Abstract
Differences between the left and right sides of the brain are present in many animal species. For instance, in humans the left cerebral hemisphere is largely responsible for language and tool use and the right for processing spatial information. Zebrafish have prominent left-right asymmetries in their epithalamus that have been associated with differential left and right eye use and navigational behavior. In wild-type (WT) zebrafish embryos, Nodal pathway genes are expressed in the left side of the pineal anlage. Shortly thereafter, a parapineal organ forms to the left of the pineal. The parapineal organ causes differences in gene expression, neuropil density, and connectivity of the left and right habenula nuclei. In embryos that have an open neural tube, such as embryos that are deficient in Nodal signaling or the cell adhesion protein N-cadherin, the left and right sides of the developing epithalamus remain separated from one another. We find that the brains of these embryos become left isomerized: both sides of the brain develop morphology and gene expression patterns that are characteristic of the left side. However, other aspects of epithalamic development, such as differentiation of specific neuronal cell types, are intact. We propose that there is a mechanism in embryos with closed neural tubes that prevents both sides from developing like the left side. This mechanism fails when the two sides of the epithalamus are widely separated from one another, suggesting that it is dependent upon a signaling protein with limited range.
Keywords: left-right asymmetry, zebrafish, epithalamus, neural tube defects, pineal gland, habenula nuclei
Introduction
Structural and functional asymmetries of the brain have long been described in humans and other vertebrates. These asymmetries are thought to be the result of the localization of specialized functions to one side or the other. One of the best-understood examples in humans is the localization of language processing to the left hemisphere. This functional asymmetry has been linked to a morphological asymmetry in the planum temporale, a region in the temporal lobe of the cerebral cortex. The morphological asymmetries in the planum temporale are detectable as early as the third trimester of fetal life and continue through adulthood (Preis et al., 1999). In normal adults, the left planum temporale has a larger volume and cellular differences such as a greater extent of myelination (Hugdahl, 2005; Steinmetz, 1996).
The zebrafish epithalamus has several prominent left-right asymmetries in neuroanatomy that are correlated with laterality in behavior. In wild-type (WT) embryos, the first known epithalamic asymmetry is the expression of genes encoding components of the Nodal signaling pathway, lefty1 (lft1), cyclops (cyc), and pitx2 in the left side of the developing pineal complex (Concha et al., 2000; Liang et al., 2000; Sampath et al., 1998). Cells in this anlage will give rise to pineal gland, located at the dorsal midline of the brain, and the parapineal organ, located to the left of the pineal (Concha et al., 2003; Gamse et al., 2003). The parapineal regulates the formation of features that differ between the left and right habenular nuclei, including higher neuropil density and expression of the potassium channel tetramerisation domain containing 12.1 (kctd12.1)gene on the left side and higher kctd12.2 expression on the right (Aizawa et al., 2007; Gamse et al., 2005; Gamse et al., 2003; Kuan et al., 2007a; Kuan et al., 2007b). The left and right habenula also develop different efferent connections. Axons from the left habenula extend to the dorsal and ventral regions of the interpeduncular nucleus (IPN) in the midbrain, while axons from the right habenula extend only to the ventral region (Gamse et al., 2005).
The habenula and IPN have both been implicated in the regulation of many behaviors including feeding, mating, and aversive learning, suggesting that the morphological asymmetries in the zebrafish brain may be important for left-right differences in such behaviors (Bianco and Wilson, 2009; Vallortigara et al., 1999). In zebrafish and many species of fish, birds, and frogs there is a tendency to use the left and right eyes differently. The right eye is used preferentially when an object is unfamiliar or complicated, and the left for familiar objects and scenes (Andrew et al., 2009; Bianco and Wilson, 2009; Miklosi and Andrew, 1999; Miklosi et al., 1997; Vallortigara et al., 1999). Laterality in eye use is correlated with asymmetries in the epithalamus and IPN. The frequent situs inversus (fsi) line of zebrafish has a high rate of situs inversus in the epithalamus and the viscera (Barth et al., 2005). Fish with reversed situs also had reversed eye use behavior when viewing themselves in a mirror or when preparing to bite a target (Barth et al., 2005). When WT fish with reversed parapineal sidedness were placed into a tank with a mirror, they had a delay in the onset of navigation around the tank and a decrease in the total distance traveled within a given time (Facchin et al., 2009).
There are many features shared between the pathways that establish the direction of asymmetry in the visceral organs and asymmetry in the zebrafish brain. Both asymmetries depend upon the function of cilia that create a leftward flow of fluid across the organizer during gastrulation and on the asymmetric expression of Nodal pathway genes on the left side (Cooke, 2004; Schier, 2009). In addition, midline tissue has a conserved role in maintaining asymmetry by preventing left signals from crossing over to the right side. In visceral asymmetry, midline floor plate and notochord form a physical barrier and also express Lft, which inhibits Nodal signaling by preventing binding of Nodal ligands to their receptors (Cooke, 2004; Schier, 2009). In the zebrafish brain, lft1 is expressed throughout the left pineal anlage, not exclusively in the midline, suggesting it may not function as a barrier. However, as in visceral asymmetry, when the mesendodermal structures beneath the developing neural tube are defective, the ventral midline of the brain is disrupted and genes that are normally on the left side are expressed bilaterally (Bisgrove et al., 2000; Concha et al., 2000; Liang et al., 2000). Interestingly, subsequent asymmetries in the parapineal and habenular nuclei are still established, but the sidedness is randomized between normal and reversed situs (Gamse et al., 2002; Gamse et al., 2003). Less is known about the role of the midline tissue in brain asymmetry. It is not clear whether tissues such as the prechordal plate act as a molecular or physical barrier. Nor is it known whether this barrier has selectivity for certain molecules.
We found that zebrafish embryos with open anterior neural tubes have a brain asymmetry phenotype that is distinct from either WT embryos or embryos with defects in ventral midline tissues. Both the left and right sides of the epithalamus developed characteristics normally associated with the left side. As in embryos with ventral midline defects, both sides of the pineal anlage expressed Nodal pathway genes. However, instead of developing randomized parapineal sidedness, a parapineal organ developed on both the right and left sides. Consistent with a role of the parapineal in influencing habenular asymmetry, both the left and right habenulae developed gene expression consistent with a left identity when a parapineal forms on both sides of the brain. In contrast, other aspects of epithalamic development, such as the differentiation of specific cell types, appeared to be unaffected. This suggests that there is a mechanism that normally prevents both sides of the epithalamus from becoming left. When the left and right sides of the epithalamus fail to meet, this signal can no longer function. Comparison of embryos with range of neural tube defects demonstrates that the penetrance of the left isomerized phenotype correlates with the distance between the left and right sides. This suggests that preventing left isomerism depends upon an extracellular signal that can only travel a discrete distance.
Methods
Zebrafish
Zebrafish stocks were maintained at 28.5°C in a 14:10 light:dark cycle according to standard procedure (Westerfield, 2000). Stocks used were the WT strain Zebrafish Danio rerio (ZDR) (Aquatica Tropicals, Plant City, FL), flh:GFPc162 (Gamse et al., 2003); foxd3:GFPzf104 (Eichele et al., 2005; Gilmour et al., 2002), n-cadherin (cdh2vu125 and cdh2p73) (Birely et al., 2005; von der Hardt et al., 2007), one eyed pinhead (oepm134 and oeptz257) (Brand et al., 1996; Schier et al., 1996), squint (sqtcz35) (Feldman et al., 1998), and sqtcz35;cycm294 (Feldman et al., 1998). WT, transgenic, and mutant embryos were obtained through natural matings (Westerfield, 2000). sqtcz35 and sqtcz35;cycm294 embryos were raised at 33°C for the first 24 hpf to increase the penetrance of the open neural tube phenotype.
Whole mount in situ hybridization (WISH)
WISH was carried out as previously described (Thisse et al., 1993). Antisense RNA probes included: serotonin N-acetyl transferase (aanat2) (Gothilf et al., 1999), exorhodopsin (exorh) (Mano et al., 1999), orthodenticle homeobox 5 (otx5) (Gamse et al., 2002), pitx2 (Tsukui et al., 1999), crestin (Rubinstein et al., 2000), lefty1/antivin (lft1/atv) (Thisse and Thisse, 1999), floating head (flh) (Talbot et al., 1995), kctd12.1/lov (Gamse et al. 2003), kctd12.2/ron (Gamse et al., 2005), growth factor independent 1.2 (gfi1.2) (Dufourcq et al., 2004).
Whole mount antibody staining
Whole mount antibody staining was performed as described (Pierce et al., 2008). The 4D2 monoclonal antibody against the N-terminus of bovine Rhodopsin was used at a dilution of 1:60 to detect Exorh protein (Noche et al., 2011). Goat anti-mouse antibody coupled to Alexa Fluor 546 (Invitrogen) was used at a dilution of 1:2000. Rabbit anti-GFP (Torrey Pines Biolabs) was used at a dilution of 1:500.
Photography
Bright field and fluorescent images were obtained on a SPOT camera or a CoolSnap ES camera connected to a Nikon Eclipse 80i microscope and on a Leica DM6000B microscope with a 20X objective. Confocal images were collected on a Zeiss/Perkin Elmer RS-3 spinning disk confocal microscope with a 40X oil-immersion objective and analyzed with Volocity software (Improvision).
mRNA Injections
Embryos from the ZDR and foxd3:GFP lines were injected with 0.63 pg to 5 pg of lft1 mRNA or GFP mRNA at the one cell stage using a Harvard Apparatus PLI-90 nitrogen picoinjector. All embryos with the same neural tube phenotype were pooled regardless of the amount of mRNA injected.
