
| 8 mm cryoablation catheter | 16 (15%) |
| Ablation during tachycardia | 67 (61%) |
| Ablation during sinus rhythm | 42 (39%) |
| First degree atrioventricular block | 3 (2.7%) |
| Permenant complete atrioventricular block | 0 (0%) |
| Incomplete right bundle branch block | 3 (2.7%) |
| Nodal rhythm | 9 (8.2%) |
| Recurrence | 1 (0.9%) |
AVNRT, atrioventricular nodal reentrant tachycardia; kg, kilogram; max, maximum; min, minimum; mm, milimeter.
The nature of AVNRT was typical in 94 patients. An atypical AVNRT was noted due to fast-slow conduction in 13 patients and slow-slow conduction in two patients. The procedures were performed using a 6-mm tip catheter in 93 patients and an 8-mm tip in 16 patients. Eighty-nine patients had had a documented SVT prior to the procedure, while 20 patients underwent an electrophysiologic study due to likely symptoms of SVT.
The acute success rate of the interventions was excellent (100%). Ablation procedures were performed during induced tachycardia in 67 patients and during sinus rhythm in 42. A single echo beat could be induced in 20 patients at the end of the procedures. None of the patients experienced recurrence during follow-up.
The mean follow-up period was 13.3 ± 5.8 months (range: 4-17 months). Fourteen patients complained of palpitation after the procedures throughout the study period. The rhythm documented on ECG, Holter ECG or event recorder during palpitation was sinus tachycardia in 13 patients. Recurrence was noted in one patient during follow-up. An ECG performed during palpitation revealed recurrent SVT. This patient had received the ablation procedure with a 6-mm tip catheter. In this patient, a second cryoablation procedure was scheduled. No recurrence was noted among the patients in the 8-mm group.
No permanent cryoablation-related complications occurred. A sudden AV conduction delay during cryoablation was detected in four patients throughout the study period. Ablation was ceased immediately when any sign of AV conduction delay was noted. In this situation, we interrupted the procedure until normalization of AV conduction. Three patients suffered from an incomplete right bundle branch block, which resolved spontaneously at the end of the procedure on the next day and 1 week later, respectively. A nodal rhythm was encountered in nine patients during the procedures. A first degree and transient 2:1 second degree AV block developed in three and two patients, respectively.
DISCUSSION
In this study, we demonstrated the outcomes of cryoablation procedures performed through the EnSite system without using fluoroscopy in a pediatric cohort with AVNRT. The acute success rate of the procedures was excellent with a very satisfying recurrence rate in the follow-up period.
Transcatheter ablation therapy has evolved as a safe and feasible treatment option in patients with AVNRT during the last 25 years.5,12,20 RF is the energy source used for slow pathway ablation in these procedures, with high success and low recurrence rates, and RF ablation is considered to be the treatment of choice in patients with AVNRT. However, the procedure is associated with a non-negligible risk of AV block and concerns have been raised with regards to its safety.5,9 Cryoablation has been introduced as an alternative method to ablation to overcome this safety issue due to its advantages, namely "cryomapping" and "cryoadhesion". Cryoablation enables the application of low energy and thus slow formation of a reversible lesion to check the position of the ablation catheter and proximity of the lesion to the AV node (cryomapping). Moreover, the freezing catheter tip firmly attaches to underlying myocardium enabling improved catheter stability (cryoadherence). Owing to these features, cryoablation has become more popular than RF ablation, with comparable success and recurrence rates and virtually no risk of AV block.5,9,21 Nonetheless, cryomapping has remained impossible with 8-mm tip cryocatheters. In our series, we paid the utmost attention to starting cryoablation far away from His and antegrade fast pathway regions using these catheters. If three attempts of 30 seconds of cryoablation were unsuccessful to cease the tachycardia (as in four patients in our series), we performed the cryoablation during sinus rhythm. We did not experience any antegrade fast pathway damage in this study. To the best of our knowledge, there have been no reports of permanent AV block due to cryoablation in pediatric patients with supraventricular arrhythmia substrates. Therefore, we only used cryoenergy in our patients with AVNRT.
