Abstract
Background
The aim of this study was to assess the acute hemodynamic effects of remote ischemic preconditioning (RIPC) on coronary perfusion pressure and coronary collateral blood flow.
Methods
A total of 17 patients with coronary heart disease with severe (70%-85%) stenosis in one or two vessels confirmed by angiography were enrolled into this study. They were randomly divided into the RIPC group (9 patients) and the control group (8 patients). Distal pressure of coronary artery stenosis before balloon dilation (non-occlusive pressure, Pn-occl) and distal coronary artery occlusive pressure (Poccl) during balloon dilation occlusion were measured in all patients. The patients in the RIPC group received three cycles of lower limb ischemia-reperfusion preconditioning (5 minutes inflation of a blood pressure cuff, followed by 5 minutes reperfusion). For controls, the cuff was not inflated. After this process, Pn-occl and Poccl were measured again in each patient.
Results
There were no significant differences in angiographic characteristics between the two groups (all p > 0.05). Troponin I (TNI) levels after percutaneous coronary intervention (PCI) were lower in the RIPC group than in the control group (p = 0.004). In the RIPC group, mean Pn-occl and Poccl were significantly increased after RIPC compared to before RIPC [(72.78 ± 10.10) mmHg vs. (79.67 ± 9.79) mmHg, p = 0.002, (20.89 ± 8.61) mmHg vs. (26.78 ± 10.73) mmHg, p = 0.001, respectively].
Conclusions
RIPC can improve distal coronary perfusion pressure and rapidly increase distal coronary occlusive pressure thereby improving coronary collateral blood flow.
Keywords: Collateral, Coronary heart disease, Ischemic preconditioning
INTRODUCTION
Myocardial protection by preconditioning was first described in a canine experimental model in 1986. In that study, several brief periods of myocardial ischemia (ischemic preconditioning) were found to substantially reduce the extent of infarction after restoration of blood flow.1 The most powerful intervention in animals to augment the benefits of reperfusion is remote ischemic conditioning (RIC), which consists of cycles of brief ischemia and reperfusion to an organ or tissue remote from the heart.2
Due to the reduced myocardial injury during coronary angioplasty and thrombolytic therapy, RIC has demonstrated clinical value. Remote preconditioning has also been recently introduced. Transient episodes of ischemia and reperfusion of organs or tissues far from the heart (such as kidneys, intestine and skeletal muscles) can not only reduce the subsequent long period of ischemic injury of the organ or tissue itself, but also protect the remote heart.3 This new concept expands the clinical application and suggests a more significant clinical role, since ischemia and reperfusion could be performed on limbs noninvasively. This study aimed to assess the acute hemodynamic effects of remote ischemic preconditioning (RIPC) on coronary perfusion pressure and coronary collateral blood flow.
MATERIALS AND METHODS
Study population
Between June 2015 and October 2015, patients with coronary heart disease confirmed by angiography were enrolled into this study. The inclusion criteria were as follows: one or two major vessels with stenosis of 70%-85% confirmed by coronary angiography, with one vessel with type A or type B lesion chosen as the target vessel. The exclusion criteria were: acute myocardial infarction within 1 month, severe stenosis of the main stem (> 50%), target vessel lesion stenosis > 85%, distal target vessel diffused lesion or severe three-vessel lesions, and patients with infectious diseases, tumors, hematological system diseases, significant congestive heart failure, serious arrhythmia, uncontrolled hypertension, and diabetic mellitus. The eligible patients were randomly divided into the RIPC group and control group. This protocol was approved by the Ethics Committee of our hospital before implementation. Written informed consent to participate in this study was obtained from each participant.
Biochemical measurements
Venous blood samples were collected on the morning of admission to the hospital and again the next morning after a percutaneous coronary intervention (PCI) to measure levels of serum troponin I (TNI). Serum levels of alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TGs), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and creatinine were measured using a clinical automated biochemistry analyzer (Hitachi HCP-7600, Japan).
