Abstract
Major depressive disorder (MDD) and bipolar disorder (BD) are common severe affective diseases. Although previous neuroimaging studies have investigated brain abnormalities in MDD or BD, the structural and functional differences between these two disorders remain unclear. In this study, we adopted a multimodal approach, combining voxel-based morphometry (VBM) and functional connectivity (FC), to study the common and distinct structural and functional alterations in unmedicated MDD and BD patients. The VBM analysis revealed that both the MDD and BD patients showed decreased gray matter volume (GMV) in the left anterior cingulate cortex (ACC_L) and right hippocampus (HIP_R) compared with the healthy controls, and the MDD patients showed decreased GMV in the left superior frontal gyrus (SFG_L) and ACC_L compared with the BD patients. Furthermore, we took these clusters as seed regions to analyze the abnormal resting-state functional connectivity (RSFC) in the patients. We found that both the MDD and BD groups had decreased RSFC between the ACC_L and the left orbitofrontal cortex (OFC_L) and that the MDD group had decreased RSFC between the SFG_L and the HIP_L, compared with the healthy controls. Our results revealed that the MDD and BD patients were more similar than different in GMV and RSFC. These findings indicate that investigating the frontal-limbic system could be useful for understanding the underlying mechanisms of these two disorders.
Keywords: Affective disorder, Multimodal, Voxel-based morphometry, Functional connectivity
Abbreviations: VBM, voxel-based morphometry; RSFC, resting-state functional connectivity; GMV, gray matter volume; R-fMRI, Resting-state fMRI; HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid; dmPFC, dorsomedial prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; ACC, anterior cingulate cortex; SFG, superior frontal gyrus; ORBmid, orbital part middle frontal gyrus; ORBsup, orbital part superior frontal gyrus; OFC, orbitofrontal cortex; HIP, hippocampus; THA, thalamus
Highlights
-
•
Both MDD and BD patients had reduced GMV in the ACC_L and HIP_R compared with HC.
-
•
MDD patients had decreased GMV in the ACC_L and SFG_L compared with BD patients.
-
•
Both BD and MDD patients had decreased ACC-OFC RSFC compared with HC.
-
•
The MDD and BD patients were more similar than different in GMV and RSFC.
1. Introduction
Major depressive disorder (MDD) and bipolar disorder (BD) are common, severe affective diseases that affect people throughout the world (Gonda et al., 2012; Murray et al., 2013). Because of the high prevalence of depressive symptoms and the similarity to MDD, BD patients are difficult to diagnose correctly in clinical practice (Grande et al., 2016). Only 20% of BD patients with a depressive episode are diagnosed accurately within the first year of seeking treatment (Hirschfeld et al., 2003) and the mean delay is about 5–10 years from onset to correct diagnosis (Baldessarini et al., 2007). These misdiagnoses lead to inappropriate treatment and devastating consequences for BD patients (Phillips and Kupfer, 2013). Fortunately, the development of neuroimaging techniques has provided opportunities to deepen our knowledge of the mechanisms underpinning affective disorders.
Neuroimaging methods have been widely adopted to study brain structural and functional alterations in affective disorders. Several studies that used voxel-based morphometry (VBM) approach have revealed abnormal gray matter volume (GMV) in MDD in various regions involved in emotion processing, such as the prefrontal cortex (PFC) (Salvadore et al., 2011), anterior cingulate cortex (ACC) (Machino et al., 2014), hippocampus (Zou et al., 2010), and amygdala (Frodl et al., 2008). In BD patients, abnormal GM has been reported primarily in the PFC (Ganzola and Duchesne, 2017), ACC (Bora et al., 2010), and temporal regions (Selvaraj et al., 2012). We noticed that most of the previous studies focused on the difference in GMV between the patients and healthy subjects, but very few studies (Redlich et al., 2014; Cintia et al., 2015) directly compared the MDD and BD patients from a structural perspective. Redlich et al. (2014) detected decreased GMV in the ACC, hippocampus, and amygdala, while Cintia et al. (2015) observed increased GMV in the dorsolateral prefrontal cortex (DLPFC), in BD patients compared with MDD patients.
Functional neuroimaging methods have also been used to study brain functional abnormality in BD and MDD patients. Dysfunction in BD or MDD patients has been detected based on resting-state fMRI (R-fMRI) (Brady et al., 2017; Zhu et al., 2018) and task-fMRI (T-fMRI) techniques (Erk et al., 2010; Townsend et al., 2013). Several previous studies (Hamilton et al., 2012b; Townsend and Altshuler, 2012; Rive et al., 2013) reported abnormal functional activation in both MDD and BD patients in the frontal-limbic system, which is believed to support emotion processing and regulation (e.g., ACC and amygdala), reward processing (e.g., OFC and striatum), and cognitive control (e.g., DLPFC and VLPFC). However, the results from the very few studies that have used fMRI to directly compare the brain activity of MDD with that of BD patients have not always been consistent. For instance, Zhang et al. (2017) found increased fractional amplitude of low-frequency fluctuations (fALFF) in the SFG and putamen, whereas Yu et al. (2017) detected lower fALFF in the MFG, MTG, and MOG in BD compared with MDD patients. Regarding emotion processing, Fournier et al. (2013) observed lower amygdala activation, while Grotegerd et al. (2014) found greater amygdala activation when responding to negative facial stimuli in BD patients compared with MDD patients.
Multimodal techniques that combine structural and functional methods may provide even more useful information for clinical diagnosis. For example, by combining cortical thickness and functional connectivity (FC) analyses in MDD patients, Späti et al. (2015) found indications that PFC thinning may impair the engagement of the ACC during depressive episodes, and Van Tol et al. (2014) found that cortical thickness of the dmPFC could be used to predict the RSFC between the dmPFC and the default mode network (e.g., the precuneus). The combination of VBM and FC also has been adopted to study schizophrenia (Zhang et al., 2015), temporal lobe epilepsy (Doucet et al., 2016), and Parkinson's disease (Canu et al., 2015). Until now, as far as we know, no prior study has investigated the brain abnormalities in BD or MDD patients by combining VBM and FC analyses.
Our goal in this study was to detect common and distinct brain structural and functional alterations between patients with BD and MDD based on brain structural and functional images. Because medication use and different subtypes of patients can confound the findings, all the patients recruited in the present study were unmedicated and the individuals with BD were type II. In the calculations, we first determined the areas with abnormal GMV and then estimated their resting-state functional connectivity (RSFC) with each voxel throughout the whole brain. Based on the structural and functional abnormalities reported in a previous study (Cardoso De Almeida and Phillips, 2013), we hypothesized that the altered regions in BD and MDD would be primarily located in the prefrontal and limbic areas involving in emotional processing.
