Abstract
Background
5-Fluorouracil (5-FU)-based chemotherapy is a conventional therapeutic approach for the treatment of patients with colorectal cancer (CRC). However, development of 5-FU resistance frequently occurs. We explored a potential method for regulating the sensitivity to 5-FU-based chemotherapy in CRC patients.
Material/Methods
Cell viability was determined by 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide (MTT) assay. Gene expression levels were detected by real-time quantitative polymerase chain reaction (RT-qPCR). Protein expression levels were evaluated by Western blot. TargetScan was used for the prediction of binding sites for miRNA in mRNAs. The interaction between mRNA 3′UTR and miRNA was verified by dual luciferase reporter assay. Tissue samples were obtained from 33 CRC patients who received surgery at Xingtai People’s Hospital.
Results
miR-106a level was associated with 5-FU sensitivity in CRC cells. Overexpression of miR-106a reduced 5-FU sensitivity of HCT116 and SW620 cells, and antagonist of miR-106a sensitized HCT116 and SW620 towards 5-FU. miR-106a overexpression decreased dual-specificity phosphatases 2 (DUSP2) expression at mRNA and protein levels in HCT116 and SW620 cells. Through downregulation of DUSP2, miR-106a elevation increased COX-2 expression and stemness-maintenance genes (SOX2 and OCT4). Furthermore, we predicted that miR-106a directly binds to 3′UTR of DUSP2 mRNA, which was confirmed by dual luciferase assay. Silencing of DUSP2 reversed elevated 5-FU sensitivity induced by miR-106a antagonist in HCT116 cells. A negative correlation was discovered between miR-106a and DUSP2 in tumor samples of CRC patients.
Conclusions
miR-106a plays an important role in mediating response to 5-FU-based chemotherapy in CRC and could serve as a potential target for CRC patients.
MeSH Keywords: Colorectal Neoplasms, Fluorouracil, MicroRNAs
Background
Colorectal cancer (CRC) is the third most commonly diagnosed cancer type in the United States, with more than 73 000 new cases reported and at least 26 000 patients dying of it annually [1]. 5-Fluorouracil (5-FU) is a widely used chemotherapy agent for clinical treatment of various cancers [2]. 5-FU, alone or in combination with other drugs, greatly improved the clinical outcomes of CRC patients [3]. However, the therapeutic efficacy of 5-FU is challenged by different responses from individuals towards drug treatment and the acquired drug resistance [4]. Several biomarkers have been discovered to predict responses to 5-FU-based chemotherapy in CRC, although their clinical application remains controversial [5,6]. Thus, it is of great importance to explore new molecular mechanisms in driving 5-FU sensitivity of CRC to fulfill unmet clinical needs.
Disruption of normal microRNAs (miRNAs) expression is frequently observed during cancer initiation and development [7]. miRNAs are small, non-coding, single-stranded RNA sequences that can post-transcriptionally regulate expression of their target genes through directly binding to complementary 3′UTR sequences [8]. miRNAs function as tumor suppressors or an oncogenes, depending on the setting [9]. Overexpression of oncogene miRNAs and decrease of tumor suppressor miRNAs expression alter cell signaling and promote cancer progression [10]. Several studies showed that expression of some specific miRNAs is associated with response to chemotherapy agents in CRC and may serve as predictors for CRC patients receiving chemotherapy [11–13].
Tumor-initiating cells account for a small proportion of tumor-forming cancer cells within a tumor [14]. With strong self-renewal ability, tumor-initiating cells confer chemotherapy resistance and lead to poor prognosis for cancer patients [15]. In CRC, metastasis during 5-FU treatment and resistance to 5-FU resulted from YAP-induced stemness in cancer cells [16]. Dual-specificity phosphatases (DUSPs) are negative regulators of MAPKs [17]. DUSP family members can be divided into 3 groups according to their distribution in cells. DUSP2 is a member of class I DUSP and is located in the nucleus [18]. DUSP2 expression is significantly reduced in many human cancers and its level is inversely correlated with cancer malignancy [19]. Downregulation of DUSP2 induces cancer stemness and increased chemoresistance/lapatinib resistance in cancer cells [20,21].