Study designand procedures
Coronary angiography and PCI data were obtained using a digital angiography machine (INOVA 2000, GE Company, USA), and coronary blood pressure was recorded using a multichannel electrophysiology recorder (Cardiolab 4000 EP Recording System, GE Company, USA). At the same time, aortic blood pressure and distal coronary blood pressure were assessed using a coronary guiding catheter (Cordis Company, USA) and 2F micro-catheter (Terumo Company, Japan), respectively. Of note, the 2F micro-catheter had its tip bent into a J shape using steam and a 0.016-inch steerable guide wire. In addition, a Doppler blood flow survey meter (BIDOP ES-100V3, Hayashidenko, Japan) was used to detect blood flow in the dorsalis pedis artery.
An injection of 8000U heparin was administrated intravenously to the patients before the PCI procedures. Two 0.016-inch guidewires were the placed into the same target vessel, one of which was used to transport a 2F micro-catheter to the area distal to stenosis for coronary blood pressure measurements and the other to transport a balloon to the area proximal to stenosis for vessel transient occlusion. Electrocardiography (ECG), blood pressure and arterial oxygen saturation (SaO2) were recorded continually and no drugs were injected during the procedure.
For each patient, a guiding catheter was used to determine the aortic blood pressure, and a 2F micro-catheter was also used to determine the coronary arterial blood pressure distal to stenosis (Pn-occl). A balloon, which had the same diameter as the target vessel, was then placed proximal to the coronary stenosis and dilated for 30 seconds to occlude blood flow completely. Coronary arterial blood pressure distal to the balloon transient occlusion (Poccl) was recorded.
RIPC and control interventions
Before the PCI procedure, the RIPC and control interventions were completed in under 2 hours. During this process, the patients in the RIPC group received ischemic preconditioning induced by oppression of a blood-pressure cuff inflated above the left thigh to totally occlude blood flow in the dorsalis pedis artery (vascular Doppler revealed no blood flow) for 5 minutes, followed by 5 minutes of deflation to allow reperfusion. This procedure was repeated 3 times. For the patients in the control group, they had a similar cuff placed around the left thigh which was not inflated. After this procedure, the aortic pressure (Pao), Pn-occl and Poccl were recorded again for all patients. During the measurements of Pn-occl, Poccl, and Pao, the heart rate was also recorded.
Statistical analysis
SPSS 19.0 (SPSS Inc., Chicago, USA) was used to analyze data. Continuous variables were reported as mean ± SD, and the paired t-test was used to compare data before and after preconditioning. Data from different groups were compared using the two-sample t-test. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. A p value < 0.05 was considered to be statistically significant.
RESULTS
Patients
The study flowchart is shown in Figure 1. Of 40 patients with coronary heart disease confirmed by angiography, 17 patients were included (9 in the RIPC group and 8 in the control group).
Figure 1.
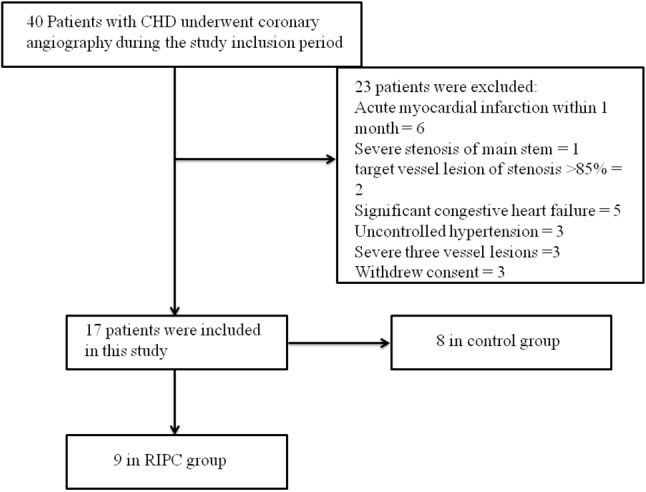
Flowchart of the study population and design. CHD, coronary heart disease; RIPC, remote ischemic preconditioning.
