Abstract
Background
Long noncoding RNAs (lncRNAs) recently have been implicated in the pathological processes of cardiovascular diseases. In this study, LncRNADisease database and PubMed database were used to screen myocardial infraction (MI)-related lncRNAs and to investigate the diagnostic role of lncRNAs in ST-segment elevation myocardial infraction (STEMI).
Material/Methods
Forty-six patients with STEMI and 40 healthy controls were included in the study. Venous blood samples acquired at different time points and the expression levels of lncRNAs in plasma were measured by qRT-PCR. In addition, other blood samples were collected before and after percutaneous coronary intervention (PCI). Correlation analysis and receiver operating characteristic (ROC) curve were used to assess the diagnosis value of the markers. All included patients were followed up for 12±1 months.
Results
Nine MI-related lncRNAs were selected from the database. The qRT-PCR results showed that the expression of hypoxia inducible factor 1A antisense RNA 2 (aHIF), member 1 opposite strand/antisense transcript 1 (KCNQ1OT1), and mitochondrial long noncoding RNA uc022bqs.1 (LIPCAR) were significantly increased in patients with STEMI compared to the control patients. The ROC curve showed that LIPCAR (AUC=0.782, 95% CI: 0.707–0.0.894) had better diagnostic accuracy. Moreover, correlation analysis indicated that LIPCAR were positively correlated with myocardial enzymes and negatively correlated with left ventricular ejection fraction. The level of LIPCAR in STEMI patients after PCI was lower (P<0.05). Multivariate regression analysis indicated that higher levels of LIPCAR were independent predictors of major adverse cardiovascular events in patients with STEMI (HR=5.93; 95% CI, 1.46–9.77; P=0.001).
Conclusions
Highly expressed LIPCAR in plasma may serve as a warning sign for the diagnosis of STEMI.
MeSH Keywords: Biological Markers; Diagnosis; Myocardial Infarction; RNA, Long Noncoding
Background
Acute myocardial infraction (AMI) is one of the most common types of coronary heart disease (CHD) clinically, characterized with multiple complications, high morbidity and high mortality [1]. The diagnosis of ST-segment elevation myocardial infraction (STEMI) mainly depends on chest pain symptoms, electrocardiogram (ECG) and myocardial enzyme. However, the survey showed that STEMI accounted for only 5% of patients with persistent chest pain [2]. ECG is susceptible to interference, such as early repolarization patterns, acute pericarditis and Brugada syndrome [3,4]. Although cardiac troponin (cTn) is the main force in the diagnosis of STEMI, it still faces the sensitivity and specificity deficit in the first few hours of STEMI due to a delayed increase of circulating levels [5,6]. In view of this, it is of great importance to explore highly sensitive and specific biomarkers for the early diagnosis of STEMI.
Long noncoding RNAs (lncRNAs) are defined as non-protein coding transcripts longer than 200 nucleotides and are closely related to various biological processes including cell growth, differentiation, cell proliferation, and apoptosis [7,8]. Recently, many studies have shown that lncRNAs can regulate the pathological process of a variety of cardiovascular diseases, including AMI [9], heart failure (HF) [10], and congenital heart disease [11]. Interestingly, plasma and serum were used as samples for gene chip screening, and some lncRNAs were described as candidate biomarkers [9,12,13]. However, studies about circulating noncoding RNA as a clinical biomarker of AMI is still in its infancy, and its stability and reliability in diagnosing AMI still requires further exploration
In this study, we selected 9 candidate MI-related lncRNAs from the LncRNADisease database and PubMed database which integrated published lncRNAs, and tested their expression levels in plasma by RT-PCR. Then we evaluated the diagnostic value of hypoxia inducible factor 1A antisense RNA 2 (aHIF), member 1 opposite strand/antisense transcript 1 (KCNQ1OT1), and mitochondrial long noncoding RNA uc022bqs.1 (LIPCAR) for STEMI patients and finally determined LIPCAR as the potential biomarker. The expression pattern of LIPCAR in the process of AMI and predictive value were also evaluated.
