Abstract
Rickettsia helvetica is an emerging human pathogen, belonging to the spotted fever group (SFG) rickettsiae, associated with generally aneruptive fever, meningitis, and sudden death in chronic perimyocarditis. In this study, we describe the detection of R. helvetica in human-parasitizing and free-living Ixodes ricinus from the Metropolitan City of Rome. The pathogen was found in a tick acquired by a woman in an urban park. The circulation of R. helvetica was further confirmed by its detection in free-living ticks from a wild green area. These findings demonstrate that urban as well as wild green areas can represent a risk of infection to humans by R. helvetica, with potentially severe sequelae. To the best of our knowledge, this is the first report of R. helvetica in the Lazio region. Large-scale studies are needed to evaluate and quantify the presence of R. helvetica and other SFG rickettsiae in the urban and periurban context and to assess the risk to humans and animals related to their frequentation.
Keywords: : Rickettsia helvetica, Ixodes ricinus, human, Rome, Italy
Introduction
Rickettsia helvetica is a member of the spotted fever group (SFG) rickettsiae. It is reported as an emerging human pathogen suspected to cause symptoms such as generally aneruptive fever, headache, arthralgia, and myalgia. R. helvetica was also associated with chronic perimyocarditis in sudden cardiac death and has been isolated from a patient with subacute meningitis (Nilsson et al. 1999b, 2010, Nilsson 2009). The pathogenic role of R. helvetica in companion animals and whether they can serve as a reservoir are still unknown. The pathogen was molecularly detected in ticks removed from dogs and cats (Boretti et al. 2009, Pennisi et al. 2015, Morganti et al. 2017). None of the animal blood samples tested by PCR resulted positive for R. helvetica (Boretti et al. 2009).
Ticks of the genus Ixodes are the main vectors and reservoirs of R. helvetica, transmitting the pathogen both transstadially and transovarially, even though it was also found in Dermacentor reticulatus (Dobec et al. 2009).
R. helvetica was first isolated in Switzerland in 1979 from Ixodes ricinus and later characterized (Beati et al. 1993).The pathogen was then reported in other European countries, North Africa, and Japan (Parola et al. 2013). Cases of aneruptive fever associated with antibodies to R. helvetica were reported in France, Italy, and Thailand (Fournier et al. 2004). In Italy, R. helvetica was first detected in I. ricinus from northeastern regions in 2002 and then reported in several wild areas of other regions (Beninati et al. 2002, Bertolotti et al. 2006, Floris et al. 2008, Maioli et al. 2012). Few studies were carried out to investigate the presence of SFG rickettsiae in Italian urban parks, with just one reporting the presence of R. helvetica (Corrain et al. 2012, Mancini et al. 2015).
With 5.4 km2 and 4.4 million inhabitants, the Metropolitan City of Rome covers almost one-third of the territory of Lazio region, Central Italy, occupying the flat area of the Roman country, the Tiber Valley, and several hilly and mountainous areas. Rome is the largest municipality of the Metropolitan City, the most populated in Italy, and the fourth most populous city in the European Union. Rome is also the greenest city in Europe, with 63.8% of its territory covered by green areas and urban parks highly frequented throughout the year. In this study, we describe the detection of R. helvetica in human-parasitizing and free-living I. ricinus from urban and wild green areas of the Metropolitan City of Rome. To the best of our knowledge, this is the first report of R. helvetica in the Lazio region.
Materials and Methods
Human-parasitizing tick
A 52-year-old woman removed a tick from herself soon after walking around in Veio Park with her dog. Veio Park is a large park located in the northern part of Rome, extending from the periurban to the urban area. The park comprises natural environments, rich in wide woodlands, creeks, and water gaps, and seminatural environments characterized by traditional extensive agriculture and outdoor farming systems. Several wild species, including badgers, foxes, fallow deer, and wild boars, live in the park. The patient, affected by autoimmune hepatitis, was treated with azathioprine in monotherapy at maintenance doses. The woman did not show any clinical signs apart from a papular lesion in the bite site on the gluteus. Two months after the tick bite, she was submitted to an indirect fluorescent antibody test to detect IgM and IgG antibodies to SFG rickettsiae resulting negative.
Free-living ticks
Free-living I. ricinus sampled by dragging in a wild green area (Tolfa Mountains) were checked for Rickettsia spp. DNA as part of a research project aimed at evaluating the presence of tick-borne pathogens (TBPs) in ticks from the province of Rome.
