Abstract
Aims: Shorter telomere length is associated with increased chronic disease risk in adulthood including diabetes mellitus and cardiovascular risk. Few studies have evaluated the relationship between telomere length change and incident disease risk in populations with a high percentage of overweight and obesity.
Results: In an urban Latina population recruited in San Francisco (n = 82) with a high prevalence of overweight and obesity (78.4%), we assessed leukocyte telomere length and telomere length change over a 1-year period in relation to obesity, chronicity of obesity, and incident metabolic disease risk 5–6 years later. We also assessed the relationship between telomere length change over a 1-year period and weight loss. There were no significant associations between baseline telomere length and socio-demographics including age and ethnicity, or current weight status. Telomere length change, however, was associated with being obese at baseline and previous years of chronic obesity. A high percentage of women who were obese at baseline were also obese the year before (90%) and 2 years before (85%). Obesity at baseline was an independent predictor for increased telomere length attrition (β = −346.9, −568.4 to −125.4; P < 0.01). Similarly, chronic obesity was associated with increased risk for accelerated attrition (β = −280.6, −518.4 to −42.8; P < 0.01).
Innovation: We speculate that accelerated attrition may be a harbinger of metabolic disease. We also found that those who had or developed hypertension had accelerated attrition [−407.4 ± 464.0 vs. −168.1 ± 643.6 (P = 0.03)].
Conclusion: In populations with chronic and long-standing obesity, telomere length attrition rate, rather than baseline telomere length may be a more sensitive indicator of health status including chronic disease development.
Keywords: : telomere length, chronic obesity, obesity, Latino
Background
Cross-sectional studies in adults indicate that components of metabolic dysfunction including insulin resistance, abdominal obesity, and hypertension are associated with shorter leukocyte telomere length1–4 and that chronic or long-term adult obesity is associated with the shortest telomere length compared with those who have had shorter periods of obesity.3 Shortened telomere length is associated with risk for development of type 2 diabetes mellitus,5 increasing progression of the metabolic syndrome6 and cardiac events including myocardial infarction.7
Many of the metabolic, cardiovascular, and obesity-associated health conditions found to be correlated with shorter telomere length in cross-sectional studies have not been consistently replicated in longitudinal studies to evaluate the relationship between telomere length change over time and health outcomes.8–10 It is not clear why there is more inconsistency with risk factors for leukocyte telomere change versus telomere length.
In our cohort of immigrant Latino women at high risk for obesity and metabolic disease, we assessed the relationship between leukocyte telomere length at baseline and change over an approximate 1-year period and obesity including the chronicity of obesity and abdominal obesity. In contrast with other studies, this study consisted of a high-risk, homogenous population with a high percentage of obesity at baseline including annual measures on the chronicity of obesity. We also assessed the relationship between telomere length change and incident development of hypertension and type 2 diabetes mellitus.
Methods
Cohort and procedures
This group of mothers was recruited prenatally at two hospitals in San Francisco in 2006–2007, with sociodemographic and health history variables assessed at the baseline prenatal visit and at annual follow-up visits thereafter until 9 years postpartum in addition to a 4- to 6-week and 6-month postpartum visit. In 2016, at the annual 9-year visit we had 164 of the original 201 women assessed (81.5%). The cohort has been described in previous publications including recruitment and data collection specifics.11,12 All procedures were approved by the Committee on Human Research (CHR), the Institutional Review Board of the University of California, San Francisco and all women provided signed consents to participate. Although the cohort was recruited prenatally, for all measures described below for this sub-study, no woman was pregnant, currently breastfeeding, or had been recently pregnant (12 months postpartum).
Telomere length
We examined telomere length by quantitative polymerase chain reaction using genomic DNA from dried blood spots in a sample of 82 women over a 1-year time point in 2010 and 2011, 4 and 5 years after recruitment of the original, pregnant cohort. The mean length from these DNA samples was determined using Southern blot analysis and compared to the T/S ratios (the ratio of telomeric product vs. single copy gene product) for these samples to convert T/S ratios to base pairs (bp). This was expressed as the following formula: base pairs = 3274 + 2413 × (T/S). The transformation from T/S ratios to base pairs was based on a set of genomic DNA samples from the human fibroblast primary cell line IMR90 at different population doublings, and with the telomerase protein subunit gene (hTERT) transfected into a lentiviral construct as previously described in our studies.12,13
The conversion formula used to transform T/S ratios to basepairs was derived from a series of DNA samples from cultured cells, not from direction comparison of Southern blot analysis and quantitative PCR in the study samples. Therefore the telomere length change reported here should be treated as relative change within this context.