Material and Methods
Participants
From July 2015 to October 2016, 50 patients diagnosed with STEMI due to typical chest pain were admitted to the Heart Center of Beijing Chaoyang Hospital. In the same period, another 40 non-AMI patients in the same hospital were selected as the control group. Before registration, data on patients’ age, height, weight, smoking history, and cardiovascular risk factors were collected. The diagnosis of STEMI was based on the criteria recommended by the European Society of Cardiology guidelines in 2012 [14], combining several parameters: ischemic symptoms, significantly elevated myocardial enzymes (cTnI and CK-MB), elevated ST-segment of ECG, pathological Q wave and narrowing ≥50% in the left main coronary artery and ≥70% in one or several of the major coronary arteries. All patients who were diagnosed with AMI were given echocardiography and immediate percutaneous coronary intervention (PCI) as soon as possible. Exclusion criteria included heart failure (HF) primarily due to severe valve disease and dilated cardiomyopathy, combined acute and chronic infection, serious liver and kidney dysfunction, malignancies and immune system diseases, and cardiac complications affecting prognosis. The study was approved by the ethics committee of Beijing Chao-Yang Hospital. All participants signed an informed consent form.
Samples collection and detection of myocardial enzymes
The blood samples were taken at the following time points (4 hours, 6 hours, 12 hours, 24 hours, 3 days and 7 days) after chest pain symptoms occurred. The rest of the participants had fasting venous blood collected on the next morning of admission. Specimens were placed in heparin anticoagulant tubes and centrifuged at 3000 rpm for 10 min to separate the plasma. The supernatant was dispensed and stored at –80°C. The concentration of cTnI was detected by enzyme linked immunosorbent assay (No. ABIN1979499, Elabscience Biotechnology Co, Wuhan, China) with a measuring range of 100–8000 pg/mL. The concentration of CK-MB was detected by ELISA (No. ABIN5067597, Elabscience Biotechnology Co, Wuhan, China) with a measuring range of 0.3–80 U/L. The absorbance was measured at 450 nm with a SpectraMax M5e multi-mode microplate reader (Molecular Devices, USA).
Quantitative real-time PCR
The total RNA was isolated from plasma using TRIzol reagent (Invitrogen, CA, USA). After quantification, cDNA was synthesized from 1 μg RNA using a TransScript-Uni cDNA Synthesis SuperMix kit (AU311-03, Transgen, Cat. Beijing, China). Reaction was performed in a GeneAmp PCR System 9700 HT Fast (Applied Biosystems, USA) for 60 min at 37°C. Real-time PCR was performed using a LightCycler 480 II Real-time PCR instrument (Roche, Switzerland) with TransStart Top Green qPCR SuperMix (AQ131-03, Transgen, Cat. Beijing, China). The reaction conditions are as follows: 95°C for 15 min, followed by 40 cycles of 95°C for 5 sec and 60°C for 32 sec. At the end of the amplification, the melt curve was performed to verify the product quality. Samples were analyzed in triplicate and included no-template controls. In this study, U6 was selected as an internal reference. Primer sequences for lncRNAs and U6 were synthesized by Generay Biotech (Shanghai, China). The specific primer sequences are shown in Supplementary Table 1. The relative gene expression is analyzed by the method of 2−ΔΔCt.
Follow-up and major adverse cardiac events
All patients were followed for 12±1 months. During the follow-up period, professionals were responsible for continuous observation the occurrence of HF, angina pectoris, recurrent infarction, readmission, and death. Follow-up mainly included outpatient visits, telephone calls, and follow-up visits. The recurrence of MI and cardiac death was defined as major adverse cardiovascular events (MACE).