2. Methods
2.1. Subjects
A total of 79 unmedicated, currently depressed patients (18–50 years old), including 36 MDD patients (17 M/26F, 27.9 ± 9.1 years old) and 43 BD II patients (20 M/16F, 30.5 ± 8.5 years old), were recruited from the Psychiatry Department of the First Affiliated Hospital of Jinan University (JNU), Guangzhou, China, from November 2013 to October 2016. The diagnoses of BD and MDD were made by two experienced psychiatrists (Y.J. and S.Z.) according to the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P). For each patient, the clinical symptoms were assessed according to the 24-item Hamilton Depression Rating Scale (HDRS) (Williams, 1988) and the Young Mania Rating Scale (YMRS) (Young et al., 1978) during the 7-day period before the scanning. The inclusion criteria for the MDD patients were a total HDRS score > 21, while for the BD patients they were a total YMRS score < 7 and a total HDRS score > 21. In this study, we excluded patients with (1) any other Axis-I psychiatric disorders or (2) a history of organic brain disorders, neurological disorders, mental retardation, cardiovascular diseases, alcohol/substance abuse, pregnancy, or any physical illness. None of the patients had ever received electroconvulsive therapy prior to participating in the study. All of the patients were either medication-naïve or not medicated for at least 6 months at the time of the scanning. Twenty-nine patients (18 MDD and 11 BD) were medication naïve because they had never been diagnosed before or did not want to take medication. While for the other recruited patients, they generally visited their physicians (psychiatrist/general practitioner) because of depressive relapse after quitting medication. Among them, 18 patients with MDD had been treated with antidepressants (duloxetine or paroxetine), while 32 patients with BD had been treated with antidepressants (duloxetine or paroxetine) and/or mood stabilizers (lithium, sodium valproate) and/or atypical antipsychotic medications (olanzapine or risperidone). For these fifty patients, they had been off-medication for at least 6 months prior to the scan and were therefore currently unmedicated.
In addition, we also recruited 47 age- and gender-matched healthy subjects (22 M/25F, 29.7 ± 9.2 years old) as the control group. The healthy controls (HC) were included if they: (1) were 18–50 years old, (2) fulfilled the diagnostic criteria of the Structured Clinical Interview for DSM-IV non-patient Edition (SCID-NP), (3) were without any current or past significant medical or neurological illness, and (4) were without a history of psychiatric illness in his/her first-degree relatives.
All subjects included in this study were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). The study was approved by the Ethics Committee of the First Affiliated Hospital of JNU. Each subject provided written consent for participation after a full written and verbal explanation of the study.
2.2. Image acquisition
All the subjects were scanned on a 3.0 T GE Discovery MR750 scanner (General Electric, Boston, MA, USA) with an eight-channel phased-array head coil in the Medical Imaging Department of the First Affiliated Hospital of JNU. High resolution brain structural images were obtained with a T1-weighted 3D Ax FSPGR BRAVO sequence. The sequence parameters were as follows: repetition time (TR) = 8.2 ms, echo time (TE) = 3.2 ms, flip angle (FA) = 12°, data matrix = 256 × 256, field of view (FOV) = 256 × 256 mm2, slice thickness = 1 mm, and 136 axial slices covering the whole brain. The R-fMRI data were acquired using a gradient-echo echo-planar imaging (EPI) sequence with the following parameters: TR = 2000 ms, TE = 25 ms, FA = 90°, data matrix = 64 × 64, FOV = 240 × 240 mm2, slice thickness = 3 mm with inter-slice gap = 1 mm, 35 interleaved axial slices, and 210 volumes. The subjects were instructed to relax with their eyes closed while remaining awake throughout the scan. The brain structural data were visually checked by two experienced radiologists (Y.W. and Y.S.) to exclude subjects with abnormal brain structure.
2.3. Voxel-based morphometry
All the T1-weighted brain structural images were processed using CAT12 (http://dbm.neuro.uni-jena.de/cat/) based on the SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), a widely used program for performing voxel-based morphometric (VBM) analyses. For the VBM analysis, we used the diffeomorphic anatomical registration through an exponentiated Lie algebra algorithm (DARTEL) (Ashburner, 2007) to improve the registration quality of the structural images (Klein et al., 2009). Briefly, the structural MRI data preprocessing procedures were as follows. First, the original individual T1-weighted images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Second, the segmented GM and WM images for all the subjects were used to create a study-specific template using DARTEL. Next, the individual segmented images were warped to the study-specific template and spatially normalized to Montreal Neurological Institute (MNI) space using modulation. Finally, the modulated GM images were smoothed with a 6-mm full width at half maximum (FWHM) isotropic Gaussian kernel. Thus, we obtained the smoothed, modulated GM image for each subject.
2.4. Resting-state functional connectivity (RSFC)
2.4.1. R-fMRI data preprocessing
All the original functional images were preprocessed using SPM12 and Data Processing & Analysis for Brain Imaging (DPABI 2.2) (Yan et al., 2016). The data were processed using the following seven steps. (1) The first 10 functional volumes were discarded for magnetization equilibrium, leaving 200 volumes for further analysis. (2) Slice-timing correction was used to correct the acquisition time delay between slices. (3) Head motion correction was performed using a six-parameter rigid-body transformation. The R-fMRI data from the subjects with excessive head movement (translation > 2 mm in any plane or rotation > 2° in any direction) were discarded. In this step, we also estimated the mean framewise displacement (FD) (Power et al., 2014) from the derivatives of the six rigid-body realignment parameters. (4) The realigned functional images were spatially normalized to standard MNI space using the DARTEL algorithm. (5) The normalized functional images were sampled to 3 × 3 × 3 mm3 and smoothed with a 4-mm FWHM isotropic Gaussian kernel. (6) Temporal band-pass filtering (0.01–0.08 Hz) was performed on the time series for each voxel. (7) A regression model was applied to regress out the nuisance variables (head motion parameters from the Friston 24-parameter model, WM signals, and CSF signals) from the time series of each voxel. The residuals were used in the subsequent resting-state functional connectivity (RSFC) analysis.
2.4.2. Seed-based RSFC analysis
The clusters showing significant group difference in GMV (ANOVA test) were selected as the seed regions of interest (ROIs) for the RSFC analysis. First, the peak coordinate for each significant cluster obtained from VBM analysis was determined and was selected to create a 5-mm radius ROI. Next, we extracted the mean time course for each given ROI and calculated its Pearson's correlation with the time course of each of the rest voxels in the whole brain to generate the r-FC map for each subject. Subsequently, we transformed the individual r-FC map into a z-FC map using a Fisher r-to-z transformation to improve the normality for each subject. Finally, we compared the between-group difference in RSFC based on these z-FC maps.
2.5. Statistical analysis
2.5.1. Demographic and clinical data
A one-way analysis of variance (ANOVA) was used to compare the differences in age and years of education across the MDD, BD, and HC groups. A χ2-test was conducted to detect group difference in gender. Additionally, two-sample t-tests were applied to estimate the differences between the MDD and BD groups in the clinical data, including age of onset, illness duration, number of episodes, HDRS scores, and YMRS scores. The statistical significance level for these analyses was set at p < .05. These statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS).
