Highlights
-
•
Relative good overall local control rate of all four different treatment groups of >75%.
-
•
Multivariate analysis shows deep seated tumors, age (<30 years) and extracompartmentally situated tumors as negative predicting markers of local outcome.
-
•
The radiotherapy alone group did not have better local control rates than the observation group (p = 0.355).
-
•
In case of recurrent disease, adjuvant radiotherapy has a definite advantage over surgery alone (p = 0.001).
-
•
Radiotherapy alone gives in 50% of cases partial or complete regression, and in 14% complete regression. Eventually 23% develop local progression.
-
•
Observation could be considered as first line treatment in patients with tumors not in close adherence to important structures and no symptoms.
-
•
Stabilization of the tumor arose after a median time of >1 year after observation, and a local recurrence or progression occurred after a median time of <3 years.
Keywords: Aggressive fibromatosis, Different treatment strategies, Systematic review
Abstract
Background
The treatment approach for aggressive fibromatosis is changing. Although surgery is the mainstay in common practice, recent literature is reporting a more conservative approach. We compared the local control rate for surgery, surgery with radiotherapy, radiotherapy alone and a wait and see policy in a systematic review.
Methods
A comprehensive search of the databases PubMed/Medline, Embase and Cochrane, of the medical literature published in 1999 till March 2017 was performed by two reviewers, including articles about extra abdominal aggressive fibromatosis without the genetical variants. A total of 671 studies were assessed for eligibility, and 37 studies were included for analysis, representing 2780 patients.
Results
The local control rates for surgery alone, surgery and radiotherapy, radiotherapy alone and observation were 75%, 78%, 85% and 78%, respectively. For patients with recurrent disease observation had a better local control rate than surgery alone (p = 0.001). In the observation group, stabilization of the tumor was seen in median 14 (range 12–35) months. The time to local recurrence in the treatment group was median 17 (range, 11–52) months.
Conclusion
A watchful conservative first line approach with just observation and closely monitoring, by means of physical examination and MRI, appears to be justified in a subgroup of patients without clinical symptoms and no possible health hazards if the tumor would progress.
Introduction
Although aggressive fibromatosis (AF) is histological classified as a low grade soft tissue sarcoma it can clinically lead to severe morbidity, functional impairment and even death when located at anatomical critical sites. The treatment approach has changed over time: surgery remained the mainstay in the treatment of AF, but other treatment modalities were explored. Due to the infiltrative pattern and the lack of a pseudocapsule, clear margins are difficult to obtain, necessitating repeated operations and causing severe cosmetic and functional morbidity. Moreover, surgery itself can evoke recurrent disease as trauma is a known predictive factor in the development of AF [1]. In the nineties, adjuvant radiotherapy was successfully applied to improve local control [2]. Radiotherapy alone was performed in selected cases, usually in patients with unresectable tumors, leading in some cases to local control or even regression [3], [4]. Systemic treatment has been reported as an effective treatment in some studies, albeit the number of patients in these studies was low and optimal drug doses and treatment duration remain unclear [5]. More recently, a wait-and-see policy has been advocated because AF has the potential to regress spontaneously [6].
Since AF has a low mortality and usually occurs in young patients, treatment morbidity in the short and long term is an important factor in the treatment decision. Due to the low incidence of AF, studies usually concern small number of patients, therefore we aimed to analyze outcome for different treatment strategies in a systematic review.
Material and method
A comprehensive computer-aided search of the databases PubMed/Medline, Embase and Cochrane, of medical literature published after 1998, was conducted in March 2017 using the search term in Pubmed/Medline was: ‘(desmoid[All Fields] OR aggressive fibromatosis[All Fields]) AND surgery[All Fields] AND English[Language] NOT case report[All Fields] Not polyposis[Title Word] NOT pediatric[All Fields]’ (in which surgery was replaced by ‘radiotherapy’ and ‘wait and see’). The search term in Embase was: “desmoid tumor”/exp AND surgery AND [english]/lim AND [1–1–1999]/sd NOT [01–3–2017]/sd NOT ‘case report’/exp NOT polyposis’ (in which surgery was replaced by ‘radiotherapy’ and ‘wait and see’). The search term in Cochrane: ‘Desmoid’. We augmented our computerised literature search by manually reviewing the reference lists of identified studies and relevant reviews. Two reviewers (JMS/MGN) independently selected studies for possible inclusion in the review by checking titles. Criteria for inclusion were: clinical studies evaluating one of the four treatment strategies in desmoid tumors/aggressive fibromatosis: 1) surgery alone, 2) surgery with adjuvant radiotherapy, 3) radiotherapy alone and 4) wait-and-see policy. Criteria for exclusion were: studies about case reports, reviews and editorials. Furthermore, we excluded all articles that studied solely children, Gardner syndrome or familial polyposis coli as subjects, because paediatric patients have a high recurrence rate and often have a different treatment strategy, and because AF in Gardner syndrome can be considered a different category due to the genetic linkage. The articles related to one anatomic region were also excluded because certain anatomic regions have their own specific biological tumor behaviour [7].
The final decision regarding inclusion was based on the full article. Two reviewers (JMS/MGN) independently assessed the eligibility of the studies. If there was any disagreement between the readers, a consensus was reached by discussion.