Statistical analysis
The data was analyzed using SPSS version 19.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, USA: IBM Corp) software and GraphPad Prism 6.0 (GraphPad Software, San Diego, CA, USA; www.graphpad.com), the normal distribution measurement data was calculated as the mean ±SD and skewed distribution data was indicated by medians and interquartile range (IQR). The Student’s t-test was used to compare 2 groups of continuous variables, and the chi-square test was performed to compare categorical data. The receiver operating characteristic (ROC) curves were performed to evaluate the diagnostic efficacy of lncRNAs. Multivariable Cox regression analysis was carried out to evaluate the predictive value of lncRNA to MACE. P<0.05 was determined to be statistically significant.
Results
Study populations
Baseline clinical characteristics of the study population are show in Table 1. Forty-six STEMI patients and 40 controls were researched the correlation of circulating lncRNAs with STEMI. Patients with STEMI showed more obesity (P=0.031), higher smoking rates (P=0.04), lower ejection fraction (P=0.024), and significantly elevated myocardial enzymes (both cTnI and CK-MB, P<0.01). However, there were no differences in age, low-density lipoprotein cholesterol (LDL-C), hypertension, and diabetes history between the STEMI group and the control group.
Table 1.
Baseline clinical characteristics of the patients.
| Variable | STEMI (n=46) | Control (n=40) | P value |
|---|---|---|---|
| Age, years | 66.35±10.08 | 64.85±13.07 | 0.41 |
| Male, % | 31 (67.4%) | 24 (60%) | 0.67 |
| BMI, kg/m2 | 28.35±8.17 | 24.79±7.33 | 0.031 |
| Smoking, % | 27 (58.7%) | 18 (45%) | 0.04 |
| Heart rate, beats/min | 79.8 (74.2, 84.4) | 76.5 (71, 84) | 0.72 |
| SBP, mmHg | 120 (110, 130) | 134 (128, 140) | 0.56 |
| DBP, mmHg | 74.2±6.34 | 72.5±11.04 | 0.38 |
| Hypertension, % | 33 (72%) | 24 (60%) | 0.59 |
| Diabetes mellitus, % | 15 (32.6%) | 12 (28.6) | 0.49 |
| LVEF, % | 55.37±10.18 | 60.04±9.64 | 0.024 |
| LDL-C, mmol/L | 2.7±0.25 | 2.83±0.65 | 0.79 |
| Gensini score | 67.21±7.19 | 20.31±4.06 | 0.022 |
| CK-MB, U/L | 77.49±5.28 | 2.96±0.41 | <0.01 |
| cTnI, ng/ml | 0.57±0.19 | 0.02±0.003 | <0.01 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; LVEF – left ventricular ejection fraction; LDL-c – low-density lipoprotein cholesterol; CK-MB – creatine kinase-MB; cTnI – cardiac troponin I. Data are presented as the Mean ±SD, median (interquartile range) or %. P<0.05 means a statistically significant difference between groups.
