Abstract
Patient: Female, 27
Final Diagnosis: Herpes Simplex Virus Type 1 (HSV-1) encephalitis
Symptoms: Fever • headache • seizure
Medication: —
Clinical Procedure: Dermal filler injection
Specialty: Neurology
Objective:
Unusual or unexpected effect of treatment
Background:
Dermal fillers are increasingly used for medical and aesthetic purposes in clinical practice. Common complications following filler injections include bruising, itching, infections, allergic reactions, and tissue necrosis. This case is the first report of Herpes simplex virus type 1 (HSV-1) encephalitis as a possible complication of dermal filler injection.
Case Report:
A 27-year-old woman with no past medical history presented with altered mental state, headaches, and seizures. She had a nasal dermal filler injection for aesthetic purpose five weeks before her acute presentation. A diagnosis of HSV-1 encephalitis was made based on brain imaging with computed tomography and magnetic resonance imaging (MRI) findings that showed bilateral frontotemporal lobe hyperintensity. Analysis of her cerebrospinal fluid (CSF) confirmed the presence of HSV-1 DNA. Despite anti-viral treatment with acyclovir, she developed postencephalitic syndrome.
Conclusions:
This case report highlights the possibility that among the complications of the use of cosmetic dermal fillers, the transmission of HSV-1 and the development of HSV-1 encephalitis should be recognized.
MeSH Keywords: Acyclovir; Encephalitis, Herpes Simplex; Status Epilepticus
Background
Cosmetic procedures are increasingly performed by clinicians, including the use of dermal fillers, which can be injected in the clinic. There are now a wide variety of fillers that are currently available for medical and cosmetic use. The complications of the use of dermal fillers have been described in the literature and include bruising, itching, infections, allergic reactions, and tissue necrosis [1,2].
In this report, a case of Herpes simplex virus type 1 (HSV-1) encephalitis as a possible complication of dermal filler injection is described. To our knowledge, this is the first report of HSV-1 encephalitis as a possible complication of the use of cosmetic dermal filler injection.
Case Report
A 27-year-old woman with no past medical history or note presented to the emergency department with a three-day history of altered mental state, headaches, and seizures. She had an injectable dermal filler into her nose, for aesthetic purpose, five weeks before her admission to hospital. One week after the dermal injection, she reported a persistent fever, headache and generalized lethargy. She had visited her general practitioner (GP), who diagnosed a viral infection and prescribed an antipyretic agent.
The patient reported that there were no skin lesions or rashes immediately after the intradermal injection procedure, and had no recent symptoms suggestive of upper respiratory tract infection, urinary tract infection, ear infection, or gastroenteritis. She denied a history of head trauma, childhood febrile seizures, or family history of epilepsy. The patient was single and had no high-risk behaviors or illicit drug abuse.
On examination during her hospital admission, her Glasgow Coma Scale (GCS) was 11/15 (Eye-opening, 3/5; Motor response, 5/5; Verbal response, 3/5). Although she had neck stiffness, she had no long tract signs, such as hyperreflexia or spasticity, and the Hoffman’s sign and Babinski sign were normal. No skin rashes were present. Examination of her cardiovascular, respiratory, and abdominal systems was normal. Her vital signs included blood pressure (BP) of 120/70 mmHg, heart rate 100 bpm, temperature 38°C, SpO2 99% on room air, random blood glucose of 5 mmol/L. Her laboratory findings were hemoglobin (Hb) 12 g/dL, white cell count (WCC) 12×109/L, platelet count 350×109/L, urea 5 mmol/L, sodium 140 mmol/L, potassium 3.6 mmol/L, creatinine 89 µmol/L, albumin 40 g/L, alanine transaminase (ALT) 40 U/L, aspartate transaminase (AST) 35 U/L.
Two hours after her emergency hospital admission, she developed status epilepticus requiring endotracheal intubation, and treatment with intravenous (IV) phenytoin at a dose of 15 mg/kg and was treated in the intensive care unit (ICU). She did not require vasopressors or oxygen therapy but was initially treated with IV ceftriaxone 2 gm twice daily and IV acyclovir 500 mg three times daily for presumed acute meningoencephalitis.
Serological tests were negative for pregnancy, drug toxicology, human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), hepatitis viruses, malaria, leptospirosis, dengue fever, and mycoplasma. Her connective tissue disease work-up was negative. Thyroid function tests were normal. Serum anti-N-methyl D-aspartate (NMDA) receptor antibody (for NMDA receptor antibody encephalitis) was negative.
