Abstract
Background
Osteoporosis is a major health risk for women worldwide. Osteoporosis is caused by an imbalance between bone resorption and formation. Hormonal imbalance and increased redox signaling cause bone deterioration.
Material/Methods
Oxidative stress was determined through assessment of ROS, lipid peroxide levels, and antioxidant activity. Inflammatory protein markers and Nrf2-related protein expressions were determined through Western blot analysis. Interleukin expressions were determined using ELSA.
Results
In the present study, we showed that supplementation of lutein protects the ovariectomized (OVX) rats against oxidative stress through its antioxidant protection. OVX rats showed an increase in oxidative stress markers. Lutein treatment significantly decreased the lipid peroxidation levels and ROS in the OVX rats. OVX rats showed inflammatory responses through NF-κB activation and increased inflammatory cytokines (TNF-α, IL-6, IL-8). Further, there was significant upregulation in osteoclast-specific marker NFATc1 in OVX rats compared to sham rats. Lutein supplementation activated Nrf2 driven antioxidant gene expression (HO-1, NQO1) and protected OVX rats against inflammatory responses.
Conclusions
We showed the critical role of Lutein in protection against osteoporosis in OVX rats by downregulation of inflammation and osteoclast-specific marker (NFATc1) expression through Nrf2 activation.
MeSH Keywords: Lutein, NF-E2 Transcription Factor, Osteoporosis, RANK Ligand, Reactive Oxygen Species
Background
Postmenopausal osteoporosis is a major health risk to the ageing women. Reduced estrogen leads to deregulation in bone metabolism causing micro-architectural deterioration through bone resorption mechanisms [1]. Osteoclast activation plays a major role in pathogenesis of osteoporosis. Hematopoietic cells of the monocyte/macrophage lineage differentiate to osteoclasts in the presence of RANKL (receptor activator of NF-κB ligand) and M-CSF (macrophage colony-stimulating factor) [2]. Various factors, including free radicals and cytokines, regulate osteoclast differentiation through osteoclast marker expressions [3]. Thus, preventing mechanisms might lessen the effects of bone resorption and osteoporosis. Hormone replacement therapy and bisphosphonate drugs have gained much attention in preventing osteoporosis; however, owing to the adverse effects, some of the drugs have been discontinued [4]. Antioxidants have gained more attention in preventing initiation of bone disease through antioxidant defense mechanisms. In this study, we explored whether lutein has a protective role against osteoporosis.
Lutein occurs in fruits and vegetables, with highest content in spinach and egg yolks. Lutein protects against age-related macular degeneration and its dietary supplementation delays disease progression. Supplementation with lutein along with zeaxanthin might protect against diabetic retinopathy [5]. Lutein regulates various inflammatory diseases through suppressing oxidative stress. Lutein administration reduced lipopolysaccharide (LPS)-induced uveitis in a mouse model through downregulating inflammatory responses [6]. A neuroprotective role of lutein has been reported in endotoxin-induced uveitis by regulating NF-κB and inflammatory cytokines [7].
In the present study, we evaluated whether lutein protects against osteoporosis by regulating inflammation and antioxidant defense mechanisms.
Material and Methods
Animals, treatments, and specimen collection
Twenty Sprague-Dawley Wistar female rats aged 3 months were used for the present study. All the procedures complied with ethics guidelines. The Institutional Animal Care and Use Committee of Yidu Central Hospital of Weifang, Shandong, China, approved the study. The rats were housed with a 12-h light/dark cycle and the temperature was maintained at 22–24°C. All rats were given standard rat chow and water ad libitum. After acclimatization, rats were divided into 4 groups: Sham, Lutein, OVX, and OVX+Lutein groups. The rats were anaesthetized with 3% sodium pentobarbital and bilateral OVX was performed in OVX and OVX+Lutein groups. Bilateral laparotomy was performed in sham rats and lutein-treated rats. After 3 weeks of wound care, Lutein and OVX+Lutein rats were administered lutein (50 mg/Kg) for 4 weeks. Sham and OVX rats were administered saline. After the treatment schedule, all the rats were deprived of food for 24 h and then sacrificed. Blood samples were centrifuged at 1800 rpm for 20 min and the serum was collected. Femurs were dissected and stored at –80°C for studies.
Lipid peroxidation
The serum and tissue lipid peroxide content were analyzed using the Lipid Peroxidation Assay Kit (Sigma Aldrich, St. Louis, MO) following the manufacturer’s instructions. The lipid peroxide levels are expressed as TBARS (nM/mg of protein).