Identify the STEMI-related lncRNA in plasma
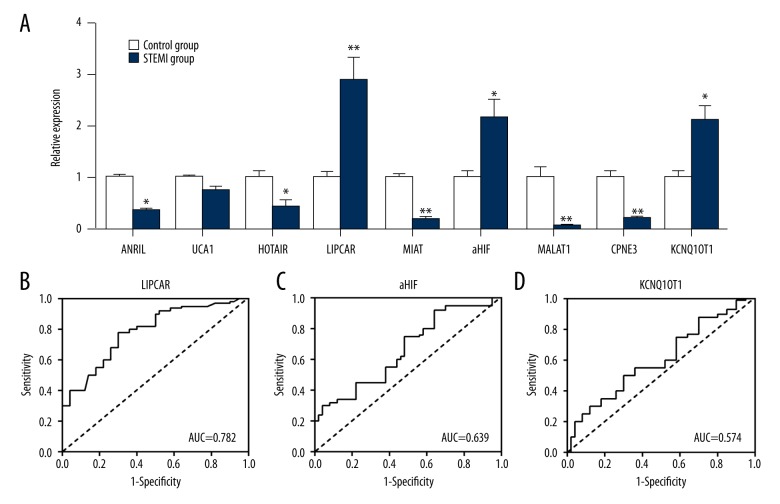
We screened a total of 9 MI-related lncRNAs from the LncRNADisease database [15] (http://cmbi.bjmu.edu.cn/lncrnadisease) and PubMed database (https://www.ncbi.nlm.nih.gov/pmc/) detected their expression levels in patient’s plasma, respectively. The results of qRT-PCR showed that the 3 lncRNAs with elevated expression were aHIF, KCNQ1OT1, and LIPCAR; and decreased expressions were HOX antisense intergenic RNA (HOTAIR), urothelial carcinoma-associated 1 (UCA1), myocardial infarction-associated transcript (MIAT), metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), aclin-dependent kinase inhibitor 2B antisense RNA 1 (ANRIL), and Gene COPINE III (CPNE3). In order to determine which lncRNA had a high diagnostic value, ROC curve was performed; LIPCAR provided the greatest predictive ability, with an AUC of 0.782 (95% CI: 0.707–0.0.894, sensitivity 82% and specificity 75%) (Figure 1).
Figure 1.
STEMI-related lncRNA in plasma. (A) The 9 MI-related candidate lncRNA were evaluated by qRT-PCR. (B) Receiver operating characteristic (ROC) curves for LIPCAR. (C) ROC curves for aHIF. (D) ROC curves for KCNQ1OT1. * P<0.05, ** P<0.01 vs. the control. STEMI – ST-segment elevation myocardial infraction; lncRNA – long noncoding RNAs; LIPCAR – mitochondrial long noncoding RNA uc022bqs.1; aHIF – hypoxia inducible factor 1A antisense RNA 2.
Expression pattern of plasma LIPCAR levels in the patients with STEMI
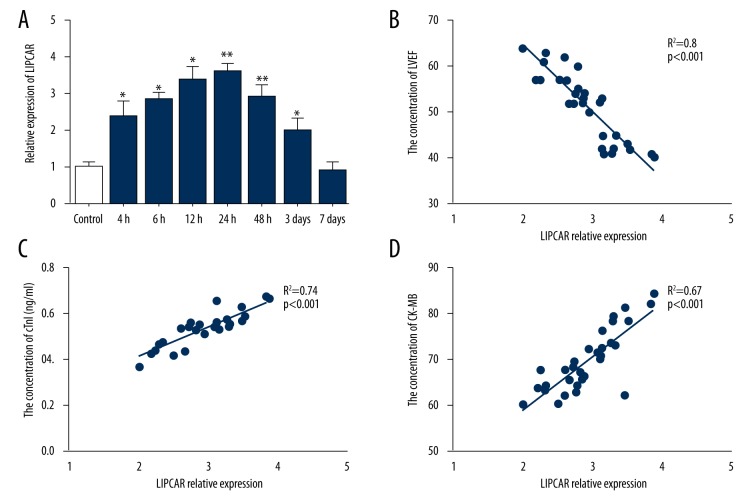
After confirming that LIPCAR was clearly associated with STEMI, we further explored the dynamic changes of LIPCAR in the circulation after MI. As show in Figure 2A, the expression of LIPCAR in STEMI was significantly increased within 4 hours of onset of symptoms compared with controls, reached a peak at 12–24 hours and gradually returned to base line levels at 7 days. Moreover, correlation analysis indicated that LIPCAR was positively correlated with cTnI (r=0.74, P<0.001) and CK-MB (r=0.67, P<0.001) and negatively related to LVEF (r=0.8, P<0.001) in patients with STEMI (Figure 2B–2D).
Figure 2.