2.5.2. Voxel-based morphometry analysis
A one-way ANOVA, calculated using SPM12, was used to detect differences in the GMV across the MDD, BD, and HC groups with age, gender, years of education, and total intracranial volume (TIV) as covariates. Correction for multiple comparisons was performed using the 3dClustSim program in Analysis of Functional NeuroImages (AFNI, https://afni.nimh.nih.gov/), and the cluster threshold was set at an uncorrected voxel-level p < .001, α = .05 in these analyses. In this way, we determined the clusters that had significant GMV differences between the three groups.
For each of these clusters, we estimated the relationship between its GMV and the clinical data in the combined BD and MDD group. We first extracted the mean GMV for a given cluster and then calculated the Pearson's partial correlation between the GMV value and each of the clinical data measures (age of onset, HDRS scores, illness duration, and number of episodes). In the calculations, we controlled age, gender, and years of education as covariates.
Subsequently, post hoc comparisons were performed to test the GMV differences between each pair of groups based on the significant clusters from the ANOVA analysis. In these calculations, we regressed out the effects of the covariates, which were gender, age, years of education, and TIV, and set the threshold for the multiple comparisons correction to be the same as the one used in the ANOVA analysis.
2.5.3. Seed-based RSFC analysis
A one-way ANOVA was performed to study the differences in RSFC values based on the z-FC maps across the MDD, BD, and HC groups. After multiple comparisons correction, we obtained the clusters that showed a significant RSFC difference between the three groups. Next, post hoc comparisons were used to determine the difference in RSFC between each pair of groups based on the results of the ANOVA analyses. For the multiple comparisons correction, we used the 3dClustSim program in AFNI and set the cluster threshold at an uncorrected voxel-level p < .001, α = .05 in these functional analyses. In these calculations, we controlled age, gender, years of education, and mean framewise displacement (FD) as covariates.
3. Results
3.1. Demographic and clinical comparisons
Table 1 lists the demographic and clinical data for all the study subjects. Eight subjects (3 BD and 5 HC) were excluded from the RSFC analysis because of excessive movement during the scanning. Therefore, the final number of subjects in the RSFC study included 40 BD, 36 MDD, and 42 HC. No significant differences were observed in gender distribution (χ2 = 2.02, p = .364), age (F = 0.87, p = .420), and years of education (F = 0.43, p = .654) across the MDD, BD, and HC groups. Additionally, no significant differences were found in age of onset (t = 1.12, p = .265), illness duration (t = −0.20, p = .846), number of episodes (t = −1.67, p = .100), HDRS scores (t = −0.67, p = .505), and YMRS scores (t = 0.18, p = .861) between the MDD and BD groups.
Table 1.
Demographic and clinical data comparisons.
| Characteristics | MDD | BD | HC | Statistics | p-value |
|---|---|---|---|---|---|
| Gender (M/F) | 20/16 | 17/26 | 22/25 | χ2 = 2.02a | .364 |
| Age (years old) | 30.7 (8.5) | 27.9 (9.1) | 29.7 (9.2) | F = 0.87b | .420 |
| Education (years) | 14.1 (3.8) | 14.3 (2.3) | 14.7 (3.4) | F = 0.43b | .654 |
| Age of onset (years old) | 26.8 (8.6) | 24.3 (10.4) | N/A | t = 1.12c | .265 |
| Illness duration (months) | 32.1 (47.1) | 34.2 (54.8) | N/A | t = −0.20c | .846 |
| Number of episodes | 1.9 (1.4) | 2.4 (1.3) | N/A | t = −1.67c | .100 |
| HDRS scores | 28.0 (4.8) | 28.7 (5.1) | N/A | t = −0.67c | .505 |
| YMRS scores | 2.6 (3.4) | 2.4 (2.3) | N/A | t = 0.18c | .861 |
Notes: Means and standard deviations (SD) are listed in the table. Abbreviations: MDD, major depressive disorder; BD, bipolar disorder; HC, healthy control; M, male; F, female; HDRS, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale; N/A, not applicable.
χ2-test.
One-way ANOVA test.
Two-sample t-test.
3.2. VBM analysis
3.2.1. Comparison of GMV between the three groups
Fig. 1a shows the clusters with significant differences in GMV between the MDD, BD, and HC groups. We identified four clusters, which were located in the left superior frontal cortex (SFG_L), left anterior cingulate cortex (ACC_L), left thalamus (THA_L), and right hippocampus (HIP_R), that showed significant group differences in GMV. The size and coordinates of these clusters are listed in Table 2. We also compared the mean GMV value of these four significant clusters between the MDD, BD, and healthy controls (Table S2 and Fig. S4).
Fig. 1.
Clusters showing significant differences in gray matter volume (GMV) between the patients with major depressive disorder (MDD), the patients with bipolar disorder (BD), and the healthy controls (HC). (a) Significant clusters obtained from the ANOVA analysis, (b) significant clusters obtained from the post hoc comparisons, and (c) significant correlations between GMV and clinical measures. These clusters were determined using the 3dClustSim correction (uncorrected p < .001, q < 0.05). The detected four clusters are located in the right hippocampus (HIP_R, C1), left anterior cingulate cortex (ACC_L, C2), left superior frontal gyrus (SFG_L, C3), and left thalamus (THA_L, C4). The orange clusters indicate positive F or t values, while blue indicates negative values.
Table 2.
Clusters with significant differences in gray matter volume (GMV) between the major depressive disorder (MDD) patients, bipolar disorder (BD) patients, and healthy controls (HC).
| Cluster index | Regions | BA | Cluster size (#voxels) | Peak MNI coordinates |
F/t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Three-group comparison | |||||||
| C1 | HIP_R | – | 168 | 27 | −12 | −12 | 11.23a |
| C2 | ACC_L | 32 | 132 | −2 | 39 | 27 | 13.53a |
| C3 | SFG_L | 8, 9 | 352 | −12 | 26 | 45 | 14.28a |
| C4 | THA_L | – | 113 | −18 | −20 | 0 | 12.81a |
| MDD < HC | |||||||
| C1 | HIP_R | – | 102 | 27 | −11 | −12 | −4.83b |
| C2 | ACC_L | 32 | 109 | −3 | 41 | 24 | −7.08b |
| C3 | SFG_L | 8, 9 | 313 | −12 | 24 | 47 | −6.00b |
| MDD > HC | |||||||
| C4 | THA_L | – | 113 | −18 | −20 | 0 | 4.95b |
| BD < HC | |||||||
| C1 | HIP_R | – | 158 | 26 | −8 | −17 | −5.03b |
| C2 | ACC_L | 32 | 108 | −2 | 39 | 27 | −4.78b |
| MDD < BD | |||||||
| C2 | ACC_L | 32 | 102 | −3 | 42 | 20 | −4.31b |
| C3 | SFG_L | 8, 9 | 270 | −9 | 27 | 38 | −4.65b |
Notes: BA, Brodmann's area; MNI, Montreal Neurological Institute; L/R, left/right hemisphere; SFG, superior frontal gyrus; ACC, anterior cingulate cortex; THA, thalamus; HIP, hippocampus. # indicates the number of voxels in each cluster; voxel size = 1.5 × 1.5 × 1.5 mm3.
The value was obtained from an ANOVA analysis.
