Abstract
Background
Cervical cancer is one of the most common female malignancies in the world. The ubiquitin-specific protease 8 (USP8) functions by removing ubiquitin from protein substrates, and its potential role in cancer development was recently uncovered in lung cancer. The aim of this study was to investigate the expression and function of USP8 in cervical squamous cell carcinoma (CSCC).
Material/Methods
Immunohistochemical staining and quantitative PCR were performed to explore the expression of USP8 in both CSCC tissues and adjacent normal cervical tissues. Univariate and multivariate analyses were conducted to evaluate the clinical significance of USP8 in CSCC. Proliferation, migration, and invasion abilities of 2 CSCC cell lines were assessed after overexpression or silencing USP8, respectively.
Results
Both the RNA and protein levels of USP8 were upregulated in CSCC tissues compared to normal cervical tissues. High expression of USP8 was correlated with advanced tumor stage and high recurrence risk. Moreover, USP8 was identified as a novel independent prognostic factor for CSCC patients. Cellular studies showed that USP8 can enhance the proliferation, migration, and invasion abilities of CSCC cells, thereby promoting tumor progression.
Conclusions
High expression of USP8 is frequent in CSCC tissues, which promotes tumor proliferation and invasion, and is correlated with a poor overall survival. Targeting USP8 may be a novel direction for drug development for CSCC therapy.
MeSH Keywords: Neoplasm Invasiveness, Prognosis, Uterine Cervical Neoplasms
Background
Cervical cancer occurs with abnormal cell growth on the cervix, and most cervical cancers are caused by human papillomavirus (HPV) infection. Cervical cancer is the third most common female malignancy in the world [1]. Although the global incidence of cervical cancer is declining due to introduction of vaccines and improvement of disease screening [2], it remains one of the leading cancer-related causes of death for women [3,4]. Current treatment strategies for cervical cancer include surgery, chemotherapy, and radiotherapy; however, many patients have tumor relapse and metastasis. The clinical outcomes of patients show completely different patterns that are determined by unique molecular events. Therefore, exploring the cellular and molecular mechanisms of cervical cancer development is essential for prognosis prediction and patient treatment.
Most protein modifications are reversible, such as ubiquitination and deubiquitination. Ubiquitination can promote protein degradation and plays critical roles in controlling protein abundance to maintain normal cellular processes. The ubiquitination balance is regulated by both ubiquitin ligases and deubiquitinases (DUBs). Ubiquitin ligase can add ubiquitin moieties onto proteins and induce protein degradation, while DUBs hydrolyze the ubiquitin from ubiquitinated substrates [5]. To date, 102 putative DUB genes had been identified in humans [6], and ubiquitin-specific proteases (USPs) is the largest DUB subfamily [7]. Dysregulation of USPs causes unbalanced protein modification and degradation, leading to disease occurrence, including cancers [8]. For example, USP7 was reported to be upregulated in lymphocytic leukemia [9], colorectal cancer [10], and ovarian cancer [11]. High expression of USP11 was correlated with poor overall survival in breast cancer [12]. However, not all USPs are oncoproteins; for example, the USP33 can repress the tumor development of breast cancer [13], colorectal cancer [14], and lung cancer [15]. It seems that the detailed roles of different USPs largely depend on tissue locations and downstream substrates.
The enzymatic activity of several DUBs, including UCH-L1, UCH-L3, USP7, and USP9X, is upregulated following HPV E6/E7 immortalization of keratinocytes, indicating their participation in growth transformation [16]. CYLD is another DUB involved in cervical cancer development by negatively regulating innate antiviral responses [17]. Although the Oncomine database showed a potential increase on the mRNA level of USP8 in cervical cancer tissues [8], its protein expression and clinical significance are completely unknown.
Here, we report our findings on the RNA and protein expression profiles of USP8 in clinical cervical squamous cell carcinoma (CSCC) tissues. Statistical analysis revealed its significant correlation with tumor progression and disease recurrence. Furthermore, by overexpressing or silencing USP8, cellular studies indicated that USP8 can directly upregulate the proliferation and metastatic abilities of CSCC cells. Our data not only identify USP8 as a novel predictive biomarker for CSCC, but also provide evidence of its potential in tumor therapy development.