Hypertension and diabetes mellitus follow-up measurements
In 2016, approximately 4–5 years after the telomere length measurements at the 9- to 10-year follow-up point, we contacted participants by phone to ask about any diagnosis of hypertension and diabetes mellitus using the questionnaire used in the National Health and Nutrition and Examination Survey.14 Additionally, we extracted body mass index (BMI) data from the most recent follow-up visit as part of our study procedures (2015 or 2016). At each follow-up visit, our trained research assistants have assessed weight using digital scales, height with portable stadiometers, and waist circumference with standard measuring tapes.
Confounders and outcome variables
Socio-demographics
Age is represented in analyses both as continuous variables, and using binary indicators of age ≥35 years to represent more advanced age. Ethnicity was also dichotomized as Mexican versus Central American based on self-report. The majority of women (97.6%) were foreign born with most born in Mexico (62.2%) and the remainder in the countries of Central America. Only 2.4% were U.S.-born women.
Weight variables
Obesity was defined as having a BMI (kg/m2) number ≥30 using Centers for Disease Control and Prevention references15 at baseline measurement (the same time that telomeres were collected, 4–5 years postpartum) and chronic obesity was defined as having obesity at minimum 1 year before baseline measurement (measured at the 3-4 year postpartum visit). Persistent obesity was defined as obesity both at the telomere length collection time period (4–5 years postpartum) and then again 4–5 years later in 2015 or 2016. Although we differentiate between chronic obesity (as obesity over a couple years) and persistent (lasting 5 years) other authors have defined chronic obesity as obesity that spans a specific time period.16,17 We also assessed weight loss and BMI category change. We focused on changes from obese to overweight/normal weight. Abdominal obesity was defined as having a waist circumference >88 cm.18
Statistical analysis
We used t-tests to investigate the relationship between telomere length at baseline or telomere length change (over the 1 year time period). Our primary predictors of interest were dichotomous including obesity, chronicity of obesity, persistent obesity, and hypertension. Other covariates that we evaluated in relationship to baseline telomere length and telomere length change included socio-demographics such as ethnicity (Mexican vs. Central American origin) and women's age. Analysis of variance was used to assess continuous predictors such as age, BMI score, and telomere length at baseline or telomere length change. Multivariable linear regression models were used to assess independent predictors for shorter telomere length and accelerated telomere length loss using variables that were significant at P < 0.05 in bivariate analysis. With a pre-set sample size of 82, we had 80% power to detect an effect size of telomere change of approximately 40 ± 38 bp if 10% of the sample had the predictor of interest (e.g., chronic obesity or persistent obesity) or 40 ± 50 bp with 20% having the predictor of interest based on previous studies that have evaluated short-term telomere length change.9,13
Results
We enrolled 82 Latina women with telomere length measured at baseline who had had additional telomere length measurements approximately 1 year later (follow-up was conducted after 12.7 ± 2.4 months).12 There was no loss to follow-up for these 82 women. Age at baseline was 31.7 ± 5.0 years. Baseline telomere length in women was 7065.09 ± 690.14 bps and mean yearly change over the 1-year period was 93.28 ± 668.47 bp (mean length 1 year later was 7159.80 ± 551.65 bp).12 Approximate monthly change was 7.77 ± 55.71 bp.
Baseline health conditions included the following: 36.5% were obese at baseline (78.4% overweight and obese) and 37.3% were obese a year later (77.3% overweight and obese). A high percentage had chronic obesity (defined as obesity at baseline and at minimum 1 year before baseline; 32.5%) and 57.7% had abdominal obesity measured at baseline. Specifically, almost all women who were obese at baseline were also obese the year before (90%) and 2 years before (85%). A very low percentage reported having hypertension (3.7%) or diabetes mellitus (3.7%) at initial original enrollment of the cohort. Of those women who were recontacted in 2016, 7.8% (6/77) reported existing or incident hypertension and 1.5% (1/65) reported developing type 2 diabetes mellitus.
Predictors of shorter telomere length and telomere length attrition
Risk factors for shorter telomere length at baseline were assessed. There were no associations between telomere length and obesity, chronic obesity, or socio-demographics (e.g., age, ethnicity, advanced age(≥ 35 years)).