Her chest X-ray and electrocardiogram (ECG) were normal. The brain magnetic resonance imaging (MRI) showed hyperintensity in the bilateral frontotemporal regions (Figure 1). Her cerebro-spinal fluid (CSF) protein was elevated at 0.6 g/L, and Herpes simplex virus type 1 (HSV-1) DNA from the CSF was detected. She had a positive serum HSV-1 IgM and a negative HSV-1 IgG test. The electroencephalogram (EEG) showed generalized theta activity with no definite alpha rhythms (Figure 2), which supported a diagnosis of mild, diffuse electrocerebral disturbance. A diagnosis of HSV-1 encephalitis was made, and she was treated with IV acyclovir for three weeks and methylprednisolone for five days. Her seizures improved with levetiracetam 250 mg twice daily, and she was transferred to the neurology ward after one week in the ICU. Following discharge from the hospital, she was free from seizures, but remained dysphasic with impaired cognitive function, but had no more seizures.
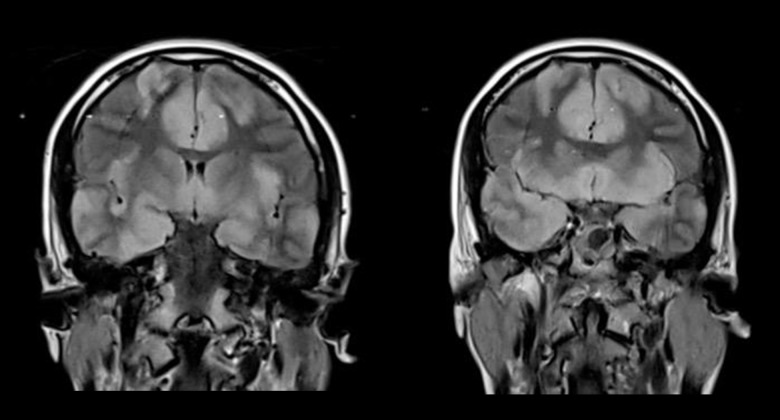
Figure 1.
T2-weighted-Fluid-Attenuated Inversion Recovery (FLAIR) brain magnetic resonance imaging (MRI) in the coronal plane. T2-weighted-Fluid-Attenuated Inversion Recovery (FLAIR) brain magnetic resonance imaging (MRI) in the coronal plane shows hyperintense and swollen cerebral cortices involving both frontal and temporal lobes, including the insular regions.
Figure 2.
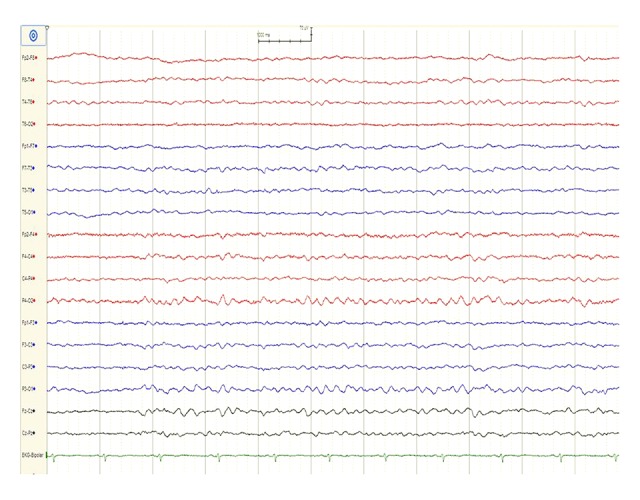
Electroencephalogram (EEG) findings. An electroencephalogram (EEG) shows theta activity with no definite alpha rhythms.
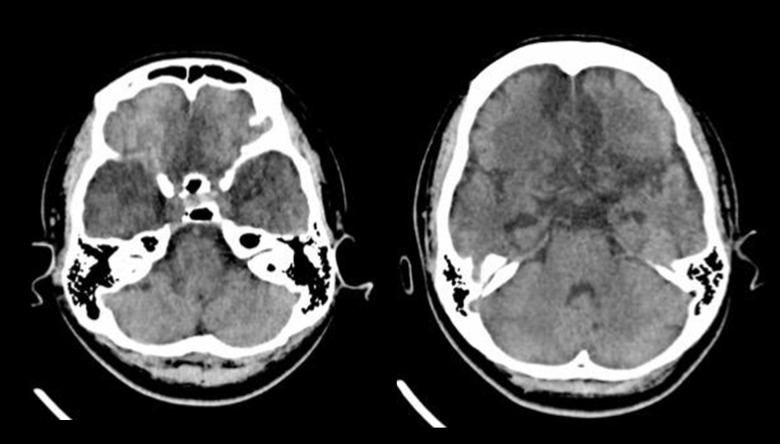
Two months following hospital discharge, the patient was re-admitted with postencephalitic syndrome (hypomania, self-injury, hyperphagia). A repeat brain CT scan showed hypodensity within the previously involved areas in the bilateral frontotemporal lobes (Figure 3). She was transferred to the psychiatry ward for further management of her behavioral symptoms. Three months later, she remained free from seizures, and her behavior was less aggressive. However, her cognitive function remained poor, she slept most of the time and had poor verbal ability. Her activities of daily living (ADLs) were heavily dependent upon her mother, including bathing, toilet hygiene, functional mobility, dressing, and grooming. Her mother refused further neuroimaging and EEG studies.
