Abstract
Background
Adropin is a peptide hormone expressed in coronary artery endothelial cells, which plays a potential endothelial protective role. We sought to assess whether serum adropin levels are correlated with the coronary slow flow phenomenon (CSFP).
Methods
We enrolled 82 patients with angiographically confirmed CSFP and 184 age-matched controls. Serum adropin levels were measured by enzyme-linked immunosorbent assay (ELISA), and coronary flow rate was assessed using thrombolysis in myocardial infarction (TIMI) frame count (TFC). CSFP was defined as a corrected TIMI-TFC greater than two standard deviations from the normal range.
Results
Serum adropin levels were significantly lower in the CSFP patients (n = 82) than in the controls (n = 184) (4.03 ± 1.99 vs. 4.86 ± 1.88 ng/ml, p = 0.001). Multivariate logistic regression analysis revealed that serum adropin was the only independent negative predictor of CSFP (odds ratio 0.758, 95% confidence interval 0.647-0.888, p = 0.001). Serum adropin levels were independently and negatively correlated with mean TFC (r = -0.387, p < 0.001).
Conclusions
We demonstrated that decreased serum adropin levels were independently associated with the presence and severity of angiographically proven CSFP. These findings suggest that serum adropin may be a potential biomarker to provide valuable information regarding the prediction of CSFP.
Keywords: Adropin, Biomarker, Cardiovascular, Coronary slow flow phenomenon, Disease
INTRODUCTION
Coronary slow flow phenomenon (CSFP) is a relatively rare angiographic finding characterized by a delayed antegrade progression of contrast agent to normal or near-normal epicardial coronary arteries.1,2 The incidence of CSFP has been reported to range from 1-7% among patients undergoing coronary angiography (CAG).1,3 More than 80% of patients with CSFP have been reported to experience frequent recurrent chest pain, almost 20% of whom require readmission following the same diagnosis.4 In addition, the presence of CSFP has been reported to be associated with various clinical events including angina pectoris, acute myocardial infarction (AMI), life-threatening arrhythmias and sudden cardiac death.5-7
Although CSFP has been known to cardiologists for decades, the potential molecular mechanism of the disease has not been fully elucidated. Many studies have indicated that endothelial dysfunction, which can be caused by many factors including inflammation, adiposity, and atherogenesis, may play a key role in the pathophysiology of CSFP.8-10 Therefore, molecules involved in the pathophysiology of endothelial dysfunction may be correlated with CSFP.
Adropin is a recently described peptide hormone encoded by the energy homeostasis-associated gene (Enho).11 Adropin is expressed in the liver and the brain, and it appears to participate in maintenance of metabolic homeostasis, lipid metabolism and insulin response.11,12 A recent study further found that adropin is also expressed in coronary artery endothelial cells and that it plays a potential endothelial protective role.13 From this viewpoint, we hypothesized that adropin deficiency may be involved in the pathophysiology of CSFP. Therefore, the aim of this study was to detect serum adropin levels in patients with CSFP and to assess the association between adropin and CSFP.
MATERIALS AND METHODS
Study population
This study was performed between May 2013 to May 2015. Over this 2-year period, of 2865 consecutive patients who underwent clinically indicated CAG in Tangdu Hospital, 82 patients who had angiographically confirmed normal coronary or near-normal (< 40% stenosis) coronary arteries and CSFP were enrolled. During the same period, 184 age- and sex-matched patients with normal coronary or near-normal coronary arteries and normal coronary flow were enrolled as controls. Participants were excluded if they had acute coronary syndrome (ACS), atrial fibrillation, sinus node dysfunction or conduction disturbance, hypertrophic cardiomyopathy, severe valvular disease, active autoimmune disorders, severe heart failure, unstable hemodynamics, suspected myocarditis or pericarditis, advanced renal or hepatic disease, malignant disease, diabetes and if they had used nitrates within 24 hx. All patients gave written informed consent before study entry. The study protocol was approved by the Ethics Committee of Tangdu Hospital.