Table 1 shows the clinical and angiographic characteristics of the patients in the two groups. No adverse events occurred during the whole trial, and all 17 patients received PCI successfully. No significant differences in age, gender, risk factors, anthropometric data, laboratory data, medication use, and angiographic variables were observed before PCI (all p > 0.05) between the two groups. After PCI, the levels of TNI were lower in the RIPC group than in the control group (p < 0.01).
Table 1. Clinical and angiographic characteristics of patients in two groups.
| RIPC (n = 9) | Control (n = 8) | p | |
| Age, years | 65.88 ± 4.99 | 65.25 ± 5.52 | 0.805 |
| Male, n (%) | 3 (33.3) | 3 (37.5) | 1.000 |
| BMI, kg/m2 | 23.44 ± 2.24 | 23.75 ± 3.33 | 0.825 |
| Heart rate, beats/min | 71.00 ± 8.53 | 70.38 ± 8.70 | 0.883 |
| Blood examination | |||
| ALT, U/L | 14.78 ± 7.01 | 13.50 ± 5.42 | 0.683 |
| TC, mmol/L | 4.46 ± 0.89 | 4.30 ± 1.10 | 0.752 |
| TG, mmol/L | 1.83 ± 0.40 | 1.92 ± 0.45 | 0.663 |
| LDL-C, mmol/L | 2.41 ± 0.48 | 2.46 ± 0.65 | 0.855 |
| HDL-C, mmol/L | 1.18 ± 0.27 | 1.01 ± 0.23 | 0.200 |
| Creatinine, μmol/L | 82.33 ± 7.43 | 82.13 ± 8.61 | 0.958 |
| TNI, ng/mL | 0 | 0 | - |
| TNI, ng/mL* | 0.21 ± 0.16 | 0.62 ± 0.31 | 0.004 |
| Medical histories | |||
| Current smokers, n (%) | 1 (11.1) | 1 (12.5) | 1.000 |
| Hypertension, n (%) | 6 (66.7) | 6 (75.0) | 1.000 |
| Diabetic mellitus, n (%) | 1 (11.1) | 2 (25.0) | 0.576 |
| Medication, n (%) | |||
| Aspirin | 9 (100.0) | 8 (100.0) | 1.000 |
| Statins | 9 (100.0) | 8 (100.0) | 1.000 |
| ACEI/ARB | 8 (88.9) | 8 (100.0) | 1.000 |
| Beta-blockers | 7 (77.8) | 6 (75.0) | 1.000 |
| Affected Vessels, n (%) | 0.153 | ||
| 1 | 3 (33.3) | 6 (75.0) | |
| 2 | 6 (66.7) | 2 (25.0) | |
| Lesion type, n (%) | 1.000 | ||
| Type A | 5 (55.6) | 4 (50.0) | |
| Type B | 4 (44.4) | 4 (50.0) | |
| Target vessel, n (%) | 0.453 | ||
| LAD | 5 (55.6) | 6 (75.0) | |
| Cx | 2 (22.2) | 1 (12.5) | |
| RC | 2 (22.2) | 1 (12.5) | |
| Segment, n (%) | 0.743 | ||
| Proximal | 3 (33.3) | 2 (25.0) | |
| Mid | 5 (55.6) | 4 (50.0) | |
| Distal | 1 (11.1) | 2 (25.0) | |
| Reference lumen diameter, mm | 2.92 ± 0.42 | 2.95 ± 0.37 | 0.889 |
| Stenosis, % | 77.89 ± 3.72 | 78.75 ± 3.92 | 0.649 |
| Lesion length, mm | 16.33 ± 3.35 | 15.75 ± 3.33 | 0.724 |
Data are presented as mean ± SD or n (%).