However, little is known about the regulation of DUSP2 by miRNAs and their involvement in 5-FU sensitivity.
Material and Methods
Patients
We analyzed the gene expression levels of DUSP2 and miR-106a in tumor tissues and the adjacent normal tissues from 33 CRC patients. Participants were enrolled at Xingtai People’s Hospital from June 2015 to May 2016. Written informed consent was obtained from each patient. The experiments were carried out under the supervision of the Ethics Committee of Xingtai People’s Hospital.
Cell culture
We obtained the 293 cell line and human CRC HCT116/SW620 cell lines from ATCC (American Type Culture Collection) and used them within 6 months. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) in an incubator at the temperature of 37°C with 5% CO2.
Treatment of 5-FU
For the treatment of 5-FU, different concentrations of 5-FU (0.5, 1, 2.5, and 5 μg/ml) or 5-FU (2.5, 5, 10, and 20 μg/ml) were added to the DMEM and incubated for 48 h. The former series of different concentrations of 5-FU (0.5, 1, 2.5, and 5 μg/ml) was used for the detection of cell viability in HCT116 cell lines and SW620 cell lines after the treatment of miR-NC antagonist or miR-106a antagonist, while the later series of different concentrations of 5-FU (2.5, 5, 10, and 20 μg/ml) was applied for the detection of cell viability in HCT116 cell lines and SW620 cell lines after the treatment of miR-NC mimics or miR-106a mimics. Cells were then used for the subsequent experiments.
MTT assay
Cell viabilities of HCT116 and SW620 cell lines were determined by MTT assay. In brief, HCT116 or SW620 cells were cultured in 96-well plates and treated with the indicated agent for the indicated time. After that, cells were stained by MTT for 2 h. Absorbance at 570 nm was read on a microplate reader after dissolving the purple precipitates with MTT detergent reagent.
RT-qPCR
Total RNA from cells and tissues was extracted by TRIzol (Invitrogen) according to the manufacturer’s instructions, then reverse-transcribed into cDNA using the PrimeScript™ RT reagent Kit. RT-qPCR was performed on a CFX96 device using SYBR® Premix Ex Taq™ II.
For determination of miRNA expression level, reverse transcription was performed using the miScript II RT kit. miR-106a expression levels in different groups were detected with an miScript SYBR Green PCR kit according to the manufacturer’s instructions.
U6 was used as an endogenous reference gene for miR-106a and GAPDH was used as an endogenous reference gene for DUSP2.
Western blot
Proteins were extracted from cells with RIPA lysis buffer (Beyotime Institute of Biotechnology, Jiangsu, China) and quantified by bicinchoninic acid method (BCA, Beyotime Institute of Biotechnology, Jiangsu, China). Equal amounts of samples (15 μg) were loaded into each lane on a sodium dodecyl sulfonate (SDS) gel (8–10%), and then transferred onto a polyvinylidene fluoride (PVDF) membrane. After blocking in 5% non-fat milk for 1 h at room temperature, the membranes were incubated in primary antibodies overnight at 4°C followed by incubation with secondary antibodies at room temperature for 1 h. Subsequently, the bands were developed by ECL Western blotting substrate and images were captured by ImageQuant LAS 4000.
Cell transfection
miR-NC mimics or miR-106a mimics (40 nM, Genepharma, Shanghai, China) was transfected by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, miR-NC mimics or miR-106a mimics and Lipofectamine 2000 were mixed in Opti-MEM medium and incubated for 20 mins at 37°C, then the cells were added to the mixture and cultured for 24 h. Transfected cells were used for subsequent experiments.