Expression pattern of plasma LIPCAR levels in the patients with STEMI. (A) Plasma LIPCAR expression was detected at different time points by qRT-PCR. (B) Correlation between LIPCAR and LVEF. (C, D) Correlation between LIPCAR and myocardial enzymes. * P<0.05, ** P<0.01 vs. the control. LIPCAR – mitochondrial long noncoding RNA uc022bqs.1; STEMI – ST-segment elevation myocardial infraction; LVEF – left ventricular ejection fraction.
Relationship between LIPCAR and the severity of coronary occlusion
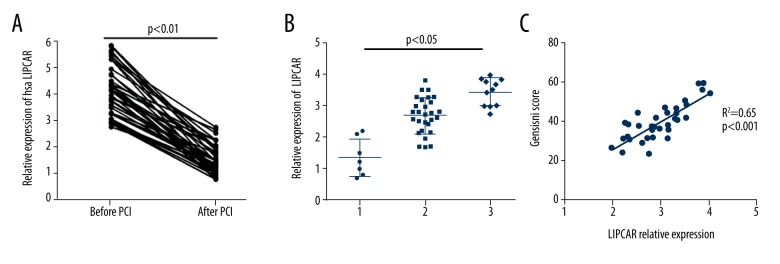
Of the 46 patients with STEMI, 7 patients had 1-branch lesions, 28 patients had 2-branch lesions, and 11 patients had 3-branch lesions. As shown in Figure 3A, LIPCAR level was significantly decreased after the occluded vessels recanalization. Subgroup analysis found that plasma LIPCAR level was obviously higher in STEMI patients with 2- and 3-branches lesions (P<0.05). Moreover, correlation analysis showed a positive relation between LIPCAR and Gensini scores (r=0.65, P<0.001), suggesting that the extent of lncRNA elevation reflects the severity of coronary stenosis (Figure 3B, 3C).
Figure 3.
Relationship between LIPCAR levels and coronary lesions. (A) The expression levels of LIPCAR before and after PCI. (B) LIPCAR expression levels in patients with different coronary lesions. (C) Correlation between LIPCAR and Gensini score. LIPCAR – mitochondrial long noncoding RNA uc022bqs.1; PCI – percutaneous coronary intervention.
Association between LIPCAR levels and MACE
In Cox regression analyses, both Gensini score and circulating LIPCAR were associated with increased risk of 1-year MACE in STEMI patients (all P values <0.05). According to the median, LIPCAR was divided into high and low levels. High LIPCAR expression level significantly increased the risk of MACE compared to low LIPCAR expression level (HR=5.93, 95% CI: 1.46–9.77, P=0.001) (Table 2).
Table 2.
Multivariate Cox regression analysis of MACE.
| Variable | HR (95% CI) | p Value |
|---|---|---|
| Gender, Male vs. Female | 1.21 (0.74–2.98) | 0.831 |
| BMI | 1.19 (0.87–4.75) | 0.062 |
| Smoking, % | 0.84 (0.44–2.13) | 0.449 |
| Hypertension, % | 1.01 (0.74–1.05) | 0.542 |
| Diabetes mellitus, % | 0.56 (1.04–3.31) | 0.150 |
| LVEF, % | 1.94 (0.81–2.57) | 0.459 |
| LDL-C, mmol/L | 1.35 (0.97–1.42) | 0.731 |
| Gensini score | 3.88 (0.52–7.66) | 0.002 |
| LIPCAR, according to the median | – | 0.026 |
| Low LIPCAR expression level | 2.14 (0.59–4.16) | 0.15 |
| High LIPCAR expression level | 5.93 (1.46–9.77) | 0.001 |
BMI – body mass index; LVEF – left ventricular ejection fraction; LDL-c – low-density lipoprotein cholesterol; LIPCAR – mitochondrial long noncoding RNA uc022bqs.1. P<0.05 means a statistically significant difference between groups.
