Abstract
Antiangiogenic therapy is a universal approach to the treatment of malignant gliomas but fails to prolong the overall survival of newly diagnosed or recurrent glioblastoma patients. Imaging biomarkers are quantitative imaging parameters capable of objectively describing biological processes, pathological changes and treatment responses in some situations and have been utilized for outcome predictions of malignant gliomas in anti-angiogenic therapy. Advanced magnetic resonance imaging techniques (including perfusion-weighted imaging and diffusion-weighted imaging), positron emission computed tomography and magnetic resonance spectroscopy are imaging techniques that can be used to acquire imaging biomarkers, including the relative cerebral blood volume (rCBV), Ktrans, and the apparent diffusion coefficient (ADC). Imaging indicators for a better prognosis when treating malignant gliomas with antiangiogenic therapy include the following: a lower pre- or post-treatment rCBV, less change in rCBV during treatment, a lower pre-treatment Ktrans, a higher vascular normalization index during treatment, less change in arterio-venous overlap during treatment, lower pre-treatment ADC values for the lower peak, smaller ADC volume changes during treatment, and metabolic changes in glucose and phenylalanine. The investigation and utilization of these imaging markers may confront challenges, but may also promote further development of anti-angiogenic therapy. Despite considerable evidence, future prospective studies are critically needed to consolidate the current data and identify novel biomarkers.
Abbreviations: 18F-FDOPA, 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine; 18F-FLT, [18F]-fluoro-3-deoxy-3-L-fluorothymidine; ADC, apparent diffusion coefficient; AVOL, arterio-venous overlap; BBB, blood brain barrier; CBF, cerebral blood flow; CBV, cerebral blood volume; CNS, central nervous system; CT, computed tomography; D-2HG, D-2-hydroxypentanedioic acid; DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; DSC-MRI, dynamic susceptibility contrast magnetic resonance imaging; DWI, diffusion-weighted imaging; FDG, fluorodeoxyglucose; fDMs, functional diffusion maps; FLAIR, fluid-attenuated inversion recovery; FSE pcASL, fast spin echo pseudocontinuous artery spin labeling; GBM, glioblastoma; Ktrans, volume transfer constant between blood plasma and extravascular extracellular space; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; nGBM, newly diagnosed glioblastoma; OS, overall survival; PET, positron emission computed tomography; PFS, progression-free survival; PWI, perfusion-weighted imaging; RANO, Response Assessment in Neuro-Oncology; rCBF, relative cerebral blood flow; rCBV, relative cerebral blood volume; rGBM, recurrent glioblastoma; ROI, region of interest; RSI, restriction spectrum imaging; SUV, standardized uptake value; TMZ, temozolomide; VAI, vessel architectural imaging; VEGF-A, vascular endothelial growth factor A; VNI, vascular normalization index.
Keywords: Imaging, Biomarkers, Anti-angiogenic, Glioma, Therapy
Highlights
-
•
Anti-angiogenic therapy only benefits specific populations of glioma patients.
-
•
Advanced imaging techniques can produce quantitative imaging biomarkers.
-
•
Physiological and metabolic parameter can predict outcome for anti-angiogenic therapy.
-
•
Larger prospective studies are needed to provide further evidence.
1. Introduction
Glioma is the most common malignant primary central nervous system (CNS) tumor, with an annual incidence of 5.26 per 100,000 individuals (Omuro and DeAngelis, 2013). The management of glioma requires a combination of surgical excision, chemotherapy, radiotherapy and targeted therapy based on the histological and molecular features of the tumor (Jiang et al., 2016); however, the survival outcome remains poor. The median overall survival (OS) rates for low-grade gliomas (WHO grade II), anaplastic gliomas (WHO grade III) and glioblastomas (GBM, WHO IV) are 159, 96 and 16 months, respectively (Buckner et al., 2016; Wick et al., 2016; Gilbert et al., 2014; Chinot et al., 2014).
Angiogenesis, a hallmark of cancer (Hanahan and Weinberg, 2000), occurs by sprouting angiogenesis, vasculogenesis, intussusception, co-opting preexisting vessels, vascular mimicry, or endothelial cells derived from cancer stem cells (Carmeliet and Jain, 2011). As a result of the universality of angiogenesis in most malignant gliomas (referring to WHO grade III and grade IV gliomas), anti-angiogenic therapy has become a critical approach in glioma therapy (anti-tumor effects of anti-angiogenic therapy in gliomas are displayed in Fig. 1). Bevacizumab, a humanized monoclonal antibody that targets vascular endothelial growth factor A (VEGF-A), was approved in 2009 for the treatment of recurrent GBM. Bevacizumab treatment has resulted in pharmacological debulking and volumetric reductions in 55% of patients with recurrent malignant gliomas, thereby leading to neurological palliation and increased progression-free survival (PFS) (Wong et al., 2011). Thus, bevacizumab is regarded as the first-line therapy for recurrent malignant gliomas in some institutes. However, bevacizumab combined with temozolomide (TMZ) and radiotherapy failed to demonstrate a significant improvement in the OS of patients with newly diagnosed glioblastoma (nGBM) in two large, multicenter, randomized, phase III clinical trials (RTOG0825 and AVAglio) (Gilbert et al., 2014; Chinot et al., 2014), and bevacizumab combined with lomustine did not provide an OS benefit over lomustine alone in recurrent glioblastoma (rGBM) patients (EORTC 26101 trial) (Wick et al., 2017). These findings suggest that the drug may not benefit all patient subsets (critical clinical trials investigating anti-angiogenic therapy in GBM are listed in Table 1). Thus, biomarkers for predicting the treatment effects and monitoring the treatment responses to anti-angiogenic therapy are urgently required. Several molecular biomarkers, such as the proneural subtype of GBM and the serum levels of MMP2 and MMP9, are correlated with the OS of patients undergone bevacizumab therapy (Sandmann et al., 2015; Tamura et al., 2017); however, use of these biomarkers has the following limitations: (1) continuous monitoring using tumor molecular markers is difficult because repeated sampling is impossible in solid tumors; (2) tumor molecular markers can hardly be obtained from patients without indications for surgery/biopsy, particularly when the detection method of circulating tumor cells and circulating DNA of glioma is not sophisticated; (3) molecular markers may not fully represent the whole tumor considering tumor heterogeneity; and (4) molecular markers of all types require distinct sampling, storage and testing techniques, which may be obstacles to implementation.
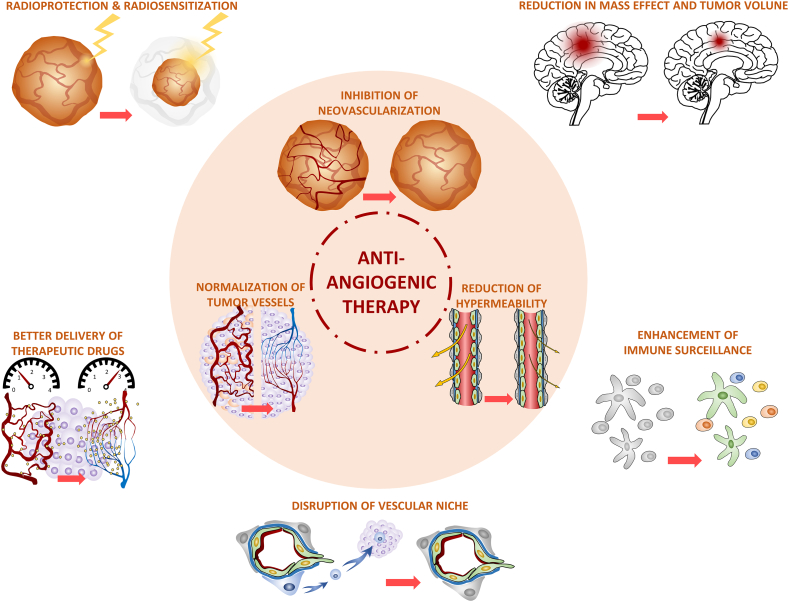
Fig. 1.
Anti-tumor effects of anti-angiogenic therapy in gliomas.
