Abstract
Background
Neurological complications are an important concern in the repair of type A aortic dissection. Supra-aortic involvement is considered to be an important risk factor for neurological injuries. However, the optimal brain protection strategy still remains controversial. The aim of the present study was to assess the efficacy and short-term results of retrograde cerebral protection techniques in the treatment of acute type A aortic dissection.
Methods
Between 2005 and 2013, 185 patients who underwent repair of acute type A aortic dissection were enrolled in this study, all of whom received retrograde cerebral perfusion. The patients were divided into two group: 102 patients who had at least one carotid artery involved as the carotid dissection group, and 83 patients who had no carotid artery involvement as the non-carotid dissection group.
Results
The mean age of the patients was 57.8 years and 69% were male. The 30-day mortality rate was 10.3%, and the overall in-hospital mortality rate was 11.9%. Eight patients (4.3%) developed new permanent neurological deficits (PNDs) including two in the non-carotid dissection group and six in the carotid dissection group. Although new PND was milder in the carotid dissection group, there was no significant difference (p = 0.248). The proportion of patients who received a coronary artery bypass graft was significantly higher in the carotid dissection group (1 vs. 8, p = 0.037).
Conclusions
According to our study, the retrograde cerebral perfusion technique is an easy and safe procedure, especially for patients with concomitant carotid dissection.
Keywords: Cerebral perfusion, Type A aortic dissection
INTRODUCTION
Acute type A aortic dissection (ATAAD) is a lethal condition requiring emergency surgery. Surgery for ATAAD is usually accompanied by high surgical mortality and morbidity. Various surgical methods and strategies have been developed to improve the short-term and long-term surgical outcomes, including access to cardiopulmonary bypass, cerebral perfusion, and extensive replacement of the aortic arch. Neurological dysfunction is one of the most concerning complications, however the optimal brain protection strategy during surgical repair of acute type A dissection still remains controversial.
The arch vessels may also be involved in the onset of type A aortic dissection. Carotid dissection can influence neurological outcomes and choice of surgical strategy. In this study, the patients were divided into two subsets according to the degree of arch vessel involvement. The primary endpoint was surgical mortality and neurological outcomes. We also reviewed our experience of ATAAD and analyzed the role of retrograde cerebral perfusion (RCP) in brain protection.
METHODS
Patients
From February 2005 to September 2013, 210 patients underwent emergency surgery for ATAAD. Twenty patients were managed with selective antegrade cerebral perfusion (ACP), and the pre-operative computed tomographic images of five patients were missing. After excluding these 25 patients, the remaining 185 patients were enrolled in this study. Data were collected and analyzed retrospectively.
The mean age at presentation was 57.8 years (range 25-87 years), 69% of the patients were male, and three patients had Marfan syndrome. The mean body mass index was 26.2 kg/m2 and 36 patients were over 70 years of age. The preoperative characteristics including demographic data and preoperative morbidities are presented in Table 1.
Table 1. Preoperative demographics and clinical characteristics.
| Variable | Non-carotid dissection group | Carotid dissection group | p value |
| Patients (numbers) | 83 | 102 | |
| Age (years) | 57.8 | 57.7 | 0.991 |
| Body mass index (kg/m2) | 26.3 | 26.1 | 0.670 |
| Gender | |||
| Male | 59 | 69 | 0.615 |
| Hypertension | 62 | 77 | 0.901 |
| Diabetes mellitus | 3 | 5 | 0.669 |
| Coronary artery disease | 2 | 7 | 0.161 |
| Afib | 5 | 5 | 0.737 |
| COPD | 2 | 1 | 0.444 |
| ESRD under hemodialysis | 0 | 2 | 0.200 |
| Old CVA | 4 | 3 | 0.505 |
| Previous type B aortic dissection | 2 | 5 | 0.377 |
| Pre-operative shock | 9 | 14 | 0.555 |
| Pre-operative conscious change | 11 | 12 | 0.760 |
| Pre-operative intubation | 6 | 13 | 0.219 |
| Pre-operative CPR | 1 | 4 | 0.257 |
Afib, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CPR, cardiopulmonary resuscitation; CVA, cerebral vascular accident; ESRD, end-stage renal disease.