Discussion
AMI is associated with progressive cardiomyocytes loss and leads to the continuous release of myocardial enzymes. Currently used markers (including troponin, CK-MB) are found to be limited by the heterogeneity of some other diseases, genetic backgrounds, and lifestyles. Accumulating evidence has shown that aberrantly regulated lncRNA is correlated with the progression of various diseases, like cardiovascular disease [16–18]. Although little is known about the origin and function of lncRNAs in circulation, their sensitive and stable differential expression in the blood of patients with cardiovascular diseases and healthy people makes them a potential biomarker [19]. The mechanism of action is likely that cardiac tissue damage leads to an additional release of lncRNAs, similar to the release of proteins.
The LncRNADisease database is a website containing about 480 lncRNA entries of experimentally supported lncRNA-disease associations, including 160 diseases, 478 entries of lncRNA interacting partners at various molecular levels. In total, 166 diseases were included, and cardiovascular diseases accounted for 10.8% [15]. Some of these lncRNAs have been identified as having significant differences in the blood of patients with AMI compared to healthy individuals [6,9,12,20].
In this study, we focused on the expression of lncRNAs in peripheral blood of STEMI patients. Nine MI-related lncRNAs were screened from the database and tested for expression by RT-PCR, of which 3 lncRNAs (aHIF, KCNQ1OT1, and LIPCAR) were significantly elevated. By modulating the stability of HIF1α messenger RNA, upregulation of aHIF has the capacity to regulate angiogenesis and anti-apoptosis [21]. KCNQ1OT1 is highly expressed in the peripheral blood of patients with AMI and is associated with ischemia-reperfusion injury [22]. However, in our study, only LIPCAR showed the optimal sensitivity and specificity for the diagnosis of AMI in ROC curve analysis. Therefore, we choose LIPCAR for further study.
Accumulated evidence supports many noncoding RNAs origin is mitochondrial. The contribution of mitochondrial lncRNA to total lncRNA pools in human left ventricle is approximately 70%, indicating that a large proportion of circulating mitochondrial lncRNAs might come from the heart [23,24]. Although it is still controversial whether LIPCAR is derived from the nucleus or mitochondria, it does not affect it as a biomarker of ventricular remodeling after MI [10,25].
Our results showed that lncRNA rapidly increased in the short term after the onset of symptoms, and its expression pattern in plasma was close to that of CK-MB. Plasma LIPCAR expression in patients with STEMI was obviously decreased after PCI, indicating that the dynamic changes of plasma LIPCAR reflects the situation of myocardial ischemia and coronary lesions. Higher expression of LIPCAR gene may increase the risk of AMI by promoting formation of coronary artery lesions. Multivariate Cox regression analysis revealed that Genisi score and LIPCAR levels were independent predictors of MACE after AMI. This result was consistent with Kumarswamy et al. findings [10]. Although we found that LIPCAR was differentially expressed between STEMI and control groups, additional studies are needed to determine the consistency of LIPCAR expression in tissues and plasma. Larger sample sizes are needed to further confirm the potential applications of LIPCAR as a novel biomarker to diagnosis AMI.
Conclusions
This study found that LIPCAR may serve as a potential biomarker of AMI, especially for STEMI, and could predict the severity and progression of CHD. However, the clinical application value and expression mechanism of LIPCAR still need further study.
Supplementary Table
Supplementary Table 1.
Primers used for qRT-PCR.