The application of anti-angiogenic therapy in gliomas results in the inhibition of neovascularization, the normalization of tumor vessels, and a reduction in hyperpermeability, which bring about multiple clinical effects, including reduction in mass effect and tumor volume; the improved delivery of therapeutic drugs; radioprotection and radiosensitization; the disruption of the vascular niche; and the enhancement of immune surveillance.
Table 1.
Critical clinical trials investigating anti-angiogenic therapy in glioblastoma.
| Author (year) | Trial | Phase | Disease | Regimen | Patient number | Median PFS (m) | Median OS (m) | Notes |
|---|---|---|---|---|---|---|---|---|
| Friedman et al. (2009) | BRAIN | II | rGBM | BEV | 85 | 4.2 | 9.2 | The first large prospective study demonstrating that BEV ± irinotecan is well tolerated and achieves certain therapeutic effects in rGBM. |
| BEV + irinotecan | 82 | 5.6 | 8.7 | |||||
| Kreisl et al. (2009) | NCI | II | rGBM | BEV | 48 | 4.0 | 7.8 | A study shows that single-agent BEV is well tolerated and has biological activity in rGBM. Data reveals that imaging biomarkers can be applied to monitor and predict treatment responses. |
| Taal et al. (2014) | BELOB | II | rGBM | BEV | 50 | 3 | 8 | A study shows promising effects of BEV + lomustine in rGBM patients. The results in the BEV + lomustine group now serve as the ‘comparison standard’ for the investigations focusing on rGBM. |
| Lomustine | 46 | 2 | 8 | |||||
| BEV + lomustine | 44 | 11 | 11 | |||||
| Wick et al. (2017) | EORTC 26101 | III | rGBM | BEV + lomustine | 288 | 4.2 | 9.1 | PFS was significantly prolonged in the BEV + lomustine group, but an OS advantage was not conferred. More adverse events were observed in the BEV + lomustine group due to the longer duration of treatment. |
| Lomustine | 149 | 1.5 | 8.6 | |||||
| Chinot et al. (2014) | AVAglio | III | nGBM | BEV + TMZ/RT | 458 | 10.6 | 16.9 | The study observed a significant improvement in PFS (P < 0.0001) but not in OS. Although the maintenance of the performance status is observed in the BEV + TMZ/RT group, a higher incidence of adverse events is also documented. |
| TMZ/RT | 463 | 6.2 | 16.8 | |||||
| Gilbert et al. (2014) | RTOG 0825 | III | nGBM | BEV + TMZ/RT | 312 | 10.7 | 15.7 | Although there is a similar increase in PFS (P < 0.01) and not in OS, a more frequent increased symptom burden and declined neurocognitive function is noticed in the BEV + TMZ/RT group. |
| TMZ/RT | 309 | 7.3 | 16.1 | |||||
| Batchelor et al. (2013b) | REGAL | III | rGBM | Cediranib | 131 | 3.1 | 8.0 | This study fails to demonstrate a benefit in PFS and OS for cediranib±irinotecan versus lomustine alone in patients with rGBM. Patients in the cediranib+lomustine arm experienced higher rates of toxicities than patients in both monotherapy arms. |
| Cediranib + lomustine | 129 | 4.2 | 9.4 | |||||
| Lomustine | 65 | 2.7 | 9.8 |
Abbreviations: rGBM, recurrent glioblastoma; nGBM, newly diagnosed glioblastoma; BEV, bevacizumab; TMZ, temozolomide; RT, radiotherapy; PFS, progression-free survival; OS, overall survival.
Imaging biomarkers are quantitative parameters that are mainly derived from computed tomography (CT), magnetic resonance imaging (MRI) or positron emission computed tomography (PET) and may be employed to objectively describe biological processes, pathological changes and treatment responses in different situations (White paper on imaging biomarkers, 2010). Imaging biomarkers have substantial clinical value in cancer management, namely, for detection, prediction, staging and treatment response assessment (White paper on imaging biomarkers, 2010). As a parallel type of marker, imaging biomarkers may compensate for the deficiencies in molecular biomarkers, thus providing a clinical basis for patient selection and therapeutic monitoring: (1) imaging can be acquired continuously to monitor therapeutic responses; (2) imaging biomarkers can be evaluated in patients without indications for surgery/biopsy given the noninvasiveness of imaging modalities; and (3) imaging can capture heterogeneity within the lesion. This article summarizes the imaging biomarkers, including the physiological and metabolic imaging biomarkers, used to predict the treatment response to anti-angiogenic therapy (e.g., bevacizumab), in order to help physicians predict and monitor the therapeutic response of malignant glioma to anti-angiogenic therapy before or during the early phase of treatment.
2. Traditional criteria in the assessment of tumor progression
In 1990, Macdonald et al. proposed postcontrast T1-weighted MRI assessment criteria for complete response, partial response, stable disease and progressive disease (Macdonald et al., 1990). Since then, MRI has been considered the universal imaging modality for monitoring treatment responses and tumor progression of malignant glioma. However, post-contrast T1-weighted MRI reflected the breakdown of the blood brain barrier (BBB) rather than the status of the tumor itself, and factors that affect the BBB can interfere with the imaging. As a result, utilization of the Macdonald criteria for evaluating anti-angiogenic therapy, particularly anti-VEGF therapy, was insufficient because of the effects of the anti-angiogenic drugs on vessels (a significant reduction in T1-weighted contrast enhancement and peritumoral edema in imaging findings, also referred to as a pseudoresponse) and the infiltrative nonenhancing growth pattern after anti-angiogenic therapy (Zuniga et al., 2009).
The Response Assessment in Neuro-Oncology (RANO) criteria were subsequently published in 2010 as an update to the Macdonald criteria and placed more emphasis on the T2-weighted and fluid-attenuated inversion recovery (FLAIR) MRI changes (Wen et al., 2010). Although T2-weighted FLAIR imaging reveals the progression of the invasive phenotype, the predictive value of T2-weighted FLAIR imaging for the effect of anti-angiogenic therapy for rGBM is limited because the reduction in the high signal on the T2-weighted FLAIR image could also be caused by bevacizumab-induced alterations in vascular permeability (Schmainda et al., 2015; Clarke and Chang, 2009). The RTOG 0625/ACRIN 6677 trial also failed to demonstrate an association between disease progression depicted on T2-weighted FLAIR images and patient outcome (Boxerman et al., 2013).
In general, traditional assessments of tumor progression based on the Macdonald and RANO criteria remain widely used in the evaluation of anti-angiogenic therapy; however, the sensitivity and accuracy of these criteria in outcome prediction face controversies.
3. Physiological parameters in anti-angiogenic therapy
While the use of classical imaging techniques presents several challenges for predicting the treatment effect in gliomas, the identification of physiological parameters acquired from advanced MRI technique that may guide targeted therapy has recently become a key research focus. Advanced MRI technique include perfusion-weighted imaging (PWI) and diffusion-weighted imaging (DWI). PWI generates physiological parameters, such as the relative cerebral blood volume (rCBV) and Ktrans (volume transfer constant between blood plasma and extravascular extracellular space), using bolus contrast tracking techniques, including dynamic susceptibility contrast MRI (DSC-MRI), dynamic contrast-enhanced MRI (DCE-MRI) and arterial spin labeling, which reflects the process of blood perfusion. DWI generates parameters such as the apparent diffusion coefficient (ADC), which based on the Brownian movement of water molecules. These parameters are closely related to the cerebral vascular structure, BBB permeability and tumor cell density and play important roles in predicting the survival of glioma patients (Burth et al., 2016), particularly those under anti-angiogenic therapy.
3.1. Cerebral blood volume imaging
Cerebral blood volume imaging is mainly generated from DSC-MRI. DSC-MRI records the dynamic process of the first pass effect in a short period of time, during which the contrast agent enters the brain tissue via cerebral vessels. DSC-MRI subsequently calculates multiple parameters, including cerebral blood flow (CBF), cerebral blood volume (CBV), mean transmit time (MTT) and time to the peak signal change (TTP) (Barker et al., 2013). rCBV, derived from DSC-MRI, provides physiological information regarding neovascularity and angiogenesis of the brain. rCBV is capable of indirectly depicting the tumor vasculature in vivo and assisting in glioma grading, metastasis recognition, and predicting chemoradiotherapy responses (Zhang et al., 2017). In a retrospective study in 2010, Sawlani et al. analyzed 16 rGBM patients who had undergone bevacizumab therapy. Calculating the hyperperfusion volume based on the rCBV, the authors determined that the magnitude of the change in the hyperperfusion volume before and after treatment was significantly related to PFS (Sawlani et al., 2010). This study was the first investigation to show the correlation between cerebral blood volume imaging and the effect of anti-angiogenic drugs.