Data Analyses
Digital images with dorsal views of the epithalamus were measured using ImageJ software. Pineal width was measured from the most lateral points of the otx5 pineal expression, regardless of whether the pineal was oval, elongated, or divided. Pineal organs were considered elongated if their width was longer than their length and their width was greater than the upper 95% confidence interval of WT pineal organ width (n = 46 WT pineal organs measured). Statistical analysis was performed using Tukey-Kramer comparison and Two-way ANOVA using JMP 10.0 software.
Results
Neural tube closure is not required for the differentiation of dorsal neural tube cells
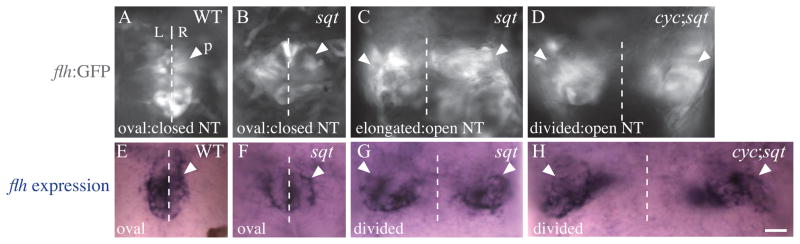
During embryonic development, the cells that will become the brain start as a flat neural plate epithelium. As development proceeds, the neural plate folds to become the neural tube. As this occurs, cells that are at the left and right edges of the neural plate converge until they fuse at the dorsal midline. Our previous work demonstrated that Nodal signaling is required for closure of the anterior neural tube in zebrafish (Aquilina-Beck et al., 2007). For instance, in WT embryos and sqt mutants with closed neural tubes, pineal precursors start in two domains that eventually fuse to form a single pineal anlage at the dorsal midline of the epithalamus (Figure 1A, B, E, F). In Nodal signaling mutants with partially open neural tubes, the pineal anlage in some cases still fuses but remains elongated along the left-right axis (Figure 1C). In embryos with more severe phenotypes, the pineal cells remain divided in two domains (Figure 1D, G, H).
Figure 1. flh is expressed in embryos with an open neural tube.
In (A, E) WT and (B, F) sqt mutant embryos with closed neural tubes, the pineal precursors (p, white arrowheads) form a single, oval domain that spans the dorsal midline (dotted lines) separating the left (L) and right (R) sides of the epithalamus. (C) In sqt embryos with mild open neural tube defects, the pineal precursors still span the dorsal midline of the brain, but the pineal anlage is elongated along the left-right axis. (D, G, H) In sqt and cyc;sqt embryos with a severe open neural tube phenotypes, the pineal precursors are present in two divided domains on the left and right sides of the brain. Dorsal views of the epithalamus in (A – D) live or (E – H) fixed embryos, anterior to the top. Scale bar = 20 μm.
We find that cells within the pineal anlage still differentiate even in embryos with severe open neural tube defects. Zebrafish that lack the Nodal signal Sqt have variable phenotypes, including neural tube phenotypes that range from indistinguishable from WT to severe (Aquilina-Beck et al., 2007). However, flh, which encodes a transcription factor for pinealocyte differentiation, is expressed at high levels in severely affected sqt mutants and in cyc;sqt double mutants, which have almost no Nodal signaling (Figure 1D, G, H, Supplemental Tables 1 and 2) (Aquilina-Beck et al., 2007).
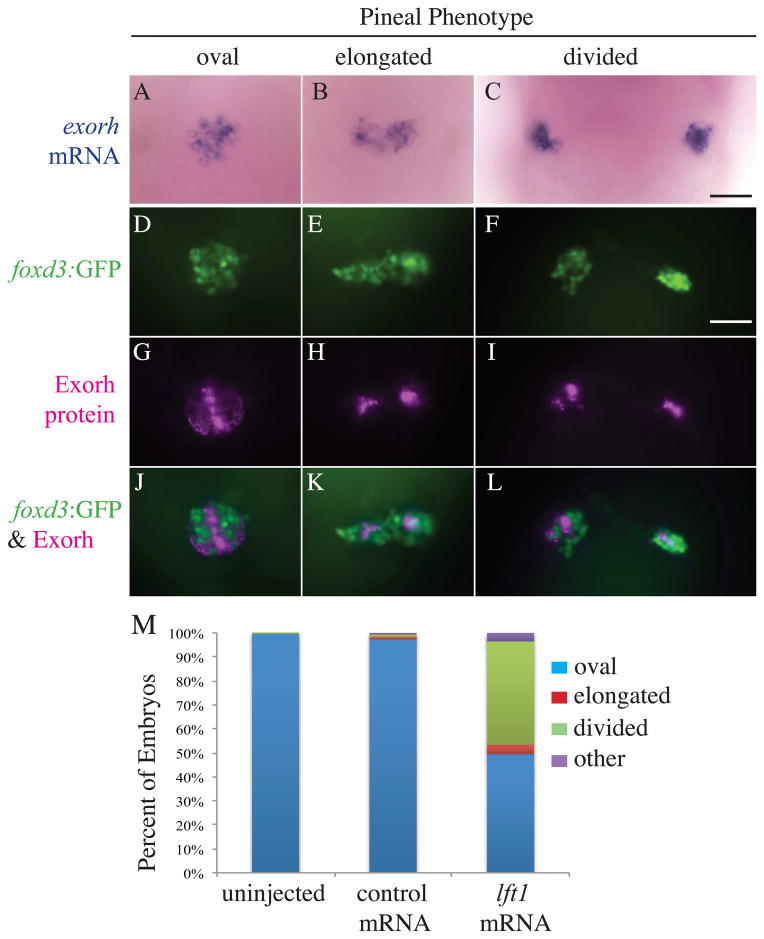
Injections of the lft1 mRNA, which encodes a Nodal inhibitor that acts at the step of ligand binding, phenocopies Nodal signaling mutants (Thisse and Thisse, 1999) (Supplemental Figure 1). Consistent with this, we find that embryos overexpressing Lft1 have a high frequency of open neural tube defects as well as other aspects of the Nodal deficient phenotype (Figure 2)(Supplemental Figure 1). We took advantage of this injection method to produce large numbers of embryos with open neural tubes for further analysis. We find that the pinealocytes in embryos with divided pineal organs initiate the expression of cell type specific genes and proteins. The center of the pineal organ is populated by photoreceptors that are responsible for detecting changes in environmental light conditions and for entraining the pineal circadian clock (Cahill, 2002; Korf et al., 1998). These photoreceptors form an oval shaped domain that is surrounded by projection neurons that extend axons to the ventral brain (Figure 2) (Wilson and Easter, 1991). In WT embryos, control injected embryos, and lft1 mRNA injected embryos with a single, oval shaped pineal, the protein Exorh, which is the major photopigment expressed in pineal photoreceptors, is expressed in the center domain of the pineal corresponding to the photoreceptor end segments (Figure 2G). Similarly, the exorh mRNA is found in an oval shaped domain that spanned across the dorsal midline (Figure 2A). In embryos with an open neural tube, both Exorh protein and exorh mRNA were expressed in either a pineal anlage that was expanded laterally or divided into two widely spaced domains (Figure 2C, I).
Figure 2. Projection neuron and photoreceptor differentiation occurs in the pineal glands of Lft1 overexpressing embryos with open neural tubes.
(A – C) The photoreceptor specific gene exorh is expressed in embryos with all three pineal phenotypes (n = 21 oval, n = 2 elongated, n = 6 divided). (D – L) Embryos were analyzed for both foxd3:GFP transgene expression and Exorh protein expression, and a representative embryo with an oval pineal (D, G, J), with an elongated pineal (E, H, K), and a divided pineal (F, I, L) are shown. (D–F) are images of the foxd3:GFP expression, (G–I) are images of the Exorh immunostaining, and (J–L) are overlays of the two expression patterns. Note that in (J–L), foxd3:GFP and Exorh are expressed in distinct regions of the pineal, suggesting the foxd3:GFP transgene is primarily found in pineal projection neurons at this stage of development (n = 36 oval, n = 4 elongated, n = 11 divided). (M) Percentage of lft1 mRNA injected embryos expressing the foxd3:GFP transgene in an oval pattern (closed anterior neural tube), or elongated or divided pattern (open neural tube). n > 120 embryos. All embryos were at 48 hpf. All images are dorsal views, anterior to the top. Scale bar = 25 μm.
The foxd3 gene is expressed in pineal projection neurons and in some pineal photoreceptors. An overlay of foxd3:GFP and Exorh immunostaining indicates that the majority of the foxd3:GFP expressing area does not overlap with the Exorh expressing area, suggesting that the foxd3:GFP transgene is primarily in projection neurons at this stage of development (Figure 2J–L). In lft1 mRNA injected foxd3:GFP transgenic embryos, the GFP signal is detected in an oval shaped domain typical of a normal pineal anlage, an elongated domain or in two divided regions (Figure 2D–F).