Dual luciferase reporter assay
DUSP2 3′UTR was amplified from cDNA of 293 and then ligated into pGL3. pGL3- DUSP2-3′UTR Mut was obtained using a site-directed mutagenesis kit.
The dual luciferase reporter assay was conducted as follows: 293 cells were first plated in 24-well plates for 24 h, then co-transfected with pGL3-DUSP2-WT or pGL3-DUSP2-3′UTR Mut and miR-NC mimics or miR-106a mimics and pRL, and the mixture was incubated at 37°C for 48 h; the assay was performed with the Dual Luciferase Assay System.
Statistical analysis
Data were analyzed by GraphPad Prism 6. Results are expressed as mean ±SD. Comparisons between different groups were conducted using the t test. Comparisons among more than 3 groups were conducted using one-way ANOVA followed by Newman-Keuls test. p<0.05 was considered as a significant difference.
Results
Overexpression of miR-106a decreases the sensitivity of CRC cells towards 5-FU
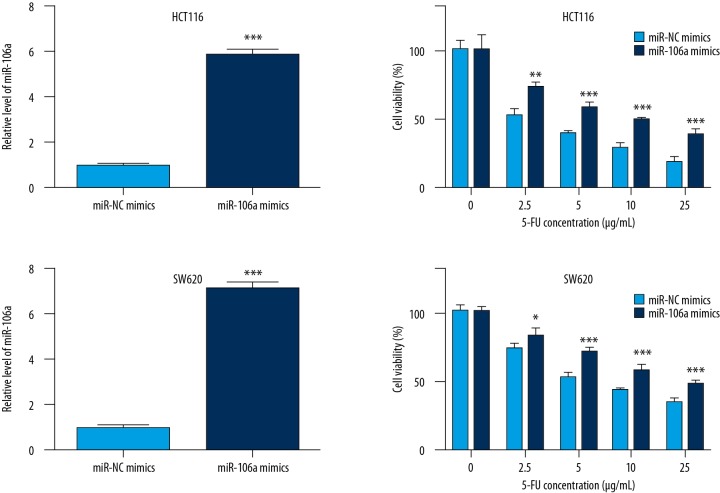
To determine the effect of miR-106a on the sensitivity of CRC cells towards 5-FU, we increased the expression of miR-106a in HCT116 cells by transfection of miR-106a mimics. Transfection of miR-106 mimics significantly elevated miR-106a levels in HCT116 cells (Figure 1A). In addition, compared with cells transfected with miR-NC mimics, transfection of miR-106a mimics reduced sensitivity of cells upon 5-FU treatment (2.5, 5, 10, and 20 μg/ml) (Figure 1B). Similarly, in another CRC cell line, SW620, transfection of miR-106 mimics significantly elevated miR-106a level (Figure 1C) and overexpression of miR-106a also led to an obvious reduction of sensitivity towards 5-FU treatment (2.5, 5, 10, and 20 μg/ml) (Figure 1D).
Figure 1.
miR-106a decreases the sensitivity of CRC cells towards 5-FU. In HCT116 cells, compared with cells transfection with miR-NC mimics, miR-106 mimics significantly elevated miR-106a level (A) and reduced sensitivity of cells upon 5-FU treatment (B). In SW620 cells, miR-106 mimics showed similar results (C, D). *, **, *** p<0.05 and p<0.01, p<0.0001, respectively.