In the surgical group, recurrent disease is described as recurrent disease after complete resection. In the radiotherapy and observation group, recurrent disease would be described after complete regression, and progressive disease after partial regression or stabilization of disease.
Statistical methods
The Fisher exact test was used to assess the significance of differences between local control rates of the different treatment modalities. Local control was defined as no recurrence or no progression of disease. The 2-sided p value was used and was considered significant if p < 0.05. This data is available in the Supplementary Table 1. The Fisher exact test is considered appropriate for independent observations; all articles describing the same study populations were excluded.
Since the treatment modalities solely radiotherapy and observation do not include surgical margins, no comparison was made within this subgroup (Supplementary Table 1). In addition, no statistical analysis of comparison was made in case the number of patients was very small.
Results
Literature search and data description
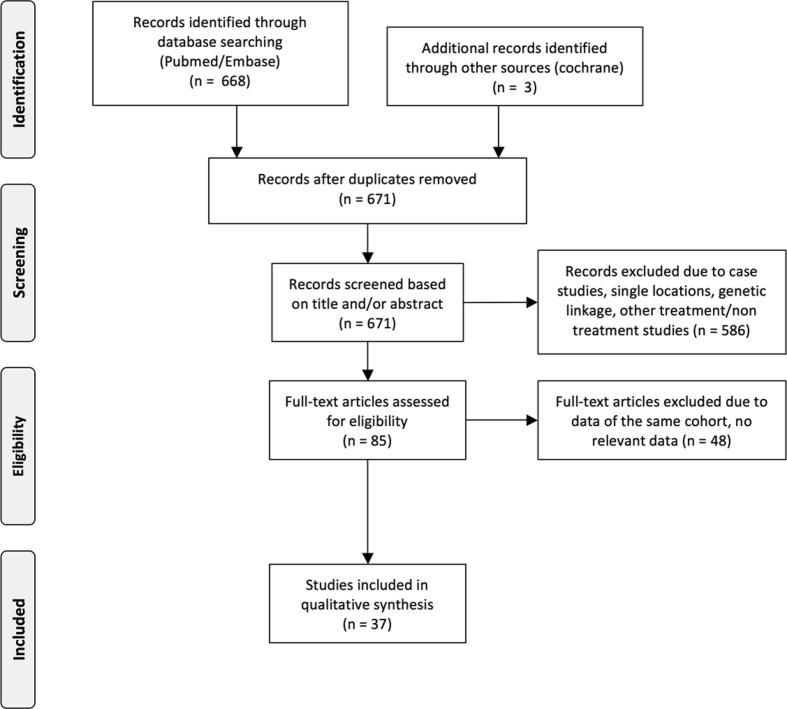
Using the search strategy, 671 studies were listed, of which 85 met the inclusion criteria based on the abstract. Finally, after reading the full text, 37 studies were included in the analysis (Fig. 1) [1], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41].
Fig. 1.
Flow diagram literature search.
The total amount of patients studied for surgery was 1670, for surgery and adjuvant radiotherapy 815, for radiotherapy alone 155 and for observation 140 (Table 1). The median radiation dose for the surgery and adjuvant radiotherapy group was 54 (3–74) Gy, and for the radiotherapy alone group 50 (30–72) Gy. The median follow up was 63 (16–150) months.
Table 1.
Overview of the number of patients, radiation dose, and follow-up for each article included in the systematic review.
| Author | Primary/recurrence (%/%) | Tumor location (%) | Surgery | Surgery + RT | RT | Observation | Radiation dose (range) Gy | Median FU (months) | |
|---|---|---|---|---|---|---|---|---|---|
| Bonvalot S [7] | 100 (primary) | abd/chest wall (42), LE (23), UE (7), HN (14), trunk (14) | 67 | 13 | – | – | 50 (4–60) | S + RT | 76 |
| Stoeckle E [8] | 65/35 | – | 92 | 7 | – | – | 50 (20–60) | S + RT | 123 |
| El-Haddad [9] | 48/52 | E (52), trunk (39), HN (9) | 6 | 41 | 4 | 3 | 50.4 (45–60) | S + RT | 88 |
| Husain Z [10] | – | LE (20), UE (40), buttock (10), Trunk (20), HN (10) | – | 10 | – | – | 50.7 (44–62) | S + RT | 48 |
| Huang K [11] | 75/25 | abd wall (50), LE (18), trunk (11), HN (18), UE (3) | 106 | 25 | – | – | (45–55) | S + RT | 102 |
| Ballo MT [12] | 45/55 | abd wall (10), HN (10), trunk (44), buttock (5), UE (18), LE (13) | 122 | 46 | 21 | – | 60 55 |
S + RT RT |
113 |
| Gronchi A [13] | 63/27 | abd wall (22), trunk (50), LE (12), UE (8), HN (8) | 172 | 40 | – | – | 57 (45–65) | S + RT | 135 |
| Duggal A [14] | 71/29 | UE (34), LE (20), trunk (31), abd wall (3), buttock (12) | 27 | 8 | – | – | 50 (10–64) | S + RT | 68* |
| Gluck I [15] | 76/24 | trunk (57), E (13), HN (20), abd/pelvis (10) | 54 | 28 | 13 | – | 56 (50–68) 50 (50–59) |
S + RT RT |
|
| JelinekJA [16] | – | abd (17), E (83) | 19 | 35 | – | – | 54 | S + RTa | 38 |