Material and Methods
Ethics statement
This study was approved by the Research Ethics Committee of Yidu Central Hospital of Weifang and Heilongjiang Province Hospital (China). Each enrolled patient provided written informed consent. All specimens were handled following relevant ethical and legal standards.
Samples and clinical characteristics
This retrospective study was conducted on a total of 128 paraffin-embedded CSCC samples, 27 fresh-resected CSCC samples, and paired nontumoral cervical samples. All patients were diagnosed and underwent tumor resection in Yidu Central Hospital of Weifang and Heilongjiang Province Hospital between 2007 and 2012. None of the patients received preoperative tumor therapy, and all final diagnoses were based on histopathological examination. The clinicopathological classifications and tumor staging were determined according to the FIGO criteria. The collected information of the patients included age, HPV infection, FIGO stage, tumor size, tumor invasion, pelvic lymph node metastasis, and disease recurrence. Detailed clinicopathological characteristics are summarized in Table 1.
Table 1.
Correlation between USP8 expression and clinicopathologic characteristics of CSCC patients.
| Variables | Cases (N) | USP8 expression in CSCC | P# | |
|---|---|---|---|---|
| Low (N=48) | High (N=80) | |||
| Age (year) | 0.713 | |||
| <47 | 72 | 26 | 46 | |
| ≥47 | 56 | 22 | 34 | |
| HPV infection | 0.116 | |||
| Negative | 18 | 10 | 8 | |
| Positive | 110 | 38 | 72 | |
| FIGO stage | <0.001* | |||
| I–II | 73 | 39 | 34 | |
| III | 55 | 9 | 46 | |
| Horizontal diffusion diameter | 0.161 | |||
| <4.0 cm | 78 | 33 | 45 | |
| ≥4.0 cm | 50 | 15 | 35 | |
| Stromal invasion depth | 0.031* | |||
| <2/3 | 59 | 28 | 31 | |
| ≥2/3 | 69 | 20 | 49 | |
| Vagina invasion | 0.095 | |||
| Negative | 103 | 35 | 68 | |
| Positive | 25 | 13 | 12 | |
| Parametrial invasion | 0.008* | |||
| Negative | 77 | 36 | 41 | |
| Positive | 51 | 12 | 39 | |
| Lymphovascular invasion | 0.012* | |||
| Negative | 81 | 37 | 44 | |
| Positive | 47 | 11 | 36 | |
| Pelvic lymph node metastasis | 0.004* | |||
| Poor | 81 | 38 | 43 | |
| Positive | 47 | 10 | 37 | |
P value was analyzed by Chi-square test or Fisher exact test;
indicates P<0.05 with statistical significance.
Immunochemistry (IHC) staining and evaluation
Immunochemistry (IHC) staining was performed as described by others [18]. Tissue sections were incubated with anti-USP8 antibody (1: 100; Abcam) overnight at 4°C, and then incubated with secondary antibody for 45 min at 37°C. PBS instead of primary antibody was used as a negative control for IHC. After final staining with a DAB substrate kit (Abcam), the staining results were reviewed by 2 independent pathologists. The IHC score was performed based on both the proportion of positively stained cells and the intensity of staining color. Briefly, the proportion of positive tumor cells was scored as 1 (0–25% positive tumor cells), 2 (25–50% positive tumor cells), 3 (50–75% positive tumor cells), and 4 (>75% positive tumor cells). The staining intensity was scored as 1 (no staining), 2 (light yellow), 3 (dark yellow), and 4 (brown). The staining score was calculated by multiplying intensity score and proportion score. Accordingly, the CSCC patients were classified into 2 groups: a high USP8 expression group (staining score ≥8) and a low USP8 expression group (staining score <8).