3.1.1. Individual rCBV values before/after treatment
In 2014, Schmainda et al. measured the rCBV value of the tumor regions of interest (ROIs) 60 days before and 20–60 days after bevacizumab treatment based on a standardized T1-weighted image before and after contrast infusion in 36 recurrent high-grade gliomas patients. The rCBV maps before and after treatment were corrected for leakage and standardized to the same intensity scale (stdRCBV). The results showed that patients with a stdRCBV less than a specific value (the value was 4400 in the study by Schmainda et al.) both before and after treatment had a significantly longer OS, and patients with a stdRCBV less than this value after treatment had a longer PFS. The median OS was 395 days for patients with a stdRCBV less than the value both before and after treatment, whereas the median OS was only 100.5 days for patients with a stdRCBV greater than the value both before and after treatment. The median OS was 291 days for patients with a stdRCBV only greater than the value before treatment and 254 days for patients with a stdRCBV only greater than the value after treatment. The results suggest that the stdRCBV value before and after treatment may predict OS and PFS in recurrent high-grade glioma patients (Schmainda et al., 2014).
Several studies further pursued methods to predict the anti-angiogenic treatment response before initiation to select responders. Kickingereder et al. investigated pre-treatment leakage-corrected whole-brain rCBV maps based on DSC-MRI, contrast-enhancing T1-weighted and T2-weighted FLAIR images of 127 rGBM patients, 71 of whom received bevacizumab treatment and 56 of whom received alkylating chemotherapy without anti-angiogenic treatment. The authors determined that the patients with a pre-treatment rCBV < 3.92 had significantly longer OS and PFS than the patients with a pre-treatment rCBV > 3.92 in the bevacizumab group, whereas the rCBV values were not associated with outcome in the alkylating chemotherapy group. Further logistic regression analysis indicated that the accuracies for predicting 6-month PFS and 12-month OS were 81.7% and 78.9%, respectively, using the pre-treatment rCBV in patients on bevacizumab (Kickingereder et al., 2015a). In 2016, Bennett et al. reported similar results that indicated the pre-treatment rCBV value was significantly associated with OS (p = .031) (Bennett et al., 2017). Liu et al. determined that GBM patients with lower pre-treatment rCBV values had more favorable outcomes than patients with higher rCBV values, and the OS of the favorable outcome group was higher with use of anti-angiogenic drugs than with use of standard therapy (Liu et al., 2017).
These studies suggest that individual rCBV values before or after anti-angiogenic treatment are correlated with clinical outcome in GBM patients. Specifically, the pre-treatment rCBV value can predict the patient survival of under anti-angiogenic therapy and is highly valuable in guiding subsequent treatment.
3.1.2. Magnitude of change in the rCBV value before and after treatment
In 2015, Schmainda et al. analyzed DSC-MRI data from the randomized multicenter phase II clinical trial RTOG0625/ACRIN6677 to determine whether the rCBV change during the early phase of treatment was predictive of outcome. One hundred twenty-three rGBM patients received bevacizumab combined with irinotecan or TMZ therapy, among which 37 patients provided at least one DSC-MRI scan that could be normalized to white matter (rCBV) or standardized into stdRCBV on a same-intensity scale. The results showed that, in general, the rCBV and stdRCBV at 2 weeks, 8 weeks and 16 weeks post-treatment were lower than the pre-treatment value. The reductions in the rCBV and stdRCBV values were substantially greater for the patients who survived for one year or more than for the other patients, and the changes of rCBV at 2 weeks and stdRCBV at 16 weeks were statistically significant. Further analysis indicated a worse OS and shorter mean survival rates for patients with positive changes in the rCBV before and after treatment than for the other patients. The percentage change at 8 weeks after treatment showed the same trend; however, the result did not reach statistical significance (Schmainda et al., 2015). In 2016, Bennett et al. also reported that changes in the rCBV value could predict the survival of patients undergone bevacizumab therapy (with or without carboplatin). MRI imaging was performed an average of 24 days before treatment and 4 weeks and 8 weeks after treatment. Patients with greater reductions in rCBV before and after treatment had significantly longer median OS and PFS than other patients (Bennett et al., 2017). Hilario et al. detected the DSC-MRI-derived leakage from the subtraction between the maximum CBV and leakage-corrected CBV and found that the reduction in leakage at the first follow-up (compared with baseline leakage) was associated with a more favorable PFS and OS (Hilario et al., 2017).
These studies confirmed that the changes in the rCBV value could be used to identify patient subgroups that would respond better to bevacizumab therapy and that these changes could be used to develop individualized treatment plans according to the response during the early phase of treatment.
3.1.3. Other methods
Parameters that reflect the CBF status may also be used to predict the survival of patients under anti-angiogenic therapy. Harris et al. reported that in addition to rCBV, a higher relative CBF (rCBF) prior to bevacizumab treatment was associated with longer OS and PFS in rGBM patients and that a greater reduction in rCBF was also associated with longer OS (Harris et al., 2015). A 2016 study by Kickingereder et al. confirmed these findings, and the authors determined that baseline normalized rCBV, rCBF and parametric response maps from normalized rCBV and rCBF were predictive of PFS and OS (Kickingereder et al., 2016).
In addition to DSC-MRI, rCBF may be acquired using the arterial spin labeling technique (Weber et al., 2006), which is useful for detecting tumor progression and recurrence (Choi et al., 2013) and predicting patient outcome (Furtner et al., 2014). Lyu et al. first predicted the survival of patients under anti-angiogenic drugs using 3D fast spin echo (FSE) pseudocontinuous artery spin labeling (pcASL) to measure CBF. The authors measured CBF before and after treatment in 16 patients with recurrent high-grade glioma receiving bevacizumab-combined chemotherapy and determined that both rCBF with the cerebellar CBF as the reference before treatment and the average magnitude of the change in CBV (aCBF) before and after treatment were significantly associated with OS and PFS. The study suggested that the FSE pcASL technique might be useful for generating predictive images (Lyu et al., 2017).
Despite considerable evidence, cerebral blood volume imaging has its limitations when predicting the treatment effect of anti-angiogenic therapy, as there may be a variance in techniques at different institutes when generating cerebral blood volume imaging. In addition, most of the derived parameters are relative parameters instead of absolute parameters, which may produce variance in standardization and normalization and hinder the generalization of the reference values across institutes. Furthermore, several studies did not support the use of CBV-associated physiological parameters to predict the treatment effects of anti-angiogenic drugs. In 2015, Stadlbaure et al. determined that although quantification of CBV could confirm the changes in cerebral perfusion during anti-angiogenic treatment, aCBV and rCBV could not distinguish a false response in rGBM. The authors suggested that the quantification of oxygenation might be a more effective approach to evaluate the effects of anti-angiogenic drugs (Stadlbauer et al., 2015). Netto et al. also reported that changes in rCBV values by ferumoxytol-based DSC-MRI could not differentiate false progression during bevacizumab treatment, which may lead to misjudgments of the patient's condition (Netto et al., 2016). Nevertheless, studies have suggested that cerebral blood volume imaging (i.e., the rCBV) may be predictive of disease recurrence and the survival of patients with anti-angiogenic therapy.
3.2. Vessel architectural imaging
Physiological parameters generated from DSC-MRI and DCE-MRI may be used to evaluate vascular structures. Since 1990, DCE-MRI has been widely used to investigate the BBB (Larsson et al., 1990). T1-weighted images can be obtained from DCE-MRI before, during and after a bolus injection of a contrast agent. The contrast agent passes through the vascular wall and aggregates in the extracellular space when the BBB is compromised or when vascular permeability is increased (Bergamino et al., 2014). The DCE-MRI-generated physiological parameters included the following: Ktrans; the volume of extravascular extracellular space per unit volume of tissue (ve); the volume of blood plasma per unit volume of tissue (vp); and the rate constant between the extravascular extracellular space and plasma (Kep) (Bergamino et al., 2014). DCE-MRI parameters, particularly Ktrans, could be applied to drug pharmacokinetic studies, predictions of tumor prognosis, and evaluations of tumor recurrence and progression (Zhang et al., 2017).