| Park HC [17] | – | E (36), HN (16), trunk (32), buttocks (16) | – | 21 | 3 | – | 48 (40–59) | S + RT, RT | 39* |
| Lev D [18] | 74/26 | UE (14), LE (16), abd wall (16), trunk (27), intra abd (14), retroperitoneal (6), HN (7) | 94 | 35 | 9 | – | (50–56) | S + RT, RT | 69 |
| Phillips SR [19] | 73/27 | abd wall (21), HN (4), trunk (42), UE (9), LE (17) buttock (7) | 73 | – | 2 | 18 | (30–72) | S + RT, RT | 63 |
| Mankin HJ [20] | – | UE (7), LE (48), trunk (34), abd wall/pelvis (11) | 185 | 39 | – | – | – | 31 | |
| Dalen BP [21] | – | abd wall (24), UE (22), LE (22), trunk (31), HN (1) | 29 | – | 1 | – | – | – | |
| Zlotecki RA [22] | 42/58 | UE (42), LE (35), trunk (7), abd (11), HN (5) | – | 65c | – | 54 (50–56) | S + RT, RT | 72 | |
| Barbier O [23] | 42/58 | UE (31), LE (58), buttock (11) | – | – | – | 26 | – | 16* | |
| Baumert BG [24] | 60/40 | 42 | 68 | – | 59 (3–74) | S + RT | 72 | ||
| Fiore M[6] | 65/35 | E (33), trunk (17), HN (4), abd wall (40), intra abd (7) | – | – | – | 83 | – | 33 | |
| Merchant NB [25] | 100 (primary) | E (49), trunk (23), abd wall (20), HN(8) | 74 | 31 | – | – | (45–65) | S + RTb | 49 |
| Nakayama T [26] | 82/18 | abd wall (18), UE (9), HN (18), LE (46), trunk (9) | 2 | – | 9 | – | – | 56 | |
| Pajaras B [27] | 90/10 | abd wall (45), intra abd (15), UE (15), HN (10), LE (10), trunk (5) | 17 | 2 | – | – | 50 (50) | S + RT | 35 |
| Pignatti G [28] | 42/58 | UE (30), LE (60), trunk (8), other (2) | 63 | 17 | 0 | 1 | (35–66) | S + RT | 134* |
| Schulz-Ertner D [29] | 43/57 | HN (8), UE (25), LE (29), abd wall (10), intra abd (10), trunk (18) | – | 26 | 2 | – | 48 (36–60) | RTb | 46 |
| Sharma V [30] | 88/12 | E (45), HN (14), Trunk (14), abd (27) | 15 | 15 | 4 | 8 | 60 (9–70) 50 (40–50) |
S + RT RT |
– |
| Shido Y [31] | – | trunk (30), UE (13), LE + buttock (57) | 30 | – | – | – | – | 89 | |
| Sorensen A [32] | – | abd(30), extra abd (70) | 44 | 28 | – | – | – | 96 | |
| Guney Y [33] | – | UE (29), LE (29), HN (14), buttock (14), trunk (14) | – | 4 | 3 | – | 51 (50–62) 50 (40–50) |
S + RT RT |
16 |
| Rudiger HA [34] | 59/41 | UE (31), LE (43), trunk (26) | – | 17 | 17 | – | 50 (24–60) | S + RT | 51* |
| Chew C [35] | 36/64 | UE (43), LE (40), HN (17) | – | 40 | 1 | 1 | – | 150 | |
| Kriz [36] | 48/52 | E (54) trunk (38) abd wall (8) | – | 37 | 15 | – | 50–60 55–65 |
S + RT RT |
44 |
| Zeng [1] | 67/33 | abd wall (27) intra abd (11) trunk (18) E (14) HN (25) buttock (4) | 184 | 39 | – | – | 38–66 | S + RT | 54 |
| Prodinger [37] | UE (49) LE (51) | 10 | 17 | – | – | 50–60 | S + RT | 65 | |
| Shin [38] | 74/26 | trunk/HN (41) E (59) | 95 | 24 | – | – | 38–70 | S + RT | 82* |
| Sri ram [39] | UE (10) HN (23) trunk (18) buttock (14) LE (35) | 48 | 19 | 5 | – | 48 | |||
| Keus [40] | 61/39 | UE (32) LE (32) HN (2) trunk (23) abd wall (11) | – | – | 44 | – | 56 | RT | 60 |
| Ergen [41] | 20/80 | UE (20) LE (45) trunk (20) intra abd (10) HN (5) | – | 18 | 2 | – | 60 (40–64) | S + RT | 77.5 |
RT = Radiotherapy, S = Surgery, FAP = Familial Adenomatous Polyposis FU: Follow-Up, E = extremity, LE = lower extremity, UE = upper extremity, HN = head and neck, abd = abdominal.
5 patients received intra operative radiotherapy.
Some patients received brachytherapy.
Number of patients receiving S + RT and RT alone.
Mean follow up.
Treatment results
The median age was 34 years. AF is more common among women than men, ratio 2:1.