Real time-qPCR
Total RNA samples from the primary clinical tissues were extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Approximately 2 μg of total RNA was used for cDNA synthesis. The USP8 primer was 5′-CGCAATCATCTCCTTCCATT-3′, and 5′-GATTTGGGAGAAGTAGCCCC-3′. The gene level of GAPDH was used as normalization control with following primers: 5′-AATGAAGGGGTCATTGATGG-3′ and 5′-AAGGTGAAGGTCGGAGTCAA-3′. The PCR condition was conducted as follows: 95°C for 10 min, followed by 44 cycles of 95°C for 15 s, 60°C for 60 min, and 65°C for 10 s. After normalized to the GAPDH expression as quality control, qPCR was carried out to evaluate the relative level of USP8 mRNA in each of the CSCC tissues compared with paired adjacent nontumorous cervical tissues [19]. All experiments were performed in triplicate.
Cell culture
SiHa and SW756 cell lines of the human cervical squamous cell carcinoma were obtained from the American Type Tissue Culture Collection (ATCC; Rockville, MD, USA). Both cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (FBS) and antibiotics [20].
Plasmid construct, siRNA, and transfection
The USP8 plasmid was constructed by inserting human USP8 coding sequence into the pcDNA3.1 construct by Genepharma (Shanghai, China), and the plasmid was sequenced before transfection. The siRNA toward USP8 was also purchased from Genepharma with the following sequence: 5′-AATCTTCAGCAGCTTATATCC-3′ (targeting 3607–3627 region) [21]. Both overexpression and silencing were performed by using Lipo2000 according to the manufacturer’s instructions. The transfection efficiency was tested by Western blot as described before [22].
Cell proliferation, migration, and invasion assays
Transfected SiHa and SW756 cells were plated in 96-well tissue culture plates, and cell growth was assessed by CCK-8 assay as previously described by others [23]. Cell migration was evaluated using a 24-well plate with Transwell inserts containing 8-μm pore size membranes [24]. A total of 200 μL cells at 5×105/mL concentration were seeded into the upper chambers, and serum-free DMEM was added into the chamber after cell adhesion. The lower chambers were supplied with 500 μL DMEM containing 10% FBS, serving as the assessment of cell migration. After incubation for 24 h, cells on the upper surface of the membranes were carefully removed with a cotton wool swab, while those that migrated to the lower side of the membrane surface were fixed and stained. The average number of migrated cells was counted from 10 random fields under a light microscope (×400). For the invasion assay, we precoated the Transwell inserts with Matrigel, and used similar procedures as described for the cell migration assay; the only difference was that cells were cultured for 48 h before taking out the Transwell insert.
Statistical
Statistical analyses were performed with SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). The associations between USP8 expression and the clinicopathologic parameters of the CSCC patients were evaluated by chi-square tests and Fisher exact tests. Kaplan-Meier and log-rank tests were conducted to identify the risk factors for CSCC recurrence. Multivariate analysis was further performed to calculate their independent prognostic roles. Data from the cellular experiments are presented as mean ±SD, and differences between groups were determined by the t test compared with control groups. Differences were considered statistically significant at P<0.05.
Results
Patient information
Clinicopathological characteristics of 128 CSCC patients are summarized in Table 1. All patients were treated with curative surgical resection and histologically diagnosed as having squamous cell carcinomas. Follow-up data were collected until 2016. Most of the patients (110/128, 85.9%) showed positive for HPV infection. There were 73 patients classified as FIGO stage I–II (57.0%), and 55 patients with FIGO stage III (43.0%). The size of CSCC was evaluated based on horizontal diffusion diameters, and 78 cases were less than 4.0 cm (49.4%). The tumor invasion to stromal, vagina, and parametrial were all retrieved (Table 1). Among them, 47 patients (36.7%) showed positive for lymphovascular invasion, and 47 patients (36.7%) were positive for pelvic lymph node metastasis.