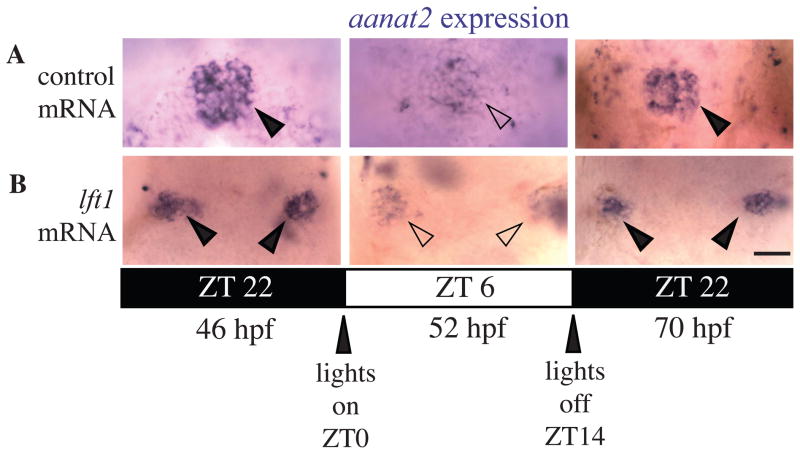
One of the main functions of the pineal organ is the rhythmic biosynthesis of the hormone melatonin, which regulates daily rest activity rhythms (Cahill, 2002; Korf et al., 1998). In zebrafish, onset of rhythmic expression of the melatonin pathway gene aanat2 depends upon the activity of pineal photoreceptors (Gothilf et al., 1999; Ziv and Gothilf, 2006; Ziv et al., 2005). In control mRNA injected embryos raised in a normal 14:10 light:dark cycle, aanat2 mRNA is expressed at low levels during the day and at high levels during the night, mirroring the nocturnal rise in melatonin (Figure 3)(Gothilf et al., 1999). We find that this rhythmic expression pattern of aanat2 is preserved in embryos with open neural tubes, suggesting that rhythmic function of the pineal has been initiated (Figure 3).
Figure 3. Rhythmic expression of aanat2 is initiated in pinealocytes of embryos with open neural tubes.
Dorsal views of embryos raised in 14:10 hour light:dark cycle fixed at indicated time points. (A) GFP mRNA injected control embryos with oval pineal glands. (B)lft1 mRNA injected embryos with divided pineal glands. Closed arrowheads indicate pineals with high aanat2 expression; open arrowheads indicate pineals with low aanat2 expression. ZT indicates time within the circadian cycle, lights turning on at ZT = 0 and lights turning off at ZT = 14. Dark bars indicate lights off and light bars indicate lights on. For each time point, n ≥ 10 for embryos with oval shaped pineal and embryos with divided pineal. Scale bar = 25μm
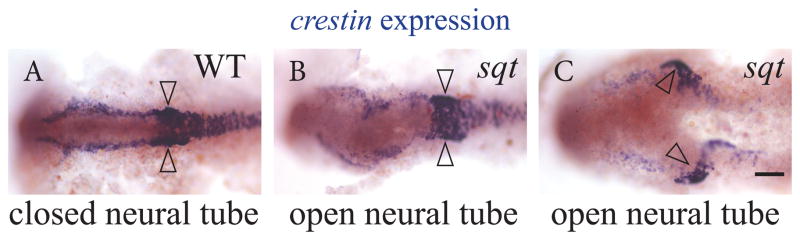
Neural crest cells are another cell type likely affected by disruption in neural tube closure. Specification of neural crest depends upon the convergence of Wnt signals from the epidermis and Bone Morphogenic Protein (BMP) signals from the dorsal neural tube. In WT embryos at the 6 somite stage, crestin is highly expressed in two rows of premigratory neural crest cells along the developing hindbrain. During somitogenesis, the crestin expression domain gradually expands rostrally and caudally along the left and right borders of the developing neural tube (Rubinstein et al., 2000) (Figure 4A). We find that in sqt mutants with divided pineal organs the two crestin expression domains still form, including the concentrated groups of cells adjacent to rhombomeres 6 (Figure 4). Consistent with the fact that the neural crest derived cranial structures are severely disrupted in Nodal signaling mutants, the neural crest cells at later stages are present, but their arrangement is very different than in their WT siblings (Supplemental Figure 2).
Figure 4. The neural crest gene crestin is expressed in embryos with an open neural tube.
sqt embryos with an open neural tube were identified by two-color WISH by first detecting flh in the pineal with a red precipitate (not shown as it is out of the focal plane of the image). The crestin signal in the neural crest cells was then detected via a purple precipitate. (A) In WT embryos, crestin is expressed in a large region next to rhombomeres 4 to 6 (open arrowheads), and in two stripes of cells on the left right sides at the borders of the folding neuroepithelium. (B and C) In sqt embryos with an open neural tube, both regions of crestin are present, although they are further apart. The experiment was repeated three times and representative images are shown. Embryos were fixed at the 8–10 somite stage. All images are dorsal views with anterior to the left. Scale bar = 50μm.
The initiation of asymmetry in the epithalamus is disrupted when the neural tube is open
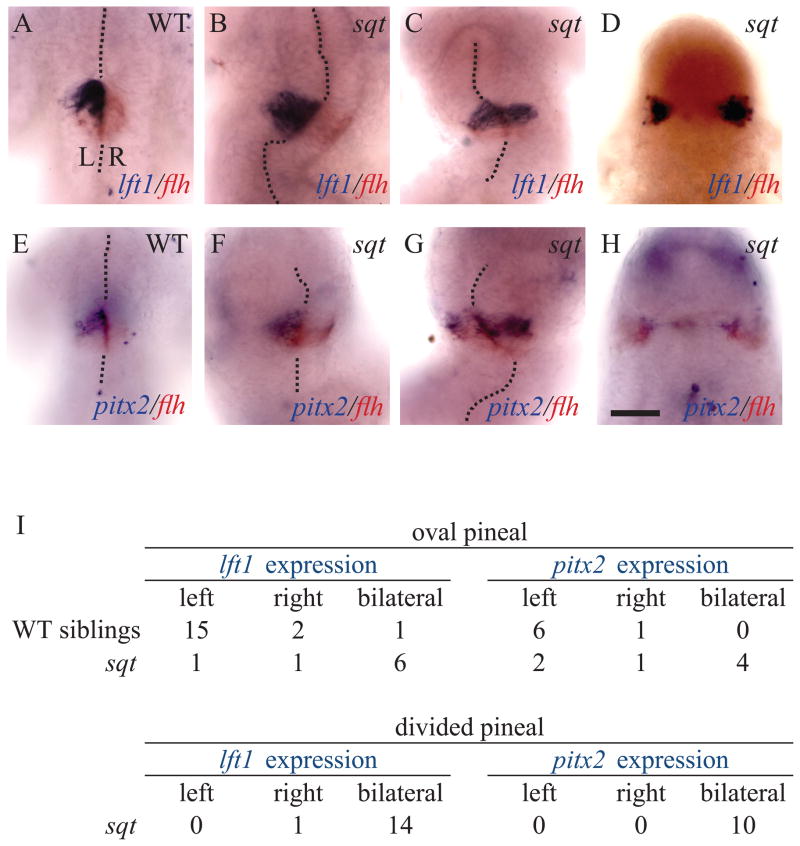
Left-sided expression of cyc, lft1 and pitx2 in the presumptive pineal organ during late somite stages is the earliest known left-right asymmetry in the zebrafish brain (Concha et al., 2000; Liang et al., 2000). In sqt mutants with oval shaped pineals, indicating closed neural tubes, both lft1 and pitx2 are most often expressed bilaterally, with a smaller proportion of embryos having normal left-sided expression (Figure 5). The presence of two phenotypes likely reflects the variability in sqt mutants, with left sided expression in embryos with normal ventral midline tissues and bilateral expression in embryos that lack these tissues. In sqt mutants with a divided pineal, the expression of lft1 and pitx2 is almost always bilateral (n = 24/25) (Figure 5D, H, I).
Figure 5. Left-sided gene expression in the developing pineal is disrupted when the neural tube does not close.
(A)Left sided expression of lft1 and bilateral expression of flh in the left pineal anlage of a WT embryo. (B) WT expression pattern of lft1 and flh in a sqt mutant with a closed neural tube. (C) Bilateral lft1 expression in a sqt mutant with a closed neural tube. (D) Expression of lft1 and flh in both the left and right sides of a sqt embryo with a divided pineal anlage. (E) WT embryo with pitx2 on the left and flh on both sides of the developing pineal. (F) sqt mutant with a WT pattern of pitx2 expression. (G) Bilateral pitx2 expression when the pineal anlage is elongated but not fully divided. (H) sqt mutant with bilateral pitx2 expression and a divided pineal anlage. (I) Quantification of lft1 and pitx2 expression patterns in embryos with closed and open neural tubes. Embryos that did not show lft1 or pitx2 gene expression because they were too young or too old were not included in the analysis. The midline of the brain is marked with dotted lines. All embryos were fixed at 26-somites stage and are shown in dorsal views with anterior to the top. The experiment was repeated three times and representative images are shown. Scale bar = 50μm.
