Abstract
Arterial calcification results in arterial stiffness and higher systolic blood pressure. Arterial calcification is prevented by the high expression of the Matrix-Gla gene (MGP) in the vascular smooth muscle cells (VSMC) of the arteries' tunica media. Originally, MGP, a gene highly expressed in cartilage and VSMC, was found to be one of the top expressed genes in the trabecular meshwork. The creation of an Mgp-lacZ Knock-In mouse and the use of mouse genetics revealed that in the eye, Mgp's abundant expression is localized and restricted to glaucoma-associated tissues from the anterior and posterior segments. In particular, it is specifically expressed in the regions of the trabecular meshwork and of the peripapillary sclera that surrounds the optic nerve. Because stiffness in these tissues would significantly alter outflow facility and biomechanical scleral stress in the optic nerve head (ONH), we propose MGP as a strong candidate for the regulation of stiffness in glaucoma. MGP further illustrates the presence of a common function affecting key glaucomatous parameters in the front and back of the eye, and thus offers the possibility for a sole therapeutic target for the disease.
Keywords: Calcification, Stiffness, Transgenic mice, Matrix Gla, Glaucoma
1. Introduction
1.1. Calcification and stiffness
Mineralization of the extracellular matrix (ECM) occurs when calcification deposits are formed into the extracellular space. Mineralization is initiated by the cell's release of membrane-bound matrix vesicles (MV) into the ECM, exposure to elevated Ca and Pi, and formation of calcium phosphate precipitates (Anderson, 2003; Kapustin et al., 2011, 2015). In the artery calcification, the majority of the calcification-competent extracellular vesicles are of exosomal origin. Once in the intercellular space, the released MVs associate with the ECM, interact with collagen and elastic fibrils, and by a still unknown mechanism serve as nucleation sites for the continuation of formation of hydroxyapatite nanocrystals (Kapustin et al., 2011, 2015; Krohn et al., 2016). Calcification is now known to be a highly regulated process. These MVs contain mineralization inducing proteins, such as alkaline phosphatase (ALP), MMP2 and annexins, as well as potent mineralization inhibitors, such Matrix Gla (MGP) and Fetuin-A (Krohn et al., 2016; Schurgers et al., 2013). Alteration in the production of these proteins by the cell would result in different levels of them being loaded into the vesicles and as a consequence, in different extents of calcification. Absence of Mgp in the arterial wall of mice results in premature death caused by massive arterial calcification (Luo et al., 1997). Matrix vesicles from vascular smooth muscle cells (VSMC) treated with calcification media show a dramatic reduction of MGP 48 h after treatment. (Kapustin et al., 2011).
Stiffness of a tissue, or resistance to deformation by a mechanical force, occurs when its cells and ECM undergo a number of biological changes which would lead to an overall compromise of its flexibility and rigidity. In the cell, stiffness is a physiological response that is required to resist an exogenous force. Stiffness is also associated with pathological conditions in a number of diseases such cardiovascular, cancer, chronic kidney disease (CKD), diabetes and glaucoma (Braunger et al., 2015; Kerr and Guerin, 2007; Mattace-Raso et al., 2006; Shim et al., 2015; Tiago et al., 2016; Visontai et al., 2005). Increased stiffness can be caused by a variety of cellular, cell-ECM and ECM hardening mechanisms. Per example, in endothelial cells lining the blood vessels, a stiffness response has been show to occur by the activation of the RhoA signaling pathway which leads to changes in cytoskeleton and focal adhesions to withstand the force (Collins et al., 2012). In these cells, an ECM signaling mechanism through integrins is also known to influence the response of mechanosensitive proteins and induce stiffness. In the vessels, stiffness induced by the sheer stress generated by the blood flow has been associated with elastin fragmentation, collagen deposition, matrix protein cross-linking, advance glycation end products, oxidative stress, angiotensin, dietary salt and a number of other factors (Zieman et al., 2005). Mineralization of the ECM leads inexorably to an increased stiffness.
Given the recent attention to the fact that stiffness in glaucomarelated tissues is associated with most, if not all glaucomatous conditions of elevated IOP (Braunger et al., 2015; Girard et al., 2009; Last et al., 2011; Nguyen et al., 2013) in this review we like to bring the reader's attention to the involvement of a single gene which would be mediating stiffness in glaucoma, as well as stiffness in the systemic arterial system. The gene is Matrix Gla (MGP), a potent mineralization inhibitor, known to be responsible for the physiological softness of cartilage, ossification of bone, and the mineralization and calcification/stiffness of arteries in the vascular system (Schurgers et al., 2013). In the eye, this gene has been found to be very abundant, and active only in the trabecular meshwork and peripapillary sclera (Borrás et al., 2015b), two tissues highly relevant to the development of glaucoma. MGP expression levels, as well as those of its activating enzyme λ-carboxylase, are reduced in the trabecular meshwork of primary open angle (POAG) patients when compared to normal, age-sex matched controls (Xue et al., 2007). Transcription of MGP is also reduced in primary human trabecular meshwork cells (HTM) treated with glaucomatous agents TGFβ1 (Vittitow and Borrás, 2004), TGFβ2, and dexamethasone (Xue et al., 2007). Mechanical forces of elevated IOP and stretch also alter expression of the MGP gene in human perfused anterior segment organ cultures and porcine trabecular meshwork (Comes and Borrás, 2009; Vittal et al., 2005; Vittitow and Borrás, 2004). In HTM cells, silencing the expression of MGP by siRNA, induces increase of the calcification marker ALP (Xue et al., 2007). When overexpressed in HTM cells, MGP reduced the calcification induction of BMP2 (Xue et al., 2006).
2. Association of vascular calcification and arterial stiffness
In the vascular system, calcification is associated with arterial stiffness. Early in 1997, before much of the calcification regulation was elucidated, experiments were conducted in rodents to demonstrate that accumulation of calcium in the media of the vessel wall would decrease aortic elasticity. The authors developed a rat model of calcium overload by treating rats with vitamin D and nicotine and produced 10–40-fold increase of calcium deposition in the elastic fibers of the media. After treatment, they measured stiffness of the arterial wall by elastic modulus, aortic pulse wave velocity (PWV) and isobaric elasticity. Their results showed that the stiffness of the arteries in the treated rats was significantly increased. Such increase was significantly correlated with the content of calcium in the wall (Niederhoffer et al., 1997). They therefore concluded that calcium overload on the medial elastic fibers is associated with decrease of aortic elasticity.