CAG and determination of CSFP
Quantitative CAG was carried out in the catheterization department of our hospital according to standard protocols. No auto-injector was used, and no nitrates were administrated before CAG. Angiograms were analyzed by two experienced interventional cardiologists blinded to the study protocol. CAG was recorded at 15 frames per second. Thrombolysis in myocardial infarction (TIMI) frame count (TFC) was determined for each major coronary artery in each patient by two independent observers using a previously reported technique.14 A third observer resolved any discrepancies. Frame counts in the left anterior descending coronary artery (LAD) were divided by a factor of 1.7 to correct for its longer length. Because the most frequently standardized filming rate is 30 frames per second, the TFCs were multiplied by 2. The standard corrected TFCs (cTFCs) for normal visualization of the coronary arteries were 21.1 ± 1.5 for the LAD, 22.2 ± 4.1 for the left circumflex artery (LCx), and 20.4 ± 3.0 for the right coronary artery (RCA) according to Gibson et al.14 CSFP was defined as a cTFC greater than two standard deviations from the normal range, while normal coronary flow was defined as a cTFC within two standard deviations of the normal range. The mean TFC for each subject was calculated by totaling the TFCs for the LAD, LCx, and RCA, and then dividing by 3.
Biochemical analysis
After an overnight fast, blood samples were obtained from all participants before CAG. Because adropin is a peptide hormone which is easily broken down by proteases, aprotinin (500 kallikrein/ml) was added before the collection of blood samples to prevent proteolysis. Blood samples were immediately centrifuged, and serum samples were stored at -80 °C until measurement. Routine laboratory parameters including triglycerides (TGs), total cholesterol (TC), fasting blood glucose (FBG), low density lipoprotein cholesterol (LDL-c), high density lipoprotein cholesterol (HDL-c), blood urea nitrogen (BUN), creatinine (Cre), and high-sensitivity C-reactive protein (hs-CRP) levels were determined in the biochemical laboratory of Tangdu Hospital. Serum adropin levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Cusabio Biotech Co., Wuhan, CN). The sensitivity of the assay was 0.08 ng/mL, and the inter- and intra-assay coefficients of variation (CV) were less than 14% and 5%, respectively. All of the serum samples were routinely analyzed by ELISA in duplicate, and the results were averaged.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± SD and compared using the unpaired t-test. Continuous variables with skewed distribution were summarized as median and quartile range and compared using the Mann-Whitney U test. The χ2 test was used to test categorical variables. Backward stepwise multivariate logistic regression was performed to assess the independent predictors of angiographically confirmed CSFP. Correlations between clinical variables and mean TFC were analyzed by Spearman correlation analysis. Multivariate linear regression analysis was performed to assess the independent predictors of mean TFC. Statistical analyses were performed using SPSS 22.0 statistical software (SPSS Inc., Chicago, Illinois, USA). Significance was defined as a p value < 0.05.
RESULTS
Baseline clinical and laboratory characteristics
Eighty-two CSFP patients were identified in 2865 consecutive patients who underwent CAG, and the incidence of CSFP was 2.86%. Baseline clinical and laboratory characteristics of the study population are shown in Table 1. Patients with CSFP had significantly higher TFC values for the LAD (30.71 ± 7.80 vs. 22.26 ± 3.59, p < 0.001), LCx (28.96 ± 8.03 vs. 20.57 ± 2.66, p < 0.001), and RCA (30.28 ± 5.84 vs. 21.83 ± 3.03, p < 0.001), as well as mean TFC (30.28 ± 5.84 vs. 21.76 ± 2.55, p < 0.001) compared to those with normal coronary flow. LAD, LCx, and RCA CSFP was involved in 78%, 39.02%, and 50% of the patients, respectively. One-, two-, and three-vessel CSFP was involved in 39.02%, 31.71%, and 29.27% of the patients, respectively.
Table 1. Baseline characteristics and angiographic findings and serum adropin levels.