Parapineal organs often form on both sides of the epithalamus when the neural tube does not close
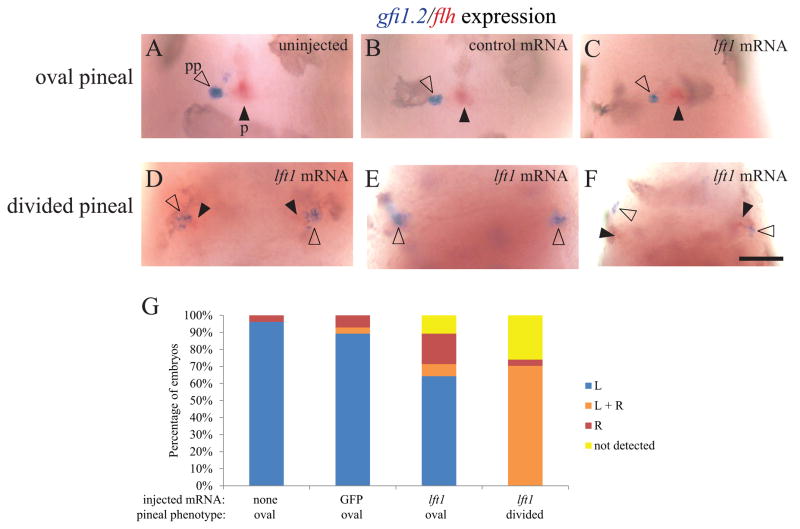
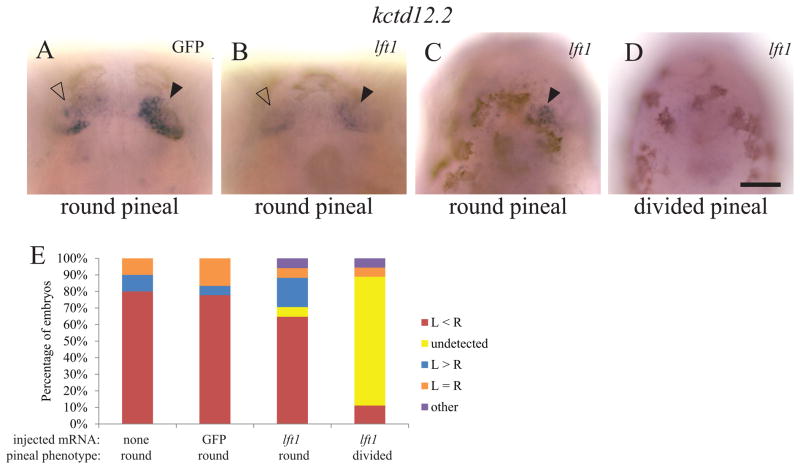
The parapineal organ forms to the left of the pineal organ in many teleost fish (Borg et al., 1983). The cells of the parapineal originate from both sides of the pineal complex anlage, and migrate leftward away from the pineal anlage shortly after the left-sided expression of Nodal pathway genes (Concha et al., 2003; Gamse et al., 2003). We find that in WT, control mRNA injected embryos and Lft1 overexpressing embryos, when the pineal organ is oval shaped, indicating a closed neural tube, more than 80% of the fish have a single gfi1.2-expressing parapineal to the left of the pineal organ (Figure 6A–C, G). In contrast, when the pineal is divided, the majority of embryos form a parapineal on both the left and right sides of the brain (n = 19/27) (Figure 6D–F, G).
Figure 6. Both the left and right sides of the brain form a parapineal when the epithalamus is divided.
foxd3:GFP homozygous embryos were injected with GFP mRNA or lft1 mRNA at the one to two cell stage. Injected embryos and their uninjected siblings were sorted by the pineal phenotype using fluorescent microscopy, fixed at 3 dpf, and then assayed for gfi1.2 in the parapineal (pp, closed arrowheads) and flh in the pineal (p, open arrowheads). (A–C) In uninjected embryos, embryos injected with control GFP mRNA, and in lft1 mRNA injected embryos with oval shaped pineals, the parapineal organ is located adjacent to left side of the pineal. (D–F) In the lft1 injected embryos with divided pineal organs (not shown, as pineal is out of the focal plane of the image), there is a region of gfi1.2 expression on both the left and right sides of the brain, suggesting two parapineal organs have formed. (G) Graph of data represented in A–F. The experiment was repeated four times and representative images are shown. n ≥ 26 for each pineal phenotype. Dorsal views, anterior to the top. Scale bar = 50 μm.
The Oep protein is a component of the Nodal receptor complex. Although our previous study did not find open neural tube defects in oep mutants (Aquilina-Beck et al., 2007), we now find they have incomplete penetrance of the elongated and divided pineal phenotypes (Supplemental Figures 3 and 4). This open neural tube phenotype is present at higher frequency in embryos carrying the null oeptz257 allele than in embryos carrying the hypomorphic oepm134 allele, indicating it increases with decreasing levels of Nodal (Supplemental Figure 3). Further, the bilateral formation of parapineal organs occurs more often in oeptz257 mutants with a divided pineal than in those with an elongated pineal (Supplemental Figure 4).
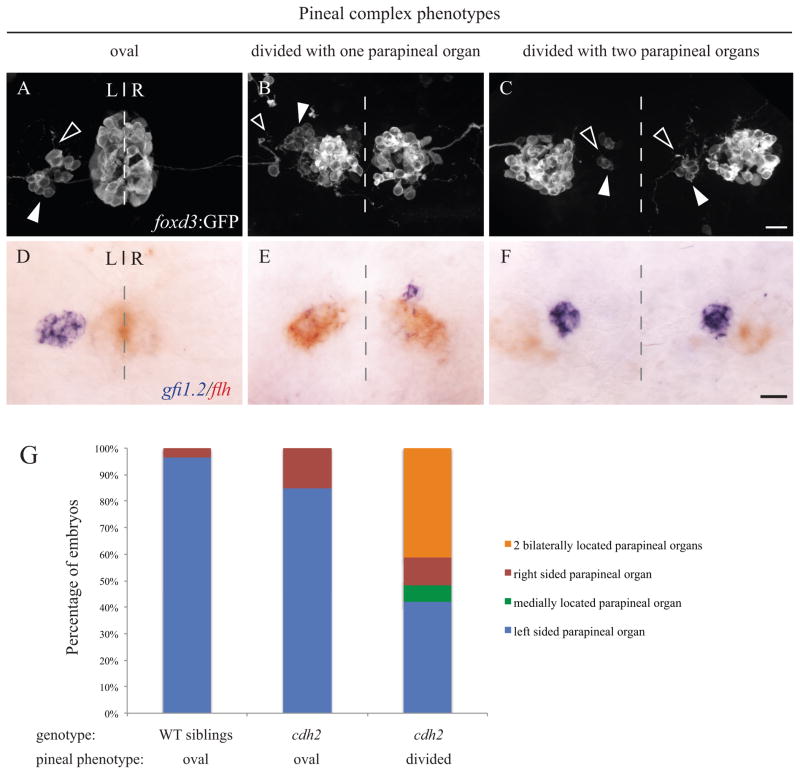
We previously reported that Nodal pathway mutants exhibit reductions in N-cadherin/Cdh2 expression and trafficking (Aquilina-Beck et al., 2007). Additionally, cdh2 mutants have neural tube closure and pineal defects comparable to embryos with disrupted Nodal signaling (Figure 7) (Aquilina-Beck et al., 2007). To characterize the left-right asymmetry phenotype in these mutants, we used high resolution imaging of embryos carrying the foxd3:GFP transgene at a stage when the GFP is expressed in both the pineal and parapineal organs (Gamse et al., 2003). As expected, in WT embryos and cdh2 mutants with a closed neural tube, the pineal organ is oval shaped and flanked on the left side by a single parapineal organ (Figure 7A). Approximately half of the cdh2 mutants with an open neural tube had a single parapineal organ and half had two parapineal organs (Figure 7G). Notably, in embryos with a divided pineal and two parapineal organs, the parapineal cells still send projections anteriorly, suggesting that these cells differentiate as neurons regardless of their location (Figure 7C).
Figure 7. n-cadherin (cdh2vu125) mutants exhibit both oval and divided pineal phenotypes.
(A – C) Confocal images of the immunofluorescence labeling of foxd3:GFP positive embryos at 2 dpf. foxd3:GFP is expressed in the pineal and parapineal organs (white arrowheads). Axonal projections from parapineal neurons are denoted with open arrowheads. (D – F) Two color in situ hybridization showing parapineal cells expressing gfi1.2 (blue) and pineal cells expressing flh (red) in 2 dpf embryos. The dashed lines represent the embryonic midline. (A, D) WT embryos have a fused, oval pineal organ and a single left sided parapineal organ. (B, E) Approximately half of the cdh2 mutants with a divided pineal organ have only a single parapineal organ. (C, F) Approximately half of the cdh2 mutants with a divided pineal organ have two bilaterally located parapineal organs. All images are dorsal views with representative images shown. All scale bars = 20 μm. (G) Graph of parapineal placement in WT siblings (n = 261), cdh2 mutants with oval shaped pineal (n = 46) and cdh2 mutants with divided pineal (n = 114).
To unambiguously distinguish between pineal and parapineal cells, we used two-color in situ hybridization with the pineal gene flh and the parapineal gene gfi1.2 (Figure 7D–F). The majority of cdh2 mutants exhibit a divided pineal phenotype (n = 114/160). In the cdh2 mutants having a divided pineal phenotype, the left isomerized phenotype was less common than in embryos that lacked Nodal signaling (49% for cdh2 versus 70% for Lft1 overexpressing embryos). Also, a left-sided parapineal organ (n = 48/114) was approximately as common as two, bilaterally located parapineal organs (n = 47/114) (Figure 7E, F, G). A minority of cdh2 mutant embryos have an oval shaped pineal (n = 46/160), and in most of these individuals the parapineal is left-sided (n = 39/46)(Figure 7).
Habenulae are left isomerized when the neural tube is open
In normal embryos and embryos with reversed asymmetry, the parapineal is always associated with the habenula nucleus with the left characteristics of high neuropil density, higher kctd12.1 expression, and lower kctd12.2 expression (Concha et al., 2003; Gamse et al., 2003). Further, when the parapineal is ablated, the habenula developed a right sided pattern of gene expression on both sides, indicating that they became right isomerized (Gamse et al., 2003). This suggests that the parapineal is responsible for producing left identity in the adjacent habenula nucleus.