In 2007, one study became the first investigation to report vascular normalization from 28 days to 4 months after the first dose of cediranib in rGBM patients who showed treatment responses (Batchelor et al., 2007). In 2014, Leu et al. analyzed CBV maps from DSC-MRI performed before and after treatment and determined that the pre- and post-bevacizumab treatment hypervascular area sizes were significantly associated with OS and PFS in rGBM patients. The authors suggested that a pre-treatment hypervascular area size >2.35cc, a post-treatment vascular area size >0.14cc and a size reduction<80% were significantly associated with poor PFS and OS (Leu et al., 2014). These findings demonstrated the potential predictive value of vascular structural changes for determining the glioma response to anti-angiogenic therapy.
3.2.1. Ktrans
Ktrans is a composite measure of permeability, capillary surface area and blood flow, and has been used to evaluate the effect of anti-angiogenic drugs in kidney cancer and lung cancer (Hsu et al., 2011; O'Connor and Jayson, 2012). In 2009, Zhang et al. became the first group to use DCE-MRI-generated Ktrans alone to predict the survival GBM patients treated with bevacizumab. The results showed that the pre-treatment Ktrans was associated with OS, whereas pre- and post-treatment changes in Ktrans were not significantly associated with OS or PFS (Zhang et al., 2009). In 2015, Kickingereder et al. specifically investigated the relationship between pre-treatment DCE-MRI-derived Ktrans and the survival of rGBM patients undergone bevacizumab. By setting Ktrans = 0.109/min as the threshold, the authors found that the PFS and OS were significantly longer in patients with lower Ktrans (Kickingereder et al., 2015b). In 2016, O'Neill et al. reported that DCE-MRI-derived Ktrans was more effective than FDG-PET and DW-MRI for predicting the GBM patient response to VEGF trap therapy (O'Neill et al., 2016).
3.2.2. Vascular normalization index
In 2009, Jain et al. treated 31 rGBM patients with cediranib and analyzed the Ktrans map by dynamic contrast-enhanced images. The researchers measured rCBV using dynamic susceptibility contrast imaging and the collagen IV levels of the peripheral blood by enzyme linked immunosorbent assay. The authors determined that the changes in Ktrans, rCBV and collagen IV levels after completing one cediranib treatment cycle (compared to the pre-treatment levels) were significantly associated with OS and PFS. The authors calculated the vascular normalization index (VNI) using the following equation:
Recursive partitioning analysis showed that the VNI values after completing one cediranib treatment cycle were significantly associated with PFS and OS and that a higher (positive) VNI value indicated prolonged PFS and OS. This study was the first investigation to show the predictive value of VNI for the survival of patients who undergone anti-angiogenic therapy (Sorensen et al., 2009).
3.2.3. Vessel architectural imaging
Jain et al. also assessed vascular normalization using a T2-weighted FLAIR image, the vascular permeability and the vessel size and measured the changes in oxygenation using vessel architectural imaging (VAI) (detailed in the subsequent paragraph). The authors determined that vascular normalization could improve tumor perfusion and thereby increase oxygenation, which could improve OS in patients with nGBM and rGBM. The authors hypothesized that anti-angiogenic therapy only benefited patients with observable vascular normalization (rather than vessel pruning) (Sorensen et al., 2012; Batchelor et al., 2013a) and that the underlying mechanism might be the higher oxygenation and more efficient drug delivery resulting from vascular normalization cause increased susceptibility of the tumor to chemotherapy. Furthermore, the tyrosine kinase inhibitor cediranib may have induced cell death. A vascular normalization-associated immune response might also play a role (Sorensen et al., 2012; Batchelor et al., 2013a). However, additional evidence is needed to prove this theory.
In 2013, Emblem et al. estimated the vessel architecture using the change in proton relaxation, which was calculated from the quotient of the contrast-enhanced gradient echo and spin echo. Typically, the gradient-echo signal peaks earlier than the spin-echo signal in areas with fast inflow of contrast agent (e.g., areas rich in arteriole-like vessels), whereas the gradient-echo signal peaks later than the spin-echo signal in areas with slow inflow of contrast agent (e.g., areas rich in venule-like vessels). When measured in a point-by point parametric plot of the gradient-echo signal and spin-echo signal, clockwise vortex can be observed if arterioles with large-caliber are included in the area, and counter-clockwise vortex can be detected in areas with large-caliber venules. The researcher demonstrated that anti-angiogenic drugs (cediranib) could decrease the vessel caliber and improve hemodynamic effects and oxygenation. Among 22 rGBM patients, 10 responders showed a relative increase in image voxels with a clockwise vortex direction (compared with the arithmetic mean of all patients), and 12 non-responders showed a relative decrease in image voxels. The responders had significantly higher OS and PFS rates than the non-responders (Emblem et al., 2013).
In 2013, LaViolette et al. measured the arterio-venous overlap (AVOL) by DSC-MRI to determine the efficacy of bevacizumab. The AVOL was calculated using binarized MELODIC-derived statistically thresholded arterial and venous maps. Eleven pairs of untreated tumor samples and normal samples were analyzed. The results showed that the total arterial volume was lower in the tumor samples than in the normal samples and that the percentage of AVOL was significantly higher in the tumor vasculature than in the normal vasculature. The median OS rate was significantly higher for recurrent high-grade glioma patients with reduced AVOL than the rate for patients with increased AVOL after bevacizumab treatment compared with the baseline (LaViolette et al., 2013).
In conclusion, vessel architectural imaging provides information on the vessel architecture, detects changes in vessel structures during anti-angiogenic therapy, and may be used to predict the treatment effect.
3.3. Diffusion coefficient imaging
DWI is another advanced MRI technique modality that is based on the Brownian movement of water. Biological macromolecules restrict the diffusion of water molecules. By applying a powerful magnetic gradient with an imaging sequence (e.g., echo planar imaging), transverse diffusion may be assessed, which, in turn, reveals the tissue integrity (Bammer, 2003). ADC, the most frequently used quantitative parameter in DWI, reflects the magnitude of water diffusion and is negatively correlated with the density of tumor cells. ADC has been applied in the settings of pseudoprogression and pseudoresponse, detecting tumor recurrence, grading tumors and monitoring the treatment effects of radiochemotherapy (Mabray et al., 2015).
3.3.1. Pre-treatment ADC values
In 2011, Pope et al. showed that the pre-treatment ADC value are associated with the effect of bevacizumab-combined radiochemotherapy in nGBM patients. The authors divided the ADC histogram into two partially overlapping normal distribution curves and discovered that patients with pre-treatment mean ADC values for the lower peak (ADCL) < 1200 mm2/s had significantly longer PFS than patients with an ADCL ≥ 1200 mm2/s (p = 0.008). Patient OS also showed the same trend (p = 0.055). However, the ADCL was not associated with PFS or OS in patients receiving standard radiochemotherapy (in which bevacizumab was added at the time of recurrence) (Pope et al., 2011). Multiple studies have subsequently suggested that the ADC value could predict the survival of rGBM patients treated with bevacizumab (Omuro et al., 2014; Rahman et al., 2014). Similarly, Wen et al. discovered that an ADC10% (ADC below this value in <10% volume) > 853 μm2/s was associated with longer PFS and OS in nGBM patients (Wen et al., 2015).