The patients were divided into two subsets according to the degree of arch vessel involvement: the carotid dissection group and the non-carotid dissection group. If a dissection flap or blood flow from the false lumen was noted in the innominate artery or the left common carotid artery, the patient was categorized into the carotid dissection group. Patients without dissection of the innominate artery and left common carotid were categorized into the non-carotid dissection group.
Definitions
Aortic dissection was diagnosed by computed tomography aortography (CTA) and was classified according to the Stanford classification which consists of two types: type A, involving the ascending aorta; and type B, involving only the aorta distal to the origin of the left subclavian artery. ATAAD was defined as dissection presenting within 14 days of symptom onset.
Neurologic complications were categorized as permanent neurologic dysfunction (PND) and temporary neurologic dysfunction (TND). PND was determined by neuroimaging findings, persistent loss of cognitive function or neurologic function that was still present at discharge from the hospital. PND after surgery was defined as new PND. The patients who had neurological dysfunction before surgery or died promptly after surgery were excluded from this endpoint. TND was defined as the occurrence of postoperative agitation, confusion, delirium, or a transient focal neurologic deficit without any evidence of new structural abnormalities on computed tomography or magnetic resonance imaging, and the resolution of all symptoms before discharge.
Prolonged mechanical ventilation (PMV) was defined according to the guidelines of the Centers for Medicare and Medicaid Services in the United States as greater than 21 days of mechanical ventilation for at least 6 hours per day. Other adverse outcomes recorded in the study included acute renal failure (ARF) requiring hemodialysis, mesenteric ischemia with surgical intervention or aortic fenestration, limb malperfusion with a loss of pulse, and isolated limb weakness or numbness. Patients with ARF after surgery who needed hemodialysis were further divided into two subsets according to the duration of hemodialysis. Patients needing hemodialysis at discharge from the hospital were assigned to the permanent hemodialysis subset, and the remainder were assigned to the transient hemodialysis subset.
Postoperatively, surgical (or 30-day) mortality and neurological complications were the major outcomes of interest in this investigation. Surgical mortality was defined as death during the first 30 days after the operation. Patients who received cardiopulmonary resuscitation (CPR) in the operating room but did not undergo surgery for ascending aorta replacement were not included in the surgical mortality group.
Operative procedures
The chest was entered by median sternotomy. All patients received surgery with a cardiopulmonary bypass, hypothermic circulatory arrest and RCP through the superior vena cava (SVC). Arterial cannulation was accessed through one of the femoral arteries. Both femoral arteries were cannulated if inadequate perfusion flow or high arterial pressure was noted. Venous drainage was performed using a double-stage cannula inserted via the right atrium. Myocardial protection was achieved with intermittent retrograde cold blood cardioplegia through the coronary sinus, and supplemented with direct antegrade coronary ostia infusion after the aorta had been opened. A left ventricular sump was inserted through the right superior pulmonary vein.
Once a rectal temperature of 16 °C was reached, the patient was placed in the Trendelenburg position, the cardiopulmonary bypass was discontinued, and circulation was arrested. The flow of RCP was maintained between 300~500 mL/minutes. The ascending aorta was replaced with an open distal anastomosis and Hemashield Dacron graft. No patient received total arch replacement in this series.
After completion of the distal reconstruction, RCP was discontinued. With the patient still in the Trendelenburg position, cardiopulmonary bypass flow was resumed until all debris and air were evacuated through the open aortic graft. Systemic warming was initiated until the body temperature reached 36 °C.
Proximal reconstruction was performed by resuspending the aortic valve, replacing the aortic valve, or via the Bentall procedure, depending on the patient’s condition. Once the procedure had been completed, the patient was gradually separated from the cardiopulmonary bypass. Hemostasis was then achieved, and the wound was closed layer by layer.