* Serum TNI levels after PCI.
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; BMI, body mass index; Cx, circumflex; HDL-C, high-density lipoprotein cholesterol; LAD, left anterior descending; LDL-C, low-density lipoprotein cholesterol; RC, right coronary; RIPC, remote ischemic preconditioning; TC, total cholesterol; TG, total triglycerides; TNI, troponin I.
Distal coronary non-occlusive pressure in the RIPC and control groups
Coronary arterial blood pressure distal to the stenosis, termed distal coronary Pn-occl, was determined in the two groups. There was no significant differences in baseline pressure between the two groups (p > 0.05). After the RIPC, the systolic blood pressure, diastolic blood pressure and mean arterial blood pressure distal to stenosis of the RIPC group were significantly elevated (p < 0.05). In addition, the diastolic blood pressure and mean arterial blood pressure distal to stenosis were significantly decreased in the patients in the control group (p < 0.05, Table 2). At the same time, during all measurements of Pn-occl, Poccl, and Pao, there were no significant changes in heart rate (all p > 0.05, Table 2, Table 3, Table 4).
Table 2. Pn-occl in two groups.
| RIPC | p | Control | p | |||
| Pre-RIPC | Post-RIPC | Pre-Interval* | Post-Interval* | |||
| cSBP, mmHg | 87.67 ± 12.00 | 95.44 ± 13.68 | 0.005 | 92.63 ± 21.25 | 90.88 ± 21.26 | 0.082 |
| cDBP, mmHg | 65.22 ± 11.32 | 75.44 ± 15.15 | 0.021 | 53.63 ± 20.85 | 51.75 ± 19.70 | 0.011 |
| cMAP, mmHg | 72.78 ± 10.10 | 79.67 ± 9.79 | 0.002 | 66.63 ± 16.12 | 64.82 ± 15.69 | 0.000 |
| Heart rate, bpm | 72.78 ± 9.90 | 71.89 ± 8.33 | 0.839 | 75.13 ± 10.59 | 71.00 ± 10.52 | 0.447 |
Data are presented as mean ± SD.
* The patients in the control group had a similar cuff placed around the left thigh which was not inflated. The interval time was 3 cycles of lower limb ischemia-reperfusion preconditioning (30 minutes).
The baseline pressure in the two groups was not significantly different. Distal coronary non-occlusive pressure in RIPC and control group.
cDBP, coronary arterial diastolic blood pressure; cMAP, coronary arterial mean blood pressure; cSBP, coronary arterial systolic blood pressure; Pn-occl, distal coronary non-occlusive pressure.
Table 3. Poccl in two groups.
| RIPC | p | Control | p | |||
| Pre-RIPC | Post-RIPC | Pre-Interval | Post-Interval | |||
| cSBP, mmHg | 26.67 ± 11.05 | 33.56 ± 10.26 | < 0.001 | 26.00 ± 21.41 | 26.50 ± 18.79 | 0.787 |
| cDBP, mmHg | 18.00 ± 8.22 | 26.11 ± 10.31 | 0.006 | 20.13 ± 18.06 | 22.00 ± 17.91 | 0.224 |
| cMAP, mmHg | 20.89 ± 8.61 | 26.78 ± 10.73 | 0.001 | 22.08 ± 19.14 | 23.50 ± 18.17 | 0.371 |
| Heart rate, bpm | 73.33 ± 8.80 | 74.89 ± 9.77 | 0.727 | 72.00 ± 8.55 | 77.13 ± 7.75 | 0.241 |
Data are presented as mean ± SD.
The baseline pressure in the two groups was not significantly different. Distal coronary occlusive pressure in RIPC and control group.
cDBP, coronary arterial diastolic blood pressure; cMAP, coronary arterial mean blood pressure; cSBP, coronary arterial systolic blood pressure; Poccl, distal coronary occlusive pressure.