USP8 expression in CSCC
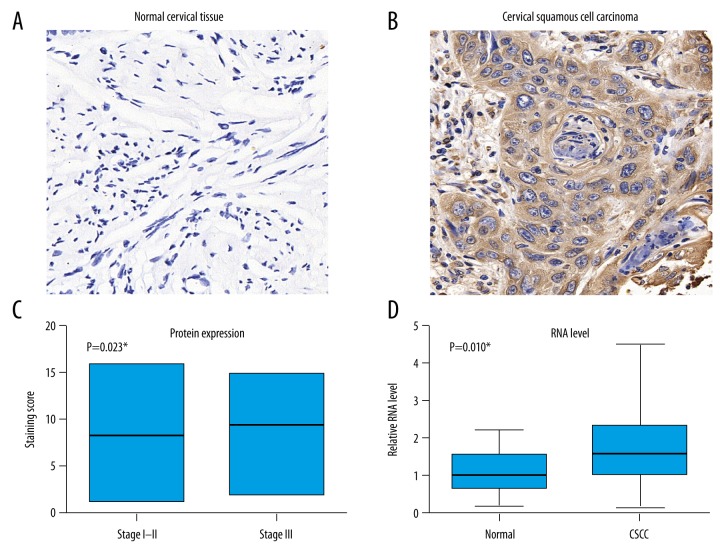
The protein expression of USP8 was assessed by IHC method. There was little protein expressed in normal cervical tissue (Figure 1A), while positive staining was observed in CSCC tumor tissues (Figure 1B). By analyzing the IHC scores among CSCC patients with different stages, we found that USP8 showed an increased expression in advanced tumors (Figure 1C). The RNA levels of USP8 in 27 fresh-frozen tissues were tested by RT-qPCR, which showed higher levels in tumor tissues than that in normal cervical tissues (Figure 1D).
Figure 1.
USP8 is upregulated in PTC tissues. Protein expression levels of USP8 in paraffined cervical tissues (A) and CSCC tissues (B) were measured by IHC, showing the positive immunoreactivity in the cytoplasm of tumor cells. (C) The mean IHC staining score of CSCC tissues were analyzed based on different FIGO stages. Patients with FIGO stage III showed significant higher levels of USP8 protein than those with stage I–II (P=0.023). (D) The mRNA levels of USP8 from 27 freshly-resected CSCC tissues and paired adjacent cervical tissues were analyzed by RT-qPCR normalized with GAPDH-mRNA level, indicating that USP8 was upregulated in tumor tissues (P=0.010).
USP8 is correlated with aggressive tumor behavior of CSCC
As shown in Table 1, all 128 CSCC patients were divided into high- or low-USP8 expression groups according to the median value of IHC score in tumor tissues. Accordingly, USP8 expression was significantly correlated with FIGO stage (P<0.001), stromal invasion depth (P=0.031), parametrial invasion (P=0.008), lymphovascular filtration (P=0.012), and lymph node metastasis (P=0.004). Therefore, it is high likely that USP8 promotes tumor progression of CSCC.
USP8 indicates higher recurrence risk of CSCC
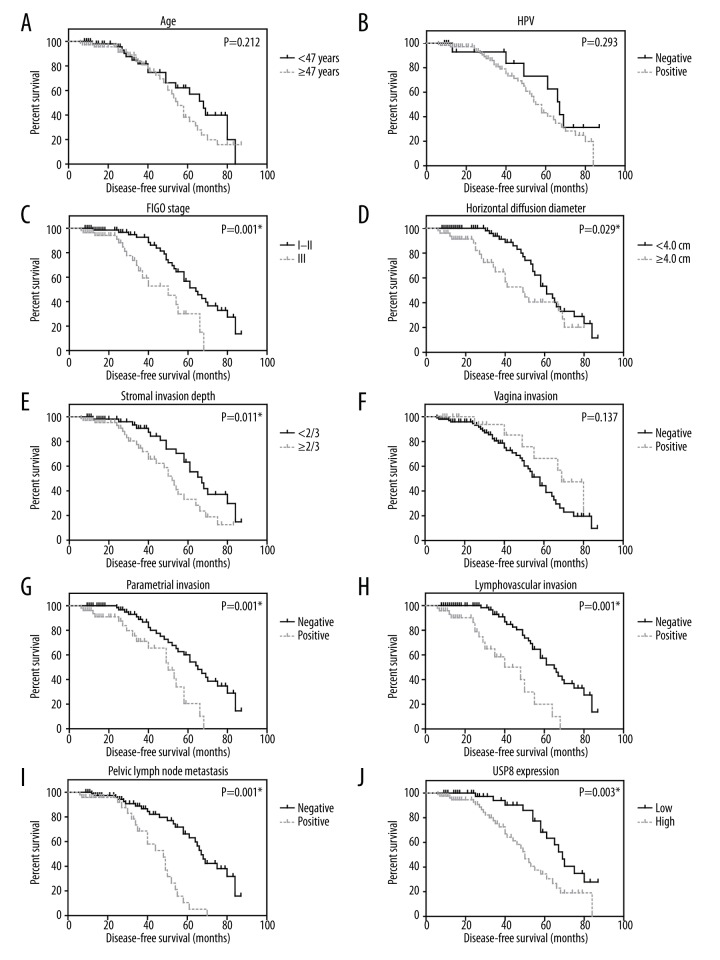
With a median follow-up period of 45 months, the 5-year disease-free survival (DFS) rate of our cohort was analyzed using Kaplan-Meier method (Figure 2). A high expression of USP8 was significantly associated with unfavorable DFS (P=0.003). USP8, advanced FIGO stage (P=0.001), and larger tumor size (P=0.029) were also correlated with high risk of disease recurrence. Patients with deeper stromal invasion (P=0.011) and parametrial involvement (P=0.001) also showed worse clinical outcomes. Other probable prognostic factors included lymphovascular filtration (P<0.001) and pelvic lymph node metastasis (P=0.003, Table 2).
