Abstract
Patient: Male, 67
Final Diagnosis: Gastric GIST
Symptoms: Chest pain • hematemesis • melena • shock
Medication: —
Clinical Procedure: Laparoscopic sleeve gastrectomy
Specialty: Surgery
Objective:
Management of emergency care
Background:
Gastrointestinal stomal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, and the stomach is the most commonly involved organ. Complete surgical resection with negative margins is the primary and only potentially curative treatment. Surgeon experience with minimally invasive gastric resections in addition to the tumor size and site has to be considered in the choice of laparoscopic or open surgical approach in order to remove the lesion.
Case Report:
A 67-year-old male patient with an history of gastric ulcer presented 2 days after an esophagogastroduodenoscopy with an incidental finding of a 30-mm gastric submucosal lesion that was not histologically defined (biopsies were taken), chest pain in association with hematemesis, and melena. An initial attempt to achieve endoscopic hemostasis with epinephrine injection was followed by the recurrence of the gastric bleeding until the presentation of hemorrhagic shock. An emergent laparoscopic sleeve gastrectomy was then performed for hemorrhage control. There were no intra- or postoperative major complications and the histological findings led to the diagnosis of a gastrointestinal stromal tumor (GIST).
Conclusions:
Laparoscopic sleeve gastrectomy is a bariatric surgical treatment of morbid obesity. This report describes the application of a bariatric procedure in a life-threatening situation and illustrates how safe and effective it can be when performed by surgeons with excellent laparoscopic skills.
MeSH Keywords: Gastrectomy; Gastrointestinal Stromal Tumors; Laparoscopy; Surgical Procedures, Minimally Invasive
Background
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and represent the 0.1–3% of all GI neoplasms [1]. They arise from the interstitial cells of Cajal, which are specialized network-forming cells distributed in the smooth muscle wall of the digestive tract with a pacemaker role. Three identified and mutually exclusive mutations provide the growth stimulation of GISTs by affecting c-KIT, PDGFRA, and BRAF genes and are found in 85%, 5%, and less than 1% of cases, respectively [2]. GISTs occur more frequently in middle-aged men, with peak incidence in the sixth decade of life. While any portion of the GI tract can be affected, they are usually detected in the stomach (60–70%). The percentage of GISTs originating in the small intestine is about 20– 30% and the remaining 5–10% occur elsewhere in the GI tract (e.g., oesophagus, colon, rectum, omentum, and peritoneum). [3]
The clinical presentation of GISTs is quite variable and depends on the size and location of the lesion. Most are asymptomatic and thus incidentally discovered during endoscopic, imaging, or surgical procedures performed for unrelated conditions. They are more likely to become symptomatic as they increase in size. Gastric GISTs are frequently associated with symptoms such as abdominal discomfort, dyspepsia, and vomiting. Larger lesions can cause upper-GI bleeding or perforation and, more rarely, obstruction. GISTs are usually solitary and have rarely invade surrounding structures or metastasize to regional lymph nodes. However, metastases to liver, bone, lung, soft tissue, and skin have been reported [4].
The diagnostic work-up for detecting lesions includes gastrointestinal endoscopy, computed tomography (CT) scan, or magnetic resonance imaging (MRI). Endoscopic ultrasound (EUS) may be helpful, as it can identify morphological details of cystic changes, necrosis, or any echogenic heterogeneity, which can be signs of malignancy. EUS is also a safe method for targeted biopsies by fine-needle aspiration.
Although the introduction of the first tyrosine kinase inhibitor (imatinib) profoundly changed the management of GISTs over the last 10 years, surgical resection with clear margins, when possible, remains the mainstay of cure. The timing of surgery and the most appropriate technique (open or laparoscopic) are defined based on multidisciplinary evaluation, with special consideration of the size and site of the tumor or the presence of a metastatic disease, in addition to the age and performance status of the patient. Imatinib is the first-line drug for use in unresectable and metastatic GISTs or may be given as neoadjuvant therapy for initially inoperable cases. For patients with imatinib-resistant GISTs, sunitinib is a second-line drug treatment and regorafenib is the third-line drug for imatinib- or sunitinib-resistant GISTs [5].
Nonetheless, the recurrence rate after radical surgery is 50% at 5 years, and more than 50% of high-risk patients will develop a recurrence within 2 years [6]. Several risk factors for malignancy have been defined for patients with GISTs, including tumor size, mitotic count, proliferating cell nuclear antigen, and proliferation index, which allow classification into very low-risk, low-risk, intermediate-risk, and high-risk groups [5].