Association of calcification with stiffness is being nowadays observed in several major systemic diseases. Patients with CKD exhibit high mortality rate which does not seem to correlate with some of their risk factors such as elevated homocysteine, hypertension or diabetes, but rather with calcification of their blood vessels. Numerous reports indicate that, in CKD patients, medial calcification in the blood vessels occurs at an early age and with great severity. Measurement of stiffness in the calcified vessels of CKD patients as well as in those with end-stage renal disease by PWV demonstrated that not only the aortic stiffness was increased but that it was a strong predictor of cardiovascular mortality (Goodman et al., 2000; Kerr and Guerin, 2007; London et al., 2003; Robinson et al., 2005). Thus authors concluded that medial calcification and arterial stiffness play a major role in the development of CKD. In another disease, chronic obstructive pulmonary disease (COPD), patients were known to have increased aortic stiffness as measured by PWV. To investigate the relationship between aortic stiffness and calcification, a clinical study of 45 COPD patients absent of diabetes, renal or cardiovascular disease were subjected to thoraco-computed tomography for the determination of quantitative aortic calcium content. Their results showed a significant correlation of PWV values with aortic calcification. It was therefore concluded that aortic calcification was related to aortic stiffness in COPD patients (John et al., 2013).
3. Vascular dysfunction and arterial stiffness in glaucoma
The association of deficiencies in the vascular system with glaucoma, the vascular theory of glaucoma, has been around since the 19th century (Girkin, 2001). This theory proposes vascular health as an alternative risk factor to elevated IOP for glaucoma (Huck et al., 2014; Wirostko et al., 2009). Their thoughts are based on observations that some patients undergo glaucoma progression despite reduced IOP, while others do not develop glaucoma in the presence of elevated IOP (Heijl et al., 2002). Strong epidemiology data indicates that the higher incidence of glaucoma in African Americans correlates with their higher incidence of cardiovascular disease (Huck et al., 2014). Several other clinical studies directly measuring systemic and ocular fluid dynamics of blood flow have reconfirmed the association of vascular dysfunctions with risk factors for glaucoma (Choi and Kook, 2015). Together, the findings would indicate that other than IOP factors, such as genetic individual response to elevated IOP (Comes and Borrás, 2009) and/or proper functioning of the arterial vessels are bound to be involved in the development of glaucoma (Resch et al., 2009).
Arterial stiffness, or loss of arterial elasticity, is an important risk factor for cardiovascular diseases (Mattace-Raso et al., 2006). Arterial stiffness in glaucoma, has been investigated in a number of clinical studies using different measuring parameters. Using a non invasive high precision ultrasound wall-tracking system, it was shown that the distensibility coefficient sensitivity of the common carotid artery was significantly reduced in the glaucoma group versus sex/age-matched controls, while the stiffness was significantly increased (Visontai et al., 2005). More recently, using carotid-femoral PWV (CF-PWV) as the stiffness marker, the carotid artery of a group of pseudoexfoliation glaucoma (PEXG) patients showed significantly higher stiffness than that of the controls (Turkyilmaz et al., 2014). Based on this association between CFPWV and PEXG, the authors proposed that the CF-PWV outcomes may be considered a risk factor for PEXG. Likewise, in a retrospective chart review, brachial-ankle PWV (baPWV) was analyzed in four groups: diabetes patients with POAG, POAG alone, normal tension glaucoma (NTG) and matched controls (Shim et al., 2015). Increased baPWV was positively associated with glaucoma in patients with diabetes, as well as in patients with only POAG and/or NTG. The authors proposed that arterial stiffness may contribute to the pathogenesis of glaucoma in diabetes patients.
4. Presence of stiffness in glaucomatous-relevant tissues
Elevated intraocular pressure (IOP) is the principal risk factor associated with the development of glaucoma (Kass et al., 2002). Physiological or elevated IOP is determined by the different resistances offered to the flow of aqueous humor by the trabecular outflow pathway (Rosenquist et al., 1989; Schuman et al., 1999). Dysfunction of this tissue by a variety of mechanisms would disrupt the facility of the aqueous humor flow and lead to pathological IOP and glaucoma. Investigators have determined the stiffness of the trabecular meshwork tissue from normal and glaucomatous patients by measuring the young elastic modulus using atomic force microscopy (AFM) (Last et al., 2011). They found that the glaucomatous trabecular meshwork was over 20-fold stiffer than the one from normal controls. Furthermore, subsequent studies by the same group identified that treatments of normal trabecular meshwork with factors/agents associated with elevated IOP and/or glaucoma, resulted also in a stiffer tissue. Dexamethasone, senescence and inhibitors of the Wnt pathway (see below), all led to higher trabecular meshwork cells stiffness (Morgan et al., 2015a, 2015b; Raghunathan et al., 2015). Other investigators have measured the specific stiffness in cells of the Schlemm's canal (SC) inner wall from normal and glaucomatous patients (Overby et al., 2014). Here the authors find that the difference in stiffness between normal and glaucomatous cells depended on the region probed with the AFM tip. When measuring with a sharp tip (20 nm tip radius), which probes the cell cortex layer, the authors find no difference in stiffness in normal versus glaucomatous cells. However, when probed with the larger, spherical AFM tips (4.5 µm and 10 µm), which probe the subcortical cytoskeleton, they find systematic differences in stiffness between glaucomatous and normal SC cells. This discrepancy is attributed to the difference of just measuring the cell cortex (sharp tip), which is thicker and stiffer than the subcortical region, versus that of probing/measuring both the cortex and the underlying cytoskeleton (larger, rounded tip). Interestingly the authors also find a correlation between SC cell stiffness and a reduced number of pores in the glaucomatous cell monolayer. They therefore concluded increased stiffness of the SC to be a potential cause of reduced pore formation.
The second tissue of the eye where stiffness plays a role in the development of glaucoma is the peripapillary sclera surrounding the optic nerve head (ONH). The degree of stiffness of the sclera has great relevance in glaucoma (Nguyen et al., 2013). The sclera is the principal load-bearing structure of the eye and its biomechanical response to elevated IOP controls the extent of force applied to the ONH (Cone-Kimball et al., 2013; Grainger et al., 1998). Deformation of the ONH due to the IOP-stresses is a clinical landmark of glaucoma and affects axons and survival of the RGC cells. The stiffness of the posterior sclera has been evaluated in several animal models. In monkeys, investigators have used a custom built pressurized apparatus that allowed the calculation of various biomechanical properties such as strain, tangent modulus and stiffness (Girard et al., 2009). Their studies concluded that older monkeys exhibit a significantly stiffer sclera than younger ones. This stiffness would lead to higher stresses, influence ONH biomechanics and affect susceptibility to glaucoma in the older age. In the mouse, it was observed that the structure of the sclera changes with pressure elevation, showing alteration in fibrillar content and orientation. These structural changes would be consistent with an increased scleral stiffness (Cone-Kimball et al., 2013; Pijanka et al., 2014).
Altogether, experimental evidence in the anterior and posterior pole of the eye reveals that stiffness is a mechanical property which influences in a key manner the development of glaucoma. Identification of gene (s) that could modulate cellular stiffness would offer an attractive target for the management of the disease.
5. Common agents that induce arterial calcification, stiffness and glaucomatous conditions
A review of the literature of agents who contribute to vascular calcification and stiffness reveals a striking similarity with those agents which traditionally have been associated with glaucoma. Among them, TGFβ, dexamethasone, the Wnt and RhoA pathways, transglutaminase 2 (TGM2) and connective tissue growth factor (CTGF).