3.3.2. Magnitude of the change in ADC values before and after treatment
In 2011, Ellingson et al. used functional diffusion maps (fDMs) and ADC characteristics to predict the survival of rGBM patients undergone bevacizumab. The authors retrospectively investigated 77 rGBM patients and calculated traditional fDMs (single threshold) and graded fDMs based on the ADC change. The results indicated that patients with larger ADC reduction volumes had worse OS rates than patients with smaller ADC reduction volumes regardless of whether the ROI was defined by T2-weighted FLAIR or T1-weighted contrast-enhancing images and whether the threshold of ADC reduction was set to a fixed value (traditional fDMs) or a range of values (graded fDMs). Specifically, patients with a volume of tissue that exhibited a decrease in the ADC within the range of 0.25 and 0.4 μm2/ms larger than the group median of 1.5 cc within T1-weighted contrast-enhancing ROIs had a significantly shorter OS than patients with a lower volume (p < 0.0001) (Ellingson et al., 2011). Similarly, Wen et al. reported that a faster reduction in the ADC was associated with an earlier recurrence of nGBM (Wen et al., 2015). In 2012, Ellingson and Pope et al. further discovered that the pre-to-post-nonlinear fDMs applied in T2-weighted FLAIR-abnormal regions provided the best sensitivity and specificity in predicting the effect of bevacizumab in patients with rGBM (Ellingson et al., 2012). Hsu et al. simultaneously showed that the pre-to-post-bevacizumab-treatment ADC reduction was positively associated with tumor volume shrinkage in recurrent low-grade gliomas; however, the relationship between ADC reduction and outcome has not been reported (Hsu et al., 2015). Galla et al. investigated 65 rGBM patients treated with bevacizumab and determined that patients with greater pre-to-post-ADC reductions had lower 1-year OS rates (Galla et al., 2017).
3.3.3. Restriction Spectrum imaging
ADCs may be augmented because of interference from the diffusion signal from edema and tumor-related necrosis (Clarke and Chang, 2009; Ellingson et al., 2014), which significantly offsets the predictive value of ADCs for bevacizumab treatment. Restriction spectrum imaging (RSI), a new type of DWI technique, may indicate the tissue microarchitecture with high conspicuity based on the anisotropic movement of water molecules in separable microscopic tissue (Brunsing et al., 2017), and has been applied in the management of prostate cancer (Brunsing et al., 2017). Dale et al. first applied RSI to intracranial imaging in 2013. In contrast to the ADC, RSI was proportional to the density of the tumor cells (White et al., 2013). In the same year, Dale et al. assessed bevacizumab-treated patients with high-grade or metastatic gliomas using both RSI and ADC. The authors discovered that RSI was less influenced by false response reactions than ADC and that RSI might serve as a more accurate modality for the evaluation of the tumor response to anti-angiogenic therapy (Kothari et al., 2013).
In 2016, Macdonald et al. retrospectively assessed 40 patients with recurrent high-grade gliomas to determine the prognostic value of RSI compared with the ADC, T1-weighted contrast-enhanced MRI and T2-weight FLAIR in bevacizumab-treated patients. The results showed that a substantial increase in RSI and a substantial decrease in ADC in the high signal areas on T2-weighted FLAIR were associated with worse OS. A change of 0.120 in the 90th percentile of RSI on T2-weighted FLAIR imaging (RSI-FLAIR90%) was determined to best dichotomize patients into 2 groups for PFS, whereas a change of 250.0 in the 10th percentile of the ADC on T2-weighted FLAIR imaging (ADC-FLAIR10%) was determined to best dichotomize patients for PFS. The authors argued that RSI could improve the accuracy of predicting PFS and OS for bevacizumab therapy compared with standard DWI, T1-weighted contrast-enhanced MRI and T2-weighted FLAIR (McDonald et al., 2016).
In general, both the ADC and RSI may be able to describe the response to anti-angiogenic drugs on the basis of the diffusion of water molecules. These parameters are easily and readily available for extensive clinical applications.
4. Metabolic imaging
Metabolic reprogramming is a major characteristic of cancer (Hanahan and Weinberg, 2011) and alters glucose, amino acid and lipid metabolism within the tumor. Altered metabolism may be detected by two basic techniques: PET imaging with radioactive tracers (11C and 18F), which is very sensitive (10−11 to 10−12 mol/L, molecular probe 1–100 ng), and magnetic resonance spectroscopy (MRS), which distinguishes the chemical and molecular components of the tumor from those of the surrounding tissue with a relatively benign microenvironment according to the radiated frequency generated by the nuclear spins of magnetic resonance nuclei (e.g., 1H, 31P, or 13C) (Kim et al., 2016).
Developments in metabolic imaging are continuously emerging in the field of glioma. D-2-hydroxypentanedioic acid (D-2HG) accumulation caused by the IDH mutation may be detected and quantified by MRS, which can identify patients with the IDH1/2 mutation, and can serve as a prognostic factor for a favorable outcome (Emir et al., 2016; Natsumeda et al., 2014). PET or MRS imaging can also differentiate pseudoprogression from true tumor recurrence based on metabolic reprogramming during tumor recurrence (Okamoto et al., 2011; Galldiks et al., 2015).
4.1. Glucose metabolic imaging
As early as the middle of the 20th century, Warburg et al. discovered differences in glucose metabolism between tumor and normal tissues (Warburg, 1956a; Warburg, 1956b), and the association between glucose metabolism and patient outcome were reported for malignant gliomas (Zukotynski et al., 2013).
In 2012, Colavolpe et al. used fluorodeoxyglucose (FDG)-PET to investigate the outcome of recurrent high-grade glioma patients treated with chemotherapy+bevacizumab+irinotecan. These authors determined that the ratio of the maximal standardized uptake value (SUV) of the tumor and contralateral normal tissue (T:CL) had the same trend as OS and PFS (Colavolpe et al., 2012). In 2016, Park et al. suggested that the 13C-lactate-to-13C-bicarbonate ratio could be used as a prognostic index for the effect of anti-angiogenic treatment in rodent models.
4.2. Amino acid metabolic imaging
Studies regarding changes in amino acid metabolism have been performed for decades. Amino acids with altered metabolism within tumors include glutamine, glutamate, methionine, aspartate and tyrosine (Kim et al., 2016), among which aspartate and phenylalanine are predictive of the effects of anti-angiogenic drugs.
1H-MRS may be used to detect aspartate metabolism through N-acetylaspartate produced by aspartate and acetyl-CoA, which is a marker of neuronal injury (Rigotti et al., 2007). In 2011, Kim et al. showed that changes in the pre-to-post-treatment ratio of N-acetylaspartate/choline could predict the 6-month overall survival of rGBM patients treated with cediranib (Kim et al., 2011). In 2013, prospective studies from the RTOG0625/ACRIN6677 trial reported that changes in the N-acetylaspartate/choline ratio and the choline/creatine ratio could predict the outcome of rGBM patients treated with bevacizumab combined with TMZ and irinotecan therapy (Ratai et al., 2013).
In 2012, Harris et al. used 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (18F-FDOPA) and [18F]-fluoro-3-deoxy-3-L-fluorothymidine (18F-FLT) PET to evaluate the treatment response of bevacizumab. Twenty-four patients with recurrent high-grade gliomas underwent 18F-FDOPA and 18F-FLT PET at 1 week before, 1–2 weeks after, and 6–9 weeks after the initiation of bevacizumab treatment. The results showed that both the median absolute values and the percentage increase in the size of the positive area could stratify patient outcome. The outcome was worse for patients with larger increases in the positive area on the post-treatment 2nd-to-1st PET scan (Harris et al., 2012). In 2014, Schwarzenberg et al. reported using 18F-FDOPA to predict the survival of rGBM patients undergone bevacizumab. The authors identified a significant pre-to-post-treatment reduction in the 18F-FDOPA-positive tumor volume. PFS and OS were significantly longer for patients with a 35% or more reduction in tumor volume that showed 18F-FDOPA uptake at the 2nd week after initiation of bevacizumab (Schwarzenberg et al., 2014).
5. Discussion
Data supporting the importance of brain imaging, with capabilities to locate the injury site, guide surgical biopsy, assist the surgical and radiotherapy protocol, and evaluate the tumor progression and response to treatment, are continuously emerging in the glioma field (Di Stefano et al., 2015). However, the prevailing imaging criteria (e.g., the RANO criteria) may be insufficient for sustained monitoring and precise outcome predictions of anti-angiogenic therapy (Schmainda et al., 2015; Clarke and Chang, 2009; Boxerman et al., 2013). Novel imaging techniques, represented by PWI (including DSC-MRI and DCE-MRI), DWI, RSI, PET, and MRS, produce quantitative parameters, such as rCBV, Ktrans, VNI, ADC, and SUV, to better illustrate the physiological conditions of glioma and better reflect the ‘true’ response to anti-angiogenic therapy. Despite the retrospective nature of current studies, specific populations of glioma patients may benefit from anti-angiogenic therapy (Sandmann et al., 2015; Tamura et al., 2017). Therefore, imaging biomarkers, which can be acquired noninvasively, may play an important role in subpopulation selection. The major imaging biomarkers and their indications for better prognosis are detailed in Table 2.