The value was estimated using a two-sample t-test.
3.2.2. Abnormal GMV in MDD patients
In this study, the MDD patients showed significantly decreased GMV in three clusters in the SFG_L, ACC_L, and HIP_R and increased GMV in one cluster in the THA_L compared with the healthy controls (Fig. 1b and Table 2).
3.2.3. Abnormal GMV in BD patients
We found uniformly significant decreased GMV in two clusters in the ACC_L and HIP_R in the BD patients compared with the healthy controls (Fig. 1b and Table 2).
3.2.4. GMV differences between MDD and BD patients
The MDD patients showed uniformly significantly decreased GMV in two clusters in the SFG_L and ACC_L compared with the BD patients (Fig. 1b and Table 2).
3.2.5. Correlations between GMV and clinical characteristics
For each cluster listed in Table 2, we estimated the correlations between its mean GMV value and the clinical variables (age of onset, HDRS scores, illness duration, and number of episodes) in the combined MDD and BD groups. We detected a significantly negative correlation between the GMV of the HIP_R and the number of episodes (r = −.35, p = .002), and the GMV of the ACC_L was negatively correlated with age of onset (r = −.33, p = .003). Then we conducted multiple comparisons correction for the number of correlation analyses. Both of these observed significant correlations remained after Bonferroni correction (p < .05).
3.3. Seed-based RSFC analysis
3.3.1. Comparison of RSFC across the three groups
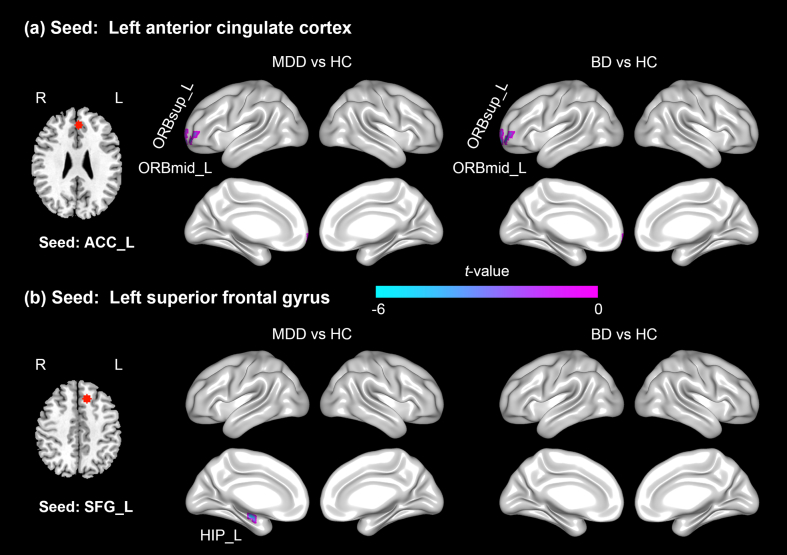
The RSFC of the ACC_L seed with two regions, the left orbital part middle frontal gyrus (ORBmid_L) and left orbital part superior frontal gyrus (ORBsup_L), showed significant differences between the three groups. The RSFC of the SFG_L seed with the HIP_L differed significantly between the three groups. However, we did not detect significant group differences in RSFC when selecting the THA_L or HIP_R as the seed region for functional connectivity. The locations of these seed regions were displayed in Fig. S2.
3.3.2. Abnormal RSFC in MDD patients
The RSFC of the ACC_L seed with two areas, the ORBmid_L and ORBsup_L, was significantly decreased in the MDD patients compared with the healthy controls (Fig. 2a and Table 3). The RSFC of the SFG_L with the cluster in the HIP_L was also decreased in the MDD patients compared with the healthy controls (Fig. 2b and Table 3).
Fig. 2.
Resting-state functional connectivity (RSFC) seeded from the ACC and SFG in the left hemisphere in either the patients with bipolar disorder (BD) or the patients with major depressive disorder (MDD) compared with the healthy controls (HC). (a) The results for the ACC_L seed region, and (b) the results for the SFG_L seed region. Both the MDD and BD patients showed significantly decreased RSFC between the ACC_L seed and two regions, the ORBsup_L and ORBmid_L. The MDD patients also displayed decreased RSFC between the seed SFG_L and the HIP_L. The negative t value indicates decreased RSFC in the patients compared with the HC. Abbreviations: ACC, anterior cingulate cortex; SFG, superior frontal gyrus; ORBmid, orbital part middle frontal gyrus; ORBsup, orbital part superior frontal gyrus; HIP, hippocampus; L, left hemisphere.
Table 3.
Abnormal resting-state functional connectivity (RSFC) seeded from the left anterior cingulate cortex (ACC_L) and the left superior frontal gyrus (SFG_L) in either the patients with major depressive disorder (MDD) or the patients with bipolar disorder (BD).
| Seed | Cluster | BA | Cluster size (#voxels) | Peak MNI coordinates |
t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ACC_L | |||||||
| MDD < HC | ORBmid_L | 10, 11 | 27 | −36 | 51 | 3 | −4.68 |
| ORBsup_L | 10, 11 | 18 | −15 | 60 | −6 | −5.37 | |
| BD < HC | ORBmid_L | 10, 11 | 41 | −30 | 54 | −6 | −5.17 |
| ORBsup_L | 10, 11 | 26 | −12 | 60 | −6 | −4.60 | |
| SFG_L | |||||||
| MDD < HC | HIP_L | – | 20 | −30 | −12 | −18 | −5.37 |
Notes: BA, Brodmann's area; MNI, Montreal Neurological Institute; ORBmid, orbital part of middle frontal gyrus; ORBsup, orbital part of superior frontal gyrus; HIP, hippocampus; L, left hemisphere. # indicates the number of voxels in each cluster; voxel size = 3 × 3 × 3 mm3.
3.3.3. Abnormal RSFC in BD patients
The RSFC of the ACC_L seed with the ORBmid_L and ORBsup_L was significantly decreased in the BD patients compared with the healthy controls (Fig. 2a and Table 3).
3.3.4. RSFC difference between MDD and BD patients
No significant RSFC differences were found between the patients with MDD and those with BD for any given seed ROI.
To validate our results, we also performed the RSFC analysis using the ROIs from both hemispheres. From these analyses, we obtained an FC pattern which is similar to those obtained from the unilateral ROIs. The results were displayed in the Table S1 and Fig. S1.
4. Discussion
In this study, we combined VBM and RSFC approaches to detect abnormal GMV and associated RSFC in patients with MDD and BD. In both the VBM and FC analysis, we detected the altered brain regions in the patients were mainly located in the frontal-limbic network. From the VBM analysis, we found that both the MDD and BD groups showed decreased GMV in the ACC_L and HIP_R and that the MDD patients showed decreased GMV in the SFG_L but increased GMV in the THA_L compared with the healthy controls. Moreover, we found decreased GMV in the ACC_L and SFG_L in the MDD patients compared with the BD patients. Using the clusters derived from the VBM analysis as seed regions, we analyzed the RSFC based on R-fMRI data and found both the MDD and BD groups had decreased RSFC between the ACC_L seed and the left OFC (ORBmid_L, ORBsup_L), and the MDD group showed decreased RSFC between the SFG_L seed and the HIP_L compared with the healthy controls.