Statistical analysis
Data were collected from chart reviews by the authors. Continuous variables are presented as means ± standard deviations. Categorical variables are presented as numbers and percentages. Independent continuous variables were compared using the unpaired Student’s t test, and categorical variables were compared using the chi-square test. All p values less than 0.05 were considered to indicate statistical significance. Statistical analysis was performed using SPSS statistical software.
RESULTS
Demographic data
There were no significant differences between the carotid dissection group and non-carotid dissection group in age, gender, hypertension, diabetes mellitus (DM), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), end-stage renal disease (ESRD), and preoperative condition (including shock, consciousness change, intubation, and CPR). Hypertension (75%) was the most common etiologic comorbidity, and a preoperative change in consciousness was present in 23 patients (12.4%).
Arch vessel involvement
Arch vessel involvement was present in 60% of the patients (Table 2). Carotid arterial dissection was found in 55% of the patients, including unilateral involvement in 33% and bilateral involvement in 22%. Among those with unilateral involvement, there was a far greater percentage of innominate artery involvement compared with left carotid artery involvement (29% vs. 4%).
Table 2. Classification of arch vessel dissection.
| Involvement of arch vessel dissection | Numbers (total = 110) |
| All three arch vessels | 24 |
| Only 1 and 2 | 17 |
| Only 1 and 3 | 14 |
| Only 2 and 3 | 3 |
| Only 1 | 42 |
| Only 2 | 2 |
| Only 3 | 8 |
1, innominate artery; 2, left common carotid artery; 3, left subclavian artery.
Cardiopulmonary bypass data and operative details
The mean cardiopulmonary bypass time, aortic cross-clamp time, and total arrest time are illustrated in Table 3. Although all of the operative times were slightly longer in the carotid dissection group, there were no significant differences between the two groups.
Table 3. Cardiopulmonary bypass data.
| Intraoperative data | Non-carotid dissection group (n = 83) | Carotid dissection group (n = 102) | p value |
| CPB time (minutes) | 224 ± 62 | 243 ± 70 | 0.056 |
| Aortic cross-clamp time (minutes) | 149 ± 51 | 163 ± 54 | 0.065 |
| Circulatory arrest time (minutes) | 40 ± 11 | 43 ± 14 | 0.206 |
CPB, cardiopulmonary bypass.
The extent of aortic replacement, performed aortic valve surgery procedures, and other associated procedures are presented in Table 4. Significantly more patients received coronary artery bypass graft (CABG) in the carotid dissection group (1 vs. 8, p = 0.037).
Table 4. Surgical procedures.
| Characteristics | Non-carotid dissection group (n = 83) | Carotid dissection group (n = 102) | p value |
| Aortic replacement | |||
| Ascending (isolated) | 82 (99%) | 98 (96%) | |
| Ascending aorta and hemiarch | 1 (1%) | 4 (4%) | 0.257 |
| Aortic valve surgery | |||
| AVR | 1 (1%) | 2 (2%) | 0.686 |
| Bentall procedure | 6 (7.2%) | 10 (9.8%) | 0.535 |
| Coronary artery bypass grafting | 1 (1.2%) | 8 (7.8%) | 0.037 |
| Bypass for mal-perfusion of extremities | 10 (12.0%) | 10 (9.8%) | 0.625 |
AVR, aortic valve replacement.
Hospital mortality
The surgical mortality rate was 10.3% (19 of 185), and the overall in-hospital mortality rate was 11.9% (22 of 185). The etiologies of all cases of in-hospital mortality are shown in Table 5. Among the cases of overall in-hospital mortality, 12 patients died as a result of cardiac failure, two died from neurological damage, two from sepsis, two from arrhythmia, two from sudden rupture of residual aorta, and two from visceral ischemia. The surgical mortality rates were 3.6% in the non-carotid dissection group (3/83) and 15.7% in the carotid dissection group (16/102), and the difference was statistically significant (p = 0.007). The predominant cause of death within 30 days was cardiac failure (n = 12).