| Name | Sequence | |
|---|---|---|
| aHIF | Forward | GGTCTGCCATCTATTACTT |
| Reverse | TCTCAGCATTATAGTCACAA | |
| KCNQ1OT1 | Forward | ATAGTAGTTGGAGACTTCA |
| Reverse | ACTGTATATTCAATGTTGGT | |
| HOTAIR | Forward | GGATGGCACTCCTAGTCTTG |
| Reverse | GTGCAGGCCTGAGTCTTAG | |
| UCA1 | Forward | ACGCTAACTGGCACCTTGTT |
| Reverse | TGGGGATTACTGGGGTAGGG | |
| LIPCAR | Forward | TAAAGGATGCGTAGGGATGG |
| Reverse | TTCATGATCACGCCCTCATA | |
| MIAT | Forward | TTTACTTTAACAGACCAGAA |
| Reverse | CTCCTTTGTTGAATCCAT | |
| MALAT1 | Forward | TTATCCTTGGAAGAGTATT |
| Reverse | TAAGAAGTCACATTATTGG | |
| ANRIL | Forward | TTGATGAGAAGAATAAGCC |
| Reverse | CTCCTTTGATGTGTGTTT | |
| CPNE3 | Forward | CCGGGGGTATACTACGGTC |
| Reverse | CTCTAGAGGGGGTAGAGG | |
| U6 | Forward | GCTTCGGCAGCACATATACTAAAAT |
| Reverse | CGCTTCACGAATTTGCGTGTCAT | |
KCNQ1OT1– member 1 opposite strand/antisense transcript 1; aHIF – hypoxia inducible factor 1A antisense RNA 2; HOTAIR – HOX antisense intergenic RNA; UCA1 – urothelial carcinoma-associated 1; LIPCAR –mitochondrial long noncoding RNA uc022bqs.1; MALAT1 – metastasis-associated lung adenocarcinoma transcript 1; MIAT – myocardial infarction-associated transcript; ANRIL – cyclin-dependent kinase inhibitor 2B antisense RNA 1; CPE3 – gene COPINE III.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by Basic Medical Research Center of Capital Medical University
References
- 1.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed) 2017;70:1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: How to use existing assays clinically and for clinical trials. Am Heart J. 2002;144:981–86. doi: 10.1067/mhj.2002.124048. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–35. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 4.Ako J, Honda Y, Fitzgerald PJ. Conditions associated with ST-segment elevation. N Engl J Med. 2004;350:1152–55. doi: 10.1056/NEJM200403113501118. [DOI] [PubMed] [Google Scholar]
- 5.Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10:623–34. doi: 10.1038/nrcardio.2013.129. [DOI] [PubMed] [Google Scholar]
- 6.Rosjo H, Varpula M, Hagve TA, et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: Distribution, associated factors, and relation to outcome. Intensive Care Med. 2011;37:77–85. doi: 10.1007/s00134-010-2051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Wu Z, Fu X, Han W. lncRNAs: Insights into their function and mechanics in underlying disorders. Mutat Res Rev Mutat Res. 2014;762:1–21. doi: 10.1016/j.mrrev.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Kazemzadeh M, Safaralizadeh R, Orang AV. LncRNAs: Emerging players in gene regulation and disease pathogenesis. J Genet. 2015;94:771–84. doi: 10.1007/s12041-015-0561-6. [DOI] [PubMed] [Google Scholar]
- 9.Yan Y, Zhang B, Liu N, et al. Circulating long noncoding RNA UCA1 as a novel biomarker of acute myocardial infarction. Biomed Res Int. 2016;2016:8072–79. doi: 10.1155/2016/8079372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumarswamy R, Bauters C, Volkmann I, et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res. 2014;114:1569–75. doi: 10.1161/CIRCRESAHA.114.303915. [DOI] [PubMed] [Google Scholar]