5.1. TGFβ2
Members of the TGFβ/bone morphogenetic protein 2 (BMP2) family have been described as potent inducers of osteogenic differentiation and calcification in mesenchymal and in vascular cells (Grainger et al., 1998; Shao et al., 2006). In our laboratory, early studies demonstrated that this property of TGFβ2 was also conserved in primary HTM cells. Treatment of HTM cells with 1 and 3 ng of TGFβ2 significantly induced the calcification marker ALP, which was concurrent with a 2.5-fold reduction in MGP and MGP's activating enzyme γ-carboxylase expression (Xue et al., 2007). Overexpression of another member of the TGFβ family, BMP2, induced also calcification in HTM cells and in living rats, where it was coupled to elevated IOP (Buie et al., 2013). However, another member of the TGFβ family, BMP7, has been shown to ameliorate rather than enhance vascular calcification in kidney disease (Mikhaylova et al., 2007). In an interesting parallel, BMP7 was shown to antagonize TGFβ2 and attenuate its effects in the trabecular meshwork (Fuchshofer et al., 2007). TGFβ is also known to induce vascular stiffness (Zieman et al., 2005) and appeared activated in a proteomic study comparing normal and stiffer trabecular meshwork ECM (Raghunathan et al., 2015). The association of TGFβ2 with glaucoma is extensive (Gottanka et al., 2004; Lütjen-Drecoll, 2005; Tripathi et al., 1994).
5.2. Dexamethasone
Prolonged cell exposure to glucocorticoids has been found to be associated with the development of calcification in vascular and human mesenchymal cells (Mori et al., 1999; Mostafa et al., 2012). Using AFM and Arterial Wave Transit Time it was shown that treatment with steroids caused stiffening of blood cells in vitro, and in aortas and elastic arteries in a chronic model of inflammation in vivo (Ko et al., 2013; Lam et al., 2007). In the trabecular meshwork, HTM cells exposure to 0.1 µM dexamethasone, significantly increased the presence of the calcification marker ALP starting at 3 days post-treatment (Xue et al., 2007). Independently, in a different laboratory, it was found that treatment with dexamethasone for 3 days resulted in a stiffness increase of 2-fold in the trabecular meshwork, and 4-fold in the laid-out ECM, when compared with the corresponding measurements in untreated cells (Raghunathan et al., 2015). It is well-established that topical ocular treatment with steroids induces elevated IOP in 30–40% of the population and in 90% of patients with POAG (Becker and Hahn, 1964; Tripathi et al., 1999).
5.3. Wnt pathway
The proteins of the Wnt signaling pathway are secreted, and transmit their signal by binding to frizzled (Frz) seven-transmembrane receptors. Upon binding, the signals are transmitted through several pathways. Activation of the Wnt canonical pathway results in de-phosphorylation and nuclear translocation of β-catenin which then interacts with transcription factors TCF/LEF for the activation of specific genes. Numerous studies support the notion that activation of the Wnt pathway is instrumental for diverting mesenchymal cells to osteoblasts (Corr, 2008; Gaur et al., 2005) and for vascular calcification (Johnson et al., 2008; Johnson and Terkeltaub, 2005; Marinou et al., 2012). For example, it was demonstrated that transglutaminase 2 (TGM2), a protein central to the calcification of VSMC, enhances calcification by activating the Wnt/β-catenin pathway (see below). Antagonist of the Wnt pathway, such as secreted-related frizzle protein 1 (sFRP1) prevents calcification and stiffness in most systems. Thus, after gene ablation, transgenic KO mice (sFRP1−/−) exhibit increased bone healing and mechanically stronger bone (Bodine and Komm, 2006; Gaur et al., 2005). Genes of the Wnt pathway and their inhibitors are also expressed and induced by elevated IOP in the trabecular meshwork (Comes and Borrás, 2009). Interestingly, contrary to what it is reported in the vascular and osteogenic fields (Bodine and Komm, 2006; Marinou et al., 2012), treatment of HTM cells with sFRP1 results in increased stiffness rather than in the expected reduction (Morgan et al., 2015b). Furthermore, intravitreal adenoviral delivery of sFRP1 to mice induced elevated IOP, which was reversed by the topical administration of an inducer of the Wnt pathway (Wang et al., 2008). In the HTM cells, β-catenin appeared to be more phosphorylated in the sFRP1 treated cultures, indicating that the sFRP1 was interfering with the activation of Wnt/β-catenin pathway which induces de-phosphorylation. However other non eye studies have shown that the sFRP1, initially seen as Wnt scavengers that prevented Wnt binding to the receptor, can bind directly to the extracellular Frz domain, and lead to the activation of the receptor (Schulte and Bryja, 2007). In addition, some members of the Wnt family, such as Wnt7b, counteract vascular calcification in atherosclerosis rather than enhancing it (Cheng et al., 2013), all to indicate that the regulation of calcification by the Wnt pathway is not fully understood. Despite this fact, it appears that the relevance of the Wnt pathway both in glaucoma and vascular diseases involves calcification and stiffness of the tissue.
5.4. RhoA pathway
RhoA is activated by force in endothelial vascular cells and this activation causes cellular stiffness (Collins et al., 2014). As in a feedback loop, RhoA activation also occurs in response to an stiffer ECM (Yeh et al., 2012) and activation of RhoA is known to be important for the cellular response to stiffness (Collins et al., 2014). The mechanism for the cellular stiffness appears to be mediated by the interaction of RhoA with integrins and the subsequent rearrangement of the cellular cytoskeleton and cellular adhesion complex (Guilluy et al., 2011). RhoA is also involved in the process of cell and ECM calcification. However, the initiation of this process by RhoA is not uniform and at times exhibits different outcomes. Thus in rat aorta organ cultures and bovine VSMC, treatment with RhoA siRNA induced ALP and calcification (Chen et al., 2010), while in human VSMC, treatment with the ROCK inhibitor Y-27632 inhibited ALP and calcification (Kizu et al., 2004). Transgenic mice overexpressing Cadherin-11 induce extensive calcification resulting in calcific aortic valve disease. Such calcification is mediated through upregulation of RhoA and it is attenuated by a ROCK inhibitor (Sung et al., 2016). Another study reports that mechanical strain promotes the production of spheroid mineralized structures in the aortic valve through a RhoA/ROCK mediated mechanism (Bouchareb et al., 2014).
In the eye, inhibition of RhoA blocked calcification induced by dexamethasone in primary HTM cells (Carabana et al., 2012). Cells treated with dexamethasone increased their endogenous ALP 4.8 ± 0.9-fold over untreated controls. Treatment with an adenoviral vector carrying dominant-negative RhoA (AdnRhoA), which inhibited RhoA by overexpressing the GTP-binding site mutated form of the molecule, reverted the dexamethasone-induced ALP to 1.9 ± 0.4- and 0.8 ± 0.9-fold (p ¼ 0.003) in a dose dependent manner. Calcification of HTM cells induced by overexpression of BMP2 (Buie et al., 2013) was also reverted by cell treatment with ROCK inhibitor Y-27632 (Carabana et al., 2012). Inhibiting the Rho pathway has been shown to reduce elevated IOP. In 1997, Y-27632 was demonstrated to be a potent smooth muscle relaxant and to correct hypertension in rats (Uehata et al., 1997). Subsequently, Y-27632 was demonstrated to reduce IOP in rabbits (Honjo et al., 2000), inhibit contraction of the trabecular meshwork (Thieme et al., 2000), increase outflow facility in rabbits and perfused porcine anterior segments (Honjo et al., 2001; Rao et al., 2001) and increase SC permeability (Kameda et al., 2012). Recently, a long term gene delivery vector carrying dnRhoA prevented elevation of nocturnal IOP in rats for one month after a single injection (Borrás et al., 2015a). Currently, some ROCK inhibitors are being tested in clinical trials (Tanihara et al., 2008).