Analysing the amount of patients with local control in relation to the total amount of patients per treatment group, the median local control rates for surgery alone, surgery and radiotherapy, radiotherapy alone and observation were 75%, 78%, 85% and 78%, respectively.
The role of surgical margins
Within the surgical group radical resections (36%) were as common as marginal resections (35%), intralesional resections were less common (11%).
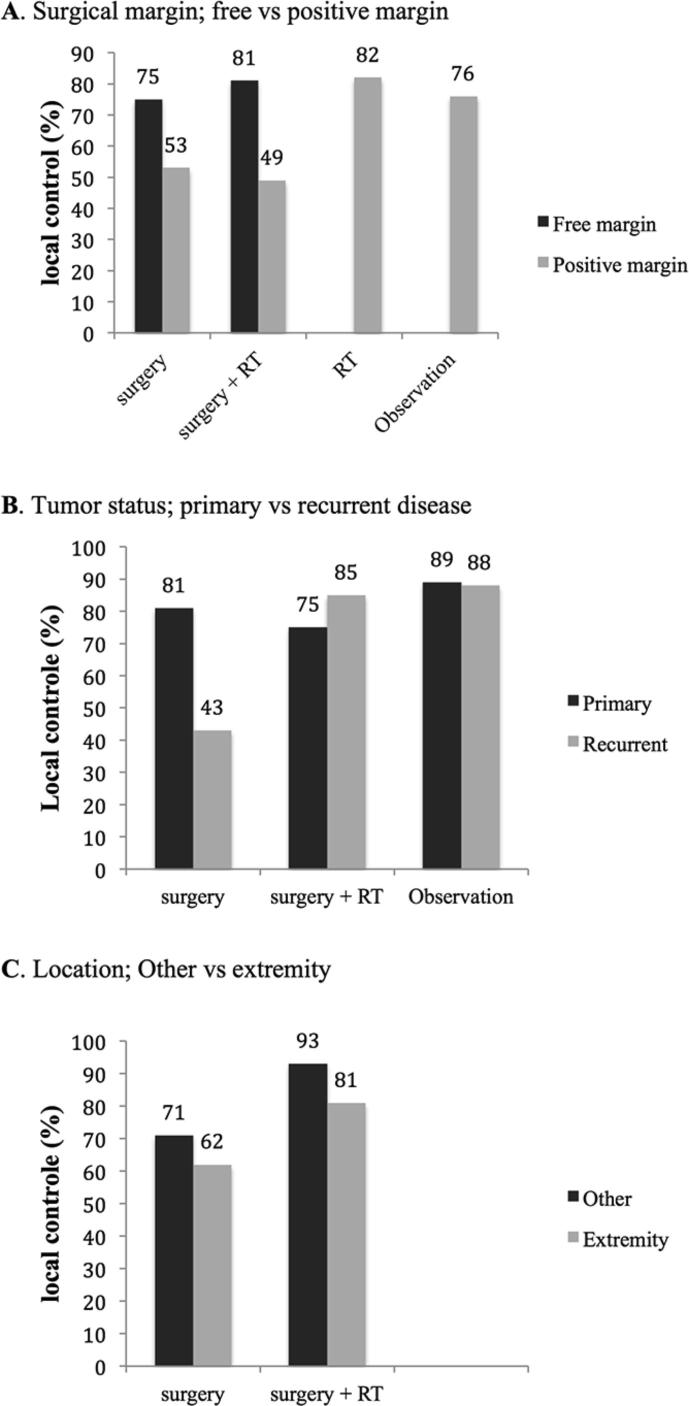
As expected, local recurrence was more common after surgery with positive margins compared to negative margins (Fig. 2A, Supplementary Table 1). Adjuvant radiotherapy after positive margins did not improve the local control rate (p = 0.549) [7], [8], [9], [11], [12], [14], [15], [17], [18], [19], [21], [24], [26], [28], [30], [31], [32], [33], [35].
Fig. 2.
(A–C) Local controle rate stratisfied per subgroup.
Between the treatment groups radiotherapy and observation, irrespective of surgical margins, no significant difference existed in terms of local control (p = 0.355).
The role of tumor status
Patients with recurrent disease had less local recurrences after being treated with adjuvant radiotherapy compared to surgery alone (p < 0.001) (Fig. 2B) [8], [10], [14], [17], [20], [22], [31], [32]. Moreover, patients who were being observed had a better local control rate than patients treated with surgery alone (p = 0.001) [8], [14], [20], [23], [26], [31]. Similar results were seen when the observation group was compared to the total surgical group with or without adjuvant radiotherapy, although this did not reach statistical significance (p = 0.063) [8], [10], [14], [17], [20], [22], [23], [26], [31], [32]. For radiotherapy alone the numbers were too small to perform statistical analysis.
The role of tumor location and size
Even though the percentage of patients with local control was higher in the group of patients treated with adjuvant radiotherapy for both tumors located at the extremities and other locations, this did not reach statistical significance (p = 0.481 and p = 0.755, respectively) (Fig. 2C) [10], [14], [17], [21], [22], [26], [32]. Regarding the radiotherapy alone and the observation group numbers were too small to analyse.