However, telomere length changes over the 1-year period were associated with obesity and chronic obesity measured at baseline. Obesity at baseline was associated with the greatest loss in telomere length (−188.8 ± 633.0 vs. 267.7 ± 595.1 in gain for those were not obese; P < 0.01; Table 1). Obesity in the year before baseline assessment (P = 0.02) and 2 years before baseline (P = 0.048; Table 1) was also associated with greater telomere length loss (Table 1).
Table 1.
Socio-Demographics, Mental Health, and Weight in Relation to Telomere Length Change in Latina Women over a 1-Year Period (Base Pairs; n = 82)
| Variable | N/total (%) | Telomere length change, years (base pairs) ±standard deviation | P |
|---|---|---|---|
| Socio-demographics | |||
| Marital status | |||
| Married or with partner | 16/81 (19.8) | 129.3 ± 459.4 | 0.80 |
| Not married | 65/81 (90.2) | 82.4 ± 717.9 | |
| Mexican ethnicity (maternal) | |||
| Yes | 52/82 (60.1) | 34.2 ± 679.7 | 0.29 |
| No (Central American) | 30/82 (36.6) | 195.8 ± 646.9 | |
| Maternal age | |||
| ≥35 | 21/82 (25.6) | 55.7 ± 667.8 | 0.21 |
| <35 | 61/82 (74.4) | 106.2 ± 673.7 | |
| Weight status | |||
| Obese at baseline | |||
| Yes | 27/74 (36.5) | −188.8 ± 633.0 | <0.01 |
| No | 47/74 (63.5) | 267.7 ± 595.1 | |
| Chronic obesity (in the year before baseline and baseline) | |||
| Yes | 25/77 (32.5) | −188.7 ± 658.9 | 0.02 |
| No | 52/77 (67.5) | 198.5 ± 645.1 | |
| Chronic obesity (in the 2 years before baseline and baseline) | |||
| Yes | 21/77 (27.3) | −158.0 ± 671.3 | 0.048 |
| No | 56/77 (72.7) | 177.2 ± 645.6 | |
| Obese 5–6 years before baseline | |||
| Yes | 17/77 (22.1) | 198.1 ± 1019.4 | 0.53 |
| No | 60/77 (77.9) | 82.0 ± 536.9 | |
| Abdominal obesity at baseline | |||
| Yes | 41/71 (57.7) | 200.6 ± 768.3 | 0.25 |
| No | 30/71 (42.3) | 9.2 ± 550.3 | |
| Chronic abdominal obesity (the year before baseline and baseline) | |||
| Yes | 34/67 (50.7) | 181.2 ± 529.5 | 0.31 |
| No | 33/67 (49.3) | 6.3 ± 824.3 | |
| Incident obesity and cardiometabolic disease | |||
| Hypertension (incident or existing) | |||
| Yes | 6/77 (7.8) | −407.4 ± 464.0 | 0.03 |
| No | 71/77 (92.2) | 168.1 ± 643.6 | |
| Diabetes (incident or existing) | |||
| Yes | 4/66 (6.1) | −227.1 ± 462.6 | 0.35 |
| No | 62/66 (93.9) | 52.1 ± 587.4 | |
| Persistent obesity at follow-up (from telomere collection to 4-5 years follow-up) | |||
| Yes | 13/69 (18.8) | 97.4 ± 229.8 | 0.92 |
| No | 56/69 (81.2) | 118.7 ± 85.1 | |
| Obese at follow-up | |||
| Yes | 17/76 (22.4) | 42.5 ± 802.2 | 0.72 |
| No | 59/76 (77.6) | 111.0 ± 640.4 | |
| Loss of weight; from obese to normal/overweight at 4-5 years follow-up | |||
| Yes | 13/69 (18.8) | −300.3 ± 424.8 | 0.01 |
| No | 56/69 (81.2) | 195.5 ± 653.6 | |
There was a greater amount of telomere length loss based on the severity of obesity with women who had BMI ≥35 kg/m2 at baseline (n = 10/74; 13.5%) having loss of −317.07 ± 184.27 versus 166.52 ± 79.06 for BMI <35 kg/m2 and BMI ≥30 kg/m2 (P = 0.03). Women who had a BMI ≥40 kg/m2 (n = 4/74; 5.4%) at baseline had even higher attrition −447.39 ± 782.70 versus 132.52 ± 627.54 compared to those with lower BMIs (P = 0.08), although results trended to statistical significance.