Tolfa Mountains are a hilly natural environment, 40 km away from Rome, characterized by bushy glades and lawns used as pastures for cattle, horses, and donkeys, with several farms scattered in the territory. Oak woods and maquis alternate with pastures and cultivated areas. Representative elements of the fauna are wild boars, roe deer, foxes, martens, hares, and many species of rodents and insectivores.
Tick identification and molecular techniques
Ticks, morphologically identified according to Manilla (1998) and Iori et al. (2005), were submitted to DNA extraction using a commercial kit (QIAamp DNA mini Kit, Qiagen, Hilden, Germany). PCR protocols targeting gltA, ompA, and ompB genes were carried out to reveal the presence of SFG rickettsiae (Scarpulla et al. 2016). Amplicons were gel purified for downstream analysis consisting of DNA classical sequencing (Big Dye terminators, v3.1, chemistry and ABI3500 capillary sequencer, Applied Biosystems). GltA and ompB sequences, of 351 and 396 bp, respectively, were analyzed (Geneious software, Biomatters Ltd.) and finally challenged in GenBank using the nBLAST algorithm.
Results
Human-parasitizing tick
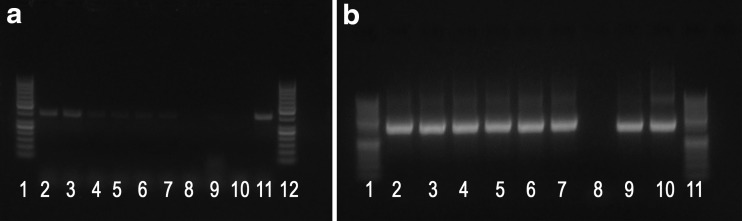
PCR assays targeting gltA and ompB yielded positive results, while no amplification was obtained for the ompA gene (Fig. 1). The nBLAST results showed that amplified gltA (KY849822) and ompB (KY951985) sequences had an identity of 100% and 99.5%, respectively (100% query coverage for both), with GenBank acc. no. KJ663745 for gltA and KJ663750 for ompB of R. helvetica previously isolated from human-parasitizing ticks in Italy.
FIG. 1.
(a) GltA. Lane 1, 12: 50 bp ladder; 2–3: sample; 4–5: 1/10 sample; 6–7: 1/100 sample; 8: negative control; 9–10: other samples; 11: positive control-381 bp. (b) OmpB. Lane 1, 11: 50 bp ladder; 2–3: sample; 4–5: 1/10 sample; 6–7: 1/100 sample; 8: negative control; 9–10: positive controls-420 bp.
Free-living ticks
A total of 42 I. ricinus were collected. R. helvetica was detected in one specimen (2.4%). GltA and ompB amplicons resulted 100% identical to those isolated in this study from the human-parasitizing tick from Veio Park (Fig. 2).
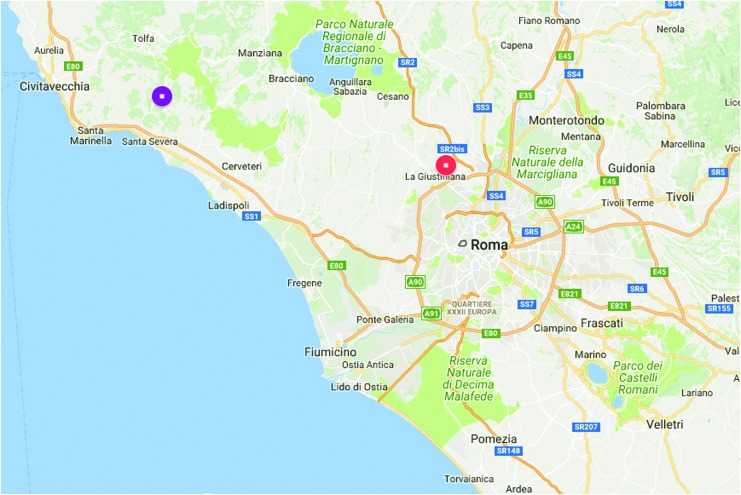
FIG. 2.
Rickettsia helvetica in human-parasitizing (Veio Park, 41.997641N 12.423329E, red dot) and free-living I. ricinus (Tolfa Mountains, 42.080594N 11.967380E, violet dot) in the Metropolitan City of Rome, Italy. Color images available online at www.liebertpub.com/vbz
Discussion
Detection of an emerging pathogen such as R. helvetica in human-parasitizing and free-living I. ricinus in the Metropolitan City of Rome raises the question of tick and tick-borne disease surveillance in urban and periurban green areas. This issue is particularly significant for at-risk people such as elderly people, children, pregnant women, or immunocompromised patients.