Prior studies have shown that the rate of freedom from recurrent AVNRT is higher in patients treated with RF compared to cryoablation. A recent review study including 14 studies with more than 2300 patients with AVNRT revealed that freedom from recurrent AVNRT was achieved in 96.5% of the patients who received RF, compared to 90.9% of those who received cryoablation after a median follow-up of 10.5 months (p < 0.001).5 In our cohort, freedom from recurrent AVNRT was achieved in 99% of the patients with a similar follow-up period. Our favorable results may be due to the number of lesions given, the eyeball formation obtained around the first effective lesion, the use of 8-mm tip catheters, and the use of an electroanatomic mapping system for guidance.
Conventionally, X-rays have been used for catheter navigation in transcatheter ablation procedures. Children are very susceptible to ionizing radiation beginning from fetal life.16 Pearce et al. demonstrated a significant association between radiation exposure and leukemia and brain tumors in children undergoing one or more computed tomography scan. This association was found to be dose dependent, and a cumulative dose of about 50 mGy was found to increase the risk of leukemia by three times.18,22 Cho et al. evaluated 23 patients with AVNRT who underwent RF ablation, and demonstrated that the patients were exposed to a mean effective dose of 20.7 (5.1-65.3) mGy of radiation during each procedure.23 Recently, electroanatomic mapping systems have been introduced to offer significant reductions in fluoroscopy in catheter ablation procedures without compromising the safety and efficacy of the procedures. These systems have been proven to be safe and feasible in pediatric and pregnant patients, increasing the accuracy of the ablation techniques and reducing radiation exposure of the fetuses, children and medical team.15,16,24 The EnSite system is capable of visualizing the real time position and orientation of an ablation catheter, creating an activation or voltage map of a cardiac chamber which can then be used to define the underlying mechanism of arrhythmias and tagging of important cardiac structures for the ablation procedure which is useful for guiding the ablative therapy.15,16,24 Fluoroscopy may be needed for guidance in particular AVNRT patients with systemic vein anomalies, however, we did not encounter any issues reaching the right atrium through femoral veins in any patient. All procedures were straightforward through the EnSite system with no significant and/ or persistent complications.
Limitations
Patients complaining of palpitation received ECG, additional 24-hour Holter ECG and event recorder monitoring for 7 days. However, it is still possible that the SVT may not have been demonstrated in some patients. In addition, the relatively short duration of the study period kept us from making certain conclusions on recurrence rates.
CONCLUSIONS
We suggest that cryoablation via an electroanatomic mapping system is safe and effective in patients with AVNRT. It has the benefits of avoiding ionizing radiation exposure for both the patient and operator, thus reducing the lifetime risk of malignancy for this population.
CONFLICT OF INTEREST
None.
FUNDING
There was no funding.
ETHICAL APPROVAL
The study protocol was approved by local ethics committee.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in this study.
REFERENCES
- 1.Drago F, Placidi S, Righi D, et al. Cryoablation of AVNRT in children and adolescents: early intervention leads to a better outcome. J Cardiovasc Electrophysiol. 2014;25:398–403. doi: 10.1111/jce.12339. [DOI] [PubMed] [Google Scholar]
- 2.Reddy CD, Silka MJ, Bar-Cohen Y. A comparison of AV nodal reentrant tachycardia in young children and adolescents: electrophysiology, ablation, and outcomes. Pacing Clin Electrophysiol. 2015;38:1325–1332. doi: 10.1111/pace.12699. [DOI] [PubMed] [Google Scholar]