Table 2.
Major imaging biomarkers and indicators for better prognosis.
| Imaging approaches | Indicators for better prognosis | Details |
|---|---|---|
| Cerebral blood volume imaging | Pre-treatment rCBV ↓ | Pre-treatment rCBV < 3.92 indicates significantly longer OS and PFS in rGBM patients with BEV (Kickingereder et al., 2015a); pre-treatment standardized rCBV less than a certain value demonstrates longer OS in rHGG patients with BEV (Schmainda et al., 2014). |
| Post-treatment rCBV ↓ | Post-treatment standardized rCBV less than the certain value suggests longer OS and PFS in rHGG patients with BEV (Schmainda et al., 2014). | |
| △rCBV ↓ | △rCBV < 0 represents better OS in rGBM patients with BEV (Schmainda et al., 2015); rGBM patients with greater rCBV reduction (△rCBV ↓) show significantly longer PFS and OS when treating with BEV ± carboplatin (Bennett et al., 2017). | |
| Vessel architectural imaging | Pre-treatment Ktrans ↓ | rGBM patients with pre-treatment Ktrans < 0.109/min reach significantly longer PFS and OS when treating with BEV(Kickingereder et al., 2015b). |
| Post-treatment VNI ↑ | Higher VNI is correlated with better PFS and OS in rGBM patients after 1 cycle of cediranib (Sorensen et al., 2009). | |
| Vascular normalization ↑ | nGBM and rGBM patients with vascular normalization after cediranib have longer OS (Sorensen et al., 2012; Batchelor et al., 2013a); rGBM patients with vascular normalization respond to cediranib and therefore reach significantly longer OS and PFS(Emblem et al., 2013). | |
| △AVOL ↓ | rHGG patients with reduced AVOL have significantly better OS than patients with increased AVOL after BEV (LaViolette et al., 2013). | |
| Diffusion coefficient imaging | Pre-treatment mean ADCL ↓ | Pre-treatment ADCL < 1200 mm2/s indicates longer PFS and OS in nGBM patients with BEV + radiochemotherapy (Pope et al., 2011). |
| △Volume of ADC ↓ | When treating with BEV, rGBM patients having a volume of tissue exhibiting a decrease in ADC within the range of 0.25 and 0.4 μm2/ms larger than 1.5 cc had significantly shorter survival than patients having a lower volume (Ellingson et al., 2011). | |
| Metabolic imaging | Post-treatment FDG T:CL ↑ | The FDG T:CL has the same trend as OS and PFS in rHGG patients with chemotherapy+BEV + irinotecan. |
| △Volume of 18F-FDOPA ↓ | PFS and OS of rGBM patients were significantly longer for patients with 35% or more reductions in tumor volume that showed 18F-FDOPA uptake after the initiation of BEV (Schwarzenberg et al., 2014). |
Abbreviations: rCBV, relative cerebral blood volume; Ktrans, volume transfer constant between blood plasma and extravascular extracellular space; VNI, vascular normalization index; AVOL, arterio-venous overlap; ADC, apparent diffusion coefficient; ADCL, ADC values for the lower peak; ADC10%, ADC below this value in <10% volume; FDG, fluorodeoxyglucose; T:CL, the ratio of the maximal standardized uptake value of the tumor and contralateral normal tissue; 18F-FDOPA, 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine; PFS, progression-free survival; OS, overall survival; rHGG, recurrent high-grade glioma; rGBM, recurrent glioblastoma; nGBM, newly diagnosed glioblastoma; BEV, bevacizumab.
Despite the reasonable evidence for utilizing imaging biomarkers in anti-angiogenic therapy, challenges remain as a result of the interactions of multiple factors, including the diversity of imaging equipment (e.g., the field strength of magnetic resonance scanners), imaging agents (e.g., the type of gadolinium-based contrast agents), imaging acquisition protocols (e.g., the administration rate of imaging agents), and post-processing techniques (e.g., leakage correction). These differences across institutions impede the replication of single-center study results and act as obstacles to launching multicenter clinical trials (Zhang et al., 2017). Although standard specifications, such as the Brain Tumor Imaging Protocol, have been put forth, significant time and effort are required prior to implementation (Ellingson et al., 2015; Welker et al., 2015; Anzalone et al., 2018). Moreover, as most studies are retrospective investigations with modest patient sizes and the study results have not been corrected for confounds that may influence outcomes (e.g., WHO status, patient age, pre-treatment tumor volume, or steroid usage), the reliability and reproducibility of existing evidence must be critically evaluated.
In addition to imaging biomarkers, several non-quantitative imaging characteristics may indicate the prognosis of patients in anti-angiogenic therapy. Nowosielski et al. identified 5 radiologic progression types (namely, classic T1, cT1 relapse, T2 diffuse, T2 circumscribed, and primary nonresponder) of nGBM patients undergoing therapy from the AVAglio trial and demonstrated that the ‘T2 diffuse’ type and frequent MGMT promoter methylation were associated with the longest PFS and OS (Nowosielski et al., 2018). Brandes et al. suggested early tumor shrinkage in T1-weighted contrast enhancement or T2-weighted FLAIR images as an indicator of prognosis in rGBM patients treated with bevacizumab (Brandes et al., 2017). Furthermore, radiomics, which extract and analyze quantitative imaging features with high throughput from medical images, may play certain roles in the guidance of anti-angiogenic therapy of glioma.
As both imaging biomarkers and molecular biomarkers can predict the treatment outcome of anti-angiogenic therapy, the correlations among these markers are notable. In the study by Pope et al., the ADCL was significantly lower in patients who were positive for MGMT promoter methylation than in patients who were negative for MGMT promoter methylation (Pope et al., 2011). Given that MGMT promoter methylation is associated with the treatment response to TMZ in nGBM patients, the predictive mechanism of established molecular biomarkers may explain the prediction principles of imaging biomarkers and thus indicate a potential approach for the detection of molecular markers using imaging parameters, which may result in a novel detection method for molecular pathology.
The investigation of imaging biomarkers may promote the development of anti-angiogenic therapy. The maximal tolerated dose of bevacizumab is approximately 15 to 20 mg/kg, and a meta-analysis has reported no beneficial difference between 5 mg/kg and 10–15 mg/kg9, which suggests that the optimal dose of bevacizumab remains unknown. Similar observations have been made regarding the bevacizumab treatment duration, which ranges from 1 cycle to several years. However, biomarkers, when validated, not only can be used to monitor therapeutic effects but also to provide guidance for the dose and duration of the anti-angiogenic therapy, which may vary from person to person, to obtain the optimal treatment effect. Moreover, the Pope et al. study indicates that among patients with a mean ADCL ≥ 1200 mm2/s, the patients who received bevacizumab combined with radiochemotherapy had significantly shorter OS than the patients who received standard radiochemotherapy (in which bevacizumab was added at the time of recurrence) (Pope et al., 2011), thus highlighting the importance of imaging biomarkers for personalized therapy. Furthermore, in consideration of the chemosensitization, radiosensitization, and immune surveillance enhancement produced by anti-angiogenic therapy, combined therapy is a future direction (Sorensen et al., 2012). Therefore, an avenue of future research would be to monitor the treatment response in joint treatment related to anti-angiogenic therapy.
Despite the abundance of evidence that supports the utilization of existing biomarkers and suggests new biomarkers, no single biomarker with sufficient sensitivity and specificity has been generally recognized for anti-angiogenic therapy of glioma because the introduction of biomarkers always lags behind the drug-developmental process. Although major studies have focused on the development of a single biomarker, a combination of several simple, easily obtainable biomarkers may provide a more efficient novel marker (e.g., the VNI in combination with Ktrans, rCBV, and collagen IV levels (Sorensen et al., 2009)). However, it should be noted that false positive findings may be a concern because of inadequate patient numbers, particularly when investigating combined markers. Thus, further prospective studies with larger cohorts are required to consolidate and confirm the current evidence, explain the mechanism of the observed changes in these biomarkers, and identify novel biomarkers.