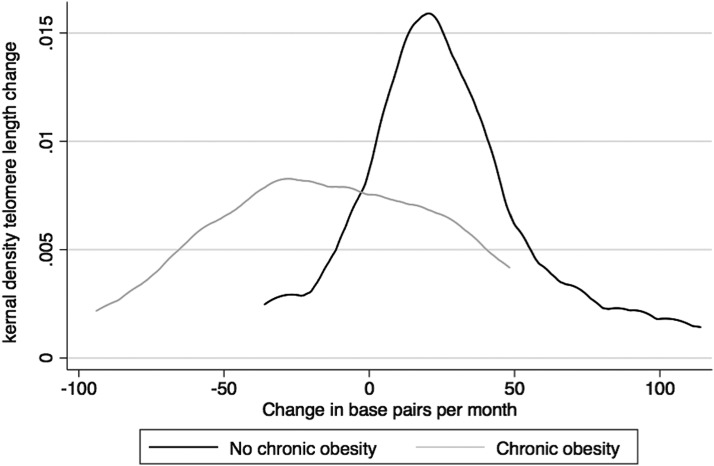
In multivariate linear regression models adjusting for telomere length at baseline and age, obesity at baseline was associated with increased telomere length loss (β = −346.9, −568.4 to −125.4; P < 0.01). Similarly, chronic obesity was associated with increased risk for accelerated telomere length loss (β = −280.6, −518.4 to −42.8; P < 0.01; Fig. 1) as was chronic obesity in the 2 years before collection of telomeres (β = −354.0, −599.7 to −108.2; P < 0.01).
FIG. 1.
Kernal density plot shows the distribution of telomere length change by chronic obesity. Black line indicates no chronic obesity and gray line indicates chronic obesity.
Telomere length as a predictor of metabolic disease and weight loss
Baseline telomere length was not associated with obesity at follow-up (P = 0.64), persistent obesity, or current or incident hypertension (P = 0.32). Telomere length loss was not associated with persistent obesity at follow-up although increased loss was associated with losing weight [moving from obese to overweight or normal weight status (−300.3 ± 424.8 vs. 195.5 ± 653.6; P = 0.01)]. Current or incident hypertension was also associated with a decrease in telomere length over the 1-year period [−407.4 ± 464.0 vs. 168.1 ± 643.6 (P = 0.03)].
Discussion
There have now been two meta-analyses on obesity and telomere length, which suggest that a concurrent relationship between obesity and telomere length is of weak magnitude (r = −0.057).19,20 However, the magnitude of effect differs by study with some finding no association possibly due to the heterogeneity of obesity in adults, with some having obesity-associated metabolic disease including diabetes mellitus and hypertension and others having obesity without any metabolic disease.19,20 This study explored the relationship between leukocyte telomere length and telomere length attrition in a primarily foreign-born cohort of Latina women with a high rate of obesity but little metabolic disease at the outset.
This study found an association between telomere attrition rate and obesity but did not find any association with obesity at baseline and telomere length in Latina women. Similarly, the rate of telomere length change was associated with incident hypertension and predictive of weight loss. It is possible that changes in telomere length are a more sensitive differentiator of development of cardiometabolic disease or changes in health status than actual leukocyte telomere length in populations with chronic obesity such as our cohort of Latina women.
The mean BMI of our cohort reported at baseline was 29.3 ± 6.6. A high percentage of our cohort was overweight or obese at enrollment (78.4%) with the vast majority of our cohort similarly overweight or obese at 5 years follow-up (80.5%). Among adults who are chronically obese, telomere length may not necessarily be predictive of overall health but telomere length changes over time may be a better marker of disease development. Specifically, we found those with the highest BMIs and extreme obesity (BMI ≥40 kg/m2) had the greatest amount of telomere length attrition.
We also found an association between accelerated telomere attrition and weight loss over the 5-year follow-up period in contrast with other studies, which have found that longer telomere length is predictive of weight loss success.2,21 Accelerated telomere shortening measured from 30 months to 2.5 years has been shown to predict disease complications in those with cancers22 and mortality in men.23 It is possible that weight loss in these Latina women may have been a marker of other disease processes, but we did not collect information on development of co-morbidities beyond metabolic disease.
Interestingly, a high percentage of women in our cohort had lengthening or maintenance of telomere length over this 1-year period as previously described.12 The only other study to our knowledge that assessed telomere length change over a short, 1-year period in women similarly found that there was little attrition over a short, 1-year period but this study was in older, postmenopausal women who may have different attrition patterns.9
In sum, leukcoyte telomere length change may be a better indicator of complications of obesity than baseline telomere length in women such as our cohort of Latina women who have a history of chronic obesity over a number of years.