Case Report
A 67-year-old man was admitted to the Emergency Department with acute onset of chest pain, vomiting blood, and emission of dark black/bloody semi-liquid and foul-smelling stools. He had developed a gastric ulcer 20 years before without any other co-morbidities except for class II obesity (BMI 36.68). His blood pressure was 155/85 mmHg, pulse rate was 100 bpm, and oxygen saturation was 99%. Electrocardiogram (ECG) and serum troponin levels ruled out an ischemic cardiac event. His hemoglobin was 9.6 g/dl.
Two days before, the patient underwent an esophagogastroduodenoscopy (OGD) to investigate recurrent digestive difficulties and discomfort after eating, which showed a 30-mm submucosal lesion in the posterior wall of the greater curvature at the gastric fundus (Figure 1) and biopsies were taken. The urgent OGD confirmed the presence of the lesion and identified the origin of bleeding in the site of the biopsy. Endoscopic hemostasis was temporarily achieved using epinephrine injection therapy. Abdominopelvic computed tomography (CT) with enhanced scans excluded any further bleeding and signs of metastatic disease (Figure 2). Nevertheless, in the following 12 h, there was a recurrence of hematemesis and melena, with a worsening of the gastric bleeding, which led to hemorrhagic shock with syncope. Heart rate was 110 bpm, blood pressure was 80/54 mmHg, and the hemoglobin level was 6.9 g/dl. Resuscitation was initiated and the patient was prepared for surgery.
Figure 1.
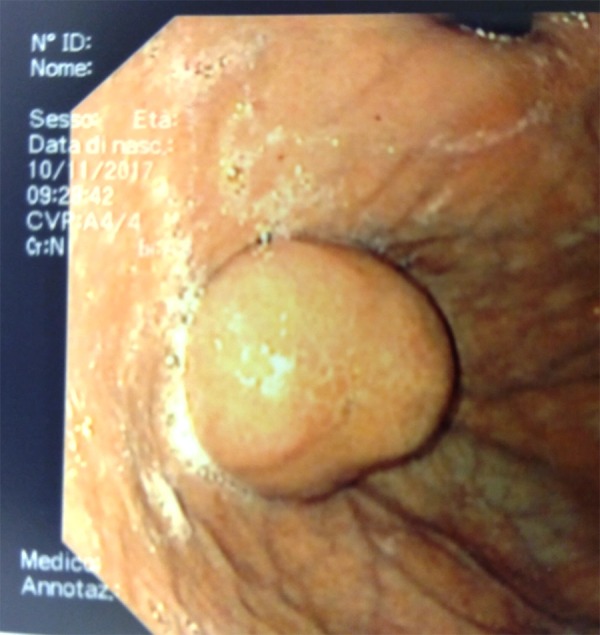
Endoscopic view: gastric submucosal neoplasm of the greater curvature at the gastric fundus.
Figure 2.
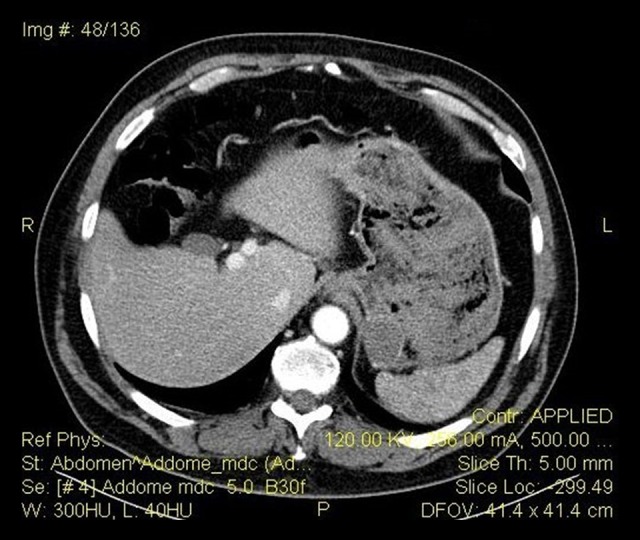
TC abdomen with medium contrast: neoplasm of the gastric fundus. No evidence of bleeding.