| Variables | Controls (n = 184) | CSFP (n = 82) | p value |
| Age (years) | 56 (50-63) | 55 (50-65) | 0.897 |
| Male (n, %) | 112 (60.87%) | 53 (64.63%) | 0.559 |
| Smoking (n, %) | 79 (42.93%) | 41 (50.00%) | 0.285 |
| BMI (Kg/mm2) | 25.27 (23.98-26.25) | 25.30 (24.37-26.42) | 0.587 |
| SBP (mm/Hg) | 131.65 ± 16.69 | 133.10 ± 17.23 | 0.517 |
| DBP (mmHg) | 81.85 ± 10.61 | 83.18 ± 11.04 | 0.350 |
| FBG (mmol/L) | 5.49 ± 0.73 | 5.50 ± 0.88 | 0.928 |
| TC (mmol/L) | 4.48 ± 1.07 | 4.47 ± 1.07 | 0.957 |
| TG (mmol/L) | 1.80 (1.29-2.65) | 1.91 (1.55-2.57) | 0.201 |
| LDL-c (mmol/L) | 2.77 ± 0.85 | 2.93 ± 0.98 | 0.204 |
| HDL-c (mmol/L) | 1.05 ± 0.22 | 1.01 ± 0.23 | 0.130 |
| BUN (mmol/L) | 5.12 ± 0.96 | 5.25 ± 0.98 | 0.776 |
| Cre (μmol/L) | 61.17 ± 10.68 | 63.90 ± 10.86 | 0.537 |
| Hs-CRP (mg/L) | 0.61 (0.43-0.87) | 0.80 (0.47-1.29)* | 0.016 |
| TIMI frame count | |||
| LAD (Corrected) | 22.26 ± 3.59 | 30.71 ± 7.80* | < 0.001 |
| LCx | 20.57 ± 2.66 | 28.96 ± 8.03* | < 0.001 |
| RCA | 21.83 ± 3.03 | 31.17 ± 8.45* | < 0.001 |
| Mean TFC | 21.55 ± 2.04 | 30.28 ± 5.84* | < 0.001 |
| Adropin (ng/ml) | 4.86 ± 1.88 | 4.03 ± 1.99 | 0.001 |
All values are mean ± SD, median with interquartile range or n, %.
BMI, body mass index; BUN, blood urea nitrogen; Cre, creatinine; CSFP, coronary slow flow phenomenon; DBP, diastolic blood pressure; FBG, fasting glucose; HDL-c, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; LDL-c, low density lipoprotein cholesterol; RCA, right coronary artery; SBP, systolic blood pressure; TC, total cholesterol; TFC, TIMI frame count; TG, triglycerides; TIMI, thrombolysis in myocardial infarction.
* p < 0.05 compared with controls.
Serum adropin levels in the study population
As shown in Table 1, the CSFP patients had significantly lower serum adropin levels compared with the controls (4.03 ± 1.99 vs. 4.86 ± 1.88 ng/ml, p = 0.001).
Multivariate logistic regression for the presence of CSFP
As shown in Table 2, multivariate logistic regression including all variables was used to assess their independent association with the presence of CSFP. The results showed that serum adropin was the only independent negative predictor of CSFP (odds ratio 0.758, 95% confidence interval 0.647-0.888, p = 0.001).
Table 2. Multiple logistic regression analysis.
| Variables | OR (95% CI) | p value |
| Age (per year) | 1.005 (0.974-1.038) | 0.741 |
| Male (no/yes) | 1.100 (0.571-2.117) | 0.776 |
| Smoking (no/yes) | 0.806 (0.440-1.475) | 0.806 |
| BMI | 0.987 (0.867-1.123) | 0.841 |
| SBP (per mm/Hg) | 1.001 (0.978-1.024) | 0.944 |
| DBP (per mmHg) | 1.018 (0.982-1.056) | 0.329 |
| FBG (per mmol/L) | 1.121 (0.771-1.629) | 0.550 |
| TC (per mmol/L) | 1.008 (0.758-1.340) | 0.956 |
| TG (per mmol/L) | 1.278 (0.914-1.785) | 0.151 |
| LDL-c (per mmol/L) | 1.150 (0.828-1.599) | 0.404 |
| HDL-c (per mmol/L) | 0.312 (0.084-1.166) | 0.083 |
| BUN (per mmol/L) | 1.136 (0.831-1.552) | 0.425 |
| Cre (per μmol/L) | 1.023 (0.996-1.051) | 0.095 |
| hs-CRP (per mg/L) | 1.083 (0.964-1.217) | 0.180 |
| Adropin (per ng/ml) | 0.758 (0.647-0.888) | 0.001 |
CI, confidence interval; OR, odds ratio; other abbreviations are as in Table 1.
Correlation of serum adropin levels and mean TFC
We performed Spearman correlation analysis to assess the correlations between variables and mean TFC. The results showed that serum adropin levels were negatively correlated with mean TFC (r = -0.387, p < 0.001, Figure 1). Multivariate linear regression was then used to adjust for potential confounders. The analysis revealed that serum adropin was still a significant negative determinant of mean TFC (t = -3.451, p = 0.001; Table 3).
Figure 1.