Table 5. Etiology of all hospital mortality.
| Classification | Non-carotid dissection group (n = 83) | Carotid dissection group (n = 102) |
| Cardiac failure | 3 | 9 |
| Neurological damage | 2 | |
| Sepsis | 1 | 1 |
| Arrhythmia | 2 | |
| Aortic rupture | 2 | |
| Visceral ischemia | 2 |
Neurological and other postoperative outcomes
Of the 185 patients, 8 (4.3%) developed new PND, including two in the non-carotid dissection group and six in the carotid dissection group. TND occurred in 25.4% of the patients. Although new PND was milder in the carotid dissection group, there was no significant difference between the two groups (p = 0.248). Among the instances of new PND, right hemispheric and left hemispheric cerebrovascular events occurred in five and three patients, respectively.
Dialysis was required postoperatively in 14 patients (7.5%), and four patients (2.2%) needed permanent hemodialysis. There were no significant differences in PMV > 3 weeks, ARF needing hemodialysis, ischemic bowel, mediastinal infection, sternal dehiscence, superficial wound infection, and re-exploratory surgery to check bleeding. There were also no significant differences in mean ventilation time, intensive care unit stay, and hospital stay between the two groups (Table 6).
Table 6. Clinical characteristics and outcomes.
| Outcome | Non-carotid dissection group (n = 83) | Carotid dissection group (n = 103) | p value |
| In-hospital mortality | 4 (2.2%) | 18 (9.7%) | 0.007 |
| 30-day mortality | 3 (1.6%) | 16 (8.7%) | 0.007 |
| Re-exploration for bleeding | 6 (3.2%) | 17 (9.2%) | 0.053 |
| New PND | 2 (1.1%) | 6 (3.2%) | 0.248 |
| TND | 20 (10.8%) | 27 (14.6%) | 0.712 |
| PMV > 3 weeks | 7 (3.8%) | 11 (5.9%) | 0.592 |
| Tracheostomy | 3 (1.6%) | 6 (3.2%) | 0.476 |
| ARF needing hemodialysis | |||
| Permanent | 1 (0.5%) | 3 (1.6%) | 0.629 |
| Transient | 3 (1.6%) | 7 (3.8%) | 0.516 |
| Ischemic bowel | 2 (1.1%) | 3 (1.6%) | 0.825 |
| Mediastinal infection | 3 (1.6%) | 3 (1.6%) | 0.797 |
| Sternal dehiscence | 2 (1.1%) | 2 (1.1%) | 0.835 |
| Superficial wound infection | 1 (0.5%) | 1 (0.5%) | 0.883 |
| Mechanical ventilation (days) | 6 | 9.5 | 0.186 |
| ICU stay (days) | 7.8 | 9.7 | 0.350 |
| Hospital stay (days) | 19.8 | 20.5 | 0.852 |
ARF, acute renal failure; ICU, intensive care unit; PMV, prolonged mechanical ventilation; PND, permanent neurologic dysfunction; TND, temporary neurologic dysfunction.
Risk factors for perioperative mortality
Supra-aortic vessel dissection, pre-operative shock, pre-operative changes in consciousness, pre-operative intubation, pre-operative CPR, long cardiopulmonary bypass time, CABG, ischemic bowel, ARF needing hemodialysis, and DM were risk factors for in-hospital mortality.
DISCUSSION
ATAAD is a lethal condition requiring emergency surgical intervention. The mortality rate can reach as high as 50% within the first 48 hours and 80% in the first 2 weeks in untreated patients.1-3 However, surgery for ATAAD is considered to be a high-risk surgery because it is associated with high rates of incidental mortality and morbidity, with a reported mortality rate after surgery ranging from 15% to 35%.1,3-5 Most complications occur due to the outflow of supraaortal, abdominal, spinal, extremity, and renal vessels which can also be affected by aortic dissection. Among the various complications that can arise, neurological injury is the most serious. The incidence of postoperative neurological complications has been reported to range from 5% to 20% in several studies.3,5 These complications can be caused by numerous factors, such as the preoperative hemodynamic status, preoperative neurologic status, perfusion technique for cardiopulmonary bypass, type of cerebral protection strategy, extent of aortic repair, and operative time. Therefore, numerous technical procedures such as methods of cerebroprotection, cannulation strategies, and aggressive surgical repair have been advocated in the last 20 years to improve postoperative outcomes. However, whether these operative techniques have a beneficial effect on outcomes is controversial. A wide range of techniques are thus currently in use.