5.5. Transglutaminase 2
Transglutaminase 2 (TGM2) is a key regulator of Pi-induced calcification. It is being extensively studied in the cardiovascular field for its role in VSMC calcification, which is associated with mortality in several diseases, including diabetes and CKD (Chen et al., 2013). TGM2 is a calcium-dependent enzyme that catalyzes the crosslinking of most ECM proteins by post-translational transor de-amidation modifications. Inside the cell, TGM2 is activated by increased intracellular calcium. Through these modifications, TGM2 regulates osteoblast differentiation and matrix mineralization in culture (Al-Jallad et al., 2006). Studies from Nurminskaya's laboratory showed that purified TGM2 added to primary VSMC binds to the LRP5 Wnt receptor, activates the β-catenin pathway and induces the TCF/LEF transcription factors (Faverman et al., 2008). Addition of TGM2 also stimulated expression of RUNX2, a key osteogenic transcription factor, and downstream targets osteocalcin and bone sialoprotein. And, inhibition of TGM2 in these cells prevented warfarin-induced calcification (Beazley et al., 2012). In vivo, TGM2 modulates mineralization. VSMC from TGM2-null mice, cannot calcify in response to hyperphosphatemia (Johnson et al., 2008), and inhibiting TGM2 by administration of quercetin prevents calcification and elastocalcinosis (Beazley et al., 2013a) in the warfarin-induced calcification mouse model.
In the glaucoma field, TGM2 has been well-characterized. It was shown to be present in the trabecular meshwork cells and tissue, and elevated in glaucoma specimens when compared to agematched controls (Fuchshofer et al., 2005; Tovar-Vidales et al., 2008, 2011; Welge-Lussen et al., 2000). TGM2 was also induced by TGFβ1 and TGFβ2 in trabecular meshwork and optic nerve astrocytes at the RNA, protein and enzymatic activity levels (Fuchshofer et al., 2005; Welge-Lussen et al., 2000). Because of the well-established fact that glaucoma is associated with increased ECM material in the glaucomatous trabecular meshwork, it has been proposed that TGM2 could be responsible for deterring ECM degradation by increasing cross-linking of its proteins (Welge-Lussen et al., 2000).
5.6. Connective tissue growth factor
Connective tissue growth factor (CTGF) is a complex, secreted matricellular protein involved in many different mechanisms (Lipson et al., 2012). Different stimuli can induce the expression of CTGF. When secreted, CTGF interacts with a variety of extracellular and cell surface proteins affecting their signaling pathways and cellular functions, all of which lead to tissue remodeling. CTGF induced osteogenic differentiation of mouse primary VSMC (Huang et al., 2013), and elevated levels of CTGF in the serum in patients of biliary atresia correlated with liver stiffness (elastography) and poor disease outcomes (Honsawek et al., 2013). Inhibition of CTGF reversed the process of fibrosis and a CTGF monoclonal antibody (FG-3019), which is in clinical development, prevents carotid artery vascular stiffness in a model of diabetic rats (Lipson et al., 2012).
In glaucomatous conditions, CTGF plays a key role. Numerous studies have demonstrated that elevated concentrations of TGFβ2, as it occurs in the aqueous humor of POAG patients, seem to be responsible for changes similar to those observed in the trabecular meshwork ECM in vivo. In vitro, it has been repeatedly shown that the observed changes in the ECM are mediated by the TGFβ2 downstream effector CTGF (Fuchshofer et al., 2011; Junglas et al., 2009). In vivo, transgenic mice overexpressing CTGF by adenoviral gene transfer or by generation of a βB1-crystallin driven CTGF cDNA exhibit elevated IOP and loss of optic nerve axons (Junglas et al., 2012). Also, SC cells from glaucoma patients which have increased levels of CTGF, were shown to be stiffer than those from matched healthy individuals (Overby et al., 2014).
Altogether, the combined evidence indicates that the same agents described to affect mineralization and stiffness in the vascular system have also been described as affecting IOP and glaucoma in the eye. The revised data findings thus support a potential central mechanism of stiffness in glaucoma which coincides with the current thinking in the field (Braunger et al., 2015). The evidence further point outs that the stiffness observed in glaucomatous manifestations could be in part triggered by the induction of mineralization of the glaucoma associated tissues.
6. The MGP gene: a master regulator of calcification and stiffness
Matrix Gla (MGP) is a 10 kD secreted protein, highly abundant in cartilage chondrocytes and in VSMCs of the tunica media of arteries (Wallin et al., 2001). Activation of MGP occurs by a posttranslational modification where its glutamic acid residues (Glu) are converted to γ-carboxyglutamic acid (Gla) by a vitamin K-dependent γ-carboxylase enzyme (Price et al., 1983). In patients, MGP polymorphisms been linked to coronary artery calcification (Crosier et al., 2009; Herrmann et al., 2000). In mice, ablation of Mgp results in massive arterial calcification and death at about 5–6 weeks of age (Zieman et al., 2005). The mechanisms by which MGP inhibits calcification in the arteries are not totally understood. After being carboxylated, MGP undergoes a conformational change and binds calcium. This conformer of carboxylated MGP binds and sequesters BMP2, a potent inducer of bone formation (Ducy and Karsenty, 2000). It is believed that MGP acts as a negative regulator of BMP2. Also, Mgp colocalizes with elastin in the arterial elastic lamina and, in the KO mice, mineral deposition and calcification lesions are seeing along the elastic lamella (elastocalcinosis). In this Mgp null mouse, calcification in the VSMC is preceded by elastin fragmentation (Beazley et al., 2013b) which appears to be the result of the induction of the elastase enzyme adipsin. An additional calcification mechanism of the Mgp KO appears to be associated with the presence of Tgm2. Mice which have ablated both genes, Mgp and Tgm2, live longer and exhibit less elastocalcinosis than the single null Mgp. Tgm2 accumulates in the calcifying aorta of the Mgp null mice (Kaartinen et al., 2007) and is a key regulator of the transformation of VSMC into osteogenic cells (Johnson et al., 2008) (see above). Ablation of Tgm2 then serves to ameliorate the calcification effects.