Table 4. Aortic pressure in two groups.
| RIPC | Control | p | |||
| Pre-RIPC | Post-RIPC | Pre-Interval | Post-Interval | ||
| SBP, mmHg | 132.22 ± 20.73 | 132.22 ± 23.34 | 140.38 ± 32.15 | 137.38 ± 20.32 | 0.875 |
| DBP, mmHg | 71.67 ± 10.14 | 75.78 ± 10.49 | 72.50 ± 9.86 | 75.38 ± 8.05 | 0.764 |
| MAP, mmHg | 91.85 ± 12.71 | 94.59 ± 13.28 | 95.13 ± 15.08 | 96.04 ± 11.29 | 0.921 |
| Heart rate, bpm | 71.44 ± 8.59 | 73.00 ± 9.60 | 71.38 ± 6.16 | 72.13 ± 11.76 | 0.981 |
Data are presented as mean ± SD.
Distal coronary occlusive pressure in the RIPC and control groups
Coronary arterial blood pressure distal to balloon occlusion, termed Poccl, was determined in the two groups. Similarly, there was no significant difference in baseline pressure (p > 0.05) between the two groups. After RIPC, the corresponding pressures were significantly elevated (all p < 0.01), while for patients in the control group, the differences were not significant (all p > 0.05, Table 3).
Aortic pressure in the RIPC and control groups
Table 4 shows Pao in two groups. Among the two groups before and after the interval, there were no significant differences in systolic blood pressure, diastolic blood pressure and mean arterial blood pressure in Pao (one-way analysis of variance, all p > 0.05).
DISCUSSION
In this preliminary study, we demonstrated that RIPC could significantly improve distal coronary Pn-occl and distal coronary Poccl. Interestingly, Pn-occl in the control group was significantly decreased. This may have been because balloon dilation for 30 seconds in the control group (to measure Poccl before the interval) may have induced myocardial ischemia, which further caused a significant drop in blood pressure. We speculate that the cardiac function was depressed after heart ischemia, and that this may have been due to decreased Pn-occl.
Experimental and human studies have shown the effectiveness of transient limb ischemia to induce distant organ protection4-8 and TNI release was substantially lower in the group receiving RIPC.9 Patients who receive intermittent limb ischemia in an ambulance during transfer for angioplasty have been shown to have a significant increase in myocardial salvage as assessed by nuclear scintigraphy.8 The effect of either ischemic preconditioning or RIPC on coronary pressures distal to stenosis has also been investigated recently. Lambiase et al.10 reported that exercise-induced myocardial ischemia had an enhanced resistance to further ischemia caused by exercise. The cardioprotection from preconditioning was assessed by collateral blood flow, and was found to be positively associated with collateral blood pressure. Furthermore, Rentrop et al. used repeated coronary artery occlusion to assess the contribution of ischemic and collateral recruitment to the development of tolerance against myocardial ischemia in patients with angiographically poor collaterals.11 This method demonstrated significantly increased pressure-derived collateral flow during repeated coronary occlusion.12 The underlying mechanisms of myocardial protection by RIPC remain to be confirmed, and the release of neurogenic and circulating factors may be involved in this process.13,14 In addition, some researchers have investigated the protective effect on coronary arteries by RIPC.8,9,13,14 However, the exact mechanisms are still unclear as to whether RIPC has a positive impact on the recruitment of coronary collateral circulation.