Decrease of miR-106a expression sensitized CRC cells towards 5-FU
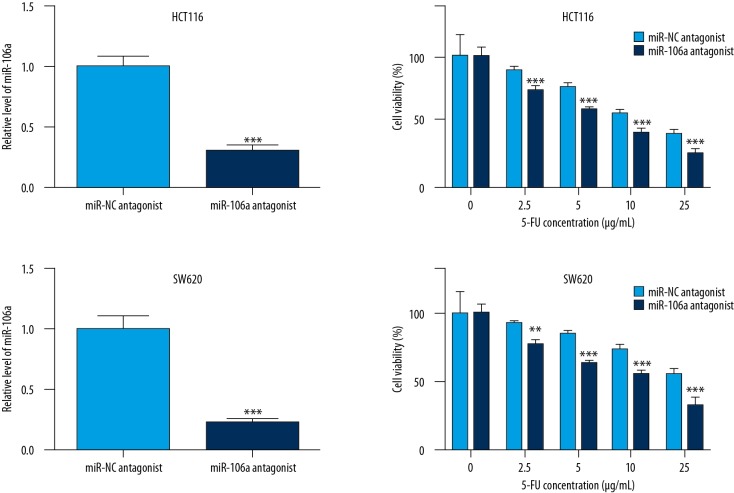
To further confirm the effect of miR-106a expression on 5-FU sensitivity in CRC cells, we assessed the cell viability with increasing concentrations of 5-FU (0.5, 1, 2.5, and 5 μg/ml) after antagonizing of miR-106a. Transfection of miR-106a antagonist significantly decreased miR-106a level in HCT116 cells (Figure 2A). As we expected, miR-106a downregulation enhanced 5-FU-induced cell viability inhibition in HCT116 cells (Figure 2B). Consistent with our observation in HCT116 cells, antagonizing of miR-106a also significantly decreased the miR-106a level (Figure 2C) and sensitized CRC cells towards 5-FU treatment in SW620 (Figure 2D).
Figure 2.
Decreased miR-106a expression sensitized CRC cells towards 5-FU. In HCT116 cells, miR-106a antagonist significantly decreased miR-106a level (A) and enhanced 5-FU-induced cell viability inhibition (B). Consistent with our observation in HCT116 cells, antagonizing of miR-106a also showed similar results in SW620 (C, D). **, *** p<0.01 and p<0.0001, respectively.
The above results indicate that miR-106a expression is associated with 5-FU sensitivity in CRC cells.
miR-106a promoted stemness of CRC cells via regulation of DUSP2
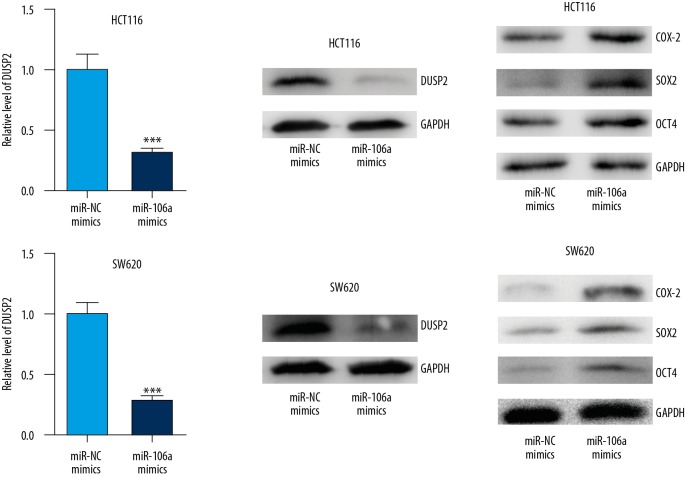
DUSP2 was recently discovered to drive stemness of CRC cells and played a pivotal role in chemotherapy resistance [21]. We observed that overexpression of miR-106a significantly decreased DUSP2 mRNA and protein expression levels in HCT116 cells (Figure 3A, 3B).
Figure 3.
miR-106 promoted stemness of CRC cells via regulation of DUSP2. In HCT116 cells, overexpression of miR-106a significantly decreased DUSP2 mRNA and protein expression levels (A, B) and elevated expression of COX-2, SOX2, and OCT4 (C). Similarly, in SW620 cells, enhanced expression of miR-106a repressed DUSP2 expression and induced COX-2, SOX2, and OCT4 protein levels (D–F). *** p<0.0001.
DUSP2 inversely controlled the expression of COX2 to modulate stemness of cancer cells [21]. Compared with cells transfected with miR-NC mimics, transfection of miR-106a mimics elevated expression of COX-2 and key regulators of stemness, SOX2 and OCT4 (Figure 3C).