In cartilage, MGP prevents osteogenic transformation of chondrocytes. An autosomal recessive rare disease, Keutel syndrome, has been associated with several mutations in the MGP gene (Tüysüz et al., 2015). Patients develop abnormal cartilage calcification, brachytelephalangism, peripheral pulmonary artery stenosis and in some, optic atrophy (Tüysüz et al., 2015). Less than 30 cases globally have been reported since its discovery in 1970 and phenotypes of the patients show clinical variability (Tüysüz et al., 2015). All the exams reported include young patients. Although very few ophthalmological exams have been described, in one case, a 6-year old patient had sudden loss of vision in both eyes at the age 3 and showed bilateral optic nerve atrophy (Hur et al., 2005). The severity of the disease is depending on the extent of the pulmonary involvement and limited reports seem to indicate that patients die at a younger age.
Inactive MGP is also associated with arterial stiffness. A recent clinical study enrolled 1001 patients in a family-based multicenter in Switzerland. Inactive MGP (dephospho-uncarboxylated MGP) was quantified in the plasma by ELISA and PWV was determined by applanation tonometry to measure stiffness. After adjusting for age and other cardiovascular factors, results showed that high levels of inactive MGP were positively associated with arterial stiffness (Pivin et al., 2015).
7. The MGP gene and MGP protein in the eye
7.1. In vitro studies in human trabecular meshwork
Earlier studies from our laboratory had identified MGP among the ten most highly expressed genes in the human trabecular meshwork (Gonzalez et al., 2000), a finding later confirmed by other investigators (Vittal et al., 2005; Wirtz et al., 2002). Subsequent studies demonstrated that trabecular meshwork in primary cells and perfused organ cultures had as much carboxylase activity as the VSMC, that the MGP was carboxylated and that the active protein was functional and able to reduce the calcification activity of BMP2 (Xue et al., 2006). Subsequently, a number of biological studies revealed that expression of MGP in primary HTM cells responded to glaucomatous insults. Thus, its expression was increased by elevated IOP at the time of homeostatic response (Vittitow and Borrás, 2004) and it was reduced by treatment with TGFβ1 (Vittitow and Borrás, 2004), TGFβ2 (Xue et al., 2007) and dexamethasone (Carabana et al., 2012). Most important, trabecular meshwork specimens from glaucoma patients exhibited decreased levels of MGP mRNA and higher levels of the calcification marker ALP when compared to age-sex matched controls (Xue et al., 2007). When the trabecular meshwork cells’ MGP expression was blocked by MGP siRNA, the levels of ALP were increased (Xue et al., 2007).
7.2. In vivo studies in transgenic mice. Generation of Mgp KO and KI
7.2.1. Mgp Knock-Out mice, Mgp−/−
As mentioned above, the Mgp-KO (Luo et al., 1997) is lethal and dies at 5–6 weeks of age. The Mgp KO provided a first glimpse on the effects of Mgp ablation in the eye but studies were limited due to the early death and compromised phenotype of the mice. Before their death at 5 weeks, mice exhibit a significant reduction in size, contorted shape and compromised breathing. Despite these limitations, unpublished experiments from our lab noted structural changes in the trabecular meshwork at the light and electron microscopy level, a moderate increase of IOP, and the increased expression of some calcification markers in the angle tissues. The detailed results of these experiments are in preparation.
7.2.2. Mgp-Cre Knock-In, MgpCreKI/CreKI
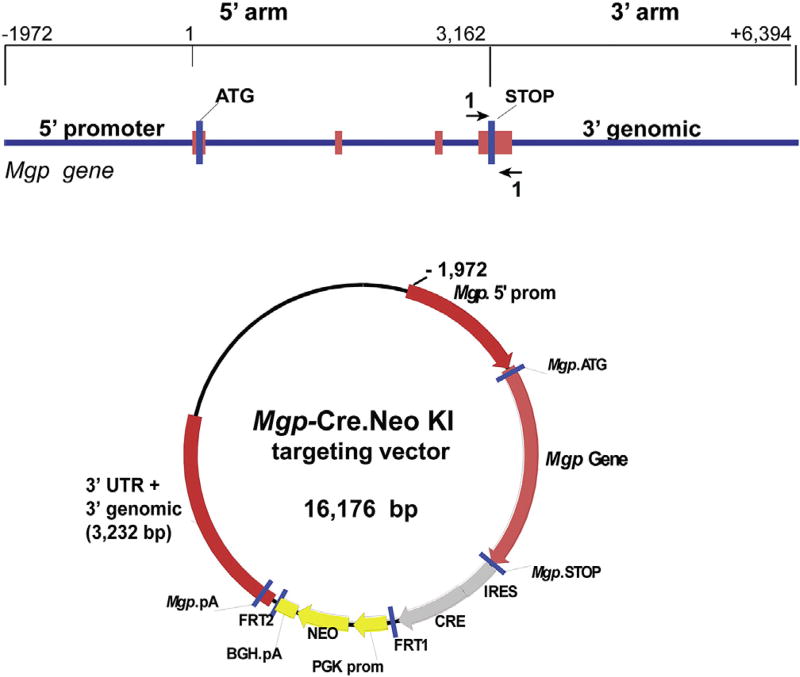
Localization and distribution of the Mgp expression in the eye are key to elucidate the mechanistic link between MGP and glaucoma. Attempts to determine the spatial and temporal patterns by in situ hybridization and immunohistochemistry were not indicative, due in part to unsuitability of the reagents used. This prompted us to use mouse genetics technology and to generate an Mgp-Cre Knock-In (Mgp-Cre KI) (Borrás et al., 2015b). In this mouse, an internal ribosomal entry site (IRES)-Cre recombinase expression cassette was inserted in between the translational Mgp STOP signal and its polyA. The Knock-In MgpCreKI was designed to express Cre recombinase only in cells where Mgp is expressed. The targeting vector is showing in Fig.1.When these mice are crossed with a mouse containing loxP sites flanking a STOP signal for a reporter gene, Cre recombination results in the deletion of the STOP and allows expression of the reporter gene. Histological assessment of the expressed reporter in their offspring does correspond to the expression of the gene driving the Cre. These Cre KI mouse lines are extremely valuable for the determination of localization of the desired gene expression in specific cell types, its temporal distribution during embryonic and adult development, and its regulation under disease conditions. An accurate determination of these parameters is essential to establish the role between MGP and glaucoma.
Fig. 1.
Diagram of the targeting strategy to generate the Mgp-Cre Knock-In (Mgp-Cre KI) mouse. Targeting vector consists of homology arms to 1972 promoter bp, exon 1, intron 1, exon 2, intron 2, exon 3, intron 3, exon 4 and 3022 bp of 3′ genomic sequences (total 8.3 kb). Sequences containing the IRES-Cre-FRT-flanked neomycin resistance gene cassette were inserted between the Mgp STOP signal and the 3′UTR. The Neo cassette was later excised by crossing the mouse with the flippase (FLP) deleter mice followed by removal of the introduced FLP gene by intercrossing the heterozygous strains. The engineered mouse, Mgp-Cre KI, renders the Cre under the transcriptional control of the Mgp gene.