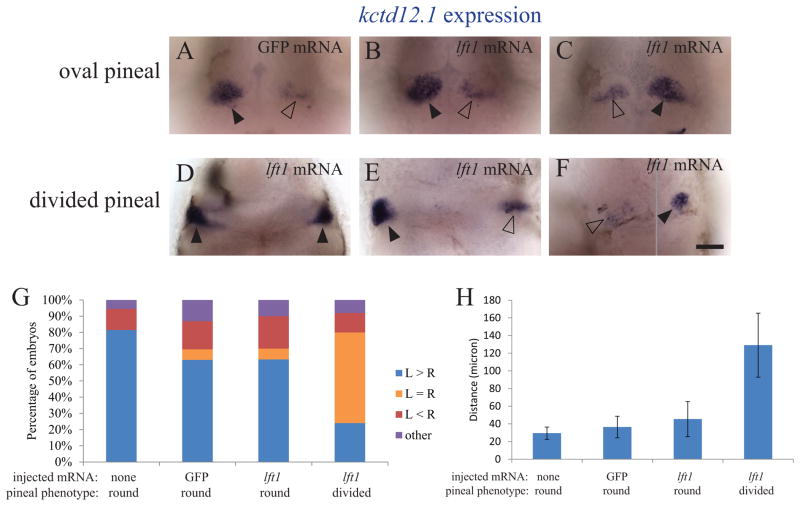
Consistent with this, we find that embryos with open neural tubes and bilateral parapineal organs often form habenular nuclei that are left isomerized (Figure 8, Supplemental Figure 5). As in earlier studies, the left-associated gene kctd12.1 is more highly expressed in the left habenula and the right-associated gene kctd12.2 is more highly expressed in the right habenula in the large majority (80%) of control injected embryos (Figures 8 and 9). The rate of reversed or abnormal kctd12.1 expression is high in all groups, consistent with earlier studies that found WT embryos have a high rate of situs inversus in the brain (Figure 8 and 9)(Liang et al., 2000).
Figure 8. kctd12.1 expression in the habenulae becomes bilateral when the neural tube does not close.
foxd3:GFP embryos injected with GFP mRNA or lft1 mRNA and uninjected siblings were sorted by the pineal phenotype, fixed at 3 dpf, and then assayed for expression of kctd12.1 in the habenula nuclei. (A – C). When the pineal is oval shaped, kctd12.1 expression pattern is higher on the left than on the right. (D – F) When the pineal is divided, the habenula can either have (D) high levels of kctd12.1 in both the left and right sides, (E) higher expression on the left, or (F) higher expression on the right. The image in F is a composite, as the two habenula were in different focal planes. Closed arrowheads indicate the habenulae with higher level of kctd12.1 expression, open arrowheads the habenulae with lower kctd12.1 expression. (G) Distribution of the kctd12.1 expression patterns in each group of embryos. The embryos categorized as “other” (< 6%) had either a single kctd12.1 expression domain near the midline or many small areas of kctd12.1 expression. n ≥ 25 for each group. (H): The distance between the two habenulae measured at the points where they were closest to each other. n ≥ 13 for each group. Avg ± standard deviation. p < 0.001 by single-factor ANOVA. A Tukey-Kramer test finds significant differences between the divided pineal group and all other groups beyond 0.01 confidence level. The experiment was repeated two times and representative images are shown. All images are dorsal views, anterior to the top. Scale bar = 50 μm.
Figure 9. kctd12.2 expression is low in both habenulae when the epthalamus is divided.
Embryos were processed as in Figure 8 but assayed instead for kctd12.2 expression. (A – C) kctd12.2 expression is higher on the right in embryos with an oval shaped pineal. (D)kctd12.2 is expressed at undetectable levels in the majority of embryos with divided pineal organs. The low or undetectable levels of kctd12.2 expression were not due to technical difficulties, as WT embryos processed in parallel had normal levels of expression. Closed arrowheads indicate the habenulae with higher level of kctd12.2 expression. Open arrowheads indicate the habenulae with lower expression. (E) The distribution of kctd12.2 expression pattern in each group of embryos. “Other” includes embryos with either a single kctd12.2 expression domain or many small areas of kctd12.2 expression. n ≥ 17 for each group. The experiment was repeated three times and representative images are shown. All images are dorsal views, anterior to the top. Scale bar = 50 μm.
When the neural tube is open, both of the habenula nuclei have a left pattern of high kctd12.1 expression at a high frequency (Figure 8D, G, Supplemental Figure 5). In contrast, kctd12.2 expression level is either extremely low or undetectable in the majority of embryos (Figure 9E, Supplemental Figure 5). In addition, when lft1 mRNA injection results in a divided pineal morphology, the average distance between the left and right habenulae is significantly increased (Figure 8H).
Abnormal morphology and positioning of epithalamic tissues
In addition to the left isomerized phenotype, other aspects of tissue morphology are also abnormal in embryos with open neural tubes. Even when both sides of the brain are developing with left characteristics, the left and right sides of the brain are typically not mirror images of one another. For instance, the left and right sides of the lft1 and pitx2 domains in the pineal anlage often have different sizes and shapes, as do the left and right habenula nuclei (Figures 5 and 8, Supplemental Figure 5).
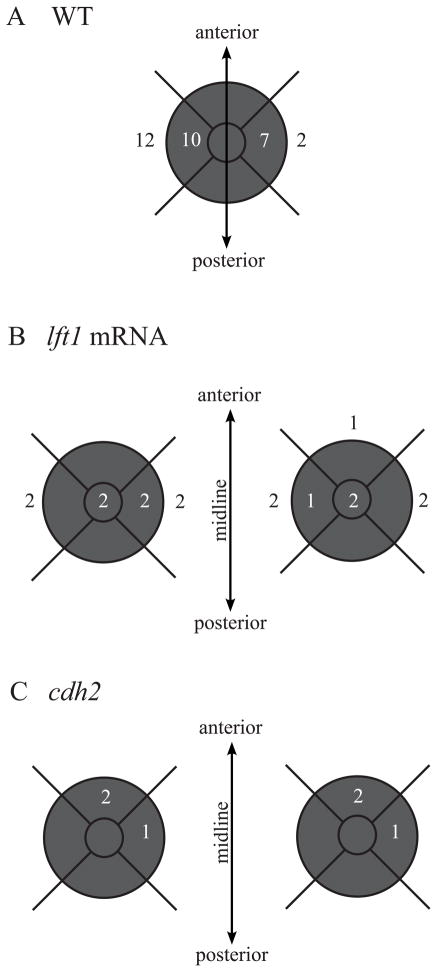
Interestingly, Nodal and N-cadherin deficient embryos with open neural tubes both have mis-positioning of their parapineal organs, but the defects are distinct between these two classes. The parapineal organs were mapped into nine positional categories within and around the left and right pineal domains (Figure 10). In the Nodal mutants, almost all parapineals (n = 15/16) are located in the same anterior-posterior position as the pineal, while the left-right position of the parapineal relative to the pineal is highly variable (Figure 10). In contrast, the parapineal staining in cdh2 mutants most often overlaps with the anterior-most area of the pineal (n = 4/6) (Figure 10). This suggests that the anterior-posterior axis is more disrupted in cdh2 mutants than in embryos that lack Nodal signaling.
Figure 10. The parapineal position is disrupted in embryos with the left isomerized phenotype.
Relative position of the parapineals to the pineal anlage in (A) WT embryos with oval pineal anlage and (B)lft1 mRNA injected embryos and (C)cdh2 mutant embryos with divided pineal anlage. Shaded areas represent the location of the pineal anlage. Numbers within the center of the shaded area represent the position of parapineals that fully overlapped the pineal anlage. Numbers within the outer perimeter of the shaded area represent the positions of parapineals that partially overlapped the pineal anlage and numbers outside the shaded area represent parapineals that did not overlap the pineal anlage. Note the high rate of situs inversus in the WT embryos (n=9/31, 30%). This high rate of reversed brain asymmetry has been observed in the other studies (Liang et al., 2000).
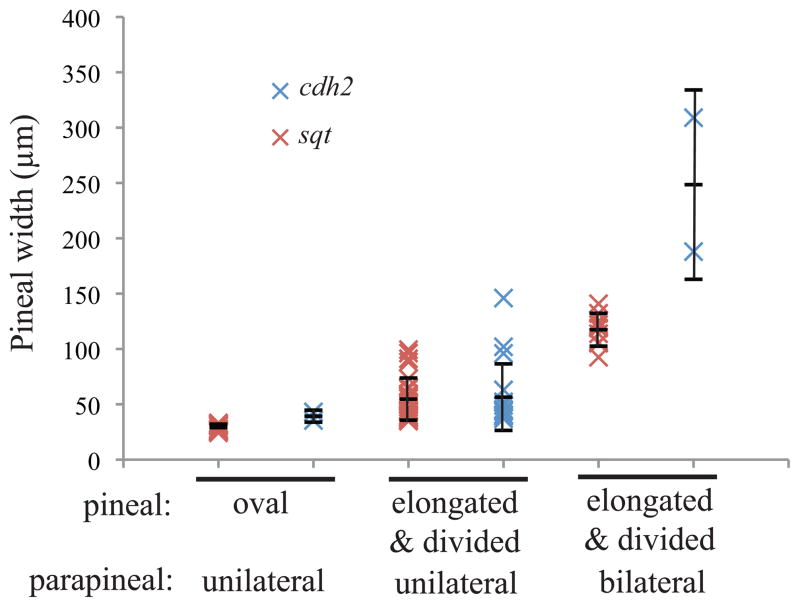
Left isomerism correlates with severity of the open neural tube phenotype
The percentage of embryos with the left isomerized phenotype is incompletely penetrant in both cdh2 mutants and Nodal deficient embryos with open neural tubes (Figures 6 and 7, Supplemental Figure 4). One possibility is that the left isomerized phenotype is dependent upon the distance between the divided pineal anlage. To test this hypothesis, we grouped the embryos based on parapineal phenotypes and measured the distance between the two most lateral points of the pineal (Figure 11). Pineal anlage were characterized as oval if their width was in the range of WT pineals, as elongated if there was a single domain that was wider than a normal pineal, and as divided if there was more than one domain. We find that the left isomerized phenotype is correlated to the severity of the open neural tube. When comparing embryos with open neural tubes, the pineal width of embryos with two bilateral parapineals is significantly greater than the pineal width in embryos with one parapineal (p < 0.0001 when Nodal deficient embryos and cdh2 mutants are analyzed separately and when both classes are analyzed together). This hypothesis could also explain the difference in penetrance of the left isomerized phenotype in embryos that lack Nodal signaling versus those that lack N-cadherin (Figures 6 and 7). The penetrance of the left isomerized phenotype is most likely lower in cdh2 mutants because more of these embryos have a mild open neural tube phenotype.