He was operated on under general anesthesia with a laparoscopic approach in a 30° reverse Trendelenburg position with legs abducted. Five trocars were placed in the upper abdominal quadrants: one 12-mm trocar above the umbilicus in the midline for the camera; three 12-mm trocars in right subcostal, subxiphoid, and left subcostal; and one 5-mm trocar in the left anterior axillary line. The preliminary exploration of the abdomen cavity showed distension of the stomach due to the bleeding, with no evidence of exophytic lesions, and ruled out signs of distant disease. Due to the location and the size of the endophytic lesion, without any further information about its histology and in order to prefer a subtotal/total gastrectomy or a more sparing treatment of the stomach, but also considering the necessity to surgically remove the source of the bleeding, we decided to perform a laparoscopic sleeve gastrectomy (LSG). The next step was an intraoperative endoscopy with a standard endoscope (Olympus GIF-Q-165®) to aspirate and lavage the stomach. It was also helpful for the following orogastric tube insertion (a 38-Fr bougie), placed against the lesser curvature to calibrate the resection as usual in an LSG.
We proceeded with the skeletonization of the greater curvature once we identified the pylorus and with the complete mobilization of the fundus and posterior gastric wall. After the inspection of the hiatal area and the endoscopic-assisted orogastric tube insertion, a gastric resection was performed using a linear stapler (Echelon Flex™ 60 Endopath®) applied alongside the calibrating bougie. The staple line was checked for complete sealing with a methylene blue dye test (negative for staple-line leak) after the placement of a nasogastric tube. It was then reinforced by nebulization with a cyanoacrylate sealant (Glubran 2®) used also to create an adhesion with the greater omentum as a chemical omentoplasty. The gastric specimen (Figure 3) was extracted in a large Endobag™ through the slightly enlarged left subcostal access. A drain was placed at the end of the procedure. Operative time was 92 min.
Figure 3.
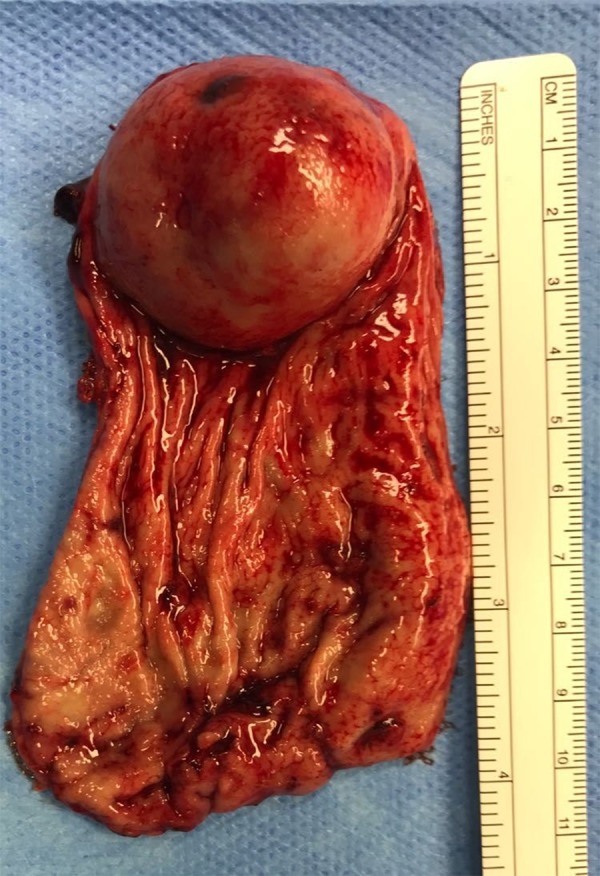
Gastric specimen: polypoid mass with hemorrhagic foci 40×35 mm in size.
The patient received a transfusion of 3 units of packed red blood cells, which brought his hemoglobin up to 9.7 g/dL and hematocrit to 30.3%.
The histopathology of the gastric specimen showed a polypoid mass with hemorrhagic foci 40×35 mm in size, without margin involvement (R0). The mitotic rate was <1/50 HP; CD34, CD117/C-kit, and smooth muscle actin were positive; and Ki67-MIB1 immunostaining indicated a low proliferative rate (count rate: 4%). The mass was diagnosed accordingly as GIST of gastric origin in the very low-risk category. In light of the pathology result, no further adjuvant chemotherapy was needed [7].
The patient improved significantly. A Gastrografin® swallow was performed at day 6 post-laparoscopy, without evidence of fistulas or spreading of the contrast agent, following which the nasogastric tube was removed and per os diet was initiated. The postoperative period was free of major complications and the patient was discharged on the 8th postoperative day. A soft diet with mashed and soft foods was prescribed. He presented a left subcostal wound infection which was opened and drained at the bedside. He also presented fever with lower urinary tract symptoms (LUTS) at postoperative day 10; the subsequently detected E. coli urinary tract infection was treated with antibiotics. The patient was subsequently seen 1 month after surgery, with no reported symptoms. He resumed a normal diet. He had lost 15 kilograms from the day of the operation (BMI 29.41).