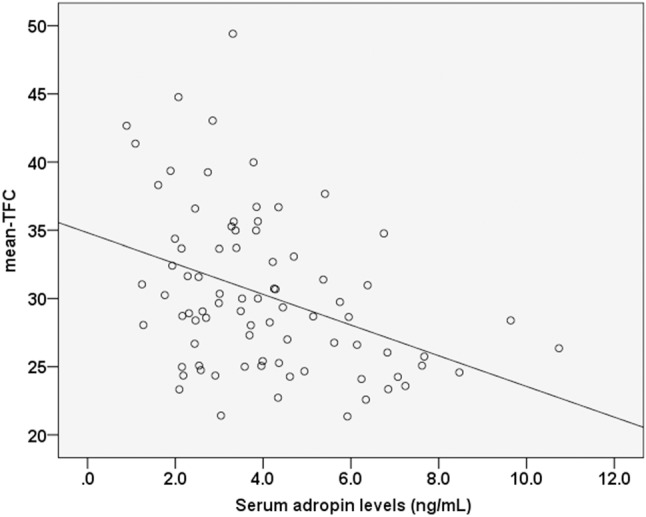
Correlations between serum adropin levels and mean-TFC. TFC, TIMI frame count.
Table 3. Multivariate linear regression analysis for the independent predictors of log (mean-TFC).
| β | t | p value | |
| Age | -0.052 | -0.458 | 0.648 |
| log (BMI) | -0.100 | -0.857 | 0.395 |
| SBP | 0.028 | 0.148 | 0.883 |
| DBP | 0.179 | 0.960 | 0.340 |
| FBG | 0.140 | 1.108 | 0.272 |
| TC | -0.019 | -0.153 | 0.879 |
| log (TG) | 0.025 | 0.222 | 0.825 |
| LDL-c | 0.049 | 0.399 | 0.691 |
| HDL-c | -0.133 | -1.13 | 0.262 |
| BUN | 0.071 | 0.600 | 0.551 |
| Cre | -0.050 | -0.440 | 0.661 |
| log (hsCRP) | 0.107 | 0.868 | 0.388 |
| Adropin | -0.418 | -3.451 | 0.001 |
All abbreviations are shown in Table 1.
DISCUSSION
To the best of our knowledge, this is the first study to evaluate the association between adropin and CSFP. There are two main findings to this study. First, the patients with CSFP had significantly lower serum adropin levels compared to the controls, and the decreased adropin levels were independently associated with the presence of CSFP. Second, among the patients with CSFP, serum adropin levels were negatively correlated with mean TFC.
CSFP is an angiographic entity characterized by delayed progression of the contrast medium during CAG. Previous studies have demonstrated the long-term prognostic significance of CSFP concomitant with an existing vascular wall abnormality in the development of future major adverse cardiovascular events.5-7 Therefore, CSFP should not be considered to be a totally benign condition and should be detected at the earliest stage.15 Although angiographic assessment is the keystone to identifying CSFP, angiographic examinations are not available in all patients because of limited healthcare budgets, especially in developing countries. In addition, angiographic examinations do not permit long-term clinical follow-up and dynamic treatment evaluation owing to their invasiveness. Therefore, biomarkers that perform well and are cost-effective to rapidly "rule out" or "rule in" strategies and those that help to triage patients into low- and high-risk treatment strategies can be integrated into clinical decision-making protocols.16
The identification of a reliable biomarker for CSFP largely depends on a better understanding of the etiology and pathogenesis underlying the disease. Although CSFP has been reported to be more common in males, smokers and patients with obesity,17 we were unable to identify significant differences between the CSFP and controls groups in terms of gender, smoking status and body mass index in the present study. These results are consistent with recent studies.15,18,19 The exception was hs-CRP, which was higher in the patients with CSFP than in the controls, which is consistent with the study of Li et al.20
The endothelium plays a crucial role in the maintenance of vascular homeostasis, and endothelial dysfunction is considered to be an important insult that can cause the initiation and progression of CSFP.8-10 Recent studies have shown that decreased adiponectin concentration and paraoxonase activity, two significant markers of endothelial dysfunction, are responsible for the etiopathogenesis of CSFP.21,22 Adropin is a secreted protein critically involved in energy homeostasis, metabolic adaptation to macronutrients, and modulation of insulin sensitivity.11,12 A recent study reported a potential endothelial protective role of adropin that is most likely mediated via upregulation of endothelial nitric oxide (NO) synthase expression through the VEGFR2-phosphatidylinositol 3-kinase-Akt and VEGFR2-extracellular signal-regulated kinase 1/2 pathways.13 In addition, the loss of NO bioavailability caused by adropin insufficiency is a cardinal feature of endothelial dysfunction that precedes the development of overt atherosclerosis and is an independent predictor of the development of SVG occlusion, as reported by Demircelik et al.23 Atherosclerosis also plays a key role in the development of CSFP.6,7 From this viewpoint, adropin may be a potential CSFP-related biomarker.