Reactive oxygen species (ROS)
Tissue homogenates were analyzed for ROS content using the DCFDA – Cellular Reactive Oxygen Species Detection Assay Kit (ab113851). The results are expressed as ROS (%) compared to sham group. The ROS levels in the control were set to 100% and relative levels are expressed.
Antioxidant enzyme activities
Antioxidant enzyme activities in the tissue homogenates were measured using Sigma Aldrich GSH (CS0260), SOD (19160), GST (CS0410), and GPx (CGP1) assay kits. The assays were performed as per the technical instructions given by the manufacturer (Sigma Aldrich, St. Louis, MO). The results are expressed as units/mg of protein.
Interleukin expressions
The serum interleukin expressions of IL-6, IL-8, and TNF-α were determined as per manufacturer’s instructions. The interleukin levels are expressed as pg/ml.
Western blot analysis
The tissue homogenates of equal protein concentration (60 ug) were separated in 12% SDS-PAGE gels. The proteins were transferred to PVDF membranes and blocked in 5% skimmed milk for 1 hr. The membrane was TBST washed 5 times. The membrane was incubated overnight at 4°C with respective primary antibodies: anti-NF-kb-p65 (ab16502), anti-Nrf-2 antibody (sc-722), anti-HO-1 antibody (sc-10789), anti-NQO1 antibody (ab34173), NFATc1 antibody (7A6), and anti-IL6 antibody (ab6672). The membrane was washed 5 times using TBST and incubated in secondary antibody, peroxidase-conjugated goat anti-mouse, or anti-rabbit IgG for 2 h. Followed by TBST washing, the membrane was visualized with the ECL system. The images were scanned for densitometric analysis using Image J software (SuperSignal; Pierce, IL).
Statistical analysis
All the experiments were performed in triplicate. The data were analyzed using one-way analysis of variance (ANOVA), Tukey’s multiple comparison tests, and GraphPad Prism. * p<0.001, when compared to sham group. +p<0.001, when compared to OVX group.
Results
Lutein reduced serum LPO and increases GSH levels
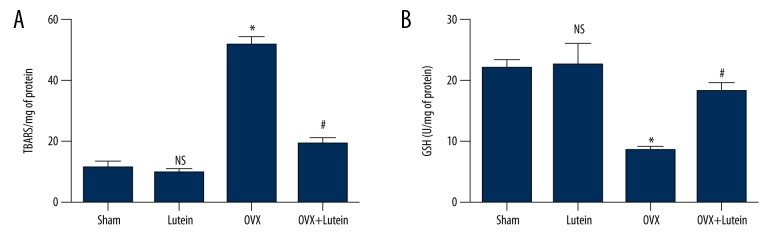
OVX rats are prone to oxidative stress and inflammation. In the present study, we found that OVX rats showed significantly higher levels of serum lipid peroxides and decreased GSH content compared to sham rats. Lutein reduced serum LPO and increased serum glutathione in ovariectomized rats (Figure 1A, B).
Figure 1.
Lutein reduces serum oxidative stress markers in OVX rats. (A) Lipid peroxide levels (TBARS/mg of protein) in serum. (B) Glutathione (U/mg of protein). * p<0.001, # p<0.001. One-way ANOVA followed by multiple comparison tests (GraphPad Prism).
Lutein reduced oxidative stress in OVX rats
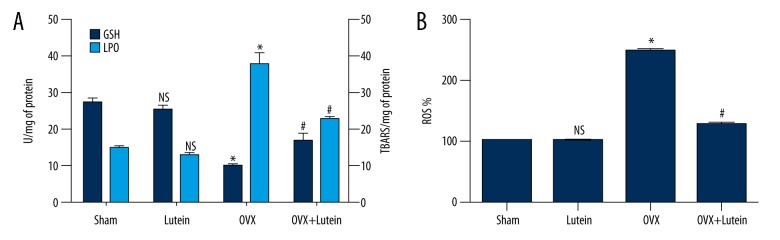
We found that femur tissue of OVX rats showed significantly increased levels of LPO and ROS levels with concomitant decline in GSH content compared to sham rats. However, lutein treatment in OVX rats increased the GSH content and decreased oxidative stress markers compared to OVX alone rats (Figure 2A, 2B).
Figure 2.
Lutein reduces oxidative stress in femur tissue of OVX rats. (A) Lipid peroxide levels (TBARS/mg of protein) and glutathione (U/mg of protein) in femur tissue. (B) Reactive oxygen species levels. * p<0.001, # p<0.001. One-way ANOVA followed by multiple comparison tests (GraphPad Prism).