Discussion
Complete surgical resection with negative margins (R0) is the criterion standard treatment for GISTs when possible [5]. Gastric GISTs require distinct surgical therapy compared to gastric adenocarcinomas because the large oncologic margins and lymphadenectomy are not necessary given the favorable disease biology of GIST [8]. Thus, in most cases, wedge resections are suitable to treat the disease and spare the stomach. However, based on the size and location of the tumor, the possibility that a subtotal or total gastrectomy must always be taken into account.
Over the last 2 decades, use of the laparoscopic approach has profoundly changed abdominal surgery. Findings in the literature demonstrate that, compared to traditional surgery, the use of laparoscopy offers several benefits in terms of perioperative complications, length of hospitalization, postoperative outcome, and resumption of normal activities. The laparoscopic approach has also been shown to be safe and feasible for gastric GISTs [9]. Nonetheless, in the most recent guidelines of the European Society for Medical Oncology, the National Comprehensive Cancer Network (NCCN), and the Asian GIST guidelines, the indication for this option has been reduced only for GISTs in favorable locations such as those in the greater curvature and anterior wall of the gastric body, fundus, and antrum [10]. Unfavorable locations include tumors in the lesser curvature of the body, fundus, and antrum, as well as the cardia and prepyloric region, where the difficulty in exposing the tumor, the risk of stenosis of the lumen postoperatively, and the risk of a non-oncological resection are obstacles to a laparoscopic approach [11]. The feasibility of laparoscopy is also influenced by the size of the tumor. The limitation of tumor size for laparoscopic surgery has traditionally been considered “diameter not more than 2 cm” but recent published data show the safety and feasibility of laparoscopic resection in large (>5 cm) and giant (>10 cm) gastric GISTs. This limit should increase in the near future and current guidelines do not state an absolute indication regarding tumor size [12]. Open surgery still plays a fundamental role in GISTs that require complex multivisceral resection, large lesions necessitating delicate tissue handling (to prevent tumor rupture or spill-age), or large abdominal incisions for specimen retrieval [9]. In addition to this, when deciding between an open or laparoscopic approach, the first consideration is that the surgeon be trained and capable of performing advanced laparoscopy.
Sleeve gastrectomy (SG) was performed for the first time in 1988 by Hess and Hess as part of a hybrid malabsorptive procedure of bariatric surgery, using biliopancreatic diversion with duodenal switch (BPD-DS). At present, laparoscopic sleeve gastrectomy (LSG) is the sole restrictive procedure also capable of changes in ghrelin (GHR), glucagon-like peptide-1 (GLP-1), and other gastrointestinal hormones that play a preeminent role in weight loss and glucose homeostasis. Despite the lack of gastrointestinal anastomosis, if compared with other bariatric procedures and the short operative time, LSG includes some key technical points such as the dissection of the stomach from the spleen and its mobilization from the pancreas and diaphragmatic crura, necessitating adequate laparoscopic surgical skills. Furthermore, the postoperative course can be affected by life-threatening complications such as gastric leak, the management of which should be accomplished in bariatric centers by specialized medical teams [13].
In this case report, the patient was admitted to the Emergency Doom for progressive onset of upper-GI bleeding following the endoscopic biopsy of a suspicious submucosal mass in the posterior wall of the greater curvature at the gastric fundus, 30 mm in size. A pathologic diagnosis of GIST may not be known before or even during surgery. Preoperative biopsy of a resectable mass is commonly performed but there are associated risks. GISTs may be soft and fragile, and biopsy may cause hemorrhage and increase the risk of tumor dissemination. In some cases, patients present with an acute abdomen requiring immediate surgery, so they are not evaluated for GIST until after the pathology report is received [14].
An initial attempt to achieve endoscopic hemostasis with epinephrine injection failed and the gastric bleeding led to hemorrhagic shock. In the management of such an acute situation, we chose to avoid any further delay of surgical intervention and used laparoscopy in approaching this abdominal emergency. The exploration of the abdomen cavity showed no evidence of gastric exophytic lesions or signs of distant disease. In light of the size of the mass and its localization in the greater curvature at the gastric fundus (confirmed by the subsequent intraoperative endoscopy), we chose to perform a laparoscopic sleeve gastrectomy (LSG), which allowed us to treat the bleeding and remove the potential GIST without opening the stomach, minimizing tumor handling and risks of rupture or dissemination. Moreover, the fact that the procedure was performed by an experienced bariatric surgeon with a well-trained team in a surgical unit performing more than 50 bariatric operations (gastric banding, sleeve gastrectomy, and Mini-Gastric bypass) per year was another relevant factor influencing selection of the surgical strategy.