4.1. Voxel-based morphometry
4.1.1. Common alterations in gray matter volume in MDD and BD
Both the MDD and BD groups showed decreased GMV in the ACC_L compared with the healthy controls (Fig. 1b and Table 2). The finding of abnormal GMV in the ACC was consistent with previous studies of MDD and BD patients (Arnone et al., 2016; Wise et al., 2016; Schmaal et al., 2017). Wise et al. (2016) compared brain structural differences across BD, MDD, and HC groups and detected significantly decreased GMV in the ACC in both the BD and MDD patient groups compared with the HC group. Thinner cortical thickness (CT) in the ACC was also reported in both MDD (Wagner et al., 2012) and BD patients (Hanford et al., 2016). The ACC has been suggested as being crucial to emotional processing (Ochsner et al., 2009; Ray and Zald, 2012), especially in evaluating emotional information. Rive et al. (2015) observed abnormal function in the ACC, specifically, decreased activation when responding to happy stimuli and increased activation when responding to sad situations, in BD patients compared with healthy controls. Similarly, Fournier et al. (2013) found increased activation in the ACC in MDD patients when responding to anger compared with healthy controls. Taken together, our finding of abnormal GMV of the ACC may be associated with the dysfunction of emotional processing in both BD and MDD patients.
In this study, hippocampal volume loss was also detected in both the MDD and BD groups (Fig. 1b and Table 2). This finding converges with several previous studies (Hibar et al., 2016; Schmaal et al., 2016). Specifically, two recent multi-site large sample analyses (Hibar et al., 2016; Schmaal et al., 2016) reported hippocampal atrophy in BD and MDD patients, respectively. As a core region of the limbic system, the hippocampus is essential for cognitive processing, such as learning and memory (Eichenbaum, 2013), and plays a key role in the pathologic mechanism of depression (Sheline, 2011). One possible explanation for the hippocampal volume loss in MDD and BD patients is that it results from prolonged exposure to stress-induced biochemical abnormalities mediated via the hypothalamic-pituitary-adrenal (HPA) axis (Sheline, 2011). Due to a greater number of glucocorticoid receptors in the hippocampus, stress-induced elevated glucocorticoid levels in the patients could result in GMV loss in the hippocampus (Frodl and O'Keane, 2013). Moreover, animal study (Musazzi et al., 2011) also supported that the decreased volume in the hippocampus may be a critical pathology in depression. The hippocampus loss was induced by abnormal enhancement of glutamate release and dendritic atrophy in rats. Thus, we inferred that structural abnormality of the HIP may be associated with the pathophysiological underpinnings and impaired cognitive function in both MDD and BD patients.
4.1.2. Gray matter volume difference between MDD and BD
The MDD patients showed decreased GMV in the ACC_L compared with the BD patients (Fig. 1b and Table 2). This result is in line with a previous study (Redlich et al., 2014) that found significantly decreased GMV in the ACC_L in an MDD group compared with a BD group and revealed that the ACC showed strong feature weights that contributed to discriminating MDD from BD patients in a classification analysis. Previous studies (Sheline et al., 2009; Myingermeys and Merge, 2016) suggested that the ACC is involved in self-referential processing, such as negative self-referential thoughts and rumination. In addition, Batmaz et al. (2013) reported that MDD patients showed more negative self-referential thoughts and ruminations than BD patients, and Kühn et al. (2012) found that the ruminations were correlated negatively with the GMV of the ACC. The greater ACC volume reduction in MDD patients than in BD patients may be associated with this greater amount of rumination.
In this study, we also found decreased GMV in the SFG_L, part of the dorsolateral prefrontal cortex (DLPFC), in the MDD patients compared with the BD patients (Fig. 1b and Table 2). This finding is consistent with a previous study (Wise et al., 2016), in which Wise et al. (2016) compared the GMV in either BD patients or MDD patients with that of healthy controls and found decreased GMV compared with HC in the DLPFC only in MDD patients, but not in BD patients. Repetitive transcranial magnetic stimulation (rTMS) to the DLPFC has been reported to be an effective tool to improve symptoms in treatment-resistant MDD patients in clinical (Carpenter et al., 2012), but whether it is effective for BD patients is controversial (Oldani et al., 2014). Moreover, Zhang et al. (2017) analyzed fALFF based on R-fMRI data and found decreased fALFF in the SFG_L in MDD patients compared with either BD patients or healthy controls. In addition, previous studies reported that the SFG hypoactivation was associated with the severity of rumination (Schiller et al., 2013), and MDD patients showed more negative ruminations than BD patients (Batmaz et al., 2013). Taken together, the GMV of the SFG_L are different between MDD and BD patients and the difference may be associated with the ruminations to some extent.
4.1.3. Altered gray matter volume only in MDD
We found increased GMV in the left thalamus in the MDD patients compared with the healthy controls (Fig. 1b and Table 2). Several studies reported altered GMV in the thalamus in MDD patients, either increased (Zhao et al., 2014; Peng et al., 2016) or decreased (Du et al., 2012), compared with healthy controls. Zhao et al. (2014) and Peng et al. (2016) found increased GMV in the thalamus in medication-free MDD patients, whereas Du et al. (2012) detected decreased GMV in the thalamus in a combined group of medicated and non-medicated MDD patients compared with a group of healthy controls. This inconsistency may indicate that medication use influences the direction of GMV changes in the thalamus in MDD patients. The thalamus, a complicated sensory information node, has been reported to be crucial to emotion, memory, and arousal (Taber et al., 2004). The structural alteration of the thalamus was considered to help account for the deficits in top-down regulation of negative emotions among individuals with MDD patients (Webb et al., 2014). In addition to structural studies, functional MRI study (Holt et al., 2016) has reported abnormal thalamus activity in emotional memory processing, and PET and SPECT meta-analysis study (Hamilton et al., 2012a) revealed that greater activity in the thalamus was correlated with enhanced processing of negative information in MDD patients. In this study, we detected no GMV alteration in the thalamus in the BD patients, which is in line with a previous study (Kempton et al., 2011), that detected decreased thalamus volume in MDD patients but not in BD patients compared with healthy controls. Therefore, our finding of increased GMV of the thalamus may be associated with the medicated-free status of the MDD patients.