Acknowledgments
Acknowledgement
The authors thank American Journal Experts for providing language help.
Funding
This work was supported by Chinese Academy of Medical Sciences [grant numbers 2016-I2M-2-001]; and 2016 PUMCH Science Fund for Junior Faculty [grant numbers pumch-2016-2.19].
Declarations of interest
None.
Contributor Information
Yu Wang, Email: ywang@pumch.cn.
Renzhi Wang, Email: wangrenzhi@pumch.cn.
Feng Feng, Email: ffeng@pumch.cn.
Wenbin Ma, Email: mawb2001@hotmail.com, mawb@pumch.cn.
References
- Anzalone N., Castellano A., Cadioli M. Brain gliomas: multicenter standardized assessment of dynamic contrast-enhanced and dynamic susceptibility contrast MR images. Radiology. 2018:170362. doi: 10.1148/radiol.2017170362. [DOI] [PubMed] [Google Scholar]
- Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45(3):169–184. doi: 10.1016/s0720-048x(02)00303-0. [DOI] [PubMed] [Google Scholar]
- Barker P.B., Golay X., Zaharchuk G. 2013. Clinical Perfusion MRI: Techniques and Applications. [Google Scholar]
- Batchelor T.T., Sorensen A.G., di Tomaso E. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor T.T., Gerstner E.R., Emblem K.E. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110(47):19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor T.T., Mulholland P., Neyns B. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett I.E., Field K.M., Hovens C.M. Early perfusion MRI predicts survival outcome in patients with recurrent glioblastoma treated with bevacizumab and carboplatin. J Neurooncol. 2017;131(2):321–329. doi: 10.1007/s11060-016-2300-0. [DOI] [PubMed] [Google Scholar]
- Bergamino M., Bonzano L., Levrero F., Mancardi G.L., Roccatagliata L. A review of technical aspects of T1-weighted dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in human brain tumors. Phys Med. 2014;30(6):635–643. doi: 10.1016/j.ejmp.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Boxerman J.L., Zhang Z., Safriel Y. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–954. doi: 10.1093/neuonc/not049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes A.A., Finocchiaro G., Zagonel V. Early tumour shrinkage as a survival predictor in patients with recurrent glioblastoma treated with bevacizumab in the AVAREG randomized phase II study. Oncotarget. 2017;8(33):55575–55581. doi: 10.18632/oncotarget.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsing R.L., Schenker-Ahmed N.M., White N.S. Restriction spectrum imaging: An evolving imaging biomarker in prostate MRI. J Magn Reson Imaging. 2017;45(2):323–336. doi: 10.1002/jmri.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner J.C., Shaw E.G., Pugh S.L. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–1355. doi: 10.1056/NEJMoa1500925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burth S., Kickingereder P., Eidel O. Clinical parameters outweigh diffusion- and perfusion-derived MRI parameters in predicting survival in newly diagnosed glioblastoma. Neuro Oncol. 2016;18(12):1673–1679. doi: 10.1093/neuonc/now122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinot O.L., Wick W., Mason W. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- Choi Y.J., Kim H.S., Jahng G.H., Kim S.J., Suh D.C. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol. 2013;54(4):448–454. doi: 10.1177/0284185112474916. [DOI] [PubMed] [Google Scholar]
- Clarke J.L., Chang S. Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep. 2009;9(3):241–246. doi: 10.1007/s11910-009-0035-4. [DOI] [PubMed] [Google Scholar]
- Colavolpe C., Chinot O., Metellus P. FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14(5):649–657. doi: 10.1093/neuonc/nos012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A.L., Fucci A., Frattini V. Detection, characterization, and inhibition of FGFR-TACC fusions in IDH wild-type glioma. Clin Cancer Res. 2015;21(14):3307–3317. doi: 10.1158/1078-0432.CCR-14-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Cloughesy T.F., Lai A. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(10):1151–1161. doi: 10.1093/neuonc/nor079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Cloughesy T.F., Lai A., Nghiemphu P.L., Pope W.B. Nonlinear registration of diffusion-weighted images improves clinical sensitivity of functional diffusion maps in recurrent glioblastoma treated with bevacizumab. Magn Reson Med. 2012;67(1):237–245. doi: 10.1002/mrm.23003. [DOI] [PubMed] [Google Scholar]
- Ellingson B.M., Kim H.J., Woodworth D.C. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. doi: 10.1148/radiol.13131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson B.M., Bendszus M., Boxerman J. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro Oncol. 2015;17(9):1188–1198. doi: 10.1093/neuonc/nov095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emblem K.E., Mouridsen K., Bjornerud A. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19(9):1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emir U.E., Larkin S.J., de Pennington N. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer Res. 2016;76(1):43–49. doi: 10.1158/0008-5472.CAN-15-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H.S., Prados M.D., Wen P.Y. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- Furtner J., Bender B., Braun C. Prognostic value of blood flow measurements using arterial spin labeling in gliomas. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0099616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla N., Chiang G., Chakraborty S. Apparent diffusion coefficient changes predict survival after intra-arterial bevacizumab treatment in recurrent glioblastoma. Neuroradiology. 2017;59(5):499–505. doi: 10.1007/s00234-017-1820-4. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Dunkl V., Stoffels G. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42(5):685–695. doi: 10.1007/s00259-014-2959-4. [DOI] [PubMed] [Google Scholar]
- Gilbert M.R., Dignam J.J., Armstrong T.S. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. The Hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harris R.J., Cloughesy T.F., Pope W.B. 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. doi: 10.1093/neuonc/nos141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.J., Cloughesy T.F., Hardy A.J. MRI perfusion measurements calculated using advanced deconvolution techniques predict survival in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2015;122(3):497–505. doi: 10.1007/s11060-015-1755-8. [DOI] [PubMed] [Google Scholar]
- Hilario A., Sepulveda J.M., Hernandez-Lain A. Leakage decrease detected by dynamic susceptibility-weighted contrast-enhanced perfusion MRI predicts survival in recurrent glioblastoma treated with bevacizumab. Clin Transl Oncol. 2017;19(1):51–57. doi: 10.1007/s12094-016-1502-4. [DOI] [PubMed] [Google Scholar]
- Hsu C.Y., Shen Y.C., Yu C.W. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55(4):858–865. doi: 10.1016/j.jhep.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Hsu C.H., Lober R.M., Li M.D. Decreased tumor apparent diffusion coefficient correlates with objective response of pediatric low-grade glioma to bevacizumab. J Neurooncol. 2015;122(3):491–496. doi: 10.1007/s11060-015-1754-9. [DOI] [PubMed] [Google Scholar]
- Jiang T., Mao Y., Ma W. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2016;375(2):263–273. doi: 10.1016/j.canlet.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Kickingereder P., Wiestler B., Burth S. Relative cerebral blood volume is a potential predictive imaging biomarker of bevacizumab efficacy in recurrent glioblastoma. Neuro Oncol. 2015;17(8):1139–1147. doi: 10.1093/neuonc/nov028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickingereder P., Wiestler B., Graf M. Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2015;121(2):373–380. doi: 10.1007/s11060-014-1644-6. [DOI] [PubMed] [Google Scholar]
- Kickingereder P., Radbruch A., Burth S. MR perfusion-derived hemodynamic parametric response mapping of bevacizumab efficacy in recurrent glioblastoma. Radiology. 2016;279(2):542–552. doi: 10.1148/radiol.2015151172. [DOI] [PubMed] [Google Scholar]
- Kim H., Catana C., Ratai E.M. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 2011;71(11):3745–3752. doi: 10.1158/0008-5472.CAN-10-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.M., Parolia A., Dunphy M.P., Venneti S. Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nat Rev Clin Oncol. 2016;13(12):725–739. doi: 10.1038/nrclinonc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothari P.D., White N.S., Farid N. Longitudinal restriction spectrum imaging is resistant to pseudoresponse in patients with high-grade gliomas treated with bevacizumab. AJNR Am J Neuroradiol. 2013;34(9):1752–1757. doi: 10.3174/ajnr.A3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl T.N., Kim L., Moore K. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson H.B., Stubgaard M., Frederiksen J.L., Jensen M., Henriksen O., Paulson O.B. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990;16(1):117–131. doi: 10.1002/mrm.1910160111. [DOI] [PubMed] [Google Scholar]