Figure 11. The differences in left isomerization frequency between sqt and cdh2 mutant embryos are caused by differences in the severity of the neural tube phenotype.
sqt cz35 mutants and cdh2p73 mutant embryos at 3 dpf were assayed for parapineal phenotype and pineal width. Each data point on the graphs indicates the result from a single embryo. The short horizontal lines indicate the average of each group, with error bars indicating standard deviation. For sqt embryos, n = 12 for oval, unilateral; n = 29 for elongated/divided, unilateral, and n = 9 for elongated/divided, bilateral. For cdh2 mutants, n = 2 for oval, unilateral, n = 17 elongated/divided, bilateral, and n = 2 for elongated/divided, bilateral.
Discussion
Communication between the left and right sides of the brain was required for development of left-right asymmetry
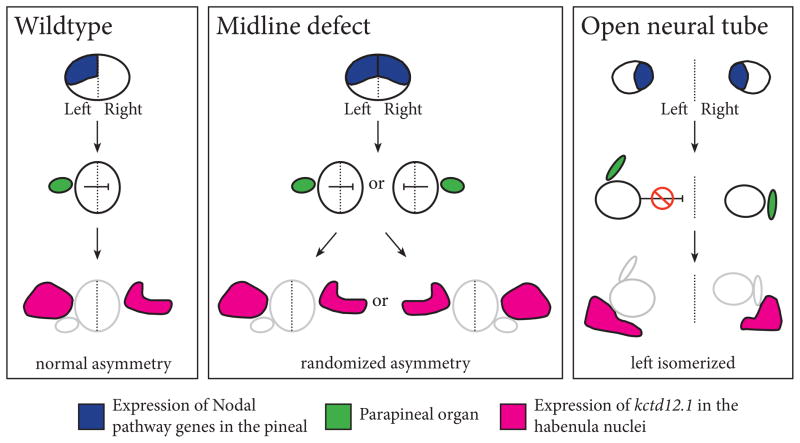
In this study, we find that all three known left-right asymmetric structures in the zebrafish epithalamus, the pineal organ, parapineal organ, and habenulae nuclei, became left isomerized when the anterior neural tube did not close (Figure 12). Several cell types that normally differentiate at the dorsal midline of the brain, including pineal photoreceptors, pineal projection neurons, cranial neural crest, parapineal cells, and the habenula nuclei still expressed cell-type-specific genes and proteins. This suggests that the changes in left-right asymmetry are not due to improper specification of dorsal cell types.
Figure 12. Model for laterality in the zebrafish epithalamus.
In WT embryos, left-right asymmetry is first apparent in the left-sided expression of the Nodal pathway genes cyc, lft1, and pitx2 in the pineal anlage. Subsequently, the parapineal is formed on the left side of the brain, adjacent to the pineal anlage. The left sided position of the parapineal then causes differences between the underlying habenula nuclei, such as the more extensive expression of leftover in the left habenula. In embryos with midline defects, the expression of cyc, lfty1, and pitx2 becomes bilateral. Despite this, the parapineal and habenula still develop left-right asymmetry, although some of the embryos have normal situs and some have situs inversus. Thus suggests that there is an inhibitory factor that prevents both sides from becoming left. In embryos with an open neural tube, all asymmetries in the epithalamus become left isomerized, suggesting that the inhibitory signal that prevents both sides from developing a left morphology is not functional.
Comparison of embryos with different classes of asymmetry defects leads to a model in which there is a signal that normally prevents both sides of the brain from developing a left identity (Figure 12). For instance, embryos with anterior midline defects, such as lack of prechordal plate mesendoderm, are initially left isomerized. However, because the neural tube is closed, this signal is still able to function, and one side of the brain develops as left and the other as right (Figure 12). In contrast, in embryos with open neural tubes, this signal cannot function because the left and right sides are separated. Consequently, both sides develop gene expression patterns and morphology characteristic of the left side. This signal likely functions before formation of the parapineal organ, as midline defective mutants have switched from being left isomerized to having asymmetry by this stage of development (Figure 12).
Further, our data strongly suggest that the signal that prevents left isomerism acts over a limited distance. The pineal widths of embryos with the left isomerized phenotype were significantly greater than those of embryos with left-right asymmetry in their epithalamus. This suggests the signal remains functional in embryos with mild open neural tube defects, but begins to fail when the distance between the left and right sides approaches 100 μm. One strong possibility is that the signal is mediated by a secreted protein. This protein normally diffuses from the left to the right side of the epithalamus and prevents the right side from developing left characteristics (Figure 12).
Although we cannot rule out other proteins, the Nodal inhibitor Lft1 itself is a good candidate to be this signal. In WT embryos, both Cyc and Lft1 are produced on the left side of the pineal anlage and then secreted (Liang et al., 2000). Cyc is able to diffuse only a few cell diameters (Chen and Schier, 2001, 2002; Muller et al., 2012), and so would remain largely confined to the left side of the pineal anlage. In contrast, Lft1 is able to diffuse over many cell diameters (Chen and Schier, 2001, 2002). Any Cyc activity that enters the right side in a normal embryo would be quickly repressed by the more efficiently diffusing Lft1.
In other areas of the embryo, including in development of left-right asymmetry of the visceral organs, Nodal and Lft proteins act in a reaction-diffusion mechanism to generate pattern (Chen and Schier, 2001, 2002; Meinhardt, 2008; Muller et al., 2012; Nakamura et al., 2006). It is possible that a reaction-diffusion mechanism also establishes the left-right axis in the zebrafish brain. In a classical reaction-diffusion mechanism, differences are created between adjacent region of the embryo by a group of cells producing both a slowly diffusing morphogen and a faster diffusing inhibitor (Gierer and Meinhardt, 1972; Meinhardt, 2008; Turing, 1990). An essential component to this model is that both the morphogen and the inhibitor are positively regulated by the activity of the morphogen. This fits well with the expression of the cyc and lft1 genes, which are both activated by Cyc signaling. In particular, if Cyc acts as this morphogen and Lft1 as this inhibitor, such a reaction-diffusion interaction could explain why embryos with midline defects eventually develop asymmetry. In embryos with midline defects, Cyc and Lft1 are produced on both sides of the pineal anlage (Figure 12) (Concha et al., 2000; Liang et al., 2000). If one side starts to express slightly more Cyc than the other side, this difference will be amplified over time. On the side that starts with slightly higher Cyc expression, Cyc activity will continue to increase and Cyc levels will become higher than Lft1. This side will become left. On the opposite side, Lft1 originating from both the left and right will inactivate the lower levels of Cyc protein, and this side will become right. This process would convert the initial left isomerism into randomized asymmetry (Gierer and Meinhardt, 1972; Meinhardt, 2008; Turing, 1990).
Although a secreted protein such as Lft1 is a good candidate for the signal that prevents left isomerism, we cannot rule out other possibilities. For instance, a contact-dependent mechanism, such as cell adhesion or lateral inhibition that is propagated across the epithalamus could also be involved in generating left-right asymmetry. Consistent with this, N-cadherin-mediated adhesion is reduced in all of the mutants we have analyzed. In the absence of N-cadherin, many aspects of polarity in the neuroepithelium are disrupted, including the oriented intercalation of cells in the neuroepithelium and spatially oriented cell divisions (Hong and Brewster, 2006; Zigman et al., 2011). Lack of N-cadherin may cause an overall lack of polarity in the neural tube that also affects left-right axis formation.
Comparison of Nodal and N-cadherin deficient phenotypes
Our previous work suggested that severely decreased expression of N-cadherin is at least in part due to the open neural tube phenotypes in Nodal signaling mutants (Aquilina-Beck et al., 2007). Thus, it was not surprising that we found left isomerized phenotypes in both classes of mutant embryos. Conversely, it is also possible that they share left isomerized phenotypes because loss of N-cadherin in some way decreases Nodal signaling. However, this is less likely, as N-cadherin mutants lack the defects that are present in embryos with only mild Nodal deficiencies, such as cylopic eyes.
However, there are several differences in the phenotypes of Nodal and N-cadherin deficient embryos. First, the penetrance of the left isomerized phenotype is lower in N-cadherin mutants versus Nodal signaling mutants with open neural tubes. As discussed above, this difference is most likely due to the difference in the severity of the open neural tube phenotype and the distance between the left and right sides of the brain. The greater severity of the open neural tube phenotype when Nodal signaling is absent suggests that other proteins involved in neural tube development, in addition to N-cadherin, are likely disrupted in the absence of Nodal signaling. Conversely, cdh2 mutants have anterior-posterior defects in parapineal positioning that are not present in Nodal deficient embryos. This suggests that N-cadherin is involved in pathways that do not intersect with Nodal signaling.