Similarly, in SW620 cells, enhanced expression of miR-106a repressed DUSP2 expression and reduced COX-2, SOX2, and OCT4 protein levels (Figure 3D–3F).
These results demonstrate that miR-106a expression is associated with stemness in CRC cells via regulation of DUSP2.
DUSP2 is a direct target of miR-106a
We next assessed whether DUSP2 is directly regulated by miR-106a. Using the TargetScan online tool, we predicted that there was a binding site for miR-106a in 3′UTR of DUSP2 mRNA (Figure 4A). Dual luciferase reporter assay showed that transfection of miR-106a mimics decreased relative luciferase activity of 293 cells transfected with DUSP2 3′UTR-WT but did not change the luciferase activity of 293 cells transfected with DUSP2 3′UTR-Mut (Figure 4B).
Figure 4.
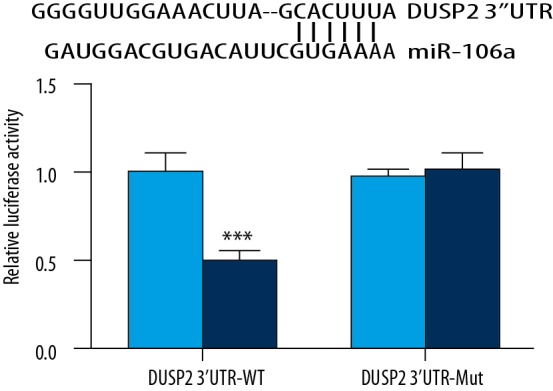
DUSP2 was targeted by miR-106a. Using the TargetScan online tool, we predicted that there was a binding site for miR-106a in 3′UTR of DUSP2 mRNA (A). Dual luciferase reporter assay showed that transfection of miR-106a mimics decreased relative luciferase activity of the 293 cell line cells transfected with DUSP2 3′UTR-WT but not DUSP2 3′UTR-Mut (B). *** p<0.0001.
These data suggest that miR-106a directly binds to 3′UTR of DUSP2 mRNA to negatively regulate its expression.
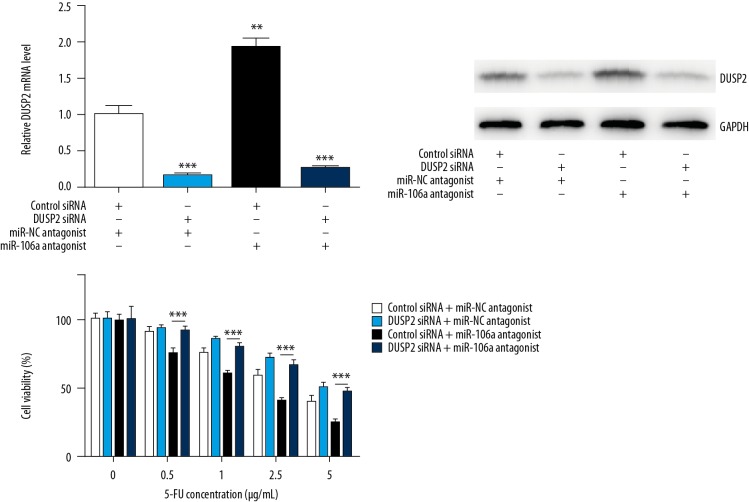
Silencing of DUSP2 abrogated miR-106 antagonist-induced high sensitivity towards 5-FU in CRC cells
To investigate whether regulation of 5-FU sensitivity by miR-106a depended on DUSP2, we assessed the cell viability upon 5-FU in CRC cells with DUSP2 silencing alone or in combination with miR-106 antagonizing. As showed in Figure 5A and B, transfection of DUPS2 siRNA or miR-106 antagonist decreased or increased DUSP2 mRNA and protein level in HCT116, respectively. Transfection of miR-106 antagonist sensitized HCT116 cells towards 5-FU treatment, and DUSP2 silencing reversed this effect (Figure 5C).