The MDD patients also showed decreased GMV in the SFG_L compared with the HC (Fig. 1b and Table 2). This finding is consistent with several previous studies (Yuan et al., 2008b; Jung et al., 2014; Peng et al., 2016). Specifically, MDD patients had a decreased GMV in the SFG that was associated with various clinical characteristics, such as first-episode medication-free (Peng et al., 2016), medicated (Jung et al., 2014), and remitted MDD patients (Yuan et al., 2008b). The SFG was considered to be important to emotional processing (Frodl et al., 2009). Frodl et al. (2009) found MDD patients had decreased activation in SFG when responding to emotional stimulus. The degree of SFG hypoactivation would be associated with the severity of rumination in MDD patients (Schiller et al., 2013). In addition, the SFG is a key region for cognitive control (Damoiseaux et al., 2006) and attention processing (Kushnir et al., 2013). The GMV loss in SFG would be correlated with the cognitive impairment (Yuan et al., 2008a) and attention deficits (Li et al., 2010) in MDD patients. Goveas et al. (2011) found the GMV deficits in SFG was correlated with the severity of depression in MDD. Moreover, using the amplitude of low-frequency fluctuations (ALFF) method, Guo et al. (2013) found the ALFF in SFG could be used to differentiate the early-onset from late-onset MDD patients, which suggested the SFG may play an important role in the pathology of major depression.
4.2. Resting-state functional connectivity
4.2.1. Common alterations in RSFC in MDD and BD
In this study, we found that both the MDD and BD patients displayed decreased RSFC within the frontal-cingulate network, mainly between the ACC_L and left orbitofrontal cortex (OFC_L) (Fig. 1a and Table 3). This finding converges with previous studies (Du et al., 2017) in which Du et al. (2017) found decreased ACC-OFC RSFC in depressed patients compared with healthy controls. The OFC in the frontal-limbic system is known to be involved in emotional processing (Stalnaker et al., 2015) and decision making (Jollant et al., 2010). In MDD patients, Frodl et al. (2010) found that the OFC-cingulate system showed decreased activation, which was related to a failure to regulate positive and negative processing, during a facial emotion matching task. Jollant et al. (2010) suggested that MDD patients showed decreased activation in the OFC when responding to risky decisions compared with controls. Additionally, the RSFC of ACC-OFC change could reflect the treatment effect of depression in patients (Baeken et al., 2017). Baeken et al. (2017) found that the FC of the ACC-OFC was positively related to a better clinical response during accelerated intermittent theta burst stimulation (aiTBS) treatment and could be used to distinguish aiTBS responders from non-responders. Thus, we suggest that abnormal ACC-OFC connectivity may be associated with dysfunction in emotion processing and decision making in depression and could reflect symptom severity to some extent.
4.2.2. Alterations of RSFC only in MDD
We found that the MDD patients displayed decreased RSFC between the SFG_L and the HIP_L compared with the healthy controls (Fig. 2b and Table 3). This result is compatible with previous studies (Tahmasian et al., 2013; Zhu et al., 2015). Specifically, Tahmasian et al. (2013) found a reduction in SFG-HIP RSFC in recurrent MDD patients and Zhu et al. (2015) found decreased SFG-HIP RSFC in older adults with subthreshold depression compared with healthy subject. The hippocampus is crucial to emotion processing and memory (Eichenbaum, 2013), and the SFG, part of the DLPFC, is a key region involved in cognitive control (Damoiseaux et al., 2006). The impaired SFG-HIP RSFC in the MDD supports a cognitive neurobiological model (Disner et al., 2011) that suggests that functional deficits in the DLPFC may impair top-down control over the limbic areas (e.g., HIP) in depression. Taken together, functional dysconnectivity of the SFG-HIP may be associated with impairment of emotion regulation in MDD patients.
Using VBM and FC analyses, we detected that brain structural and functional alterations in the patients with MDD and BD. The significantly altered regions were mainly located in the frontal-limbic system, which is believed closely relating to the clinical symptoms, such as emotional dysregulation, cognitive impairment, and rumination. These findings were in accordance with recent studies from different modalities (Price and Drevets, 2010; Du et al., 2012; Redlich et al., 2014; Peng et al., 2016; Chen et al., 2017; Du et al., 2017; Zhang et al., 2017). Using VBM analysis, Redlich et al. (2014) found that both MDD and BD patients had GMV abnormalities in the ACC, HIP, and frontal regions, compared with the healthy group. The GMV alterations in the frontal-limbic system were also reported in structural meta-analyses in MDD patients (Du et al., 2012; Peng et al., 2016). In fMRI studies, Zhang et al. (2017) detected MDD had decreased ALFF in the SFG and BD patients had increased ALFF in the putamen, compared with healthy group, and Du et al. (2017) found decreased ACC-OFC RSFC in MDD patients. Moreover, in diffusion tensor imaging (DTI) studies, Chen et al. (2017) observed decreased fractional anisotropy (FA) in the corpus callosum (CC), anterior limb of the internal capsule (ALIC), and SFG in MDD patients compared with healthy controls. In addition, genetic studies also revealed the frontal-limbic abnormalities in depression (Price and Drevets, 2010). Taken together, our findings suggest that the frontal-limbic system may be critical to the pathology of both BD and MDD and need further study in the future.
5. Limitations
The present study has some potential limitations. First, the sample size was relatively small. A larger independent sample is needed to examine the reproducibility of these findings. Second, because about 10–20% of patients initially diagnosed with MDD may eventually turn out to have BD (Woo et al., 2015), we cannot be certain whether some patients we diagnosed with MDD would turn out to have BD in the future. This may have affected the results. However, none of the MDD patients in our study had a family history of BD. Additionally, we tracked the illness states of the MDD patients in this study and found that none of them had switched to BD before the submission of this manuscript. Third, the subjects included in the VBM and RSFC analysis were not exactly the same. Because of the excessive head motion, eight subjects' functional data were excluded before the RSFC analysis. We repeated the VBM analysis that discarded these subjects' structural data and found similar results. Fourth, because this was a cross-sectional study, we cannot determine the causal relationship between the disorders and the brain structural and functional alterations. Longitudinal studies may help to clarify this question.
6. Conclusion
In summary, using a multimodal approach, we detected shared and specific brain abnormalities in BD and MDD patients. The structural and functional results we detected were primarily located in the frontal-limbic network. Compared to the healthy controls, both the BD and MDD patients displayed decreased gray matter volume in the ACC and hippocampus and decreased functional connectivity between the ACC and OFC. We also found decreased gray matter volume in the ACC and SFG in the MDD patients compared with the BD patients. Our results revealed that the MDD and BD patients were more similar than different in gray matter volume and functional connectivity. These findings suggested that the frontal-limbic system might be useful for understanding the underlying mechanisms of affective disorders.
The following are the supplementary data related to this article.
Supplementary material
Acknowledgments
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (81371535, 81471654, 81428013, 81671670, 81501456, 81471650, and 81428013); Planned Science and Technology Project of Guangdong Province, China (2014B020212022); and Planned Science and Technology Project of Guangzhou, China (201508020004, 20160402007, and 201604020184). The funding organizations played no further role in study design, data collection, analysis and interpretation, or paper writing. We also appreciate the content and English editing assistance of Drs. Rhoda E. and Edmund F. Perozzi.
Competing interest statement
The authors declare that they have no competing financial interests.
Contributor Information
Ying Wang, Email: johneil@vip.sina.com.
Ruiwang Huang, Email: ruiwang.huang@gmail.com.