For analyzing tumor size, we used the recurrence free survival instead of actual number of patients since more articles noted local control rate this way. Tumors over 5 cm [13], [26], had a worse recurrence free survival than smaller tumors, irrespective of treatment. In the observation group no difference in five years recurrence free survival was observed for tumor size [11] (Table 2).
Table 2.
Recurrence free survival of the different treatment modalities with respect to tumor size.
| Surgery |
Surgery + Radiotherapy |
Radiotherapy |
Observation |
|||||
|---|---|---|---|---|---|---|---|---|
| 5RFS | 10RFS | 5RFS | 10RFS | 5RFS | 10RFS | 5RFS | 10RFS | |
| <5 cm | 94 | 60, 94 | – | 84 | – | 100 | 44, 52 | – |
| ≥5 cm | 72 | 63, 66 | – | 69 | – | 68 | 60, 52 | – |
RFS = Recurrence free survival.
Time to recurrence or stabilization of disease
The median time to local recurrence including all treatment groups as noted in 15 articles was 17 (range, 11–52) months. Two articles noted a mean recurrence time of 16 and 20 months.
For the observational treatment group, three studies described the median time to stabilization of the tumor, which was 14 (range, 12–35) months. The median time to tumor growth in this treatment group was 32 (range, 14–38) months.
Multivariate analysis
A multivariate analysis was performed in eight studies. Prognostic factors predicting a negative outcome were large size (>4 or 5 cm), tumor location (limb, other locations than abdominal wall), positive surgical margins, deep seated tumors, age (<30 years), surgical treatment without adjuvant radiotherapy, recurrent disease and extracompartmentally situated tumors.
Complications and deaths due to treatment
In nine studies 14 patients were described who died of treatment or disease related causes, which is <1% of all treated patients. Since most articles did not describe the actual cause of death, it is not certain if any patient died due to the tumor itself.
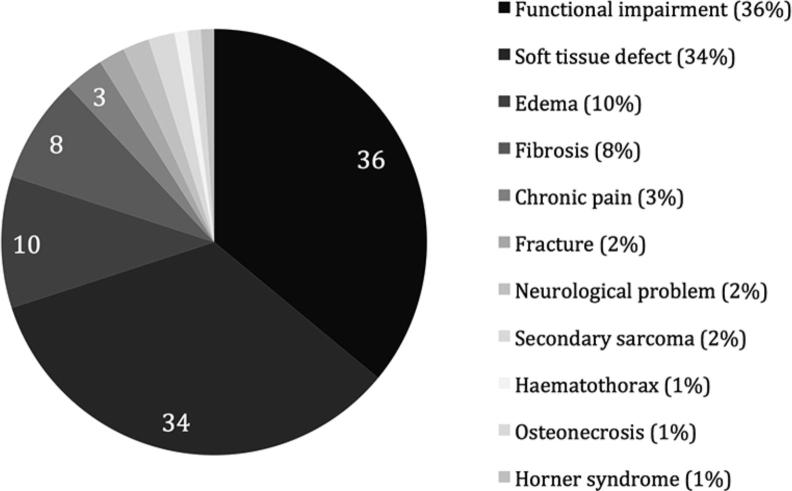
Fig. 3 describes the complications related to treatment. Soft tissue defects ranged from light dermatitis, most commonly caused by radiotherapy, to severe skin necrosis (in one case necessitating admittance to the intensive care). Severe treatment related complications were described in four patients who developed a secondary sarcoma (fibrosarcoma, angiosarcomas, MPNST) in the radiation field.
Fig. 3.
Treatment related complications in percentage.
Discussion
In this systematic analysis we looked at the outcome of patients treated with surgery with four different treatment strategies with regard to local recurrence rates. Irrespective of treatment modality, the local control rate was good, with over 75% local control in each treatment group. This finding is in concordance with recently published data [42].
Adjuvant radiotherapy is in many cases used to lower the risk of local recurrence in case of positive margins. In this review, contrary to previous findings of Nuyttens et al. [2] and Janssen et al. [42], no significant advantage for adjuvant radiotherapy was observed regarding local control [7], [8], [10], [11], [14], [17], [19], [24], [26], [28], [31], [32], [35]. There is however, a strong effect for adjuvant radiotherapy in recurrent disease, comparatively to the results of Janssen et al. [42]. An international survey in Europe showed that recurrences after radiation tend to develop most commonly at the field border or in areas receiving less than 50 Gy [24]. This implies wide field margins and high radiation doses in order to achieve a better local control rate. Although the radiation dose in this analysis varied, most institutions used ≥50 Gy.
Recently the EORTC carried out a multicenter prospective phase 2 trial to determine the tumor response in patients with inoperable aggressive fibromatosis using 56 Gy radiotherapy. Keus et al. reported a good local control rate of 82% [40]. In the majority of cases this meant partial regression (36%) or stabilized disease (41%), only in a few cases complete regression (14%) of the tumor was observed. Interestingly, even after three years response was observed on MRI. Despite this good result, eventually 23% developed local progression, even after initial response. In two patients treatment could not be continued due to extensive toxic effect of radiation. The complication rate in Nuyttens et al. [2] was reported in over one fifth of patients, and in Keus et al. [40] around one third. Only a small percentage (5%) developed severe skin toxic effects of grade 3/4 [40]. The link between the radiation dose and the risk of local progression/recurrence is debated, but some argue a better local control rate at high doses of 56 Gy [21]. However, the incidence of complications increased parallel with the dose given. Although the majority of complications is not severe and reversible, some severe complications including fractures and secondary sarcomas occurred, which were also observed in this review. More common complications were functional impairment and soft tissue defects (70% of all complications), of which the latter was in most cases reversible. The overall death rate is very low, with less than 1% of patients dying either due to the disease or treatment complications.