Limitations
These findings are preliminary in nature given the small size of our cohort (n = 82). Furthermore, incident cardiometabolic disease development was collected via self-report in a telephone interview, and future studies should confirm by chart review. Our sample also had a relatively narrow age range, comprised of women of reproductive age with parity ≥1. Future studies should assess the impact of obesity and cardiometabolic disease in a heterogeneity of age ranges. Additional studies should also be conducted in larger samples to be able to ascertain smaller effects.
Author Disclosure Statement
None of the authors had any conflicts of interest.
References
- 1.Nordfjall K, Eliasson M, Stegmayr B, et al. . Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682–2689 [DOI] [PubMed] [Google Scholar]
- 2.Cui Y, Gao Y-T, Cai Q, et al. . Associations of leukocyte telomere length with body anthropometric indices and weight change in Chinese women. Obesity (Silver Spring) 2013;21:2582–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, Parks CG, DeRoo LA, et al. . Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev 2009;18:816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masi S, Gkranias N, Li KW, et al. . Association between short leukocyte telomere length, endotoxemia, and severe periodontitis in people with diabetes: A cross-sectional survey. Diabetes Care 2014;37:1140–1147 [DOI] [PubMed] [Google Scholar]
- 5.Willeit P, Raschenberg J, Heydon EE, et al. . Leucocyte telomere length and risk of diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS One 2104;9:e112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revesz D, Milaneschi Y, Verhoeven JE, et al. . Telomere length as a marker of cellular ageing is associated with prevalence and progression of metabolic syndrome. J Clin Endocrinol Metab 2014;99:4607–4615 [DOI] [PubMed] [Google Scholar]
- 7.Brouilette S, Singh RK, Thompson JR, et al. . White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003;23:842–846 [DOI] [PubMed] [Google Scholar]
- 8.Bendix L, Thinggard M, Fenger M, et al. . Longitudinal changes in leukocyte telomere length and mortality in humans. J Gerontol Bio Sci Med 2014;69:231–239 [DOI] [PubMed] [Google Scholar]
- 9.Puterman E, Lin J, Krauss J, et al. . Determinants of telomere attrition over 1 year in healthy older women: Stress and health behaviors matter. Mol Psychiatry 2015;20:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kark JD, Goldberger N, Kimura M, et al. . Energy intake and leukocyte telomere length in young adults. Am J Clin Nutr 2012;95:479–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojcicki JM, Holbrook K, Lustig RH, et al. . Chronic maternal depression is associated with reduced weight gain in Latino infants from birth to 2 years of age. PLoS One 2011;6:e16737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojcicki JM, Shiboski S, Heyman MB, et al. . Telomere length change plateaus at 4 years of age in Latino children: Associations with baseline length and maternal change. Mol Genet Genomics 2016;291:1379–1389 [DOI] [PubMed] [Google Scholar]
- 13.Farzaneh-Far R, Lin J, Epel E, et al. . Telomere length trajectory and its determinants in person with coronary artery disease: Findings from the heart and soul study. PLoS One 2010;5:e8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for Disease Control NHANES. Hypertension questionnaire. www.cdc.gov/nchs/data/nhanes/2015-6/questionnaires/BPQ-1.pdf accessed April27, 2018
- 15.Kuczmarski RJ, Ogden CL, Guo SS, et al. . 2000. CDC growth charts for the United States: Methods and development. Vital Health Stat 11 2002:1–190 [PubMed] [Google Scholar]
- 16.Cai L, Lubitz J, Flegal KM, et al. . The predicted effects of chronic obesity in middle age on Medicare costs and mortality. Med Care 2010;48:510–517 [DOI] [PubMed] [Google Scholar]
- 17.Suglia SF, Clark CJ, Gary-Webb TL. Adolescent obesity, change in weight status and hypertension. Racial/ethnic variations. Hypertension 2013;61:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, et al. . Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 19.Mundstock E, Sarria EE, Zatti H, et al. . Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring) 2015;23:2165–2174 [DOI] [PubMed] [Google Scholar]
- 20.Müezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: A systematic review and meta-analysis. Obes Rev 2014;15:192–201 [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Calzon S, Molere A, Marcos A, et al. . Telomere length as a biomarker for adiposity change after a multidisciplinary intervention in overweight/obese adolescents: The EVASYON study. PLoS One 2014;9:e89828l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggan C, Risques R, Alfano C, et al. . Change in peripheral blood leukocyte telomere length and mortality in breast cancer survivors. J Natl Cancer Inst 2014;106:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epel ES, Merkin SS, Cawthon R, et al. . The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2008;1:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]