- 3.Ko JK, Deal BJ, Strasburger JF, et al. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 4.Hanash CR, Crosson JE. Emergency diagnosis and management of pediatric arrhythmias. J Emerg Trauma Shock. 2010;3:251–260. doi: 10.4103/0974-2700.66525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santangeli P, Proietti R, Di Biase L, et al. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol. 2014;39:111–119. doi: 10.1007/s10840-013-9842-2. [DOI] [PubMed] [Google Scholar]
- 6.Blaufox AD, Rhodes JF, Fishberger SB. Age related changes in dual AV nodal physiology. Pacing Clin Electrophysiol. 2000;23:477–480. doi: 10.1111/j.1540-8159.2000.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 7.Karakurt C, Türköz R, Sarıtaş B, et al. Huge left atrial pseudoaneurysm in a 5-month-old baby presented with supraventricular tachycardia. Acta Cardiol Sin. 2016;32:108–111. doi: 10.6515/ACS20150514A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasano T, Takahashi K, Sugiyama K. Gene therapy for cardiac arrhythmias. Acta Cardiol Sin. 2013;29:226–234. [PMC free article] [PubMed] [Google Scholar]
- 9.Oster ME, Yang Z, Stewart-Huey K, et al. Radiofrequency ablation versus cryoablation for atrioventricular nodal re-entrant tachycardia in children: a value comparison. Cardiol Young. 2017;27:224–228. doi: 10.1017/S1047951116000299. [DOI] [PubMed] [Google Scholar]
- 10.Amasyali B, Kilic A, Kabul K, et al. Atrioventricular nodal reentrant tachycardia ablation with radiofrequency energy during ongoing tachycardia: is it feasible? Postep Kardiol Inter. 2014;10:301–307. doi: 10.5114/pwki.2014.46775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karadeniz C, Akdeniz C, Turan O, Tuzcu V. Cryoablation of septal accessory pathways in children: midterm results. Pacing Clin Electrophysiol. 2014;37:1095–1099. doi: 10.1111/pace.12442. [DOI] [PubMed] [Google Scholar]
- 12.Krause U, Backhoff D, Klehs S, et al. Catheter ablation of pediatric AV nodal reentrant tachycardia: results in small children. Clin Res Cardiol. 2015;104:990–997. doi: 10.1007/s00392-015-0868-6. [DOI] [PubMed] [Google Scholar]
- 13.Apiyasawat S, Prasertwitayakij N, Ngarmukos T, et al. Feasibility, efficacy, and safety of radiofrequency catheter ablation for cardiac arrhythmias: a twelve-year experience in Thailand. J Med Assoc Thai. 2010;93:272–277. [PubMed] [Google Scholar]
- 14.Scaglione M, Ebrille E, Caponi D, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol. 2013;36:1460–1467. doi: 10.1111/pace.12183. [DOI] [PubMed] [Google Scholar]
- 15.Tuzcu V. Significant reduction of fluoroscopy in pediatric catheter ablation procedures: long-term experience from a single center. Pacing Clin Electrophysiol. 2012;35:1067–1073. doi: 10.1111/j.1540-8159.2012.03472.x. [DOI] [PubMed] [Google Scholar]
- 16.Tuzcu V, Gul EE, Erdem A, et al. Cardiac interventions in pregnant patients without fluoroscopy. Pediatr Cardiol. 2015;36:1304–1307. doi: 10.1007/s00246-015-1181-x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JN, Hornik CP, Li JS, et al. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation. 2014;30:161–167. doi: 10.1161/CIRCULATIONAHA.113.005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertram H, Windhagen-Mahnert B, Bökenkamp R, et al. Abbreviated combined anatomical/electrophysiological approach for catheter ablation of atrioventricular nodal reentrant tachycardia in children. Cardiol Young. 2001;11:182–187. doi: 10.1017/s1047951101000087. [DOI] [PubMed] [Google Scholar]
- 20.Jackman WM, Beckman KJ, McClelland JH. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992;327:313–318. doi: 10.1056/NEJM199207303270504. [DOI] [PubMed] [Google Scholar]
- 21.Papagiannis J, Papadopoulou K, Rammos S. Cryoablation versus radiofrequency ablation for atrioventricular nodal reenterant tachycardia in children: long term results. Hellenic J Cardiol. 2010;51:122–126. [PubMed] [Google Scholar]
- 22.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho HO, Park HS, Choi HC. Radiation dose and cancer risk of cardiac electrophysiology procedures. Int J Arrhythm. 2015;16:4–10. [Google Scholar]
- 24.Bhakta D, Miller JM. Principles of electroanatomic mapping. Indian Pacing Electrophysiol J. 2008;8:32–50. [PMC free article] [PubMed] [Google Scholar]