Figure 2.
USP8 expression level is helpful in predicting disease recurrence of CSCC. Kaplan-Meier survival analyses were performed based on all pathological characteristics and USP8 protein levels, respectively. Retrieved factors included: age (A), HPV infection (B), FIGO stage (C), horizontal diffusion diameter (D), stromal invasion depth (E), vaginal invasion (F), parametrial invasion (G), lymphovascular invasion (H), pelvic lymph node metastasis (I), and USP8 protein expression (J).
Table 2.
The disease-free survival (DFS) was assessed by Kaplan-Meier univariate analysis.
| Variables | Cases (N) | 5-year DFS rate (%) | Mean DFS survival (months) | P# |
|---|---|---|---|---|
| Age (year) | 0.212 | |||
| <47 | 72 | 62.2% | 61.6±3.9 | |
| ≥47 | 56 | 38.2% | 55.5±3.2 | |
| HPV infection | 0.293 | |||
| Negative | 18 | 73.1% | 64.5±6.1 | |
| Positive | 110 | 43.2% | 56.9±2.7 | |
| FIGO stage | 0.001* | |||
| I–II | 73 | 56.7% | 63.5±2.8 | |
| III | 55 | 30.1% | 46.1±3.6 | |
| Horizontal diffusion diameter | 0.029* | |||
| <4.0 cm | 78 | 53.3% | 63.0±2.8 | |
| ≥4.0 cm | 50 | 40.5% | 49.6±4.2 | |
| Stromal invasion depth | 0.011* | |||
| <2/3 | 59 | 63.3% | 64.6±3.4 | |
| ≥2/3 | 69 | 33.1% | 51.5±3.3 | |
| Vagina invasion | 0.137 | |||
| Negative | 103 | 44.3% | 56.1±2.8 | |
| Positive | 25 | 66.3% | 65.6±5.3 | |
| Parametrial invasion | 0.001* | |||
| Negative | 77 | 60.1% | 63.3±3.0 | |
| Positive | 51 | 20.5% | 46.7±3.3 | |
| Lymphovascular invasion | <0.001* | |||
| Negative | 81 | 57.4% | 64.1±2.7 | |
| Positive | 47 | 20.0% | 41.9±3.9 | |
| Pelvic lymph node metastasis | <0.001* | |||
| Poor | 81 | 66.2% | 65.0±3.0 | |
| Positive | 47 | 10.5% | 44.0±2.8 | |
| USP8 expression | 0.003* | |||
| Low | 48 | 68.3% | 67.9±3.4 | |
| High | 80 | 34.4% | 51.6±3.3 |
P value was analyzed by log-rank test;
indicates P<0.05 with statistical significance.