Lutein improved antioxidant status and suppressed cytokine expression in OVX rats
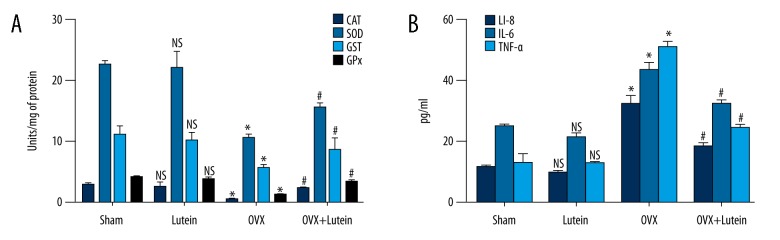
OVX rats showed lower levels of antioxidant enzyme activity compared to sham rats. After lutein treatment in OVX rats, there was significantly increased antioxidant status compared to OVX rats. Further, significantly increased interleukin levels of IL-6, IL-8, and TNF-a in OVX rats were downregulated in rats after being treated with lutein (Figure 3A, 3B).
Figure 3.
Lutein improves antioxidant status and suppresses interleukin expressions. (A) Antioxidant enzyme activities (U/mg of protein). (B) Interleukin expression pg/ml of serum (IL-6, IL-8, TNF-α). * p<0.001, # p<0.001. One-way ANOVA followed by multiple comparison tests (GraphPad Prism).
Lutein suppressed osteoclast markers and upregulated Nrf-2-driven protein expressions
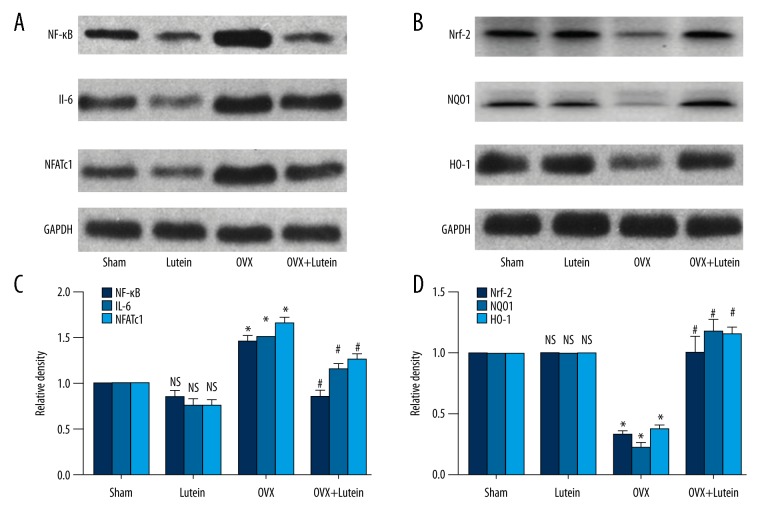
We found that OVX rats showed increased expressions of osteoclast-specific protein expressions, including NF-KB, NFATc1, and IL-6, compared to sham rats. These OVX rats also showed decreased Nrf-2 protein expression and lower expression of downstream genes such as HO-1 and NQO1. Treatment with lutein in OVX rats showed a significant decline in inflammatory proteins and NFATc1 expressions. Further, there was a significant increase in Nrf-2 and target genes in OVX rats treated with lutein (Figure 4A–4D).
Figure 4.
Lutein induces Nrf-2 expression and suppresses osteoclast-specific markers. (A) Western blot of NF-κB, IL-6, and NFATc1 expressions. (B) Densitometry analysis of NF-κB, IL-6, and NFATc1 expressions. (C) Western blot of Nrf-2-dependent protein expressions. (D) Densitometry graph of Nrf-2-dependent protein expression (HO-1, NQO1). * p<0.001, # p<0.001. One-way ANOVA followed by multiple comparison tests (GraphPad Prism).
Discussion
Women above age 50 years are at higher risk for developing osteoporosis because of low estrogen levels [8]. In the present study, we show that lutein administration in an OVX rat model significantly reduced oxidative stress and osteoclast-specific markers through increasing Nrf-2 expression.
Osteoporosis in postmenopausal women causes serious bone disorders, with increased risks for fractures [9]. Estrogens regulate osteoclast differentiation through receptor activator of NF-κB and osteoprotegerin (OPG). These hormones act downstream of RANKL signaling and mediate osteoclast apoptosis. In the absence of estrogen, increased RANKL expression and pro-inflammatory cytokine release promote osteoporosis [10]. In the present study, we found increased oxidative stress with a rise in lipid peroxides and ROS levels in OVX rats. Along with these changes, we also noticed significant downregulation of antioxidant activities. Oxidative stress promotes bone loss. Consistent reports show antioxidant decline and increased ROS activity during osteoporosis [11,12]. Previously, was reported to improve endogenous antioxidant status and reduce inflammatory responses in photo-stressed mice [13].