Around ten years ago the first reports about a wait and see policy were published. Due to the fact that data are usually small due to a relatively low incidence of AF, concerning 3% of all soft tissue sarcomas [43]. This analysis pooled data to determine whether or not conservative treatment reaches acceptable local control rates compared to surgical treatment. The majority of patients in this analysis were still treated with surgery (surgical treatment n = 2485 vs. non surgical treatment n = 295), with about one sixth of institutions describing radiotherapy alone and/or observation.
In most institutions a selection is performed for patient undergoing more conservative therapy. In case of radiotherapy alone, patients usually had large tumors, or tumors in close adherence to important structures that limited radical surgery [12], [15], [18], [30]. Patients considered for observation usually had a tumor, that in case of growth, was still eligible for surgery and had no major clinical symptoms [6], [7], [19], [23]. Only one study used a routinely first line conservative approach for all patients presenting to the institution [6] with a relatively good local control rate of 65%. Of the patients with primary disease, 35% had progressive disease, and in 32% of these patients surgical treatment was finally necessary. Interestingly, the progression free survival rate of patients with primary tumors was 47% and with local recurrence 54%. Stabilization of the tumor arose after a median time of >1 year after observation, and a local recurrence or progression occurred after a median time of <3 years, which means that patients should be regularly observed within the first five years. If sudden progression does develop, treatment should be re-evaluated.
Surprisingly, the treatment groups of radiotherapy alone and observation had a relative similar local control rate as the surgery group. One reason could be that surgery itself is a stimulant for tumor growth. Interestingly, the radiotherapy alone group did not have better local control rates than the observation group (p = 0.355). It should be noted that there is a selection bias favouring the observation group, due to the selection of tumors with a less aggressive pattern. In addition, the follow up of the two largest studies using primary observation was mean 16 months and median 33 months, while the follow up of the largest studies with radiotherapy only was median 56 months.
For primary tumors the local control rate did not seem to be influenced by the choice of treatment. The opposite is true for recurrent disease, in which adjuvant radiotherapy has a definite advantage over surgery alone (p = 0.001). This could be explained by the more aggressive nature of recurrent disease.
Based on this systematic review no preference of treatment could be indicated based on tumor location (extremity vs other locations), although outcome of patients with tumors located at the extremities was worse. Especially patients with large tumors located at the extremities have a worse local outcome, regardless of the surgical margins [13].
Patients with a tumor size larger than 5 cm had a worse local outcome, independent of the type of treatment except for the observation group.
In addition to the variables mentioned in the previous section, other studies that performed multivariate analysis showed that deep seated tumors, age (<30 years) and extracompartmentally situated tumors were negative predicting markers of local outcome. Similar predicting markers were also found in other soft tissue sarcomas [35], [44], [45].
It is important to note that pooling of the data led to large sample sizes, however, when analyzing the subgroups, the sample sizes diminished due to lack of reported data items. In addition, selection and reporting bias occurred due to the retrospective design of most included studies.
Meta-analyses of the trial results were considered, but were deemed not feasible because the heterogeneity of the patients, tumor characteristics and interventions, were too great to allow for pooling of data.
Conclusion
With consideration of previously mentioned weaknesses of this study and careful interpretation of the results, a watchful waiting approach as a first line option could be justified, in addition with closely monitoring by means of physical examination and MRI during at least five years of follow up, in a subgroup of patients without clinical symptoms and no possible health hazards if the tumor would progress, and taking into account that a considerable group of patients eventually does need surgical treatment. More data is needed to confirm a conservative approach as a safe treatment for AF, especially in smaller patient subgroups.
In case of recurrent disease, adjuvant radiotherapy with a dose ≥50 Gy has a definitive advantage over surgery alone. A multidisciplinary sarcoma team should finally make the decision with respect to the treatment options.
Conflict of interest statement
Authors declare that there is no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.03.001.