The major finding of the present study is that serum adropin was an independent negative predictor of CSFP. This result suggests that serum adropin may be a potential biomarker for the presence of CSFP. This finding is in accordance with a previous study by Celik et al., in which a deficiency of adropin was associated with cardiac syndrome X (CSX).24 Although the features of CSFP are not the same as CSX, endothelial dysfunction and disorders of microvascular homeostasis are common pathophysiologies of the two diseases.25
We also assessed the relationship between serum adropin levels and coronary flow rate using the TIMI-TFC method. We found that serum adropin levels were negatively correlated with TIMI-TFC in the patients with CSFP, and the correlation was still significant after adjusting for potential confounders in multivariate linear regression analysis. This result suggests that serum adropin may also be a potential biomarker for reflecting the severity of CSFP. However, the association between serum adropin levels and TIMI-TFC was moderate, and further studies are needed to investigate its clinical significance.
This study has several limitations that should be considered. These limitations relate primarily to its cross-sectional design and the relatively small study population. The cross-sectional nature of the study does not allow conclusions to be drawn about the true prognostic value and molecular mechanisms underlying the relationship between adropin and CSFP. Therefore, the results of the present study should be confirmed in multi-center prospective longitudinal studies with a larger study population. Second, CSFP in the present study was diagnosed by visual assessment of CAG, which cannot provide sufficient information regarding true coronary blood flow. This study lacks intravascular ultrasound or combined pressure and flow examinations, as well as evaluations of coronary endothelial function using acetylcholine. Third, a lack of short- and long-term follow-up of the CSFP patients was another limitation. Fourth, we did not evaluate correlations between adropin and other indicators of endothelial function such as intercellular adhesion molecule-1, tumor necrosis factor-α, P-selectin, and interleukin-6. Such evaluations could provide more valuable information on the disease-promoting role of adropin signaling pathways in CSFP and truly reveal the unique nature of adropin.
CONCLUSIONS
In conclusion, we demonstrated that decreased serum adropin levels were independently associated with the presence and severity of angiographically proven CSFP. These findings suggest that adropin in serum may be a potential biomarker to provide valuable information regarding CSFP prediction. Further studies are necessary to substantiate the potential significance of adropin in monitoring CSFP.
Acknowledgments
This work was supported by Fujian Natural Science Foundation for Young Scholars (2013J05114) and the National Natural Science Foundation of China (81600289).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Tambe AA, Demany MA, Zimmerman HA, et al. Angina pectoris and slow flow velocity of dye in coronary arteries--a new angiographic finding. Am Heart J. 1972;84:66–71. doi: 10.1016/0002-8703(72)90307-9. [DOI] [PubMed] [Google Scholar]
- 2.Çetin M, Kiziltunc E, Elalmış ÖU, et al. Predictive value of neutrophil lymphocyte ratio and platelet lymphocyte ratio in patients with coronary slow flow. Acta Cardiol Sin. 2016;32:307–312. doi: 10.6515/ACS20150119I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beltrame JF, Turner SP, Leslie SL, et al. The angiographic and clinical benefits of mibefradil in the coronary slow flow phenomenon. J Am Coll Cardiol. 2004;44:57–62. doi: 10.1016/j.jacc.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Ma C, Zhang Y, et al. Assessment of left and right ventricular diastolic and systolic functions using two-dimensional speckle-tracking echocardiography in patients with coronary slow-flow phenomenon. PLoS One. 2015;23:e0117979. doi: 10.1371/journal.pone.0117979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horjeti B, Goda A. Acute ischemia manifestation in a patient with coronary slow flow phenomenon. J Electrocardiol. 2012;45:277–279. doi: 10.1016/j.jelectrocard.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins BM, Stavrakis S, Rousan TA, et al. Coronary slow flow--prevalence and clinical correlations. Circ J. 2012;76:936–942. doi: 10.1253/circj.cj-11-0959. [DOI] [PubMed] [Google Scholar]