References
- Arnone D., Job D., Selvaraj S., Abe O., Amico F. Computational META-analysis of statistical parametric maps in major depression. Hum. Brain Mapp. 2016;37(4):1393–1404. doi: 10.1002/hbm.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baeken C., Duprat R., Wu G., De Raedt R., van Heeringen K. Subgenual anterior cingulate-medial orbitofrontal functional connectivity in medication-resistant major depression: a neurobiological marker for accelerated intermittent theta burst stimulation treatment? Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2017;2(7):556–565. doi: 10.1016/j.bpsc.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Baldessarini R.J., Tondo L., Baethge C.J., Lepri B., Bratti I.M. Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Bipolar Disord. 2007;9(4):386–393. doi: 10.1111/j.1399-5618.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Batmaz S., Kaymak S.U., Soygur A.H., Ozalp E., Turkcapar M.H. The distinction between unipolar and bipolar depression: a cognitive theory perspective. Compr. Psychiatry. 2013;54(7):740–749. doi: 10.1016/j.comppsych.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Yücel M., Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol. Psychiatry. 2010;67(11):1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Brady R.O., Margolis A., Masters G.A., Keshavan M., Öngür D. Bipolar mood state reflected in cortico-amygdala resting state connectivity: a cohort and longitudinal study. J. Affect. Disord. 2017;217:205–209. doi: 10.1016/j.jad.2017.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canu E., Agosta F., Sarasso E., Volonte M.A., Basaia S. Brain structural and functional connectivity in Parkinson's disease with freezing of gait. Hum. Brain Mapp. 2015;36(12):5064–5078. doi: 10.1002/hbm.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso De Almeida J.R., Phillips M.L. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol. Psychiatry. 2013;73(2):111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L.L., Janicak P.G., Aaronson S.T., Boyadjis T., Brock D.G. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- Chen G., Guo Y., Zhu H., Kuang W., Bi F. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: a voxel-based meta-analysis of diffusion tensor imaging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;76:179–187. doi: 10.1016/j.pnpbp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Cintia D., Duran F.L.S., Zanetti M.V., Santos L.C., Murray R.M. A population-based morphometric MRI study in patients with first-episode psychotic bipolar disorder: comparison with geographically matched healthy controls and major depressive disorder subjects. Bipolar Disord. 2015;13(1):28–40. doi: 10.1111/j.1399-5618.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Doucet G.E., He X., Sperling M., Sharan A., Tracy J.I. Gray matter abnormalities in temporal lobe epilepsy: relationships with resting-state functional connectivity and episodic memory performance. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M.Y., Wu Q.Z., Yue Q., Li J., Liao Y. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;36(1):11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Du L., Zeng J., Liu H., Tang D., Meng H. Fronto-limbic disconnection in depressed patients with suicidal ideation: a resting-state functional connectivity study. J. Affect. Disord. 2017;215:213–217. doi: 10.1016/j.jad.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: remembering the choices. Neuron. 2013;77(6):999–1001. doi: 10.1016/j.neuron.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Mikschl A., Stier S., Ciaramidaro A., Gapp V. Acute and sustained effects of cognitive emotion regulation in major depression. J. Neurosci. 2010;30(47):15726–15734. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J.C., Keener M.T., Almeida J., Kronhaus D.M., Phillips M.L. Amygdala and whole-brain activity to emotional faces distinguishes major depressive disorder and bipolar disorder. Bipolar Disord. 2013;15(7):741–752. doi: 10.1111/bdi.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013;52(4):24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Frodl T., Koutsouleris N., Bottlender R., Born C., Jäger M. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol. Psychiatry. 2008;13(12):1093–1101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Frodl T., Scheuerecker J., Albrecht J., Kleemann A.M., Mã¼Ller-Schunk S. Neuronal correlates of emotional processing in patients with major depression. World J. Biol. Psychiatry. 2009;10(3):202–208. doi: 10.1080/15622970701624603. [DOI] [PubMed] [Google Scholar]
- Frodl T., Bokde A.L., Scheuerecker J., Lisiecka D., Schoepf V. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol. Psychiatry. 2010;67(2):161–167. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Ganzola R., Duchesne S. Voxel-based morphometry meta-analysis of gray and white matter finds significant areas of differences in bipolar patients from healthy controls. Bipolar Disord. 2017;19(2):74–83. doi: 10.1111/bdi.12488. [DOI] [PubMed] [Google Scholar]
- Gonda X., Pompili M., Serafini G., Montebovi F., Campi S. Suicidal behavior in bipolar disorder: epidemiology, characteristics and major risk factors. J. Affect. Disord. 2012;143(1–3):16–26. doi: 10.1016/j.jad.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Goveas J.S., Espeland M.A., Hogan P., Dotson V., Tarima S. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women's Health Initiative MRI Study. J. Affect. Disord. 2011;132(1–2):275–284. doi: 10.1016/j.jad.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande I., Berk M., Birmaher B., Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561–1572. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [PubMed] [Google Scholar]
- Grotegerd D., Stuhrmann A., Kugel H., Schmidt S., Redlich R. Amygdala excitability to subliminally presented emotional faces distinguishes unipolar and bipolar depression: an fMRI and pattern classification study. Hum. Brain Mapp. 2014;35(7):2995–3007. doi: 10.1002/hbm.22380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.B., Liu F., Xun G.L., Hu M.R., Guo X.F. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;40(1):153–159. doi: 10.1016/j.pnpbp.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hanford L.C., Nazarov A., Hall G.B., Sassi R.B. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18(1):4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- Hibar D.P., Westlye L.T., van Erp T.G., Rasmussen J., Leonardo C.D. Subcortical volumetric abnormalities in bipolar disorder. Mol. Psychiatry. 2016;21(12):1710–1716. doi: 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld R.M., Lewis L., Vornik L.A. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J. Clin. Psychiatry. 2003;64(2):161–174. [PubMed] [Google Scholar]
- Holt R.J., Graham J.M., Whitaker K.J., Hagan C.C., Ooi C. Functional MRI of emotional memory in adolescent depression. Dev. Cogn. Neurosci. 2016;19(C):31–41. doi: 10.1016/j.dcn.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F., Lawrence N.S., Olie E., O'Daly O., Malafosse A. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. NeuroImage. 2010;51(3):1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Jung J., Kang J., Won E., Nam K., Lee M.S. Impact of lingual gyrus volume on antidepressant response and neurocognitive functions in major depressive disorder: a voxel-based morphometry study. J. Affect. Disord. 2014;169(169):179–187. doi: 10.1016/j.jad.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Salvador Z., Munafò M.R., Geddes J.R., Simmons A. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch. Gen. Psychiatry. 2011;68(7):675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Klein A., Andersson J., Ardekani B.A., Ashburner J., Avants B. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Vanderhasselt M.A., De R.R., Gallinat J. Why ruminators won't stop: the structural and resting state correlates of rumination and its relation to depression. J. Affect. Disord. 2012;141(2–3):352–360. doi: 10.1016/j.jad.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Kushnir V., Menon M., Balducci X.L., Selby P., Busto U. Enhanced smoking cue salience associated with depression severity in nicotine-dependent individuals: a preliminary fMRI study. Int. J. Neuropsychopharmacol. 2013;16(5):997–1008. doi: 10.1017/S1461145710000696. [DOI] [PubMed] [Google Scholar]