Estrogen deficiency negatively regulates inflammatory responses through T cell activation, cytokine release, and upregulated RANKL expressions. Appropriate levels of these factors are essential for osteoclast activity, as deregulated levels after ovariectomy failed to induce osteoclast differentiation in rodents [14]. We showed increased cytokine expressions after ovariectomy as compared to sham rats. We also observed several osteoclast-related protein expressions were increased, including NF-κB, IL-6, and NFATc1. NFATc1, a prime osteoclast-regulating protein, was upregulated in an ovariectomy rat model [15]. Transcription factor NFATc1 mediates osteoclast differentiation through osteoclast-specific gene expressions. RANKL-RANK signaling promotes activation of downstream signaling involving NF-κB and MAPK, suggesting that coordinated functions mediate osteoclast differentiation [16]. Lutein administration to the ovariectomy rats significantly prevented osteoclast-related protein expressions and subsequently enhanced the antioxidant pool. Lutein treatment after ovariectomy induced Nrf-2 activation and increased the expressions of downstream genes, including NQO1 and HO-1. Thus, Nrf2 activation might be involved in preventing inflammation and osteoclast marker expressions through suppression of oxidative stress. Lutein suppressed LPS-induced inflammatory cytokine expressions (IL-6, IL-1β, and TNF-α) in ex-vivo-treated peripheral blood mononuclear cells isolated from coronary artery disease patients [17]. Neuroprotective effects of lutein against LPS-induced inflammation in microglia are regulated through Nrf-2 activation and suppression of the NF-κB pathway [18].
Conclusions
These results show that lutein can prevent osteoporosis in a rat model by suppressing oxidative stress and osteoclast-specific protein expressions.
Footnotes
Source of support: Departmental sources
References
- 1.Eastell R, O’Neill TW, Hofbauer LC, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:16069. doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- 2.Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 3.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimalawansa SJ. Prevention and treatment of osteoporosis: Efficacy of combination of hormone replacement therapy with other antiresorptive agents. J Clin Densitom. 2000;3:187–201. doi: 10.1385/jcd:3:2:187. [DOI] [PubMed] [Google Scholar]
- 5.Neelam K, Goenadi CJ, Lun K, et al. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol. 2017;101:551–58. doi: 10.1136/bjophthalmol-2016-309814. [DOI] [PubMed] [Google Scholar]
- 6.He RR, Tsoi B, Lan F, et al. Antioxidant properties of lutein contribute to the protection against lipopolysaccharide-induced uveitis in mice. Chin Med. 2011;6:38. doi: 10.1186/1749-8546-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanai K, Hatta T, Nagata S, et al. Luteolin attenuates endotoxin-induced uveitis in Lewis rats. J Vet Med Sci. 2016;78:1229–35. doi: 10.1292/jvms.16-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demontiero O, Vidal C, Duque G. Aging and bone loss: New insights for the clinician. Ther Adv Musculoskelet Dis. 2012;4:61–76. doi: 10.1177/1759720X11430858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–25. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anandarajah AP. Role of RANKL in bone diseases. Trend Endocrinol Metabol. 2009;20:88–94. doi: 10.1016/j.tem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Ha H, Kwak HB, Lee SW, Jin, et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res. 2004;301:119–27. doi: 10.1016/j.yexcr.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Rodríguez MA, Ruiz-Ramos M, Correa-Muñoz E, Mendoza-Núñez VM. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet Disord. 2007;19:124. doi: 10.1186/1471-2474-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamoshita M, Toda E, Osada H, et al. Lutein acts via multiple antioxidant pathways in the photo-stressed retina. Sci Rep. 2016;22:30226. doi: 10.1038/srep30226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–94. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao HB, Lin H, Wu JB, Lin WC. Kinsenoside prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis by regulating classical NF-κB pathways. Osteoporos Int. 2013;24:1663–76. doi: 10.1007/s00198-012-2199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Chung RWS, Leanderson P, Lundberg AK, Jonasson L. Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis. 2017;262:87–93. doi: 10.1016/j.atherosclerosis.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Wu W, Li Y, Wu Y, et al. Lutein suppresses inflammatory responses through Nrf2 activation and NF-κB inactivation in lipopolysaccharide-stimulated BV-2 microglia. Mol Nutr Food Res. 2015;59:1663–73. doi: 10.1002/mnfr.201500109. [DOI] [PubMed] [Google Scholar]