- 7.Wozakowska-Kapłon B, Niedziela J, Krzyzak P, et al. Clinical manifestations of slow coronary flow from acute coronary syndrome to serious arrhythmias. Cardiol J. 2009;16:462–468. [PubMed] [Google Scholar]
- 8.Luo C, Liu D, Wu G, et al. Effect of enhanced external counterpulsation on coronary slow flow and its relation with endothelial function and inflammation: a mid-term follow-up study. Cardiology. 2012;122:260–268. doi: 10.1159/000339876. [DOI] [PubMed] [Google Scholar]
- 9.Signori LU, Quadros AS, Sbruzzi G, et al. Endothelial function in patients with slow coronary flow and normal coronary angiography. Clinics (Sao Paulo) 2012;67:677–680. doi: 10.6061/clinics/2012(06)22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Chen X, Li S, et al. Nicorandil improves myocardial function by regulating plasma nitric oxide and endothelin-1 in coronary slow flow. Coron Artery Dis. 2015;26:114–120. doi: 10.1097/MCA.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar KG, Trevaskis JL, Lam DD, et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 2008;8:468–481. doi: 10.1016/j.cmet.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesh Kumar K, Zhang J, Gao S, et al. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring) 2012;20:1394–1402. doi: 10.1038/oby.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovren F, Pan Y, Quan A, et al. Adropin is a novel regulator of endothelial function. Circulation. 2010;122:S185–S192. doi: 10.1161/CIRCULATIONAHA.109.931782. [DOI] [PubMed] [Google Scholar]
- 14.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 15.Cakmak HA, Aslan S, Yalcin AA, et al. Relationship between serum visfatin levels and coronary slow-flow phenomenon. Herz. 2015;40:921–928. doi: 10.1007/s00059-015-4313-4. [DOI] [PubMed] [Google Scholar]
- 16.Vasan RS. Biomarkers of cardiovascular disease. Molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz H, Demir I, Uyar Z. Clinical and coronary angiographic characteristics of patients with coronary slow flow. Acta Cardiol. 2008;63:579–584. doi: 10.2143/AC.63.5.2033224. [DOI] [PubMed] [Google Scholar]
- 18.Doğan M, Akyel A, Çimen T, et al. Relationship between neutrophil to lymphocyte ratio and slow coronary flow. Clin Appl Thromb Hemost. 2015;21:251–254. doi: 10.1177/1076029613498814. [DOI] [PubMed] [Google Scholar]
- 19.Canga A, Cetin M, Kocaman SA, et al. Increased serum resistin levels in patients with coronary slow-flow phenomenon. Herz. 2013;38:773–778. doi: 10.1007/s00059-013-3758-6. [DOI] [PubMed] [Google Scholar]
- 20.Li JJ, Qin XW, Li ZC, et al. Increased plasma C-reactive protein and interleukin-6 concentrations in patients with slow coronary flow. Clin Chim Acta. 2007;385:43–47. doi: 10.1016/j.cca.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Selcuk H, Selcuk MT, Temizhan A, et al. Decreased plasma concentrations of adiponectin in patients with slow coronary flow. Heart Vessels. 2009;24:1–7. doi: 10.1007/s00380-008-1074-5. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz A, Gur M, Yilmaz R, et al. Association of paraoxonase activity and coronary blood flow. Atherosclerosis. 2008;197:257–263. doi: 10.1016/j.atherosclerosis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Demircelik B, Cakmak M, Nazli Y, et al. Adropin: a new marker for predicting late saphenous vein graft disease after coronary artery bypass grafting. Clin Invest Med. 2014;37:E338–E344. doi: 10.25011/cim.v37i5.22014. [DOI] [PubMed] [Google Scholar]
- 24.Celik A, Balin M, Kobat MA, et al. Deficiency of a new protein associated with cardiac syndrome X; called adropin. Cardiovasc Ther. 2013;31:174–178. doi: 10.1111/1755-5922.12025. [DOI] [PubMed] [Google Scholar]
- 25.Fragasso G, Chierchia SL, Arioli F, et al. Coronary slow-flow causing transient myocardial hypoperfusion in patients with cardiac syndrome X: long-term clinical and functional prognosis. Int J Cardiol. 2009;137:137–144. doi: 10.1016/j.ijcard.2008.06.070. [DOI] [PubMed] [Google Scholar]