- LaViolette P.S., Cohen A.D., Prah M.A. Vascular change measured with independent component analysis of dynamic susceptibility contrast MRI predicts bevacizumab response in high-grade glioma. Neuro Oncol. 2013;15(4):442–450. doi: 10.1093/neuonc/nos323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu K., Enzmann D.R., Woodworth D.C. Hypervascular tumor volume estimated by comparison to a large-scale cerebral blood volume radiographic atlas predicts survival in recurrent glioblastoma treated with bevacizumab. Cancer Imaging. 2014;14:31. doi: 10.1186/s40644-014-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.T., Achrol A.S., Mitchell L.A. Magnetic resonance perfusion image features uncover an angiogenic subgroup of glioblastoma patients with poor survival and better response to antiangiogenic treatment. Neuro Oncol. 2017;19(7):997–1007. doi: 10.1093/neuonc/now270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y., Liu S., You H. Evaluation of recurrent high-grade gliomas treated with bevacizumab: A preliminary report of 3D pseudocontinuous artery spin labeling. J Magn Reson Imaging. 2017;46(2):565–573. doi: 10.1002/jmri.25558. [DOI] [PubMed] [Google Scholar]
- Mabray M.C., Barajas R.F., Cha S. Modern brain tumor imaging. Brain Tumor Res Treat. 2015;3(1):8–23. doi: 10.14791/btrt.2015.3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald D.R., Cascino T.L., Schold S.C., Cairncross J.G. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- McDonald C.R., Delfanti R.L., Krishnan A.P. Restriction spectrum imaging predicts response to bevacizumab in patients with high-grade glioma. Neuro Oncol. 2016;18(11):1579–1590. doi: 10.1093/neuonc/now063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsumeda M., Igarashi H., Nomura T. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: a study by 3.0-tesla magnetic resonance spectroscopy. Acta Neuropathol Commun. 2014;2:158. doi: 10.1186/s40478-014-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netto J.P., Schwartz D., Varallyay C., Fu R., Hamilton B., Neuwelt E.A. Misleading early blood volume changes obtained using ferumoxytol-based magnetic resonance imaging perfusion in high grade glial neoplasms treated with bevacizumab. Fluids Barriers CNS. 2016;13(1):23. doi: 10.1186/s12987-016-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowosielski M., Ellingson B.M., Chinot O.L. Radiologic progression of glioblastoma under therapy - an exploratory analysis of AVAglio. Neuro Oncol. 2018;20(4):557–566. doi: 10.1093/neuonc/nox162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor J.P., Jayson G.C. Do imaging biomarkers relate to outcome in patients treated with VEGF inhibitors? Clin Cancer Res. 2012;18(24):6588–6598. doi: 10.1158/1078-0432.CCR-12-1501. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Shiga T., Hattori N. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann Nucl Med. 2011;25(3):213–220. doi: 10.1007/s12149-010-0450-2. [DOI] [PubMed] [Google Scholar]
- Omuro A., DeAngelis L.M. Glioblastoma and other malignant gliomas: A clinical review. Jama. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Omuro A., Beal K., Gutin P. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. doi: 10.1158/1078-0432.CCR-14-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill A.F., Qin L., Wen P.Y., de Groot J.F., Van den Abbeele A.D., Yap J.T. Demonstration of DCE-MRI as an early pharmacodynamic biomarker of response to VEGF Trap in glioblastoma. J Neurooncol. 2016;130(3):495–503. doi: 10.1007/s11060-016-2243-5. [DOI] [PubMed] [Google Scholar]
- Pope W.B., Lai A., Mehta R. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol. 2011;32(5):882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman R., Hamdan A., Zweifler R. Histogram analysis of apparent diffusion coefficient within enhancing and nonenhancing tumor volumes in recurrent glioblastoma patients treated with bevacizumab. J Neurooncol. 2014;119(1):149–158. doi: 10.1007/s11060-014-1464-8. [DOI] [PubMed] [Google Scholar]
- Ratai E.M., Zhang Z., Snyder B.S. Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677. Neuro Oncol. 2013;15(7):936–944. doi: 10.1093/neuonc/not044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti D.J., Inglese M., Gonen O. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR Am J Neuroradiol. 2007;28(10):1843–1849. doi: 10.3174/ajnr.A0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann T., Bourgon R., Garcia J. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. doi: 10.1200/JCO.2015.61.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawlani R.N., Raizer J., Horowitz S.W. Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging--pilot study. Radiology. 2010;255(2):622–628. doi: 10.1148/radiol.10091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmainda K.M., Prah M., Connelly J. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–888. doi: 10.1093/neuonc/not216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmainda K.M., Zhang Z., Prah M. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015;17(8):1148–1156. doi: 10.1093/neuonc/nou364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberg J., Czernin J., Cloughesy T.F. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. doi: 10.1158/1078-0432.CCR-13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A.G., Batchelor T.T., Zhang W.T. A "vascular normalization index" as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A.G., Emblem K.E., Polaskova P. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer A., Pichler P., Karl M. Quantification of serial changes in cerebral blood volume and metabolism in patients with recurrent glioblastoma undergoing antiangiogenic therapy. Eur J Radiol. 2015;84(6):1128–1136. doi: 10.1016/j.ejrad.2015.02.025. [DOI] [PubMed] [Google Scholar]
- Taal W., Oosterkamp H.M., Walenkamp A.M. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- Tamura R., Tanaka T., Miyake K., Yoshida K., Sasaki H. Bevacizumab for malignant gliomas: current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017;34(2):62–77. doi: 10.1007/s10014-017-0284-x. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- Weber M.A., Zoubaa S., Schlieter M. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology. 2006;66(12):1899–1906. doi: 10.1212/01.wnl.0000219767.49705.9c. [DOI] [PubMed] [Google Scholar]
- Welker K., Boxerman J., Kalnin A., Kaufmann T., Shiroishi M., Wintermark M.A.S.F.N.R. Recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. Am J Neuroradiol. 2015;36(6):E41–E51. doi: 10.3174/ajnr.A4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen P.Y., Macdonald D.R., Reardon D.A. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- Wen Q., Jalilian L., Lupo J.M. Comparison of ADC metrics and their association with outcome for patients with newly diagnosed glioblastoma being treated with radiation therapy, temozolomide, erlotinib and bevacizumab. J Neurooncol. 2015;121(2):331–339. doi: 10.1007/s11060-014-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White paper on imaging biomarkers Insights Imaging. 2010;1(2):42–45. doi: 10.1007/s13244-010-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N.S., McDonald C.R., Farid N., Kuperman J.M., Kesari S., Dale A.M. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using "restriction spectrum imaging": quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013;34(5):958–964. doi: 10.3174/ajnr.A3327. s951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W., Roth P., Hartmann C. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neurol Oncol. 2016;18(11):1529–1537. doi: 10.1093/neuonc/now133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W., Gorlia T., Bendszus M. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. doi: 10.1056/NEJMoa1707358. [DOI] [PubMed] [Google Scholar]
- Wong E.T., Gautam S., Malchow C., Lun M., Pan E., Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. doi: 10.6004/jnccn.2011.0037. [DOI] [PubMed] [Google Scholar]
- Zhang W., Kreisl T., Solomon J. Paper presented at: Proc. Intl. Soc. Mag. Reson. Med. 2009. Acute effects of bevacizumab on glioblastoma vascularity assessed with DCE-MRI and relation to patient survival. [Google Scholar]
- Zhang J., Liu H., Tong H. Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: recent advances and current challenges. Contrast Media Mol Imaging. 2017;2017:7064120. doi: 10.1155/2017/7064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukotynski K.A., Fahey F.H., Vajapeyam S. Exploratory evaluation of MR permeability with 18F-FDG PET mapping in pediatric brain tumors: a report from the Pediatric Brain Tumor Consortium. J Nucl Med. 2013;54(8):1237–1243. doi: 10.2967/jnumed.112.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga R.M., Torcuator R., Jain R. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]