Outcomes of patients with ATAAD presenting with brain injury
Neurological symptoms are a common initial presentation at the onset of ATAAD and have been reported to affect approximately 17% to 40% of such patients.2,6 It is difficult to accurately estimate the incidence as it is not possible to conduct a complete neurological examination in these critically ill patients. In this study, 23 patients (12.4%) had preoperative changes in consciousness. Although we presumed that such changes would be more frequent in the carotid dissection group, there was no significant difference between the two groups, possibly due to multiple pathogenetic mechanisms which may affect conscious status in patients with ATAAD. In addition, cerebral blood flow can be affected by factors including the severity of carotid arterial obstruction, hypotension secondary to cardiac tamponade, brain embolism from thrombus in the false lumen, and/or a combination of these factors.7,8
The existence of preoperative brain injuries in the initial presentation of ATAAD presents a dilemma with respect to the decision to conduct an emergency operation, because the systemic anticoagulation required for cardiopulmonary bypass might make the neurological injury worse.7,8 Although the mortality rate is still higher in patients with preoperative brain injuries, better outcomes have been demonstrated with surgical interventions than with medical treatment.7 In this study, 23 patients had preoperative changes in consciousness, of whom 11 (48%) achieved complete recovery of neurologic status, and four (17%) had variable degrees of neurological dysfunction after surgery. The mortality rate in these patients was 35%.
Arch vessel involvement and perioperative neurological complications
Arch vessel involvement was found in 60% of all patients in the present study. Carotid arterial dissection was present in 55% of the patients, including unilateral involvement in 33% and bilateral involvement in 22%. Among the cases with unilateral involvement, dissection of the innominate artery was present in 29%. In the patients with new PND, the majority of strokes were hemispheric and predominantly right-sided (62.5%), matching the frequency reported in previous studies.2,6 The dominance of right hemispheric stroke could be explained by a higher incidence of innominate artery dissection.
Postoperative neurological complications are a frequent cause of morbidity/mortality, and the reported incidence ranges from 5% to 20%.3,5 The occurrence of neurological dysfunction after aortic surgery has been shown to increase the duration of ventilator support, intensive care unit stay, hospital stay, and rate of in-hospital mortality.9 Although supra-aortic involvement is considered to be an important risk factor for neurological injuries, there was no significant difference in the incidence of new PND between the two groups as we had expected. This suggests that other perioperative factors such as hypothermic circulatory arrest, strategy of cerebral protection, not touching the carotid arteries and/or microemboli may also play essential roles in perioperative stroke.6
Reported risk factors for PND include age (> 60 years), severity of atherosclerotic diseased vessel, site of arterial cannulation, prolonged circulatory arrest, inadequate brain protection during circulatory arrest, embolic incidents, and cerebral malperfusion secondary to carotid dissection.3,9,10 In contrast to PND, TND has been shown to be directly related to age, duration of hypothermic circulatory arrest, and inadequate brain protection.3,10
Over the past few decades, various cerebral protection methods have been developed to reduce the incidence of neurological complications, including deep hypothermic circulatory arrest (DHCA), RCP, and ACP. However, the optimal strategy remains controversial.