7.3. Localization of Mgp expression in the eye by using the Cre KI mouse line
7.3.1. Generation of MgpCreKI/+; R26RlacZ/+ Mgp-lacZ KI mouse line
Crosses of the Mgp-Cre KI mouse with the reporter mouse line R26R-lacZ (Soriano, 1999), resulted in the generation of MgpCreKI/+; R26RlacZ/+ (Mgp-lacZ KI) mouse line. The reporter here was the LacZ gene whose activity was measured by enzyme histochemistry on a X-gal substrate. In this study (Borrás et al., 2015b), a total of 43 experimental double heterozygous Mgp-lacZ KI showed functional Cre recombination in all eyes from ages 1–8 months obtained from different breeding pairs and from two founders. The blue β-gal staining indicative of high levels of Cre activity was intense and consistently present in just two areas of the eye globe, one on the anterior segment and the second in the posterior pole (Fig. 2).
Fig. 2.
Mgp Cre-mediated lacZ expression in the glaucoma associated tissues of the eye. A) left: meridional paraffin sections from the eyes of 4–6 weeks Mgp-LacZ KI (MgpCreKI/+; R26RlacZ/+) mice assayed for β-galactosidase activity and counterstained with hematoxylin and eosin. Mgp driven expression is very abundant in the trabecular meshwork region (TM) and peripapillary region of the ONH. Right: Mgp driven expression in the sclera is localized to the internal layer, increases from mid to posterior and reaches high levels at the peripapillary site around the ON. B) meridional paraffin sections from the eye of control mice (Mgp+/+; R26RlacZ/+).
7.3.2.. Expression in the anterior sement
The first area comprised the trabecular meshwork region, extending to the ciliary muscle and the supra-scleral zone above the SC. Given the function of the MPG gene in all other body tissues, the presence of this gene in the outflow pathway and outflow surrounding regions would suggest a role and a need to maintain the appropriate softness and elasticity required to facilitate aqueous humor flow. Its expression not only in the trabecular meshwork, but also on the ciliary muscle and immediate sclera above the canal further highlights the relevance of this gene in those tissues involved in the regulation of IOP.
7.3.3.. Expression in the posterior segment
The second main site of Mgp expression included the sclera tissue, in particular the peripapillary region surrounding the optic nerve. Blue cells are shown first on the mid-sclera and are restricted to the layer immediately underneath of the choroid (Borrás et al., 2015b). The expression increases towards the posterior sclera and exhibits the highest intensity and expansion at the peripapillary sclera supporting the optic nerve (Borrás et al., 2015b). These results seem to indicate that the peripapillary region appears to be in need of a strong protection against becoming stiff/calcified, and that it is using the Mgp protein as one of the potential mechanisms to avoid higher stiffness. Thus, taking into account the localization of expression of the Mpg gene and its function, it could be suggested that the Mgp encoded protein could be affecting both the outflow facility and the biomechanical properties of the ONH region.
8. Sclera stiffness and myopia
The presence of Mgp expressing cells starting at the mid-sclera of the Mgp-lacZ mouse together with the fact of the high expression of this gene in cartilage, would agree with reports that the sclera of many vertebrates contains a cartilaginous cell layer (Gottlieb et al., 1990; O'Steen and Brodish, 1990; Yoshitomi and Boorman, 1990). Chondrocytes have been identified in the sclera of myopia models in chick (Kusakari et al., 1997, 2001). It would seem logical to think that a reduction of MGP in the sclera would tend to calcify the tissue, affect its rigidity and have an influence in the development of myopia. Interestingly sclera calcification has been amply reported in the literature since as early as 1958 by David Cogan (Cogan et al., 1958), and it has been associated with aging and disease conditions since then (Patrinely et al., 1982; Wong et al., 1979). In one example in Fisher rats, the scleral cartilage is converted to bone, and 95% of the rats show a calcified sclera (O'Steen and Brodish, 1990). The authors speculate then that scleral bone formations could compromise optic circulation and influence photoreceptor death during aging. The presence of the Mgp gene in the sclera is providing the first explanation of a likely mechanism responsible for the long observed eye sclera calcification.
In another turn, numerous population studies have associated high myopia as a risk factor of POAG (Marcus et al., 2011). Similarly, many studies have demonstrated that visual field loss progression is greater in POAG patients with high myopia, than in POAG patients without myopia (Lee et al., 2008). It would be an attractive idea to consider that expression of MGP could be beneficious for glaucoma not only by facilitating outflow and maintaining in check the stiffness of the ONH but also by ameliorating hardening of the sclera and high myopia.
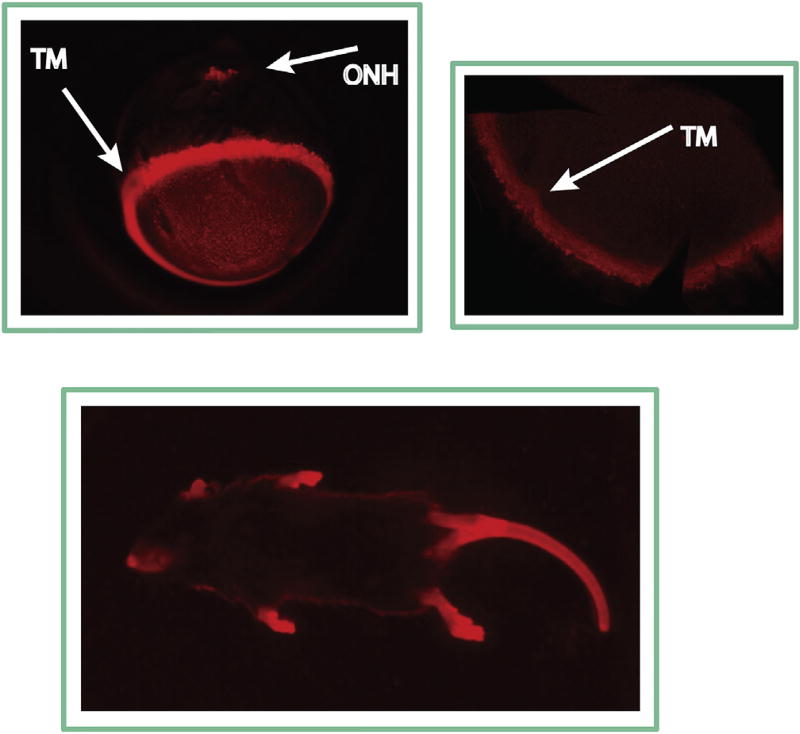
9. Validation of the Cre-directed Mgp expression
Validation of the localization of expression of Mgp in the mouse eye was shown in an independent study where the Mgp-Cre KI mouse was crossed with a different reporter strain (R26R-tdTomato) to generate MgpCreKI/+; R26RtdTomato/+ (Mgp-tdTomato KI). The reporter mouse here carried the STOP signal for the red fluorescent protein variant (tdTomato) flanked by loxP sites. In this study, analysis of whole mounts of the eye of the double heterozygous showed a specific positive red fluorescence on the limbus, indicative of the trabecular meshwork fluorescent tissue underneath. Likewise, the red fluorescence was also observed on the posterior pole at the optic nerve head region (Fig. 3). In the fluorescent images of the whole animal, red fluorescent cartilage tissues could be also observed, further validating that the Cre activity is specifically directed to the established Mgp expressed tissues (Fig. 3). Because the half-life/photostability of the reporter proteins is 24–48 h for lacZ and 1 h for tdTomato (Martin et al., 2012; Smith et al., 1995), the protein marker expression observed in these studies represents the true transcription of the gene.