In the case reported in this study, the woman did not develop any infection, despite being treated with immunosuppressive drugs, likely due to the low rickettsial load in the tick or its early removal. A similar occurrence was observed also in a study carried out in northeastern Italy on 19 people bitten by infected ticks, in which none of them showed any symptom or sign of illness during a period of 4 months after the bite, confirming that the duration of tick attachment may be used to establish the risk of disease transmission. In the mentioned study, I. ricinus resulted the most involved species in human attacks (Sanogo et al. 2003). Indeed, feeding on a wide range of hosts, including humans, and being involved in the transmission of several zoonotic pathogens, I. ricinus has high sanitary relevance.
R. helvetica infection can be insidious to diagnose due to the lack of a rash typical of other SFG rickettsiae; in addition, in rickettsial diseases, antibodies are not detectable before the second week of illness, hence direct detection of the pathogen is suggested. PCR assays help in rapid diagnosis, but since the ompA gene does not allow detection of R. helvetica, it is advisable to choose other targets such as gltA and ompB (Roux et al. 1996).
Isolation of R. helvetica in the Metropolitan City of Rome suggests that physicians must also investigate this pathogen in febrile patients without rashes recently visiting urban or periurban green areas. In this study, the pathogen was found in free-living I. ricinus with 2.4% prevalence. The reported prevalence of R. helvetica in I. ricinus in Italy was between 1.5% and 23.4%, while prevalence of 2.5% and from 16.0% to 36.8% are reported in France and in Sweden, respectively (Parola et al. 1998, Nilsson et al. 1999a, Corrain et al. 2012, Maioli et al. 2012, Tomassone et al. 2013).
In the reported case, the tick was presumably acquired directly by the woman in the urban park, her dog being free from ectoparasites and regularly treated with antiparasitic compounds. Whether dogs become rickettsiemic and develop long-lasting rickettsiemia able to allow the transmission of bacteria to a vector has not been fully clarified for all SFG rickettsiae. Nonetheless, dogs live in close association with humans and could play an important role in maintaining zoonotic foci, increasing infected tick populations in the peridomestic environment (Uspensky et al. 2002, Nicholson et al. 2010, Eremeeva and Dasch 2015). The pathogenic role of R. helvetica and other SFG rickettsiae in dogs is still unclear, but should not be overlooked (Boretti et al. 2009).
Furthermore, this study suggests that the potential risk of transmission of TBPs in urban and periurban parks characterized by seminatural and natural areas can be even greater than in wild habitats due to high human and canine frequentation and the coexistence of domestic and wild animals, potential reservoirs for several TBPs. Large-scale studies are needed to evaluate and quantify the presence of SFG rickettsiae in urban and periurban green areas and to assess the risk of infection to humans and animals.
Acknowledgments
Part of this work falls within the research project IZS LT 13/10 RC funded by the Italian Ministry of Health. The authors wish to thank Luigina Di Giampietro for graphic assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- Beati L, Péter O, Burgdorfer W, Aeschlimann A, et al. . Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of rickettsiae. Int J Syst Bacteriol 1993; 43:521–526 [DOI] [PubMed] [Google Scholar]
- Beninati T, Lo N, Noda H, Esposito F, et al. . First detection of spotted fever group Rickettsiae in Ixodes ricinus from Italy. Emerg Infect Dis 2002; 9:983–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti L, Tomassone L, Tramuta C, Grego E, et al. . Borrelia lusitaniae and spotted fever group rickettsiae in Ixodes ricinus (acari: ixodidae) in Tuscany, central Italy. J Med Entomol 2006; 43:159–165 [DOI] [PubMed] [Google Scholar]
- Boretti FS, Perreten A, Meli ML, Valentino C, et al. . Molecular investigations of Rickettsia helvetica infection in dogs, foxes, humans, and Ixodes ticks. Appl Environ Microbiol 2009; 75:3230–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrain R, Drigo M, Fenati M, Menandro ML, et al. . Study on ticks and tick-borne zoonoses in public parks in Italy. Zoonoses Public Health 2012; 59:468–476 [DOI] [PubMed] [Google Scholar]