DHCA was introduced by Griep and colleagues in 1975. It provides 30 to 40 minutes of circulatory arrest, however beyond this time brain protection has been shown to be inadequate.10 Deep hypothermia has several disadvantages. First, it requires prolonged cardiopulmonary bypass periods for cooling and rewarming. Second, deep hypothermia and a prolonged cardiopulmonary bypass time impairs coagulopathy and causes severe bleeding.1,9 Third, systemic vasoconstriction caused by deep hypothermia can diminish organ perfusion and affect cerebral autoregulation.5
Cerebral perfusion strategies have subsequently been developed to prolong the safe duration of circulatory arrest. Ueda et al. in 1990 introduced the method of RCP through the SVC as an adjunct to brain protection.1,10 RCP offers a number of advantages. First, it provides oxygen and substrates to the brain and removes toxic waste produced by ischemia. Second, it can remove air and solid emboli from the arterial branches of the arch. Third, it can avoid any maneuvers to the arch vessel which could be atheromatic, dissected, and friable. Fourth, no catheter is used in the surgical field, so the surgeon can work unimpeded.10,13 Although RCP has been in use for more than two decades, the true benefits of RCP have not been formally confirmed.9,14 Over the past decade or so, ACP has become a more popular approach than RCP, despite the good results achieved with the latter.4
Although selective antegrade perfusion of the brain with cold blood was introduced by Bachet et al. in 1986,1,10 it has only relatively recently gained wide acceptance in most centers.1 ACP offers several advantages, including: a) it preserves the natural flow direction, allows for a higher pressure, and probably provides metabolically adequate perfusion; and b) it extends the safe time of systemic circulatory arrest to 30~60 minutes.4 However, some potentially harmful aspects should be considered when using ACP. First, rupture of dissection septum of the carotid artery, and dislodging debris and air during manipulation of supra-aortic vessels are concerns during insertion of a catheter or snare of the vessels.14 This is particularly important for ATAAD because the vascular tissue is friable. Second, right carotid artery stenosis or malfunction of the circle of Willis may lead to cerebral malperfusion in unilateral ACP.4 Third, the additional lines may clutter the surgical field.4 In addition, it is important to remember that some patients with aortic dissection are absolutely not suitable for clamping or snaring of the carotid arteries (Figure 1).
Figure 1.
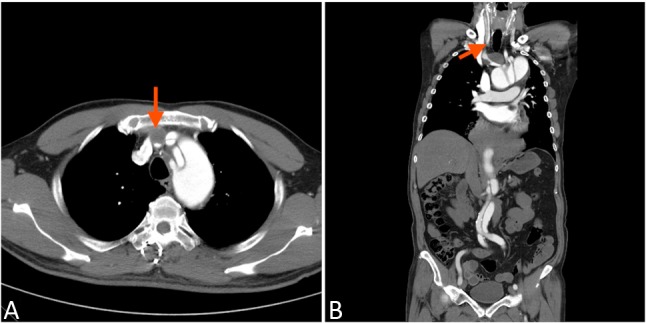
Arch vessel involvement. The CTA revealed the arch vessel involvement of the acute type A aortic dissection. Thrombosis of innominated artery (red arrow) was also noted. Any attempt to control those dissected artery during acute stage, clamping or snaring, is not reasonable.
The optimal strategy and temperature management for cerebral protection during acute aortic dissection type A surgery remain controversial.1,4 Both ACP and RCP have been shown to result in greater improvements in reducing mortality and PND than DHCA. In contrast to RCP, ACP has been shown to be beneficial in reducing the occurrence of TND.1,3 Although several clinical comparative studies between ACP and RCP have been conducted, it is not apparent which provides the best brain perfusion strategy for patients with ATAAD.10,15 Moreover, most previous studies are nonrandomized studies, and ATAAD was not the focus of the investigations.
According to the published literature, the incidence of PND in patients with ATAAD undergoing ACP or RCP ranges from 4% to 24%1,3-5,9-11 and 4% to 12%,1,3,10 respectively. The incidence of TND in patients who receive ACP has been reported to range from 2.5% to 33%3,5,9-11 and from 19% to 40%3,10 for patients who receive RCP group.
The overall incidence of new PND in the present study was 4.3%, which is lower than that in most previous reports. Although the occurrence of new PND was higher in the carotid dissection group, there was no significant difference between the two groups. We consider that dissection of supra-aortic vessels is an important risk factor for perioperative stroke. During the acute stage of dissection the dissected vessel is fragile, and therefore we do not suggest any manipulation of the supra-aortic vessels. RCP can avoid the potential risk of embolic complications that may arise from the antegrade route.12 According to our experience, RCP is an easy and safe procedure for surgical repair of ATAAD requiring open distal anastomosis.