Improvements in distal coronary Pn-occl and Poccl, which confirm a progressive adaptation to RIPC, stimulate the recruitment of collateral channels in humans12 A previous study used trans-stenotic pressure to assess the function of stenosis in a few centers15 Many of the physiological phenomena underlying coronary flow regulation have been studied in conscious and unconscious animal preparations where there is great freedom in instrumentation and intervention.16 The pathophysiological roles of Pn-occl and Poccl may explain why pressure measurements are useful when assessing coronary flow. In functional terms, the two major determinants of coronary flow are coronary arterial pressure and myocardial oxygen consumption.17 It has been reported that at constant oxygen consumption, coronary flow is relatively independent of arterial pressure, which is referred to as coronary autoregulation.18 Of note, coronary autoregulation is not perfect, and it can correspond to horizontal plateaus in the autoregulation range. Therefore, coronary arterial pressure can be used as a parameter for predicting coronary flow. Occluded coronary arteries treated by PCI may be crossed with an over-the-wire balloon, which may allow for measurements of Pn-occl and Poccl. The pressure distal to the occluded segment of the culprit coronary artery (Poccl) is generated by collateral circulation from the feeding coronary artery supplied by the systemic circulation.19 Thus, Poccl is similar to the pressure at the distal end of the collateral vessels.
Intracoronary distal pressure measurements during vessel occlusion could be used to quantitatively assess coronary collateral circulation. Pijls et al. suggested that the collateral flow index could be measured by the physiologic derivation of collateral flow from coronary Poccl.19 Distal coronary pressure during balloon occlusion is similar to the pressure at the distal end of the collateral vessels, and Pn-occl reflects the resistance to flow along the epicardial vessel and can be used to assess arteriolosclerosis and microcirculation dysfunction.20 Poccl provides retrograde flow that partly nourishes the ischemic myocardium subtended by the occluded culprit artery in short-term effects, and it may also provide supporting evidence according to significant differences in troponin index. Furthermore, Pn-occl-guided PCI has been shown to improve clinical outcomes and procedural cost effectiveness. A low Pn-occl may reflect a no-reflow phenomenon,which can guide the progress of various therapies to improve the microcirculation.21
RIPC (four cycles of 5 min of ischemia followed by 5 min of reperfusion of the arm) can protect against endothelial dysfunction induced by subsequent long-lasting ischemia in the other arm.5-8 The endothelial protection effect of preconditioning has also been reported in other studies.13,14,22-24 The underlying mechanism may involve many signaling processes. This pertains to humoral factors, blood cells, neurohumoral mediators and presumably a complex interplay. Hypoxic NO signaling may be associated with the course of RIPC, and this may require an S-nitrosation modification of mitochondrial elements in particularly. Naturally, many previously proposed mechanisms will work alongside the proposed mechanism based on NO.25 In addition, in a porcine model, RIPC, induced by transient limb ischemia, could reduce coronary resistance and increase coronary blood flow,26 and this effect has been detected in healthy volunteers.22 However, another study found that although myocardial salvage was increased in the intervention group compared to the control group, a significant difference in corrected TIMI frame count between the two groups was not detected.8 As an important index to assess blood flow of the main stem of coronary arteries, corrected TIMI frame count cannot represent blood flow of the collateral circulation. Moreover, with regards to time, transient limb ischemia in this study was induced after ST-elevation myocardial infarction (after myocardial ischemia), which was termed preconditioning. The mechanisms and effectiveness of coronary artery protection by ischemic stimuli remain to be clarified in future studies.