Figure 3.
Plain (non-contrast) computed tomography (CT) brain imaging two months after magnetic resonance imaging (MRI). Follow-up plain (non-contrast) computed tomography (CT) brain imaging shows hypodensity within the previously involved areas in the bilateral frontotemporal lobes with ex vacuo dilatation of the temporal horns of the lateral ventricles indicating volume loss. These imaging findings indicate encephalomalacia.
Discussion
Fillers are increasingly used for medical and aesthetic purposes in clinical practice, and are mainly applied to improve the appearance of the face and other body parts, correct volume defects, and improve facial contours, including of the nose [1]. Common complications following filler injections include bruising, itching, infections, allergic reactions, and tissue necrosis [2].
Herpes simplex virus type 1 (HSV-1) is a member of the Herpesviridae family of viruses and commonly infects humans, including the lips, as herpes labialis (‘cold sore’), also causes herpetic skin lesions of the finger or thumb (whitlow), and herpetic keratitis. Due to its affinity for nerve cells, HSV-1 can cause meningitis or encephalitis. Following an initial infection, the HSV viruses lie dormant in the dorsal root ganglia and may become reactivated at a later stage. Local trauma, systemic stress or immunosuppression can induce viral reactivation [3]. The incidence of HSV-1 reactivation following dermal filler injection is reported to be less than 1.45% [4]. The reactivation of the viruses is believed to be due to direct damage to the nerve axon by a needle during an aesthetic procedure. Tissue manipulation and the inflammatory process may play a role in this process. Typically, HSV-1 reactivation presents with mucocutaneous lesions, usually as ulcers or blisters.
To the best of our knowledge, HSV-1 encephalitis occurring after dermal filler injection has not been previously reported in the literature. The pathogenesis of HSV-1 encephalitis in this present case was thought to be due to the reactivation of the HSV-1 virus following the filler injection, with the involvement of the cranial nerves.
HSV-1 encephalitis is one of the common causes of fatal encephalitis, and it carries a mortality rate up to 30% [5]. It is frequently characterized by the rapid onset of fever, headache, seizures, focal neurologic signs, and impaired consciousness. The most reliable method for diagnosing HSV-1 encephalitis is by detection of viral DNA in the CSF by the use of molecular diagnostic methods, including the polymerase chain reaction (PCR). Temporal lobe lesions demonstrated by MRI are typical of this form of encephalitis. Classically, CSF may show lymphocytic pleocytosis and elevated protein level. Patients who survive an episode of HSV-1 encephalitis may have post-encephalitic syndrome [6], as shown in the present case, and have residual neurological sequelae. Antiviral prophylaxis for HSV infections is recommended in high-risk patient groups and immunocompromised individuals. Acyclovir, as first-line treatment, or valaciclovir as second-line treatment, could be started one to two days prior to the cosmetic procedure and continued for five to seven days as prophylaxis for reactivation of HSV-1 [7].
Conclusions
This case report highlights the possible complication of Herpes simplex virus type 1 (HSV-1) encephalitis, following cosmetic nasal dermal filler injection. HSV-1 encephalitis is a devastating disease with significant morbidity and mortality. It is important to consider antiviral prophylaxis in high-risk and immunocompromised groups before administering dermal fillers.
Acknowledgments
Authors would like to thank the staff of the Neurology Unit, Department of Medicine, Universiti Kebangsaan Malaysia Medical Center.
Footnotes
Conflict of interest
None.
References:
- 1.Sánchez-Carpintero I, Candelas D, Ruiz-Rodríguez R. Dermal fillers: [Types, indications, and complications] Actas Dermosifiliogr. 2010;101(5):381– 93. doi: 10.1016/s1578-2190(10)70660-0. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 2.Lafaille P, Benedetto A. Fillers: Contraindications, side effects and precautions. J Cutan Aesthet Surg. 2010;3(1):16–19. doi: 10.4103/0974-2077.63222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinde B. Herpesviruses: Latency and reactivation – viral strategies and host response. J Oral Microbiol. 2013:5. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim B. Herpes reactivation after injection of dermal fillers. ANZ J Surg. 2013;83:998. doi: 10.1111/ans.12405. [DOI] [PubMed] [Google Scholar]
- 5.Levitz RE. Herpes simplex encephalitis: A review. Heart Lung. 1998;27:209–12. doi: 10.1016/s0147-9563(98)90009-7. [DOI] [PubMed] [Google Scholar]
- 6.Hart RP, Kwentus JA, Frazier RB, Hormel TL. Natural history of Klüver-Bucy syndrome after treated herpes encephalitis. South Med J. 1986;79:1376–78. doi: 10.1097/00007611-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Sherman RN. Avoiding dermal filler complications. Clin Dermatol. 2009;27:S23–32. [Google Scholar]