- 11.Gu M, Zheng A, Tu W, et al. Circulating LncRNAs as novel, non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Cell Physiol Biochem. 2016;38:1459–71. doi: 10.1159/000443088. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Liu Y, Guo S, et al. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol Biochem. 2017;44:1497–508. doi: 10.1159/000485588. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, Su X, Liu C, et al. Prognostic value of plasma long noncoding RNA ANRIL for in-stent restenosis. Med Sci Monit. 2017;23:4733–39. doi: 10.12659/MSM.904352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Wang Z, Wang D, et al. LncRNADisease: A database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41:D983–86. doi: 10.1093/nar/gks1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao H, Yang JJ, Hu W, et al. Noncoding RNA as regulators of cardiac fibrosis: Current insight and the road ahead. Pflugers Arch. 2016;468:1103–11. doi: 10.1007/s00424-016-1792-y. [DOI] [PubMed] [Google Scholar]
- 17.Leeper NJ, Maegdefessel L. Non-coding RNAs: Key regulators of smooth muscle cell fate in vascular disease. Cardiovasc Res. 2018;114(4):611–21. doi: 10.1093/cvr/cvx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung A, Natarajan R. Noncoding RNAs in vascular disease. Curr Opin Cardiol. 2014;29:199–206. doi: 10.1097/HCO.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busch A, Eken SM, Maegdefessel L. Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann Transl Med. 2016;4:236–46. doi: 10.21037/atm.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Cong Y, Gao X, Wang Y, Lin P. Differential expression profiles of long non-coding RNAs as potential biomarkers for the early diagnosis of acute myocardial infarction. Oncotarget. 2017;8:88613–21. doi: 10.18632/oncotarget.20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Feng G, Wang Y, et al. Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: Implications for TAA pathogenesis. Int J Clin Exp Pathol. 2014;7:7643–52. [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Dai Y, Yan S, et al. Down-regulation of lncRNA KCNQ1OT1 protects against myocardial ischemia/reperfusion injury following acute myocardial infarction. Biochem Biophys Res Commun. 2017;491:1026–33. doi: 10.1016/j.bbrc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Rackham O, Shearwood AM, Mercer TR, et al. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA. 2011;17:2085–93. doi: 10.1261/rna.029405.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ro S, Ma HY, Park C, et al. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res. 2013;23:759–74. doi: 10.1038/cr.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn GW., 2nd LIPCAR: A mitochondrial lnc in the noncoding RNA chain? Circ Res. 2014;114:1548–50. doi: 10.1161/CIRCRESAHA.114.304028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Primers used for qRT-PCR.
| Name | Sequence | |
|---|---|---|
| aHIF | Forward | GGTCTGCCATCTATTACTT |
| Reverse | TCTCAGCATTATAGTCACAA | |
| KCNQ1OT1 | Forward | ATAGTAGTTGGAGACTTCA |
| Reverse | ACTGTATATTCAATGTTGGT | |
| HOTAIR | Forward | GGATGGCACTCCTAGTCTTG |
| Reverse | GTGCAGGCCTGAGTCTTAG | |
| UCA1 | Forward | ACGCTAACTGGCACCTTGTT |
| Reverse | TGGGGATTACTGGGGTAGGG | |
| LIPCAR | Forward | TAAAGGATGCGTAGGGATGG |
| Reverse | TTCATGATCACGCCCTCATA | |
| MIAT | Forward | TTTACTTTAACAGACCAGAA |
| Reverse | CTCCTTTGTTGAATCCAT | |
| MALAT1 | Forward | TTATCCTTGGAAGAGTATT |
| Reverse | TAAGAAGTCACATTATTGG | |
| ANRIL | Forward | TTGATGAGAAGAATAAGCC |
| Reverse | CTCCTTTGATGTGTGTTT | |
| CPNE3 | Forward | CCGGGGGTATACTACGGTC |
| Reverse | CTCTAGAGGGGGTAGAGG | |
| U6 | Forward | GCTTCGGCAGCACATATACTAAAAT |
| Reverse | CGCTTCACGAATTTGCGTGTCAT | |
KCNQ1OT1– member 1 opposite strand/antisense transcript 1; aHIF – hypoxia inducible factor 1A antisense RNA 2; HOTAIR – HOX antisense intergenic RNA; UCA1 – urothelial carcinoma-associated 1; LIPCAR –mitochondrial long noncoding RNA uc022bqs.1; MALAT1 – metastasis-associated lung adenocarcinoma transcript 1; MIAT – myocardial infarction-associated transcript; ANRIL – cyclin-dependent kinase inhibitor 2B antisense RNA 1; CPE3 – gene COPINE III.