In recent years, cannulation of the axillary artery has considerably reduced the incidence of PND by reducing the risk of malperfusion and retrograde embolization from the abdominal and thoracic aorta caused by femoral cannulation. Because all of our patients received femoral cannulation, we could not compare it to other forms of arterial cannulation. Nevertheless, our study did not reveal a higher incidence of neurological dysfunction. Another concern is that the diameter of the axillary artery is smaller in East Asians. Femoral cannulation is thus faster and easier.
Mortality
The International Registry of Acute Aortic Dissections (IRAD) and several international registries report a high in-hospital mortality rate of 15% to 35% in patients with ATAAD.3,9,16 Postoperative neurological dysfunction and ischemic visceral organ failure are the most frequently encountered problems and are closely associated with significant perioperative mortality and morbidity.
At present, the 30-day mortality rate ranges from 12% to 27% in patients who receive ACP,1,3-5,9,10 and 16% to 23% for RCP.1,3,10 An age > 70 years, hypotension or shock, cardiac tamponade, pre-operative CPR, and myocardial ischemia or infarction are important predictors of patient outcomes.12
In the present study, the 30-day mortality rate was 10.3% and the overall in-hospital mortality rate was 11.9%. Both results are lower than in most recently reported studies. In our study, the risk factors for mortality were supra-aortic vessel dissection, pre-operative shock, pre-operative changes in consciousness, pre-operative intubation, pre-operative CPR, long cardiopulmonary bypass time, CABG, ischemic bowel, ARF needing hemodialysis, and DM.
In recent studies, old age was not considered to be a limitation for surgery, with a reported mortality rate in patients > 70 years ranging from 13% to 37%.12 In our series, 36 patients were > 70 years of age, five (13.8%) of whom died. Eight patients were > 80 years of age, of whom one (12.5%) died after surgery.
The carotid dissection group had higher in-hospital and 30-day mortality rates, which were not due to adverse neurological events (Table 5 and 6). We could not make any definite conclusions from the present data because of the small number of cases of mortality. However, concomitant surgical procedures could also increase the surgical risk. CABG has been reported to increase mortality,12 and this relationship was demonstrated again in our study. A high 30-day mortality rate (40%) has been reported in patients with ATAAD presenting as ST-segment elevation myocardial infarction.17 The number of patients who received CABG was significantly higher in the carotid dissection group (1 vs. 8, p = 0.037), and the most common etiology of all in-hospital mortality was cardiac failure in the carotid dissection group. One possible explanation is that those patients had a more complex extent of dissection and the potential risk of myocardial ischemia. The poor outcome may be due to the underlying disease rather than the procedure itself.12 CABG at the time of surgery was performed more commonly in the patients who died compared with those who survived (18.2% vs. 3.1%), and it was a risk factor for mortality. This finding supports the abovementioned theory. Type A aortic dissection complicated with carotid dissection may implicate a higher degree of medial degeneration or an outer dissection plane of the medial layer. This will result in greater dissection of the major branches with malperfusion, ischemic events or delayed aorta rupture, indicating more severe disease. Further studies are needed to elucidate the possible reason for this finding.
Limitations
The main limitation of the current study is the retrospective study design. In addition, there was no control group of patients who underwent another cerebral perfusion modality.
CONCLUSIONS
ATAAD is a life-threatening event associated with many major morbidities and mortality requiring immediate surgical repair. Surgical treatment of type A aortic dissection consists primarily of replacing the ascending aorta to prevent expansion of the dissection and rupture into the pericardial sac. The goal of surgical intervention is operative survival. Neurological complications are another important concern. Supra-aortic involvement is considered to be an important risk factor for neurological injuries. However, the optimal brain protection strategy still remains controversial. According to our study, the RCP technique is an easy and safe procedure during repair of ATAAD, especially for patients with concomitant carotid dissection. As a result, we do not favor total arch replacement for ATAAD. Dissection of the carotid artery is also a sign of the complex extent of dissection, and myocardial ischemia should be kept in mind. Our study findings confirm that old age is not a limitation for aggressive treatment.
DECLARATION OF CONFLICT OF INTEREST
All of the authors declare no conflicts of interest.