- Li C.T., Lin C.P., Chou K.H., Chen I.Y., Hsieh J.C. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. NeuroImage. 2010;50(1):347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Machino A., Kunisato Y., Matsumoto T., Yoshimura S., Ueda K. Possible involvement of rumination in gray matter abnormalities in persistent symptoms of major depression: an exploratory magnetic resonance imaging voxel-based morphometry study. J. Affect. Disord. 2014;168:229–235. doi: 10.1016/j.jad.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Murray C.J., Vos T., Lozano R., Naghavi M., Flaxman A.D. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380(9859):2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- Musazzi L., Racagni G., Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem. Int. 2011;59(2):138–149. doi: 10.1016/j.neuint.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Myingermeys I., Merge G. Brain networks sub-serving self-referential processing in depression. Eur. Psychiatry. 2016;33:S45. [Google Scholar]
- Ochsner K.N., Ray R.R., Hughes B., Mcrae K., Cooper J.C. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol. Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldani L., Altamura A.C., Abdelghani M., Young A.H. Brain stimulation treatments in bipolar disorder: a review of the current literature. World J. Biol. Psychiatry. 2014;17(7):482–494. doi: 10.3109/15622975.2014.984630. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peng W., Chen Z., Li Y., Jia Z., Gong Q. Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J. Affect. Disord. 2016;199:114–123. doi: 10.1016/j.jad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Kupfer D.J. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381(9878):1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84(1):320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–261. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich R., Almeida J.J., Grotegerd D., Opel N., Kugel H. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222–1230. doi: 10.1001/jamapsychiatry.2014.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rive M.M., Van R.G., Veltman D.J., Phillips M.L., Schene A.H. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37(10):2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rive M.M., Mocking R.J., Koeter M.W., Van W.G., de Wit S.J. State-dependent differences in emotion regulation between unmedicated bipolar disorder and major depressive disorder. JAMA Psychiatry. 2015;72(7):687–696. doi: 10.1001/jamapsychiatry.2015.0161. [DOI] [PubMed] [Google Scholar]
- Salvadore G., Nugent A.C., Lemaitre H., Luckenbaugh D.A., Tinsley R. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. NeuroImage. 2011;54(4):2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller C.E., Minkel J., Smoski M.J., Dichter G.S. Remitted major depression is characterized by reduced prefrontal cortex reactivity to reward loss. J. Affect. Disord. 2013;151(2):756–762. doi: 10.1016/j.jad.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Veltman D.J., Erp T.G.M.V., Sämann P.G., Frodl T. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2016;21(6):806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Hibar D.P., Sämann P.G., Hall G.B., Baune B.T. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry. 2017;22(6):900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S., Arnone D., Job D., Stanfield A., Farrow T.F. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14(2):135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I. Depression and the hippocampus: cause or effect? Biol. Psychiatry. 2011;70(4):308–309. doi: 10.1016/j.biopsych.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späti J., Hänggi J., Doerig N., Ernst J., Sambataro F. Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacology. 2015;40(7):1640–1648. doi: 10.1038/npp.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker T.A., Cooch N.K., Schoenbaum G. What the orbitofrontal cortex does not do. Nat. Neurosci. 2015;18(5):620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber K.H., Wen C., Khan A., Hurley R.A. The limbic thalamus. J. Neuropsychiatry Clin. Neurosci. 2004;16(2):127–132. doi: 10.1176/jnp.16.2.127. [DOI] [PubMed] [Google Scholar]
- Tahmasian M., Knight D.C., Manoliu A., Schwerthöffer D., Scherr M. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front. Hum. Neurosci. 2013;7(5):639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J., Altshuler L.L. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14(4):326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- Townsend J.D., Torrisi S.J., Lieberman M.D., Sugar C.A., Bookheimer S.Y. Frontal-amygdala connectivity alterations during emotion down-regulation in bipolar I disorder. Biol. Psychiatry. 2013;73(2):127–135. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol M., Li M., Metzger C.D., Hailla N., Horn D.I. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol. Med. 2014;44(10):2053–2065. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- Wagner G., Schultz C.C., Koch K., Schachtzabel C., Sauer H. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J. Psychiatr. Res. 2012;46(11):1449–1455. doi: 10.1016/j.jpsychires.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Webb C.A., Weber M., Mundy E.A., Killgore W.D.S. Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol. Med. 2014;44(13):2833–2843. doi: 10.1017/S0033291714000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.W. A structured interview guide for the Hamilton depression rating scale. Arch. Gen. Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Wise T., Radua J., Via E., Cardoner N., Abe O. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry. 2016;22(10):1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo Y.S., Shim I.H., Wang H.R., Song H.R., Jun T.Y. A diagnosis of bipolar spectrum disorder predicts diagnostic conversion from unipolar depression to bipolar disorder: a 5-year retrospective study. J. Affect. Disord. 2015;174:83–88. doi: 10.1016/j.jad.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: Data Processing & Analysis for (resting-state) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yu H.L., Liu W.B., Wang T., Huang P.Y., Jie L.Y. Difference in resting-state fractional amplitude of low-frequency fluctuation between bipolar depression and unipolar depression patients. Eur. Rev. Med. Pharmacol. Sci. 2017;21(7):1541–1550. [PubMed] [Google Scholar]
- Yuan Y., Zhu W., Zhang Z., Bai F., Yu H. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol. Psychiatry. 2008;64(6):541–544. doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhu W., Zhang Z., Bai F., Yu H. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol. Psychiatry. 2008;64(6):541–544. doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng J., Fan X., Guo X., Guo W. Dysfunctional resting-state connectivities of brain regions with structural deficits in drug-naive first-episode schizophrenia adolescents. Schizophr. Res. 2015;168(1–2):353–359. doi: 10.1016/j.schres.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Zhang K., Liu Z., Cao X., Yang C., Xu Y. Amplitude of low-frequency fluctuations in first-episode, drug-naïve depressive patients: a 5-year retrospective study. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0174564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.J., Du M.Y., Huang X.Q., Lui S., Chen Z.Q. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol. Med. 2014;44(14):2927–2937. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
- Zhu X., Li R., Wang P., Li J. Aberrant functional connectivity of the hippocampus in older adults with subthreshold depression. Psych. J. 2015;3(4):245–253. doi: 10.1002/pchj.60. [DOI] [PubMed] [Google Scholar]
- Zhu J., Lin X., Lin C., Zhuo C., Yu Y. Selective functional dysconnectivity of the dorsal-anterior subregion of the precuneus in drug-naive major depressive disorder. J. Affect. Disord. 2018;225:676–683. doi: 10.1016/j.jad.2017.08.084. [DOI] [PubMed] [Google Scholar]
- Zou K., Deng W., Li T., Zhang B., Jiang L. Changes of brain morphometry in first-episode, drug-naïve, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol. Psychiatry. 2010;67(2):186–188. doi: 10.1016/j.biopsych.2009.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material