Abstract
A technique for the prevention of staphylococcal adhesion by electrical current exposure was investigated. Teflon coupons were exposed to a continuous flow of 103 cfu/ml Staphylococcus epidermidis with or without 2000 microA DC electrical current delivered by electrodes on opposite sides of a coupon, touching neither each other nor the coupon. A mean 3.46 (SD, 0.20) and 5.70 (SD, 1.03) log10 cfu/cm2 were adhered to the non-electrical current exposed coupons after 4 h and 24 h, respectively. A mean 2.46 (SD, 0.31) and 1.47 (SD, 0.73) log10 cfu/cm2 were adhered after 4 h and 24 h with exposure to 2000 microA electrical current delivered by graphite electrodes. A mean 2.21 (SD, 0.14) and 0.55 (SD, 0.00) log10 cfu/cm2 were adhered after 4 h and 24 h with exposure to 2000 microA electrical current delivered by stainless steel electrodes. Electrical current may be useful in the prevention of staphylococcal adhesion to biomaterials.
Keywords: Electrical current, Staphylococcus epidermidis, Biofilm, Electricidal effect
OBJECTIVES
Some of the most refractory modern bacterial diseases are those associated with medical devices in which the associated microorganisms grow in well-developed, adherent biofilms. Bacteria in biofilms exhibit dramatically reduced susceptibility to antimicrobial agents as compared to their planktonic forms (1, 2). Given the failure of conventional antimicrobics in the management of most biofiim-associated infections, novel and innovative preventive approaches are warranted (3,4). The development of biofilm-related infections begins with the adhesion of the microorganism to a surface, mediated by the Van der Waals forces, acid base interactions, and electrostatic forces. The electrostatic force between bacteria and the biomaterial is generally repulsive since most biomaterial surfaces and bacterial cells are negatively charged (5). It has been proposed that repulsive forces may be enhanced by the application of electric current, provoking surface detachment of bacterial biofilms (6). Such electrical current exposure could be suitable for inhibiting biofilm-associated infection.
We have previously demonstrated that in vitro exposure to electrical current delivered by electrodes on opposite sides of a biofilm-laden coupon (touching neither each other nor the coupon) reduced numbers of viable bacteria in established mature Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms, a phenomenon we termed the “electricidal effect” (7). We also showed that electrical current is active against S. epidermidis biofilms in a rabbit foreign body infection model when delivered using a stainless steel electrode in the medullary cavity of the tibia as the cathode and a stainless steel wire wrapped around the outside of the tibia as the anode (8). The aim of the present study was to determine the effect of exposure to low-intensity (i.e., 2000 microA) DC electrical current on S. epidermidis biofilm formation using a similar model to that described in (7).
METHODS
S. epidermidis Xen 43 was studied. A modification of a previously described in vitro model was used (9). Briefly, the Mayo Division of Engineering designed and fabricated two 8-channel current generator/controllers and sixteen polycarbonate test chambers to deliver a continuous flow of fresh media (with or without, bacteria), with or without electrical current, to 12.5 mm diameter × 1 mm Teflon coupons. The electrodes were stainless steel or graphite cylinders 1.5 mm diameter × 55 mm long, with 1 cm of electrode extended above the chamber for the purpose of connecting the electrode to the current generator. For each experiment, coupons were placed in a chamber, in a vertical position in a plane perpendicular to the plane formed by the electrodes. A semi-synthetic medium supplemented with glucose (7) was pumped continuously (3 ml/h) through each chamber. Electrical current (0 or 2000 microA DC) was passed from anode to cathode in the chamber continuously for each test. Each chamber continuously exposed coupons to a 103 cfu/ml logarithmic phase S. epidermidis innoculum plus 0 or 2000 microA DC electrical current. Electrical current was applied for up to 24 h at room temperature. After 4 h or 24 h exposure, coupons were aseptically removed from chambers, rinsed of planktonic bacteria, and placed in sterile tubes containing 2 mL of trypticase soy broth. Adherent biofilm bacteria were removed by vortexing for 30 s and then sonicated in an ultrasound bath (40 KHz, 320 mW/cm2) for 5 min. Suspensions of dislodged and disaggregated biofilms were serially diluted and 100 μl of each dilution was spread on the surface of blood agar plates. After incubation at 37°C to 5% CO2 for 48 h, colonies were counted on plates growing 10 cfu to 100 cfu and the cfu/cm2 was calculated. Each test was performed in sextuplicate and expressed as mean log10cfu/cm2 coupons surface. We performed a one way analysis of variance with two levels of electrical current exposure (i.e., 0 or 2000 microA), to determine if exposure to electrical current had any effect on biofilm formation. Tests were two sided; p-values <0.05 were considered statistically significant. Analysis was performed using SAS software (version 9; SAS Institute, Inc., Cary, NC, USA).