We conducted multivariate analysis to investigate the independent prognostic effects. We introduced all factors with P<0.05 into the Cox regression model, except for FIGO stage due to its close interaction with other factors such as lymph node metastasis. In multivariate analysis, high expression of USP8 was identified as an unfavorable prognostic factor (HR=2.518, 95% CI=1.328±4.772, P=0.005). In addition, parametrial invasion (P=0.002), lymphovascular filtration (P=0.009), and pelvic lymph node metastasis (P<0.001, Table 3) were also demonstrated to independently predict worse outcome.
Table 3.
Multivariate analysis of disease-free survival.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Horizontal diffusion diameter | 1.253 | 0.593±2.646 | 0.554 |
| Stromal invasion depth | 1.852 | 0.936±3.662 | 0.077 |
| Parametrial invasion | 2.934 | 1.472±5.847 | 0.002* |
| Lymphovascular invasion | 2.945 | 1.317±6.585 | 0.009* |
| Pelvic lymph node metastasis | 3.526 | 1.781±6.979 | <0.001* |
| USP8 expression | 2.518 | 1.328±4.772 | 0.005* |
Indicates P<0.05 with statistical significance.
USP8 promotes CSCC progression by enhancing tumor invasion capacity
The endogenous expression of USP8 in 2 CSCC cell lines were detectable, including SiHa and SW756 cells. Extrinsic regulation of USP8 expression in SiHa and SW756 cells were achieved by transfecting pcDNA-USP8 plasmids for overexpression or USP8-siRNA for knockdown (Figure 3A, 3B). Function assays, including proliferation, migration, and invasion, were performed to explore the effect of USP8 on CSCC progression. CCK-8 cell viability tests showed that USP8 can promote tumor cell proliferation, although statistical significance was not achieved (Figure 3C, 3D). However, both the migration and invasion capacities of CSCC cells were upregulated by USP8 overexpression (Figure 3E–3H). In contrast, both of the 2 cell lines showed attenuated cell invasion behavior after silencing USP8 (P<0.05).
Figure 3.
USP8 promotes proliferation, migration, and invasion of CSCC cells. The transfection efficiencies of USP8-overexpression and USP8-siRNA were tested in SiHa (A) and SW756 (B) cell lines by Western blot analysis. After verifying the transfection efficiency, transfected cells were enrolled into proliferation assay by CCK-8 method (C, D), migration assay by Transwell (E, F), and invasion assay by Matrigel-Transwell (G, H), respectively. The detailed assay procedures are described in the Methods section. Tumor biological data showed that USP8 overexpression has little effect on cell proliferation, but significantly promotes the migration and invasion of both SiHa and SW756 cells, whereas USP8-siRNA showed the opposite effects.
Discussion
Cervical cancer is one of the most common female cancers globally, and most patients have disease recurrence. Therefore, it would be of great clinical significance to be able to accurately evaluate disease recurrence at an early stage, which would enable early prediction and help direct therapy.
Recent studies have focused on mapping the molecular alterations in malignancies, including the RNA level, protein level, and protein modifications. As the largest deubiquitinase family, more and more USPs were reported to participate in tumor development and progression. The most-reported tumor-related role of USP8 is in lung cancers. For example, it can regulate the expression and half-life of EGFR in early lung cancer [25]. The stabilization effect of USP8 on EGFR was achieved by modulating its intracellular trafficking and recycling [26]. As one of the most important receptor tyrosine kinases, EGFR has been reported to be dysregulated in multiple tumor types. Additionally, high expression of USP8 is responsible for gefitinib resistance during lung cancer treatment [27]. Knockdown of USP8 selectively kills gefitinib-resistant lung cancer cells but shows little toxicity toward normal cells. Consistently, the expression of EGFR was decreased by genetic silencing of USP8. Furthermore, USP8 inhibitor remarkably attenuated the viability of either gefitinib-resistant or gefitinib-sensitive lung cancer cells. Therefore, USP8 exerts its tumor-promoting effects in lung cancer as least partially by stabilizing EGFR signaling pathways. Besides the expression dysregulation, mutations of USP8 gene are common in corticotroph tumors and may be associated with unfavorable biochemical outcomes after surgical treatment [28,29], but the underlying mechanisms remain unclear.