Figure 5.
Silencing of DUSP2 abrogated miR-106 antagonist-induced high sensitivity towards 5-FU in CRC cells. DUPS2 siRNA or miR-106 antagonist decreased or increased DUSP2 mRNA and protein level in HCT116, respectively (A, B). miR-106 antagonist sensitized HCT116 cells towards 5-FU and DUSP2 silencing reversed this effect (C). *** p<0.0001.
These results indicate that miR-106a reduced 5-FU sensitivity via regulation of DUSP2 expression in CRC.
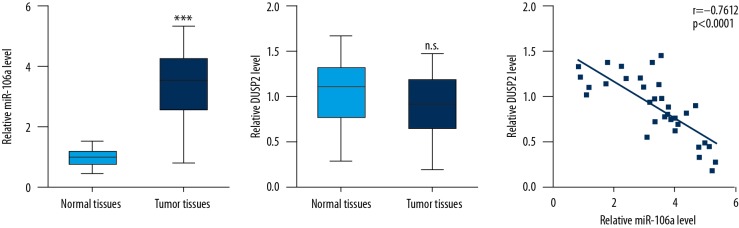
Expression of miR-106a was inversely correlated with DUSP2 mRNA levels in tumor tissues from CRC patients
RT-qPCR was used to detect miR-106a and DUSP2 mRNA levels in tumor tissues and matched normal tissues from CRC patients. Consistent with many previous reports [22,23], remarkable elevation of miR-106a levels was observed in tumor tissues compared with their counterparts (Figure 6A). There was no significant difference in DUSP2 mRNA levels between tumor tissues and matched normal tissues (Figure 6B). Interestingly, analysis of DUSP2 and miR-106a levels suggests that their expression was inversely associated with each other (Figure 6C).
Figure 6.
miR-106a level was inversely correlated with DUSP2 level in CRC tumor tissues. Remarkable elevation of miR-106a levels was observed in tumor tissues compared with their counterparts (A). There was no significant difference in DUSP2 mRNA levels between tumor tissues and normal tissues (B). DUSP2 and miR-106a level were inversely associated with each other (C). *** p<0.0001. n.s. , no significant difference.
Thus, the results from samples of CRC tumors further proved our finding of a regulatory relationship between miR-106a and DUSP2.
Discussion
Resistance to chemotherapy drugs frequently occurs in clinical treatment of CRC patients. Although there were several papers describing the different expression patterns between tumor cells from drug-sensitive patients and drug-resistant patients, the underlying molecular mechanism driving chemoresistance has remained unclear. Here, we demonstrated that miR-106a desensitized CRC cells towards 5-FU treatment. Mechanistically, miR-106a negatively regulates DUSP2 expression and thus enhances cell stemness.
A previous study indicated that miR-106a functions as a tumor suppressor or an oncogene in different cancer types, depending on cellular context [24]. In CRC, miR-106a was highly expressed in metastatic CRC cells, and promoted cell migration via repression of TGFBR2 [25]. In renal carcinoma cells, miR-106a inhibited IRS2 expression to induce cell growth arrest [26]. In particular, dysregulation of miR-106a was associated with the chemoresistance in ovarian cancer, gastric cancer, and hepatocellular carcinoma via regulation of target genes in selected cell types [27–29]. miR-106a was one of the most significantly differentially expressed miRNAs after detection and analysis of 742 miRNAs expression in plasma samples of CRC patients from both responders and non-responders towards 5-FU-based chemotherapy [30]. Overexpression of miR-106a reduced sensitivity of CRC cells towards 5-FU treatment, while antagonizing of miR-106a sensitized CRC cells towards 5-FU exposure. These data confirm that miR-106a negatively regulates 5-FU sensitivity of CRC cells.