RESUITS
Mean S. epidermidis log10 cfu/cm2 and standard deviations after 4 h and 24 h exposure to the bacterium inoculum plus 0 or 2000 microA, electrical current delivered through stainless steel or graphite electrodes are shown in Figure 1. A time-dependent reduction in biofilm formation was observed, with lower viable cell counts when electrical current was applied for a longer time. A mean 3.46 (SD, 0.20) and 5.70 (SD, 1.03) log10 cfu/cm2 were adhered to the non-electrical current exposed coupons after 4 h and 24 h, respectively. A mean 2.46 (SD, 0.31) and 1.47 (SD, 0.73) log10 cfu/cm2 were adhered after 4 h and 24 h exposure to 2000 microA electrical current delivered by graphite electrodes, A mean 2.21 (SD, 0.14) and 0.55 (SD, 0.00) log10 cfu/cm2 were adhered after 4 h and 24 h exposure to 2000 microA electrical current delivered by stainless steel electrodes (Fig. 1). Following exposure to 2000 microA electrical current delivered by stainless steel electrodes for 24 h, all plate counts for quantitative culture were negative. However, bacterial growth was observed with subculture of the broth in which coupons were incubated after the vortexing sonication procedure. We detected statistically significant differences (p<0.01) between no electrical current exposure and electrical current exposure after 24 h for both of the studied electrode compositions. Electrical current delivered through stainless steel electrodes yielded numerically but non-statistically significantly greater biofilm reductions than current delivered through graphite electrodes.
Fig. 1 -.
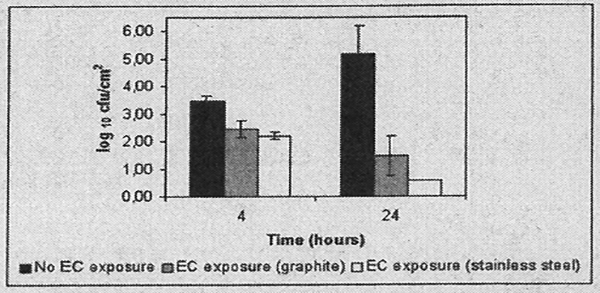
Mean log10 cfu/cm2 and standard deviation rot coupons exposed to a logarithmic phase Staphylococcus epidermieik inculum plus 0 or 2000 microA elctrical current (EC) delivered through stainless steel or graphite electrodes.
DISCUSSION
In this study, we demonstrated that S. epidermids adhesion to Teflon coupons is prevented by exposure to electrical current. This strategy may be effective in the prevention of staphylococcal adhesion to biomaterials. Van der Borden et al (10) demonstrated that a variety of adherent Staphylococcus strains could he stimulated to detach from surgical stainless steel by the application of low DC electrical currents (25–125 microA). In a follow-up manuscript (11), the effect of DC electrical currents (60 and 100 microA) and block currents (60 and 100 microA with a 50% duty cycle, 1 Hz) against staphylococcal biofilms in the late stages of formation were studied. The block currents yielded higher detachment percentages than DC current alone due to the electro-osmotic fluid flow. Bacteria remaining on the surface after current application were tes viable than they were prior to the carrent application, as demonstrated by confocal laser scanning microscopy. Stainless steel electrodes have been most commonly studied, but carbon, platinum, and gold electrodes have also been used. Using the previously described in vitro model, we demonstrated that electrode composition plays a role in the in vitro electricidal effect against established biofilms (12). In this study, we have demonstrated that it is possible to impair S. epidermidis biofilm development by applying electrical currents.
ACKNOWLEDGEMENTS
The authors thank Xenogen Corp. for providing Xen 43 and the Mayo Division of Engineering for designing and fabricating the current generator/controllers and test chambers.
Financial support: The project described was supported by RO1 AI0191594 and R21 AI061407 from NIAID/NIH, and ROI AR056647 from NiAMS/NIH.
Footnotes
Conflict of interest: None
REFERENCES
- 1.Anwar H, Dasgupta MK, Costerton JVV. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chernother. 1990; 34(11); 2043–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton JW, Ellis B, Lam K, Johnson F, Khoury At. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother 1994; 38(12); 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslarn S, Darouiche RO. Role of antibiofilm-antimicrobial agents in controlling device-related infections. Int J Artif Organs. 2011; 34(9): 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Staden AD, Dicks LM. Calcium orthophosphate-based bone cements (CPCs): Applications, antibiotic release and alternatives to antibiotics. J Appl Biomater Funct Mater. 2012; 26; 10(1): 2–11. [DOI] [PubMed] [Google Scholar]
- 5.Jucker BA, Harms H, Zehnder AJ. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maitophilia 70401 to glass and Teflon. j Bacteriol, 1996; 178(18): 5472–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poortinga AT, Bos R, Busscher HJ. Controlled electrophoretic deposition of bacteria to surfaces for the design of biofilms. Biotechnol Bioeng, 2000; 67(1): 117–120. [DOI] [PubMed] [Google Scholar]
- 7.Del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother, 2009; 53(1): 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Pozo JL, Rouse MS, Euba G, et al. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother. 2009; 53(10): 4064–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother, 2009; 53(1): 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Borden AJ, van der Mei HC Busscher HJ. Electric-current-induced detachment of Staphylococcus epidermidis strains from surgical stainless steel. J Biomed Mater Res B Appl Biomater. 2004; 68B(2); 160–164. [DOI] [PubMed] [Google Scholar]
- 11.van der Borden AJ, van der Mei HC, Busscher Hj. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis.Biomaterials. 2005; 26(33): 6731–6735. [DOI] [PubMed] [Google Scholar]
- 12.Del Pozo JL, Rouse MS, Fernandez Sampedro M, Steckelberg JM, Patel R. Electrode composition and electrical enhancement of rifampin activity against methicillin resistant Staphylococcus aurreus (MRSA) biofilms. 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) September 17–20, 2007, Chicago, IL, USA. [Google Scholar]