Our study, for the first time, shows that USP8 RNA and protein levels are elevated in CSCC tumor tissues compared to normal cervical tissues, and higher USP8 levels are correlated with advanced FIGO stages. These primary findings suggest that USP8 promotes CSCC progression, and thus encouraged us to further investigate its clinical significance. Subsequent statistical analyses identified USP8 as an unfavorable independent biomarker for CSCC recurrence. Moreover, we conducted cellular studies and found that USP8 can significantly upregulate the migration and invasion processes of CSCC cell lines. Our data are consistent with the recent findings that USP8 directly deubiquitylates and stabilizes the long isoform of FLICE-like inhibitory protein (FLIPL) in cervical cancer cell line ME-180, which was derived from the metastatic site of epidermoid carcinoma. FLIPL is a kind of death receptor modulating cell apoptosis. High expression of USP8 can therefore inhibit extrinsic apoptosis by stabilizing FLIPL [30] . However, although overexpressing USP8 in SiHa and SW756 cells enhanced cell proliferation, it did not reach statistical significance according to our data. In addition to clinical findings, cellular studies showed that USP8 can regulate Smoothened signaling [31] and CXCR4 signaling [32] in other cell models, both of which play critical roles in various tumor types. Therefore, it is high likely that the tumor-related role of USP8 in cervical cancer may have cross-talk with the signaling molecule discussed above.
The malignant phenotype of tumor cells is largely characterized by their invasion to nearby tissues and metastasis to distant lesions. One of the treatment strategies for tumors is inhibiting metastasis activity. Therefore, our findings on the effect of USP8 in CSCC suggest its potential role as a therapeutic target. As with disease-free survival, it is reasonable to surmise that USP8 is also associated with overall survival of CSCC patients, but this needs to be supported by further clinical evidence. Taking into consideration the role of USP8 in sensitizing chemotherapy for lung cancer, the possible use of USP8 inhibitor to treat CSCC deserves further investigation.
Our study has some limitations. First, the clinical data were derived from a single medical center in China, and therefore may have regional and ethnic bias. It will be necessary to confirm the general role of USP8 in multiple medical centers from different regions in further studies. Secondly, we did not explore the detailed molecular signaling pathways of USP8 in promoting cervical cancer progression, although we verified its role based on cellular effects. Thirdly, in vivo evidence will be essential to validate our findings, such as studies using the nude-mouse transplanted tumor model.
Conclusions
In conclusion, we demonstrated that USP8 in cancer tissue is an independent prognostic biomarker of CSCC, and high USP8 in CSCC can enhance cell invasion. Our findings expand the understanding of the clinical significance of USP8 in CSCC, which may help guide targeted treatment and prevent recurrence of OSCC.
Footnotes
Conflict of interest
None declared.
Source of support: Departmental sources
References
- 1.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adv Nutr. 2016;7(2):418–19. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloch Z, Yasmeen N, Li Y, et al. Knowledge and awareness of cervical cancer, human papillomavirus (HPV), and HPV vaccine among HPV-infected Chinese women. Med Sci Monit. 2017;23:4269–77. doi: 10.12659/MSM.903370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarella S, Lortet-Tieulent J, Plummer M, et al. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur J Cancer. 2013;49(15):3262–73. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11(14):1245–56. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- 6.Nijman SM, Luna-Vargas MP, Velds A, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123(5):773–86. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695(1):189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Hussain S, Zhang Y, Galardy P. DUBs and cancer: The role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle. 2009;8(11):1688–97. doi: 10.4161/cc.8.11.8739. [DOI] [PubMed] [Google Scholar]
- 9.Carrà G, Panuzzo C, Torti D, et al. Inhibition OF USP7 induces selective cancer cell death in chronic lymphocytic leukemia. Clinical Lymphoma, Myeloma and Leukemia. 2016;16:S49–50. [Google Scholar]