Differentiation of dorsal cell fates in neuroepithelium persisted in embryos with neural tube defects
Our results demonstrated that pineal and neural crest cells differentiate even in embryos with severe open neural tube phenotypes. The dorsal neural tube is the source of many morphogens such as Bone Morphogenic Protein 4, which sets up the dorsal ventral axis of the neural tube, and Wnts, which are involved in specifying neural crest cells. Thus, it is somewhat surprising that differentiation of dorsal cell types is so normal. However, this finding is consistent with the fact that expression of the transcription factor flh, which is required for the differentiation both pineal photoreceptors and projection neurons, is expressed in mutants that lack Nodal signaling (Masai et al., 1997). In addition, we have found that wnt1 is expressed in the dorsal cells of embryos with open neural tubes (Aquilina-Beck et al., 2007; Masai et al., 1997).
Our results also demonstrated that initiation of pineal rhythmic function occurs in embryos with open anterior neural tubes. In mammals, the pineal organ is not directly light sensitive. Instead, environmental lighting conditions are detected by retinal ganglion cells that reset circadian rhythms within the suprachaismatic nucleus (SCN) in the hypothalamus. The SCN controls rhythms in the pineal through a multisynaptic pathway. In contrast, rhythms in the zebrafish pineal are likely started by the detection of a dark-to-light transition by pineal photoreceptors. Pineal rhythms initiate in embryos that lack eyes or an SCN, but not in embryos that lack expression of the light sensitive pineal protein Period2 (Kennedy et al., 2004; Noche et al., 2011; Ziv and Gothilf, 2006; Ziv et al., 2005). Thus, our finding that rhythmic gene expression in the pineal are present suggests that the differentiated photoreceptors are functional in embryos with open anterior neural tubes.
Supplementary Material
Supplemental Figure 1: Lefty1 mRNA induces Nodal signaling deficient phenotypes in WT embryos. (A) Normal phenotype (indistinguishable from WT). (B) Class I (cyclopia, loss of some somites, similar to sqt mutants with mild phenotypes). (C) Class II (cyclopia, loss of over half of somites, similar to sqt mutants with severe phenotypes). (D) Class III (cyclopia, complete loss of somites, similar to cyc;sqt double mutants). (E) Percentage of embryos injected with 5 pg lefty1 mRNA with each phenotypic class. These phenotypes are based on the original characterization of Lft1 overexpression phenotypes in zebrafish (Thisse and Thisse, 1999). Scale bar = 200μM
Supplemental Figure 2: neural crest gene crestin is expressed in embryos with an open neural tube at the 26 somite stage. (A) At the 26 somite stage, crestin is expressed in the premigrating and migrating neural crest in the WT siblings and in sqt mutants with cyclopic eye and (B) a oval shaped pineal (C) divided pineal. Open arrowheads labeled with r2, r4 and r6 indicate presumed rostral cranial neural crest cell migration streams, which originate from rhombomeres 2, 4 and 6, respectively. The experiment was repeated three times and representative images are shown. n > 50 for WT siblings and n = 20 for sqt mutants. All images are dorsal views with anterior to the left. Scale bar = 50μm.
Supplemental Figure 3: oeptz257 mutant phenotypes include divided pineal. (A) WT siblings and (B) a minority of homozygous oeptz257 mutants have an oval shaped pineal phenotype. (C, D) The majority of oeptz257 mutants have an elongated or divided pineal phenotype, indicating the neural tube is open. (E) Comparison of number and percentage of WT, oepm134, and oeptz257 embryos with each pineal phenotype. Embryos are between the 25 somite stage and 24 hpf. The experiment was repeated 2 times and representative images are shown. All images are dorsal views with anterior to the top. Scale bar = 50μm.
Supplemental Figure 4: oeptz257 mutants exhibit both fused and divided pineal phenotypes. (A – C) Two color in situ hybridization shows parapineal cells expressing gfi1.2 (blue) and pineal cells expressing otx5 (red) in 2.5 dpf embryos. The dashed lines represent the embryonic midline. (A) WT embryos have an oval shaped pineal (p, closed arrowheads) organ and a single left sided parapineal organ (pp, open arrowheads). (B) Some oeptz257 mutants have an elongated pineal organ with a single parapineal organ in the middle of the pineal complex. (C) Other oeptz257 mutants have a divided pineal organ and two bilaterally located parapineal organs. All images are dorsal views with representative images shown. Scale bar = 50μm. (D) Graph depicting parapineal placement seen in WT siblings (n = 20), oeptz257 mutants with an oval shaped pineal (n = 9), oeptz257 mutants with an elongated pineal (n = 7) and oeptz257 mutants with divided pineal (n = 6).
Supplemental Figure 5: kctd12.1 and kctd12.2 expression patterns in the habenulae in oeptz257 embryos are similar to the patterns in lft1 mRNA injected embryos. (A) Normal asymmetric expression of kctd12.1 in a WT sibling. (B, C) Bilateral high levels of kctd12.1 expression in oeptz257 embryos. Note that in (B), although the left sided kctd12.1 expressing area is smaller, the strength of the staining is not weaker. (D) The percentage of different kctd12.1 expression patterns in oeptz257 mutant and their WT siblings. (E) The percentage of different kctd12.2 expression patterns in oeptz257 and their WT siblings. The experiment was repeated twice and representative images are shown. n ≥ 11 for each group. All images are dorsal views, anterior to the top. Scale bar = 50 μm.
Highlights.
Zebrafish embryos have prominent left-right asymmetries in the epithalamus
Left and right sides of epithalamus remain separated when the neural tube is open
Both sides of the epithalamus develop as “left” in embryos with open neural tubes
A short range signal normally prevents the right side from developing like the left
Acknowledgments
Our thanks to Dr. Radhika Atit and Dr. Ronald Conlon for their valuable comments in this manuscript. Drs. Marnie Halpern, Rachel Brewster, Thomas Schilling, Patrick Blader generously shared reagents. This work was supported in part by research grant 1 RO1 HD054523 (to JTG and JOL) and a University of Minnesota Grant in Aid of Research and Scholarship (to JOL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa H, Goto M, Sato T, Okamoto H. Temporally regulated asymmetric neurogenesis causes left-right difference in the zebrafish habenular structures. Dev Cell. 2007;12:87–98. doi: 10.1016/j.devcel.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Andrew RJ, Dharmaretnam M, Gyori B, Miklosi A, Watkins JA, Sovrano VA. Precise endogenous control of involvement of right and left visual structures in assessment by zebrafish. Behav Brain Res. 2009;196:99–105. doi: 10.1016/j.bbr.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Aquilina-Beck A, Ilagan K, Liu Q, Liang JO. Nodal signaling is required for closure of the anterior neural tube in zebrafish. BMC Dev Biol. 2007;7:126. doi: 10.1186/1471-213X-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr Biol. 2005;15:844–850. doi: 10.1016/j.cub.2005.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco IH, Wilson SW. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birely J, Schneider VA, Santana E, Dosch R, Wagner DS, Mullins MC, Granato M. Genetic screens for genes controlling motor nerve-muscle development and interactions. Dev Biol. 2005;280:162–176. doi: 10.1016/j.ydbio.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- Borg B, Ekstrom P, Van Veen T. The parapineal organ of teleosts. Acta Zoologica. 1983;64:211–218. [Google Scholar]
- Brand M, Heisenberg CP, Warga RM, Pelegri F, Karlstrom RO, Beuchle D, Picker A, Jiang YJ, Furutani-Seiki M, van Eeden FJ, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development. 1996;123:129–142. doi: 10.1242/dev.123.1.129. [DOI] [PubMed] [Google Scholar]
- Cahill GM. Clock mechanisms in zebrafish. Cell Tissue Res. 2002;309:27–34. doi: 10.1007/s00441-002-0570-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–610. doi: 10.1038/35079121. [DOI] [PubMed] [Google Scholar]
- Chen Y, Schier AF. Lefty proteins are long-range inhibitors of squint-mediated nodal signaling. Curr Biol. 2002;12:2124–2128. doi: 10.1016/s0960-9822(02)01362-3. [DOI] [PubMed] [Google Scholar]
- Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, Kimelman D, Nicolson T, Grunder S, Gomperts M, Clarke JD, Wilson SW. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Cooke J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol Rev Camb Philos Soc. 2004;79:377–407. doi: 10.1017/s1464793103006298. [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Rastegar S, Strahle U, Blader P. Parapineal specific expression of gfi1 in the zebrafish epithalamus. Gene Expr Patterns. 2004;4:53–57. doi: 10.1016/s1567-133x(03)00148-0. [DOI] [PubMed] [Google Scholar]
- Eichele T, Specht K, Moosmann M, Jongsma ML, Quiroga RQ, Nordby H, Hugdahl K. Assessing the spatiotemporal evolution of neuronal activation with single-trial event-related potentials and functional MRI. Proc Natl Acad Sci U S A. 2005;102:17798–17803. doi: 10.1073/pnas.0505508102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchin L, Burgess HA, Siddiqi M, Granato M, Halpern ME. Determining the function of zebrafish epithalamic asymmetry. Philos Trans R Soc Lond B Biol Sci. 2009;364:1021–1032. doi: 10.1098/rstb.2008.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Kuan YS, Macurak M, Brosamle C, Thisse B, Thisse C, Halpern ME. Directional asymmetry of the zebrafish epithalamus guides dorsoventral innervation of the midbrain target. Development. 2005;132:4869–4881. doi: 10.1242/dev.02046. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet. 2002;30:117–121. doi: 10.1038/ng793. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Thisse C, Thisse B, Halpern ME. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130:1059–1068. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- Gierer A, Meinhardt H. A theory of biological pattern formation. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Maischein HM, Nüsslein-Volhard C. Migration and Function of a Glial Subtype in the Vertebrate Peripheral Nervous System. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Gothilf Y, Coon SL, Toyama R, Chitnis A, Namboodiri MA, Klein DC. Zebrafish serotonin N-acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology. 1999;140:4895–4903. doi: 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–3905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Symmetry and asymmetry in the human brain. European Review. 2005;13:119–133. [Google Scholar]