LSG was performed by standardized technique. Aerosolized cyanoacrylate sealant Glubran 2® was used as a reinforcement of the staple line to prevent leaks and bleeding, for which there was expected to be higher risk in this case due to the increased intragastric pressure caused by the previous bleeding. The sealant also helped to create an adhesion between the staple line and the greater omentum, which is needed for chemical omentoplasty, restoring the anatomy with a further staple-line reinforcement [15].
The postoperative period was uneventful for major complications, and the ones that occurred were classified as being no more that group II according to the Clavien-Dindo classification. In light of the postoperative pathological result of a gastric GIST in the very low-risk category, no further adjuvant chemotherapy was needed. During the follow-up at 1 month after the operation, the patient no reported symptoms and he had lost 15 kg (BMI 29.41) body weight. This enabled us to conclude that LSG provided a complete surgical removal of the tumor in addition to a good outcome in terms of weight loss in a class II obesity patient.
Conclusions
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors of the gastrointestinal tract. Surgery is the mainstay of therapy for GISTs and the only potentially curative treatment when possible. The role for laparoscopy in the resection of GISTs continues to expand, providing oncologic outcomes comparable to the open approach with significant advantages in terms of perioperative complications, length of hospitalization, and postoperative outcome of the patients. This report describes the application of a laparoscopic bariatric procedure in a life-threatening situation following the presentation of a gastric GIST with an acute abdomen. It can be concluded that in the proper setting with an experienced surgeon, together with a well-trained team, emergent laparoscopy is feasible, effective, safe, and beneficial.
Footnotes
Conflict of interest
None.
References:
- 1.Rossi CR, Mocellin S, Mancarelli R, et al. Gastrointestinal stromal tumours: From a surgical to a molecular approach. Int J Cancer. 2003;107:171–76. doi: 10.1002/ijc.11374. [DOI] [PubMed] [Google Scholar]
- 2.Agaimy A, Terracciano LM, Dirnhofer S, et al. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol. 2009;62:613–16. doi: 10.1136/jcp.2009.064550. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell MJ, Punnoose S, Viswanath YKS, et al. Gastrointestinal stromal tumours (GISTs): An insight into clinical practice with review of literature. Frontline Gastroenterol. 2017;8:19–25. doi: 10.1136/flgastro-2015-100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888–95. doi: 10.1002/jso.21930. [DOI] [PubMed] [Google Scholar]
- 5.Lim KT, Tan KY. Current research and treatment for gastrointestinal stromal tumors. World J Gastroenterol. 2017;23(27):4856–66. doi: 10.3748/wjg.v23.i27.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandrioli M, Mastrangelo L, Masetti M, et al. Characterization of malignant gastrointestinal stromal tumors a single center experience. J Gastrointest Oncol. 2017;8(6):1037–45. doi: 10.21037/jgo.2017.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum Pathol. 2002;33:466–77. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 8.Koh YX, Goh BKP. Minimally invasive surgery for gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017;2:108. doi: 10.21037/tgh.2017.11.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh YX, Chok AY, Zheng HL, et al. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549–60. doi: 10.1245/s10434-013-3051-1. [DOI] [PubMed] [Google Scholar]
- 10.Liao GQ, Chen T, Qi XL, et al. Laparoscopic management of gastric gastrointestinal stromal tumors: A retrospective 10-year single-center experience. World J Gastroenterol. 2017;23(19):3522–29. doi: 10.3748/wjg.v23.i19.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CM, Chen QF, Lin JX, et al. Can laparoscopic surgery be applied in gastric gastrointestinal stromal tumors located in unfavorable sites? A study based on the NCCN guidelines. Medicine. 2017;96:14. doi: 10.1097/MD.0000000000006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CM, Park S. Laparoscopic techniques and strategies for gastrointestinal GISTs. J Vis Surg. 2017;3:62. doi: 10.21037/jovs.2017.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Luca M, Formisano G, Santonicola A. Bariatric and metabolic surgery: Indications, complications and revisional procedures. Springer; 2016. [Google Scholar]
- 14.Demetri GD, Mehren MV, Antonescu CR, et al. NCCN Task Force Report: Update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8(Suppl. 2):S1–41. doi: 10.6004/jnccn.2010.0116. quiz S42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martines G, Digennaro R, De Fazio M, Capuano P. Cyanoacrylate sealant compared to fibrin glue in staple line reinforcement during laparoscopic sleeve gastrectomy. Pilot prospective observational study. G Chir. 2017;38(1):50–52. doi: 10.11138/gchir/2017.38.1.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