REFERENCES
- 1.Wiedemann D, Kocher A, Dorfmeister M, et al. Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg. 2013;146:647–655. doi: 10.1016/j.jtcvs.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 2.Hyland MH, Holloway RG. Pearls & Oy-sters: a stroke of luck: detecting type A aortic dissection by MRA. Neurology. 2011;76:31–33. doi: 10.1212/WNL.0b013e31820d6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forteza A, Martín C, Centeno J, et al. Acute type A aortic dissection: 18 years of experience in one center (Hospital 12 de Octubre). Interact Cardiovasc Thorac Surg. 2009;9:426–430. doi: 10.1510/icvts.2009.203976. [DOI] [PubMed] [Google Scholar]
- 4.Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German registry for acute aortic dissection type A (GERAADA). Circulation. 2011;124:434–443. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- 5.Bakhtiary F, Dogan S, Zierer A, et al. Antegrade cerebral perfusion for acute type A aortic dissection in 120 consecutive patients. Ann Thorac Surg. 2008;85:465–469. doi: 10.1016/j.athoracsur.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Gaul C, Dietrich W, Friedrich I, et al. Neurological symptoms in type A aortic dissections. Stroke. 2007;38:292–297. doi: 10.1161/01.STR.0000254594.33408.b1. [DOI] [PubMed] [Google Scholar]
- 7.Eusanio MD, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:213–221. doi: 10.1016/j.jtcvs.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 8.Tsukube T, Hayashi T, Kawahira T, et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124:163–167. doi: 10.1161/CIRCULATIONAHA.110.011551. [DOI] [PubMed] [Google Scholar]
- 9.Qian H, Hu J, Du L, et al. Modified hypothermic circulatory arrest for emergent repair of acute aortic dissection type a: a single-center experience. J Cardiothorac Surg. 2013;8:125–132. doi: 10.1186/1749-8090-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolakis E, Koletsis EN, Dedeilias P, et al. Antegrade versus retrograde cerebral perfusion in relation to postoperative complications following aortic arch surgery for acute aortic dissection type A. J Card Surg. 2008;23:480–487. doi: 10.1111/j.1540-8191.2008.00587.x. [DOI] [PubMed] [Google Scholar]
- 11.Haldenwang PL, Wahlers T, Himmels A, et al. Evaluation of risk factors for transient neurological dysfunction and adverse outcome after repair of acute type A aortic dissection in 122 consecutive patients. Eur J Cardiothorac Surg. 2012;42:115–120. doi: 10.1093/ejcts/ezs412. [DOI] [PubMed] [Google Scholar]
- 12.Andrew JM, Mundy J, Pinto N, et al. Contemporary results following surgical repair of acute type A aortic dissection (AAAD): a single centre experience. Heart Lung Circ. 2010;19:665–672. doi: 10.1016/j.hlc.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Augoustides JG, Floyd TF, McGarvey ML, et al. Major clinical outcomes in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest: quantification of organ-based perioperative outcome and detection of opportunities for perioperative intervention. J Cardiothorac Vasc Anesth. 2005;19:446–452. doi: 10.1053/j.jvca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Hata M, Sezai A, Yoshitake I, et al. Clinical trends in optimal treatment strategy for type A acute aortic dissection. Ann Thorac Cardiovasc Surg. 2010;16:228–235. [PubMed] [Google Scholar]
- 15.Sugiura T, Imoto K, Uchida K, et al. Comparative study of brain protection in ascending aorta replacement for acute type A aortic dissection: retrograde cerebral perfusion versus selective antegrade cerebral perfusion. Gen Thorac Cardiovasc Surg. 2012;60:645–648. doi: 10.1007/s11748-012-0142-z. [DOI] [PubMed] [Google Scholar]
- 16.Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 17.Wang JL, Chen CC, Wang CY, et al. Acute type A aortic dissection presenting as ST-segment elevation myocardial infarction referred for primary percutaneous coronary intervention. Acta Cardiol Sin. 2016;32:265–272. doi: 10.6515/ACS20150424J. [DOI] [PMC free article] [PubMed] [Google Scholar]