Collateral artery growth has been considered to be a potent natural defense mechanism to prevent death and myocardial infarction in occlusive artery disease, and the stimulation of collateral circulation has been applied to clinical applications.26-28 Coronary collateral circulation plays an important role in patients who lost indications for PCI and surgery.29-31 We first showed that the RIPC induced by lower limb ischemia and reperfusion had an acute effect to elevate Poccl, which included systolic blood pressure, diastolic blood pressure and mean arterial blood pressure in the patients with coronary artery disease. Meanwhile, Pao was not affected by RIPC. RIPC may become a new topic in the treatment of coronary artery disease since stimulation of collaterals has generated such interest.29
This study also showed that RIPC could increase distal coronary perfusion pressure (Pn-occl). Reduced coronary resistance and increased coronary blood flow by RIPC has been reported previously,13,22-29 and these findings may explain the increased distal coronary perfusion pressure in this study. Based on our preliminary data, RIPC appeared to have a protective effect on coronary perfusion pressure and coronary collateral circulation. Whether these effects are associated with the final myocardial effects remains unclear.29,30 Timely treatment with thrombolytic drugs for acute coronary syndrome has been developed based on the observation that early reperfusion is crucial to reduce damage. The evidence of coronary collateral circulation responsiveness to RIPC suggests that, despite severe stenosis, coronary vasoactive phenomena related to preconditioning are preserved. Some studies have evaluated the role of RIC in reducing ischemia/reperfusion injury. Indeed, our results are similar to those reported by Lansky et al.,32 who only managed to show a significant short-term effect on post-PCI troponin release. In addition, Wang et al.33 recently showed that RIPC reduced the incidence of MI type 4a in patients undergoing elective PCI, but failed to show a significant effect on CRP level or subsequent cardiovascular events. The potential clinical use of RIPC seems to be positive. High-quality random clinical trials with a larger sample size are needed to verify the clinical usefulness of RIPC.
Study limitations
Since this study was a small sample trial, the results and conclusions may be limited. In addition, pressure wire, which is much thinner than the 2F micro-catheter, was not used to measure intracoronary pressures. The size of a 2F micro-catheter could partly obstruct the vessel and underestimate the true intracoronary pressure. Furthermore, we observed a decrease in Pn-occl in the controls. This phenomenon maybe explained by significant heart ischemia induced by prolonged balloon dilation. The underlying mechanism should be explored in greater detail in future studies.
CONCLUSIONS
In summary, this study showed that RIPC induced by an inflated blood pressure cuff could improve distal coronary perfusion pressure and rapidly increase distal coronary occlusive pressure thereby improving coronary collateral blood flow. Distal coronary perfusion pressure and coronary collateral flow were found to be effective in the application of RIPC in humans. Further large-scale population studies are needed to verifythis finding.
CONFLICTS OF INTEREST
The authors have declared that no conflicts of interest exist.
REFERENCES
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Bulluck H, Hausenloy DJ. Ischaemic conditioning: are we there yet? Heart. 2015;101:1067–1077. doi: 10.1136/heartjnl-2014-306531. [DOI] [PubMed] [Google Scholar]
- 3.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 4.Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–1279. doi: 10.1016/s0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- 5.Cheung MM, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 6.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 7.Venugopal V, Hausenloy DJ, Ludman A, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–1571. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 8.Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 9.Auguadro C, Scalise F, Manfredi M, et al. The prognostic role of troponin I elevation after elective percutaneous coronary intervention. J Cardiovasc Med (Hagerstown) 2015;16:149–155. doi: 10.2459/JCM.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 10.Lambiase PD, Edwards RJ, Cusack MR, et al. Exercise-induced ischemia initiates the second window of protection in humans independent of collateral recruitment. J Am Coll Cardiol. 2003;41:1174–1182. doi: 10.1016/s0735-1097(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 11.Billinger M, Fleisch M, Eberli FR, et al. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? J Am Coll Cardiol. 1999;33:1027–1035. doi: 10.1016/s0735-1097(98)00674-3. [DOI] [PubMed] [Google Scholar]