Fig. 3.
Mgp Cre-mediated tdTomato expression in the Mgp-tdTomato KI (MgpCreKI/+; R26RtdTomato/+) mice. Tissues with Mgp driven expression are fluorescent red. Top: Images from the whole eye (left) and dissected anterior segment (right) showing intense fluorescence on the trabecular meshwork and ONH area. Bottom: Image of a whole mouse photographed with a UV-transilluminator box. Cartilage tissues, where Mgp is abundantly expressed show intense red fluorescence, validating the Mgp driven expression.
10. Concluding thoughts
Together, all evidence regarding calcification, stiffness, the role of MPG in the vascular system, its numerous glaucoma-associated properties and its localization to the most relevant eye tissues, supports that this protein is a strong candidate for the regulation of stiffness in glaucoma. The original findings of the gene's high abundance in the trabecular meshwork tissue coupled with the confirmation of the presence of its activating enzyme and the actual active protein, were the first signs that led us into the path of investigating its purpose in the eye and its involvement in modulating outflow. In vitro and ex-vivo work showed that all results, from regulation by elevated IOP to inverse correlation with calcification in the tissue from glaucoma patients, matched with those expected from a glaucoma relevant gene/protein. Thus, MGP was regulated by elevated IOP, and calcification markers were upregulated in the trabecular meshwork of POAG patients. MGP expression levels were reduced in the trabecular meshwork of the same POAG patients where calcification was increased. MGP levels were also reduced in trabecular meshwork in cells treated with TGFβ and dexamethasone, and ablation of MGP by siRNA induced calcification. On the other side, the available morphology data appears to be inconsistent with the presence of calcification in the trabecular meshwork. Despite an extensive number of morphological studies in this tissue, there has been no reports on the presence of calcific plaques as those described in the sclera, albeit no similar calcification assays have been reported. In contrary, there are reports of increased stiffness in the trabecular meshwork. Although as described above, stiffness can occur independently of calcification, the high levels of an active calcification inhibitor in the tissue, and its reduction in glaucoma, makes logical to assume that calcification is at least in part responsible for the observed stiffness.
Continuing effort to support the relevance of MGP function's in glaucoma would need to come from experiments in vivo. In rats, induction of calcification of the trabecular meshwork results in elevation of IOP and degeneration of RCC, two landmarks of glaucoma. In mice, the generation of the Mgp-Cre KI strains reveals that MGP is not only localized to the trabecular meshwork region, but to the peripapillary sclera surrounding the ONH. Because of the relevance of the stiffness in this region to withstand the mechanical insults transmitted by elevated IOP, this second site localization raises the level of the importance of MGP in glaucoma. Although experiments with the Mgp-KO are limited due the early death of the mouse, conditional KOs would be able to elucidate whether a non lethal Mgp deletion in glaucoma-related tissues would significantly affect the development of the disease. Generation of such floxed Mgp mice are in progress in our laboratory. Lastly, the fact that a reduction in MGP would also affect ocular vascular stiffness and potentially myopia, indicates that this protein could address most the risk factors which have been associated with the disease in the clinical setting. The uniqueness of a single gene ameliorating the stiffness in the anterior, posterior segment as well as in the eye vasculature would make MGP a highly desirable therapeutic target for glaucoma.
Acknowledgments
I am indebted to National Eye Institute for supporting our research through past and current grants (EY13126, EY11906 and EY026220) and to the Research to Prevent Blindness for an unrestricted grant to the Department of Ophthalmology. I thank the members of the lab for their hard work and the UNC Animal Core Facility for its role in generating the mouse strains. And finally I thank David L. Epstein for his teachings, discussions, intensity, and ability to infuse on us the passion to unravel new mechanisms of glaucoma.
References
- Al-Jallad HF, Nakano Y, Chen JL, McMillan E, Lefebvre C, Kaartinen MT. Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MC3T3-E1 osteoblast cultures. Matrix Biol. 2006;25:135–148. doi: 10.1016/j.matbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Anderson HC. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- Beazley KE, Banyard D, Lima F, Deasey SC, Nurminsky DI, Konoplyannikov M, Nurminskaya MV. Transglutaminase inhibitors attenuate vascular calcification in a preclinical model. Arterioscler. Thromb. Vasc. Biol. 2013a;33:43–51. doi: 10.1161/ATVBAHA.112.300260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-mediated activation of beta-catenin signaling has a critical role in warfarin-induced vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2012;32:123–130. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazley KE, Reckard S, Nurminsky D, Lima F, Nurminskaya M. Two sides of MGP null arterial disease: chondrogenic lesions dependent on transglutaminase 2 and elastin fragmentation associated with induction of adipsin. J. Biol. Chem. 2013b;288:31400–31408. doi: 10.1074/jbc.M113.495556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Hahn KA. Topical corticosteroids and hereditary in primary openangle glaucoma. Am. J. Ophthalmol. 1964;57:543–551. doi: 10.1016/0002-9394(64)92500-0. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Komm BS. Wnt signaling and osteoblastogenesis. Rev. Endocr. Metab. Disord. 2006;7:33–39. doi: 10.1007/s11154-006-9002-4. [DOI] [PubMed] [Google Scholar]
- Borrás T, Buie LK, Spiga MG, Carabana J. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA Ophthalmol. 2015a;133:182–190. doi: 10.1001/jamaophthalmol.2014.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás T, Smith MH, Buie LK. A novel Mgp-Cre knock-in mouse reveals an anticalcification/antistiffness candidate gene in the trabecular meshwork and peripapillary scleral region. Invest. Ophthalmol. Vis. Sci. 2015b;56:2203–2214. doi: 10.1167/iovs.15-16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchareb R, Boulanger MC, Fournier D, Pibarot P, Messaddeq Y, Mathieu P. Mechanical strain induces the production of spheroid mineralized microparticles in the aortic valve through a RhoA/ROCK-dependent mechanism. J. Mol. Cell Cardiol. 2014;67:49–59. doi: 10.1016/j.yjmcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Braunger BM, Fuchshofer R, Tamm ER. The aqueous humor outflow pathways in glaucoma: a unifying concept of disease mechanisms and causative treatment. Eur. J. Pharm. Biopharm. 2015;95:173–181. doi: 10.1016/j.ejpb.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Buie LK, Karim MZ, Smith MH, Borrás T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest. Ophthalmol. Vis. Sci. 2013;54:5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabana J, Xue, Borrás T. Statins and dominant-negative RhoA inhibit dexamethasone-induced calcification in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2012;53 ARVO abstract E-3247. [Google Scholar]