Appendix A. Supplementary data
References
- 1.Zeng W.G., Zhou Z.X., Liang J.W. Prognostic factors for desmoid tumor: a surgical series of 233 patients at a single institution. Tumour Biol. 2014;35:7513–7521. doi: 10.1007/s13277-014-2002-1. [DOI] [PubMed] [Google Scholar]
- 2.Nuyttens J.J., Rust P.F., Thomas C.R., Jr Turrisi AT,3rd. Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: A comparative review of 22 articles. Cancer. 2000;88:1517–1523. [PubMed] [Google Scholar]
- 3.Sherman N.E., Romsdahl M., Evans H. Desmoid tumors: a 20-year radiotherapy experience. Int J Radiat Oncol Biol Phys. 1990;19:37–40. doi: 10.1016/0360-3016(90)90131-3. [DOI] [PubMed] [Google Scholar]
- 4.Kamath S.S., Parsons J.T., Marcus R.B. Radiotherapy for local control of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 1996;36:325–328. doi: 10.1016/s0360-3016(96)00321-5. [DOI] [PubMed] [Google Scholar]
- 5.Janinis J., Patriki M., Vini L. The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol. 2003;14:181–190. doi: 10.1093/annonc/mdg064. [DOI] [PubMed] [Google Scholar]
- 6.Fiore M., Rimareix F., Mariani L. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–2593. doi: 10.1245/s10434-009-0586-2. [DOI] [PubMed] [Google Scholar]
- 7.Bonvalot S., Eldweny H., Haddad V. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34:462–468. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Stoeckle E., Coindre J.M., Longy M. A critical analysis of treatment strategies in desmoid tumours: a review of a series of 106 cases. Eur J Surg Oncol. 2009;35:129–134. doi: 10.1016/j.ejso.2008.06.1495. [DOI] [PubMed] [Google Scholar]
- 9.El-Haddad M., El-Sebaie M., Ahmad R. Treatment of aggressive fibromatosis: the experience of a single institution. Clin Oncol. 2009;21:775–780. doi: 10.1016/j.clon.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Husain Z., Benevenia J., Uglialoro A.D. An evaluation of brachytherapy and external beam radiation used with wide-margin surgical resection in the treatment of extra-abdominal desmoid tumors. Am J Orthop. 2011;40:E78–E82. [PubMed] [Google Scholar]
- 11.Huang K., Fu H., Shi Y.Q. Prognostic factors for extra-abdominal and abdominal wall desmoids: a 20-year experience at a single institution. J Surg Oncol. 2009;100:563–569. doi: 10.1002/jso.21384. [DOI] [PubMed] [Google Scholar]
- 12.Ballo M.T., Zagars G.K., Pollack A. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158–167. doi: 10.1200/JCO.1999.17.1.158. [DOI] [PubMed] [Google Scholar]
- 13.Gronchi A., Casali P.G., Mariani L. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21:1390–1397. doi: 10.1200/JCO.2003.05.150. [DOI] [PubMed] [Google Scholar]
- 14.Duggal A., Dickinson I.C., Sommerville S., Gallie P. The management of extra-abdominal desmoid tumours. Int Orthop. 2004;28:252–256. doi: 10.1007/s00264-004-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluck I., Griffith K.A., Biermann J.S. Role of radiotherapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 2011;80:787–792. doi: 10.1016/j.ijrobp.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Jelinek J.A., Stelzer K.J., Conrad E. The efficacy of radiotherapy as postoperative treatment for desmoid tumors. Int J Radiat Oncol Biol Phys. 2001;50:121–125. doi: 10.1016/s0360-3016(00)01570-4. [DOI] [PubMed] [Google Scholar]
- 17.Park H.C., Pyo H.R., Shin K.H., Suh C.O. Radiation treatment for aggressive fibromatosis: findings from observed patterns of local failure. Oncology. 2003;64:346–352. doi: 10.1159/000070292. [DOI] [PubMed] [Google Scholar]
- 18.Lev D., Kotilingam D., Wei C. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25:1785–1791. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- 19.Phillips S.R., A'Hern R., Thomas J.M. Aggressive fibromatosis of the abdominal wall, limbs and limb girdles. Br J Surg. 2004;91:1624–1629. doi: 10.1002/bjs.4792. [DOI] [PubMed] [Google Scholar]
- 20.Mankin H.J., Hornicek F.J., Springfield D.S. Extra-abdominal desmoid tumors: a report of 234 cases. J Surg Oncol. 2010;102:380–384. doi: 10.1002/jso.21433. [DOI] [PubMed] [Google Scholar]
- 21.Dalen B.P., Bergh P.M., Gunterberg B.U. Desmoid tumors: a clinical review of 30 patients with more than 20 years’ follow-up. Acta Orthop Scand. 2003;74:455–459. doi: 10.1080/00016470310017785. [DOI] [PubMed] [Google Scholar]
- 22.Zlotecki R.A., Scarborough M.T., Morris C.G. External beam radiotherapy for primary and adjuvant management of aggressive fibromatosis. Int J Radiat Oncol Biol Phys. 2002;54:177–181. doi: 10.1016/s0360-3016(02)02926-7. [DOI] [PubMed] [Google Scholar]
- 23.Barbier O., Anract P., Pluot E. Primary or recurring extra-abdominal desmoid fibromatosis: assessment of treatment by observation only. Orthop Traumatol Surg Res. 2010;96:884–889. doi: 10.1016/j.otsr.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Baumert B.G., Spahr M.O., Von Hochstetter A. The impact of radiotherapy in the treatment of desmoid tumours. An international survey of 110 patients. A study of the Rare Cancer Network. Radiat Oncol. 2007;2:12. doi: 10.1186/1748-717X-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchant N.B., Lewis J.J., Woodruff J.M. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer. 1999;86:2045–2052. [PubMed] [Google Scholar]