DUSP2 inhibited CRC cell stemness and its downregulation was closely linked to chemoresistance [19,21]. Several reports showed that downregulation of DUSP2 in cancer cells was mediated by HIF-1α-induced transcriptional repression [31]. The role of miRNAs in regulation of DUSP2 in cancer was not studied before. We found that overexpression of miR-106a led to a decrease of DUSP2 at both mRNA and protein levels in CRC cells. A negative correlation of miR-106a levels and DUSP2 levels was observed in tumor tissues from CRC patients. Dual luciferase assay showed that DUSP2 was a direct target of miR-106a. In addition, elevation of miR-106a increased the expression of SOX2 and OCT4, which are 2 key regulators of cell stemness. More importantly, silencing of DUSP2 abolished miR-106a antagonist-induced hypersensitivity of CRC cells towards 5-FU treatment. Collectively, these data show that miR-106a directly represses DUSP2 expression and thus reduce 5-FU sensitivity of CRC cells.
Conclusions
In conclusion, we have identified miR-106a as a key regulator of 5-FU sensitivity in CRC cells. miR-106a can negatively regulate DUSP2 expression and enhance cell stemness. Our work indicates that miR-106a might be a promising therapeutic target for chemotherapy of CRC patients.
Interestingly, there were interactions between long non-coding RNAs (lncRNA) and miRNAs [32]. Moreover, the effects of lncRNAs in cancer have been studied recently. For instance, Tan et al. reported that upregulated expression of SPRY4-IT1 predicts poor prognosis in CRC [33]. Therefore, we will perform further studies to identify the lncRNAs that can target miR-106a and regulate the progression of CRC.
Footnotes
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–38. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 3.Marks EI, Tan C, Zhang J, et al. Regorafenib with a fluoropyrimidine for metastatic colorectal cancer after progression on multiple 5-FU-containing combination therapies and regorafenib monotherapy. Cancer Biol Ther. 2015;16:1710–19. doi: 10.1080/15384047.2015.1113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta. 2006;1766:184–96. doi: 10.1016/j.bbcan.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hsu HC, Liu YS, Tseng KC, et al. Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int J Colorectal Dis. 2013;28:1535–46. doi: 10.1007/s00384-013-1721-x. [DOI] [PubMed] [Google Scholar]
- 6.Findlay VJ, Wang C, Nogueira LM, et al. SNAI2 modulates colorectal cancer 5-fluorouracil sensitivity through miR145 repression. Mol Cancer Ther. 2014;13:2713–26. doi: 10.1158/1535-7163.MCT-14-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesse M, Arenz C. MicroRNA maturation and human disease. Methods Mol Biol. 2014;1095:11–25. doi: 10.1007/978-1-62703-703-7_2. [DOI] [PubMed] [Google Scholar]
- 8.Ardekani AM, Naeini MM. The role of microRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161–79. [PMC free article] [PubMed] [Google Scholar]
- 9.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas J, Ohtsuka M, Pichler M, Ling H. MicroRNAs: Clinical relevance in colorectal cancer. Int J Mol Sci. 2015;16:28063–76. doi: 10.3390/ijms161226080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T, Gao F, Zhang XP. miR-203 enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Oncol Rep. 2015;33:607–14. doi: 10.3892/or.2014.3646. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Geng L, Talmon G, Wang J. MicroRNA-520g confers drug resistance by regulating p21 expression in colorectal cancer. J Biol Chem. 2015;290:6215–25. doi: 10.1074/jbc.M114.620252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Y, Wang J, Yung VY, et al. MicroRNA-135b regulates apoptosis and chemoresistance in colorectal cancer by targeting large tumor suppressor kinase 2. Am J Cancer Res. 2015;5:1382–95. [PMC free article] [PubMed] [Google Scholar]