- Dobec M, Golubic D, Punda-Polic V, Kaeppeli F, et al. . Rickettsia helvetica in Dermacentor reticulatus ticks. Emerg Infect Dis 2009; 15:98–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremeeva ME, Dasch GA. Challenges posed by tick-borne rickettsiae: Eco-epidemiology and public health implications. Front Public Health 2015; 3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris R, Yurtman AN, Margoni FE, Mignozzi K, et al. . Detection and identification of Rickettsia species in the northeast of Italy. Vector Borne Zoonotic Dis 2008; 8:777–782 [DOI] [PubMed] [Google Scholar]
- Fournier PE, Allombert C, Supputamongkol Y, Caruso G, et al. . Aneruptive fever associated with antibodies to Rickettsia helvetica in Europe and Thailand. J Clin Microbiol 2004; 2:816–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iori A, Di Giulio A, De Felice S. d'Italia Zecche. In: Crignoli G, Rolando ed. Mappe parassitologiche. Napoli, Italy; 2005:1–199 [Google Scholar]
- Maioli G, Pistone D, Bonilauri P, Pajoro M, et al. . Etiological agents of rickettsiosis and anaplasmosis in ticks collected in Emilia-Romagna region (Italy) during 2008 and 2009. Exp Appl Acarol 2012; 57:199–208 [DOI] [PubMed] [Google Scholar]
- Mancini F, Ciccozzi M, Lo Presti A, Cella E, et al. . Characterization of spotted fever group Rickettsiae in ticks from a city park of Rome, Italy. Ann Ist Super Sanità 2015; 51:284–290 [DOI] [PubMed] [Google Scholar]
- Ixodida Manilla G. Acari. In: Calderini ed. Fauna d'Italia. Bologna, Italy; 1998:1–280 [Google Scholar]
- Morganti G, Gavaudan S, Canonico C, Ravagnan S, et al. . Molecular survey on Rickettsia spp., Anaplasma phagocytophilum, Borrelia burgdorferi Sensu Lato, and Babesia spp. in Ixodes ricinus Ticks infesting dogs in Central Italy. Vector Borne Zoonotic Dis 2017; 17:743–748 [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, et al. . The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010; 26:205–212 [DOI] [PubMed] [Google Scholar]
- Nilsson K. Septicaemia with Rickettsia helvetica in a patient with acute febrile illness, rash and myasthenia. J Infect 2009; 58:79–82 [DOI] [PubMed] [Google Scholar]
- Nilsson K, Elfving K, Pahlson C. Rickettsia helvetica in patient with meningitis, Sweden, 2006. Emerg Infect Dis 2010; 16:490–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K, Lindquist O, Liu AJ, Jaenson TG, et al. . Rickettsia helvetica in Ixodes ricinus ticks in Sweden. J Clin Microbiol 1999a; 37:400–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K, Lindquist O, Påhlson C. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet 1999b; 354:1169–1163 [DOI] [PubMed] [Google Scholar]
- Parola P, Beati L, Cambon M, Raoult D. First isolation of Rickettsia helvetica from Ixodes ricinus ticks in France. Eur J Clin Microbiol Infect Dis 1998; 17:95–100 [DOI] [PubMed] [Google Scholar]
- Parola P, Paddock CD, Socolovschi C, Labruna MB, et al. . Update on tick-borne Rickettsioses around the world: A geographic approach. Clin Microbiol Rev 2013; 4:657–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi MG, Persichetti MF, Serrano L, Altet L, et al. . Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit Vectors 2015; 8:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 1996; 34:2058–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanogo YO, Parola P, Shpynov S, Camicas JL, et al. . Genetic diversity of bacterial agents detected in ticks removed from asymptomatic patients in northeastern Italy. Ann NY Acad Sci 2003; 990:182–190 [DOI] [PubMed] [Google Scholar]
- Scarpulla M, Barlozzari G, Marcario A, Salvato L, et al. . Molecular detection and characterization of spotted fever group rickettsiae in ticks from Central Italy. Ticks Tick Borne Dis 2016; 7:1052–1056 [DOI] [PubMed] [Google Scholar]
- Tomassone L, Grego E, Auricchio D, Iori A, et al. . Lyme borreliosis spirochetes and spotted fever group rickettsiae in ixodid ticks from Pianosa island Tuscany archipelago, Italy. Vector Borne Zoonotic Dis 2013; 13:84–91 [DOI] [PubMed] [Google Scholar]
- Uspensky I, Ioffe-Uspensky I. The dog factor in brown dog tick Rhipicephalus sanguineus (Acari:Ixodidae) infestations in and near human dwellings. Int J Med Microbiol 2002; 291(Suppl 33):156–163 [DOI] [PubMed] [Google Scholar]