- 26.Nakayama T., Tsuboyama T., Toguchida J. Natural course of desmoid-type fibromatosis. J Orthop Sci. 2008;13:51–55. doi: 10.1007/s00776-007-1187-1. [DOI] [PubMed] [Google Scholar]
- 27.Pajares B., Torres E., Jimenez B. Multimodal treatment of desmoid tumours: the significance of local control. Clin Transl Oncol. 2011;13:189–193. doi: 10.1007/s12094-011-0639-4. [DOI] [PubMed] [Google Scholar]
- 28.Pignatti G., Barbanti-Brodano G., Ferrari D. Extraabdominal desmoid tumor. A study of 83 cases. Clin Orthop Relat Res. 2000;375:207–213. [PubMed] [Google Scholar]
- 29.Schulz-Ertner D., Zierhut D., Mende U. The role of radiation therapy in the management of desmoid tumors. Strahlenther Onkol. 2002;178:78–83. doi: 10.1007/s00066-002-0900-4. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V., Chetty D.N., Donde B. Aggressive fibromatosis–impact of prognostic variables on management. S Afr J Surg. 2006;44:6–8. [PubMed] [Google Scholar]
- 31.Shido Y., Nishida Y., Nakashima H. Surgical treatment for local control of extremity and trunk desmoid tumors. Arch Orthop Trauma Surg. 2009;129:929–933. doi: 10.1007/s00402-008-0750-3. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen A., Keller J., Nielsen O.S., Jensen O.M. Treatment of aggressive fibromatosis: a retrospective study of 72 patients followed for 1–27 years. Acta Orthop Scand. 2002;73:213–219. doi: 10.1080/000164702753671830. [DOI] [PubMed] [Google Scholar]
- 33.Guney Y., Hicsonmez A., Andrieu M.N., Kurtman C. Outcome of aggressive fibromatosis treated with radiation therapy. Scott Med J. 2007;52:11–14. doi: 10.1258/rsmsmj.52.4.11. [DOI] [PubMed] [Google Scholar]
- 34.Rudiger H.A., Ngan S.Y., Ng M. Radiation therapy in the treatment of desmoid tumours reduces surgical indications. Eur J Surg Oncol. 2010;36:84–88. doi: 10.1016/j.ejso.2009.07.183. [DOI] [PubMed] [Google Scholar]
- 35.Chew C., Reid R., O'Dwyer P.J. Evaluation of the long term outcome of patients with extremity desmoids. Eur J Surg Oncol. 2004;30:428–432. doi: 10.1016/j.ejso.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Kriz J., Eich H.T., Haverkamp U. Radiotherapy is effective for desmoid tumors (aggressive fibromatosis) – long-term results of a German multicenter study. Oncol Res Treat. 2014;37:255–260. doi: 10.1159/000362398. [DOI] [PubMed] [Google Scholar]
- 37.Prodinger P.M., Rechl H., Keller M. Surgical resection and radiation therapy of desmoid tumours of the extremities: results of a supra-regional tumour centre. Int Orthop. 2013;37:1987–1993. doi: 10.1007/s00264-013-1942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin S.H., Ko K.R., Cho S.K. Surgical outcome of desmoid tumors: adjuvant radiotherapy delayed the recurrence, but did not affect long-term outcomes. J Surg Oncol. 2013;108:28–33. doi: 10.1002/jso.23343. [DOI] [PubMed] [Google Scholar]
- 39.Sri-Ram K., Haddo O., Dannawi Z. The outcome of extra-abdominal fibromatosis treated at a tertiary referral centre. Eur J Surg Oncol. 2012;38:700–705. doi: 10.1016/j.ejso.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Keus R.B., Nout R.A., Blay J.Y. Results of a phase II pilot study of moderate dose radiotherapy for inoperable desmoid-type fibromatosis–an EORTC STBSG and ROG study (EORTC 62991–22998) Ann Oncol. 2013;24:2672–2676. doi: 10.1093/annonc/mdt254. [DOI] [PubMed] [Google Scholar]
- 41.Ergen S.A., Tiken E.E., Oksuz D.C. The role of radiotherapy in the treatment of primary or recurrent desmoid tumors and long-term results. Balkan Med J. 2016;33:316–321. doi: 10.5152/balkanmedj.2016.140560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen M.L., van Broekhoven D.L., Cates J.M. Meta-analysis of the influence of surgical margin and adjuvant radiotherapy on local recurrence after resection of sporadic desmoid-type fibromatosis. Br J Surg. 2017;104:347–357. doi: 10.1002/bjs.10477. [DOI] [PubMed] [Google Scholar]
- 43.Plukker J.T., van Oort I., Vermey A. Aggressive fibromatosis (non-familial desmoid tumour): therapeutic problems and the role of adjuvant radiotherapy. Br J Surg. 1995;82:510–514. doi: 10.1002/bjs.1800820424. [DOI] [PubMed] [Google Scholar]
- 44.Carneiro A., Bendahl P.O., Engellau J. A prognostic model for soft tissue sarcoma of the extremities and trunk wall based on size, vascular invasion, necrosis, and growth pattern. Cancer. 2011;117:1279–1287. doi: 10.1002/cncr.25621. [DOI] [PubMed] [Google Scholar]
- 45.Rydholm A., Gustafson P. Should tumor depth be included in prognostication of soft tissue sarcoma? BMC Cancer. 2003;3:17. doi: 10.1186/1471-2407-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.