- 14.Kong Y, Peng Y, Liu Y, et al. Twist1 and Snail link Hedgehog signaling to tumor-initiating cell-like properties and acquired chemoresistance independently of ABC transporters. Stem Cells. 2015;33:1063–74. doi: 10.1002/stem.1955. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Fu Q, Du Y, et al. MicroRNA as regulators of cancer stem cells and chemoresistance in colorectal cancer. Curr Cancer Drug Targets. 2016;16:738–54. doi: 10.2174/1568009616666151118114759. [DOI] [PubMed] [Google Scholar]
- 16.Touil Y, Igoudjil W, Corvaisier M, et al. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837–46. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 18.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3:REVIEWS3009. doi: 10.1186/gb-2002-3-7-reviews3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin SC, Chien CW, Lee JC, et al. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J Clin Invest. 2011;121:1905–16. doi: 10.1172/JCI44362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakashev SV, Reginato MJ. Hypoxia/HIF1alpha induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2. Oncotarget. 2015;6:1967–80. doi: 10.18632/oncotarget.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou PC, Li YH, Lin SC, et al. Hypoxia-induced downregulation of DUSP-2 phosphatase drives colon cancer stemness. Cancer Res. 2017;77:4305–16. doi: 10.1158/0008-5472.CAN-16-2990. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Huo Z, Song X, et al. mir-106a regulated cell proliferation and apoptosis of colon cancer through targeting the PTEN/PI3K/AKT signaling pathway. Oncol Lett. 2018;15:3197–201. doi: 10.3892/ol.2017.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng L, Zhang Y, Liu Y, et al. MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J Transl Med. 2015;13:252. doi: 10.1186/s12967-015-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie X, Liu HT, Mei J, et al. miR-106a promotes growth and metastasis of non-small cell lung cancer by targeting PTEN. Int J Clin Exp Pathol. 2015;8:3827–34. [PMC free article] [PubMed] [Google Scholar]
- 25.Feng B, Dong TT, Wang LL, et al. Colorectal cancer migration and invasion initiated by microRNA-106a. PLoS One. 2012;7:e43452. doi: 10.1371/journal.pone.0043452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Y, Zhang H, He X, et al. miR-106a inhibits the proliferation of renal carcinoma cells by targeting IRS-2. Tumour Biol. 2015;36:8389–98. doi: 10.1007/s13277-015-3605-x. [DOI] [PubMed] [Google Scholar]
- 27.Huh JH, Kim TH, Kim K, et al. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. Br J Cancer. 2013;109:452–61. doi: 10.1038/bjc.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang Y, Shen H, Li H, et al. miR-106a confers cisplatin resistance by regulating PTEN/Akt pathway in gastric cancer cells. Acta Biochim Biophys Sin (Shanghai) 2013;45:963–72. doi: 10.1093/abbs/gmt106. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Li Y, Hou Y, et al. The PDGF-D/miR-106a/Twist1 pathway orchestrates epithelial-mesenchymal transition in gemcitabine resistance hepatoma cells. Oncotarget. 2015;6:7000–10. doi: 10.18632/oncotarget.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kjersem JB, Ikdahl T, Lingjaerde OC, et al. Plasma microRNAs predicting clinical outcome in metastatic colorectal cancer patients receiving first-line oxaliplatin-based treatment. Mol Oncol. 2014;8:59–67. doi: 10.1016/j.molonc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu MH, Lin SC, et al. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J Pathol. 2011;225:390–400. doi: 10.1002/path.2963. [DOI] [PubMed] [Google Scholar]
- 32.Jalali S, Bhartiya D, Lalwani MK, et al. Systematic transcriptome wide analysis of lncRNA-miRNA interactions. PLoS One. 2013;8:e53823. doi: 10.1371/journal.pone.0053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan W, Song ZZ, Xu Q, et al. Up-regulated expression of SPRY4-IT1 predicts poor prognosis in colorectal cancer. Med Sci Monit. 2017;23:309–14. doi: 10.12659/MSM.898369. [DOI] [PMC free article] [PubMed] [Google Scholar]