- 10.An T, Gong Y, Li X, et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131:29–39. doi: 10.1016/j.bcp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Wang H, Tian L, Li H. Expression of USP7 and MARCH7 is correlated with poor prognosis in epithelial ovarian cancer. Tohoku J Exp Med. 2016;239(3):165–75. doi: 10.1620/tjem.239.165. [DOI] [PubMed] [Google Scholar]
- 12.Bayraktar S, Barrera AMG, Liu D, et al. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. 2013;19(1):10. doi: 10.1097/PPO.0b013e3182801b3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuasa-Kawada J, Kinoshita-Kawada M, Rao Y, Wu JY. Deubiquitinating enzyme USP33/VDU1 is required for Slit signaling in inhibiting breast cancer cell migration. Proc Natl Acad Sci. 2009;106(34):14530–35. doi: 10.1073/pnas.0801262106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into β-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen P, Kong R, Liu J, et al. USP33, a new player in lung cancer, mediates Slit-Robo signaling. Protein Cell. 2014;5(9):704–13. doi: 10.1007/s13238-014-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolén U, Kobzeva V, Gasparjan N, et al. Activity profiling of deubiquitinating enzymes in cervical carcinoma biopsies and cell lines. Mol Carcinog. 2006;45(4):260–69. doi: 10.1002/mc.20177. [DOI] [PubMed] [Google Scholar]
- 17.Massoumi R. CYLD: A deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7(2):285–97. doi: 10.2217/fon.10.187. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Liu Z, Li K, et al. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32(9):1570–80. doi: 10.1111/jgh.13749. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Qiu S, Shao N, Zheng J. Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt/NF-kappaB signaling pathway. Med Sci Monit. 2018;24:11–18. doi: 10.12659/MSM.905360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno E, Iura T, Mukai A, et al. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16(11):5163–74. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Yuan L, Zhang Q, et al. Resveratrol attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Int Immunopharmacol. 2015;28(1):578–87. doi: 10.1016/j.intimp.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Hou S, Du P, Wang P, et al. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol. 2017;19(9):1107–16. doi: 10.1007/s12094-017-1646-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y, Shiba-Ishii A. Ubiquitin-specific protease 8 is a novel prognostic marker in early-stage lung adenocarcinoma. Pathol Int. 2017;67(6):292–301. doi: 10.1111/pin.12546. [DOI] [PubMed] [Google Scholar]
- 26.Kasahara K, Aoki H, Kiyono T, et al. EGF receptor kinase suppresses ciliogenesis through activation of USP8 deubiquitinase. Nat Commun. 2018;9(1):758. doi: 10.1038/s41467-018-03117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byun S, Lee SY, Lee J, et al. USP8 is a novel target for overcoming gefitinib resistance in lung cancer. Clin Cancer Res. 2013;19(14):3894–904. doi: 10.1158/1078-0432.CCR-12-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Rivas LG, Theodoropoulou M, Puar TH, et al. Somatic USP8 mutations are frequent events in corticotroph tumor progression causing Nelson’s tumor. Eur J Endocrinol. 2018;178(1):59–65. doi: 10.1530/EJE-17-0634. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Rivas LG, Osswald A, Knosel T, et al. Expression and mutational status of USP8 in tumors causing ectopic ACTH secretion syndrome. Endocr Relat Cancer. 2017;24(9):L73–77. doi: 10.1530/ERC-17-0054. [DOI] [PubMed] [Google Scholar]
- 30.Jeong M, Lee EW, Seong D, et al. USP8 suppresses death receptor-mediated apoptosis by enhancing FLIPL Stability. 2017;36(4):458–70. doi: 10.1038/onc.2016.215. [DOI] [PubMed] [Google Scholar]
- 31.Xia R, Jia H, Fan J, et al. USP8 promotes smoothened signaling by preventing its ubiquitination and changing its subcellular localization. PLoS Biol. 2012;10(1):e1001238. doi: 10.1371/journal.pbio.1001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berlin I, Higginbotham KM, Dise RS, et al. The deubiquitinating enzyme USP8 promotes trafficking and degradation of the chemokine receptor 4 at the sorting endosome. J Biol Chem. 2010;285(48):37895–908. doi: 10.1074/jbc.M110.129411. [DOI] [PMC free article] [PubMed] [Google Scholar]