- 12.Cribier A, Korsatz L, Koning R, et al. Improved myocardial ischemic response and enhanced collateral circulation with long repetitive coronary occlusion during angioplasty: a prospective study. J Am Coll Cardiol. 1992;20:578–586. doi: 10.1016/0735-1097(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 13.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2003;107:218–219. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 14.Kharbanda RK, Peters M, Walton B, et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001;103:1624–1630. doi: 10.1161/01.cir.103.12.1624. [DOI] [PubMed] [Google Scholar]
- 15.Meier B, Luethy P, Finci L, et al. Coronary wedge pressure in relation to spontaneously visible and recruitable collaterals. Circulation. 1987;75:906–913. doi: 10.1161/01.cir.75.5.906. [DOI] [PubMed] [Google Scholar]
- 16.Tp VDH, Nolte F, Rolandi MC, et al. Coronary pressure-flow relations as basis for the understanding of coronary physiology. J Mol Cell Cardiol. 2012;52:786–793. doi: 10.1016/j.yjmcc.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Alella A, Williams FL, Bolene-Williams C, Katz LN. Interrelation between cardiac oxygen consumption and coronary blood flow. Am J Physiol. 1955;183:570–582. doi: 10.1152/ajplegacy.1955.183.3.570. [DOI] [PubMed] [Google Scholar]
- 18.Mosher P, Ross J, Jr., Mcfate PA, Shaw RF. Control of coronary blood flow by an autoregulatory mechanism. Circ Res. 1964;14:250–259. doi: 10.1161/01.res.14.3.250. [DOI] [PubMed] [Google Scholar]
- 19.Meisel SR, Frimerman A, Blondheim DS, et al. Relation of the systemic blood pressure to the collateral pressure distal to an infarct-related coronary artery occlusion during acute myocardial infarction. Am J Cardiol. 2013;111:319. doi: 10.1016/j.amjcard.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 20.S S, J E, IS M, et al. Development and validation of a new adenosine-independent index of stenosis severity from coronary wave-intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392–1402. doi: 10.1016/j.jacc.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Pijls NH, van Son JA, Kirkeeide RL, et al. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87:1354–1367. doi: 10.1161/01.cir.87.4.1354. [DOI] [PubMed] [Google Scholar]
- 22.Zhou K, Yang B, Zhou XM, et al. Effects of remote ischemic preconditioning on the flow pattern of the left anterior descending coronary artery in normal subjects. Int J Cardiol. 2007;122:250–251. doi: 10.1016/j.ijcard.2006.11.079. [DOI] [PubMed] [Google Scholar]
- 23.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, et al. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Broadhead MW, Kharbanda RK, Peters MJ, MacAllister RJ. KATP channel activation induces ischemic preconditioning of the endothelium in humans in vivo. Circulation. 2004;110:2077–2082. doi: 10.1161/01.CIR.0000144304.91010.F0. [DOI] [PubMed] [Google Scholar]
- 25.Rassaf T, Totzeck M, Hendgen-Cotta UB, et al. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu M, Konstantinov IE, Kharbanda RK, et al. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol (Oxf) 2007;190:103–109. doi: 10.1111/j.1748-1716.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 27.Ahn A, Frishman WH, Gutwein A, et al. Therapeutic angiogenesis: a new treatment approach for ischemic heart disease--Part II. Cardiol Rev. 2008;16:219–229. doi: 10.1097/CRD.0b013e3181620e50. [DOI] [PubMed] [Google Scholar]
- 28.Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Tong G, Yang J, et al. Effect of hepatocyte growth-promoting factors on myocardial ischemia during exercise in patients with severe coronary artery disease. Int Heart J. 2009;50:291–299. doi: 10.1536/ihj.50.291. [DOI] [PubMed] [Google Scholar]
- 30.Wang NF, Xu J, Xia Q, et al. Mechanism of cardioprotection induced by noninvasive limb ischemic preconditioning. Zhonghua Yi Xue Za Zhi. 2009;89:1999–2002. [PubMed] [Google Scholar]
- 31.Huang CL, Jen HL, Huang WP, et al. The impact of fractional flow reserve-guided coronary revascularization in patients with coronary stenoses of intermediate severity. Acta Cardiol Sin. 2017;33:353–361. doi: 10.6515/ACS20170202B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansky AJ, Stone GW. Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ Cardiovasc Interv. 2010;3:602–610. doi: 10.1161/CIRCINTERVENTIONS.110.959080. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Yan J, Li L, Su Q. The effect of remote ischemic preconditioning in patients undergoing elective percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. Exp Clin Cardiol. 2014;20:1411–1435. [Google Scholar]