- Kennedy BN, Stearns GW, Smyth VA, Ramamurthy V, van Eeden F, Ankoudinova I, Raible D, Hurley JB, Brockerhoff SE. Zebrafish rx3 and mab21l2 are required during eye morphogenesis. Dev Biol. 2004;270:336–349. doi: 10.1016/j.ydbio.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Korf HW, Schomerus C, Stehle JH. The pineal organ, its hormone melatonin, and the photoneuroendocrine system. Adv Anat Embryol Cell Biol. 1998;146:1–100. doi: 10.1007/978-3-642-58932-4. [DOI] [PubMed] [Google Scholar]
- Kuan YS, Gamse JT, Schreiber AM, Halpern ME. Selective asymmetry in a conserved forebrain to midbrain projection. J Exp Zool B Mol Dev Evol. 2007a;308:669–678. doi: 10.1002/jez.b.21184. [DOI] [PubMed] [Google Scholar]
- Kuan YS, Yu HH, Moens CB, Halpern ME. Neuropilin asymmetry mediates a left-right difference in habenular connectivity. Development. 2007b;134:857–865. doi: 10.1242/dev.02791. [DOI] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- Mano H, Kojima D, Fukada Y. Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland. Brain Res Mol Brain Res. 1999;73:110–118. doi: 10.1016/s0169-328x(99)00242-9. [DOI] [PubMed] [Google Scholar]
- Masai I, Heisenberg CP, Barth KA, Macdonald R, Adamek S, Wilson SW. floating head and masterblind regulate neuronal patterning in the roof of the forebrain. Neuron. 1997;18:43–57. doi: 10.1016/s0896-6273(01)80045-3. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Models of biological pattern formation: from elementary steps to the organization of embryonic axes. Curr Top Dev Biol. 2008;81:1–63. doi: 10.1016/S0070-2153(07)81001-5. [DOI] [PubMed] [Google Scholar]
- Miklosi A, Andrew RJ. Right eye use associated with decision to bite in zebrafish. Behav Brain Res. 1999;105:199–205. doi: 10.1016/s0166-4328(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Miklosi A, Andrew RJ, Savage H. Behavioural lateralisation of the tetrapod type in the zebrafish (Brachydanio rerio) Physiol Behav. 1997;63:127–135. doi: 10.1016/s0031-9384(97)00418-6. [DOI] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336:721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell. 2006;11:495–504. doi: 10.1016/j.devcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Noche RR, Lu P-N, Goldstein-Kral L, Glasgow E, Liang JO. Circadian rhythms in the pineal organ persist in zebrafish larvae that lack ventral brain. BMC Neuroscience. 2011;12 doi: 10.1186/1471-2202-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce LX, Noche RR, Ponomareva O, Chang C, Liang JO. Novel functions for Period 3 and Exo-rhodopsin in rhythmic transcription and melatonin biosynthesis within the zebrafish pineal organ. Brain Res. 2008;1223:11–24. doi: 10.1016/j.brainres.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis S, Jancke L, Schmitz-Hillebrecht J, Steinmetz H. Child age and planum temporale asymmetry. Brain Cogn. 1999;40:441–452. doi: 10.1006/brcg.1998.1072. [DOI] [PubMed] [Google Scholar]
- Rubinstein AL, Lee D, Luo R, Henion PD, Halpern ME. Genes dependent on zebrafish cyclops function identified by AFLP differential gene expression screen. Genesis. 2000;26:86–97. doi: 10.1002/(sici)1526-968x(200001)26:1<86::aid-gene11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sampath K, Rubinstein AL, Cheng AM, Liang JO, Fekany K, Solnica-Krezel L, Korzh V, Halpern ME, Wright CV. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal morphogens. Cold Spring Harbor perspectives in biology. 2009;1:a003459. doi: 10.1101/cshperspect.a003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–178. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Steinmetz H. Structure, functional and cerebral asymmetry: in vivo morphometry of the planum temporale. Neurosci Biobehav Rev. 1996;20:587–591. doi: 10.1016/0149-7634(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Trevarrow B, Halpern ME, Melby AE, Farr G, Postlethwait JH, Jowett T, Kimmel CB, Kimelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallon J, Chandraratna RA, Chien K, Blumberg B, Evans RM, Belmonte JC. Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci U S A. 1999;96:11376–11381. doi: 10.1073/pnas.96.20.11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. 1953. Bull Math Biol. 1990;52:153–197. doi: 10.1007/BF02459572. discussion 119–152. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Regolin L, Pagni P. Detour behaviour, imprinting and visual lateralization in the domestic chick. Brain Res Cogn Brain Res. 1999;7:307–320. doi: 10.1016/s0926-6410(98)00033-0. [DOI] [PubMed] [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. 2000. [Google Scholar]
- Wilson SW, Easter SS., Jr Stereotyped pathway selection by growth cones of early epiphysial neurons in the embryonic zebrafish. Development. 1991;112:723–746. doi: 10.1242/dev.112.3.723. [DOI] [PubMed] [Google Scholar]
- Zigman M, Trinh le A, Fraser SE, Moens CB. Zebrafish neural tube morphogenesis requires Scribble-dependent oriented cell divisions. Curr Biol. 2011;21:79–86. doi: 10.1016/j.cub.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv L, Gothilf Y. Period2 expression pattern and its role in the development of the pineal circadian clock in zebrafish. Chronobiol Int. 2006;23:101–112. doi: 10.1080/07420520500464551. [DOI] [PubMed] [Google Scholar]
- Ziv L, Levkovitz S, Toyama R, Falcon J, Gothilf Y. Functional development of the zebrafish pineal gland: light-induced expression of period2 is required for onset of the circadian clock. J Neuroendocrinol. 2005;17:314–320. doi: 10.1111/j.1365-2826.2005.01315.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Lefty1 mRNA induces Nodal signaling deficient phenotypes in WT embryos. (A) Normal phenotype (indistinguishable from WT). (B) Class I (cyclopia, loss of some somites, similar to sqt mutants with mild phenotypes). (C) Class II (cyclopia, loss of over half of somites, similar to sqt mutants with severe phenotypes). (D) Class III (cyclopia, complete loss of somites, similar to cyc;sqt double mutants). (E) Percentage of embryos injected with 5 pg lefty1 mRNA with each phenotypic class. These phenotypes are based on the original characterization of Lft1 overexpression phenotypes in zebrafish (Thisse and Thisse, 1999). Scale bar = 200μM
Supplemental Figure 2: neural crest gene crestin is expressed in embryos with an open neural tube at the 26 somite stage. (A) At the 26 somite stage, crestin is expressed in the premigrating and migrating neural crest in the WT siblings and in sqt mutants with cyclopic eye and (B) a oval shaped pineal (C) divided pineal. Open arrowheads labeled with r2, r4 and r6 indicate presumed rostral cranial neural crest cell migration streams, which originate from rhombomeres 2, 4 and 6, respectively. The experiment was repeated three times and representative images are shown. n > 50 for WT siblings and n = 20 for sqt mutants. All images are dorsal views with anterior to the left. Scale bar = 50μm.
Supplemental Figure 3: oeptz257 mutant phenotypes include divided pineal. (A) WT siblings and (B) a minority of homozygous oeptz257 mutants have an oval shaped pineal phenotype. (C, D) The majority of oeptz257 mutants have an elongated or divided pineal phenotype, indicating the neural tube is open. (E) Comparison of number and percentage of WT, oepm134, and oeptz257 embryos with each pineal phenotype. Embryos are between the 25 somite stage and 24 hpf. The experiment was repeated 2 times and representative images are shown. All images are dorsal views with anterior to the top. Scale bar = 50μm.
Supplemental Figure 4: oeptz257 mutants exhibit both fused and divided pineal phenotypes. (A – C) Two color in situ hybridization shows parapineal cells expressing gfi1.2 (blue) and pineal cells expressing otx5 (red) in 2.5 dpf embryos. The dashed lines represent the embryonic midline. (A) WT embryos have an oval shaped pineal (p, closed arrowheads) organ and a single left sided parapineal organ (pp, open arrowheads). (B) Some oeptz257 mutants have an elongated pineal organ with a single parapineal organ in the middle of the pineal complex. (C) Other oeptz257 mutants have a divided pineal organ and two bilaterally located parapineal organs. All images are dorsal views with representative images shown. Scale bar = 50μm. (D) Graph depicting parapineal placement seen in WT siblings (n = 20), oeptz257 mutants with an oval shaped pineal (n = 9), oeptz257 mutants with an elongated pineal (n = 7) and oeptz257 mutants with divided pineal (n = 6).
Supplemental Figure 5: kctd12.1 and kctd12.2 expression patterns in the habenulae in oeptz257 embryos are similar to the patterns in lft1 mRNA injected embryos. (A) Normal asymmetric expression of kctd12.1 in a WT sibling. (B, C) Bilateral high levels of kctd12.1 expression in oeptz257 embryos. Note that in (B), although the left sided kctd12.1 expressing area is smaller, the strength of the staining is not weaker. (D) The percentage of different kctd12.1 expression patterns in oeptz257 mutant and their WT siblings. (E) The percentage of different kctd12.2 expression patterns in oeptz257 and their WT siblings. The experiment was repeated twice and representative images are shown. n ≥ 11 for each group. All images are dorsal views, anterior to the top. Scale bar = 50 μm.