- Chen NX, Chen X, O'Neill KD, Atkinson SJ, Moe SM. RhoA/Rho kinase (ROCK) alters fetuin-A uptake and regulates calcification in bovine vascular smooth muscle cells (BVSMC) Am. J. Physiol Ren. Physiol. 2010;299:F674–F680. doi: 10.1152/ajprenal.00730.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NX, O'Neill K, Chen X, Kiattisunthorn K, Gattone VH, Moe SM. Transglutaminase 2 accelerates vascular calcification in chronic kidney disease. Am. J. Nephrol. 2013;37:191–198. doi: 10.1159/000347031. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Shao JS, Behrmann A, Krchma K, Towler DA. Dkk1 and MSX2- Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2013;33:1679–1689. doi: 10.1161/ATVBAHA.113.300647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kook MS. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed. Res. Int. 2015;2015:141905. doi: 10.1155/2015/141905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan DG, Hurlbut CS, Kuwabara T. Crystalline calcium sulphate (gypsum) in scleral plaques of a human eye. J. Histochem. Cytochem. 1958;6:142–145. doi: 10.1177/6.2.142. [DOI] [PubMed] [Google Scholar]
- Collins C, Guilluy C, Welch C, O'Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 2012;22:2087–2094. doi: 10.1016/j.cub.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Osborne LD, Guilluy C, Chen Z, O'Brien ET, III, Reader JS, Burridge K, Superfine R, Tzima E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat. Commun. 2014;5:3984. doi: 10.1038/ncomms4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes N, Borrás T. Individual molecular response to elevated intraocular pressure in perfused postmortem human eyes. Physiol. Genomics. 2009;38:205–225. doi: 10.1152/physiolgenomics.90261.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone-Kimball E, Nguyen C, Oglesby EN, Pease ME, Steinhart MR, Quigley HA. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis. 2013;19:2023–2039. [PMC free article] [PubMed] [Google Scholar]
- Corr M. Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis. Nat. Clin. Pract. Rheumatol. 2008;4:550–556. doi: 10.1038/ncprheum0904. [DOI] [PubMed] [Google Scholar]
- Crosier MD, Booth SL, Peter I, Dawson-Hughes B, Price PA, O'Donnell CJ, Hoffmann U, Williamson MK, Ordovas JM. Matrix Gla protein polymorphisms are associated with coronary artery calcification in men. J. Nutr. Sci Vitaminol. (Tokyo) 2009;55:59–65. doi: 10.3177/jnsv.55.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney Int. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Birke M, Welge-Lussen U, Kook D, Lütjen-Drecoll E. Transforming growth factor-beta 2 modulated extracellular matrix component expression in cultured human optic nerve head astrocytes. Invest. Ophthalmol. Vis. Sci. 2005;46:568–578. doi: 10.1167/iovs.04-0649. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Ullmann S, Zeilbeck LF, Baumann M, Junglas B, Tamm ER. Connective tissue growth factor modulates podocyte actin cytoskeleton and extracellular matrix synthesis and is induced in podocytes upon injury. Histochem. Cell Biol. 2011;136:301–319. doi: 10.1007/s00418-011-0844-9. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Girard MJ, Suh JK, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest. Ophthalmol. Vis. Sci. 2009;50:5226–5237. doi: 10.1167/iovs.08-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girkin CA. Strategies for neuroprotection. J. Glaucoma. 2001;10:S78–S80. doi: 10.1097/00061198-200110001-00028. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Epstein DL, Borrás T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest. Ophthalmol. Vis. Sci. 2000;41:3678–3693. [PubMed] [Google Scholar]
- Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lütjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest. Ophthalmol. Vis. Sci. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Joshi HB, Nickla DL. Scleral changes in chicks with form-deprivation myopia. Curr. Eye Res. 1990;9:1157–1165. doi: 10.3109/02713689009003472. [DOI] [PubMed] [Google Scholar]
- Grainger DJ, Metcalfe JC, Grace AA, Mosedale DE. Transforming growth factor-beta dynamically regulates vascular smooth muscle differentiation in vivo. J. Cell Sci. 1998;111(Pt 19):2977–2988. doi: 10.1242/jcs.111.19.2977. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Swaminathan V, Garcia-Mata R, O'Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat. Cell Biol. 2011;13:722–727. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Herrmann SM, Whatling C, Brand E, Nicaud V, Gariepy J, Simon A, Evans A, Ruidavets JB, Arveiler D, Luc G, Tiret L, Henney A, Cambien F. Polymorphisms of the human matrix gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2000;20:2386–2393. doi: 10.1161/01.atv.20.11.2386. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Honda YYBYNS. Effects of rho-associated protein kinase inhibitor, Y-27632, on intraocular pressure and aquesus humor dynamics in the rabbit eye. Invest. Ophthalmol. Vis. Sci. 2000;41 ARVO abstract E-2715. [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- Honsawek S, Udomsinprasert W, Chirathaworn C, Anomasiri W, Vejchapipat P, Poovorawan Y. Correlation of connective tissue growth factor with liver stiffness measured by transient elastography in biliary atresia. Hepatol. Res. 2013;43:795–800. doi: 10.1111/hepr.12015. [DOI] [PubMed] [Google Scholar]
- Huang J, Huang H, Wu M, Li J, Xie H, Zhou H, Liao E, Peng Y. Connective tissue growth factor induces osteogenic differentiation of vascular smooth muscle cells through ERK signaling. Int. J. Mol. Med. 2013;32:423–429. doi: 10.3892/ijmm.2013.1398. [DOI] [PubMed] [Google Scholar]
- Huck A, Harris A, Siesky B, Kim N, Muchnik M, Kanakamedala P, Amireskandari A, Abrams-Tobe L. Vascular considerations in glaucoma patients of African and European descent. Acta Ophthalmol. 2014;92:e336–e340. doi: 10.1111/aos.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur DJ, Raymond GV, Kahler SG, Riegert-Johnson DL, Cohen BA, Boyadjiev SA. A novel MGP mutation in a consanguineous family: review of the clinical and molecular characteristics of Keutel syndrome. Am. J. Med. Genet. A. 2005;135:36–40. doi: 10.1002/ajmg.a.30680. [DOI] [PubMed] [Google Scholar]
- John M, Hussain S, Prayle A, Simms R, Cockcroft JR, Bolton CE. Target renal damage: the microvascular associations of increased aortic stiffness in patients with COPD. Respir. Res. 2013;14:31. doi: 10.1186/1465-9921-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ. Res. 2008;102:529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Terkeltaub RA. External GTP-bound transglutaminase 2 is a molecular switch for chondrocyte hypertrophic differentiation and calcification. J. Biol. Chem. 2005;280:15004–15012. doi: 10.1074/jbc.M500962200. [DOI] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bosl M, Bosserhoff A, Kostler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Junglas B, Yu AH, Welge-Lussen U, Tamm ER, Fuchshofer R. Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 2009;88:1065–1075. doi: 10.1016/j.exer.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Kaartinen MT, Murshed M, Karsenty G, McKee MD. Osteopontin upregulation and polymerization by transglutaminase 2 in calcified arteries of Matrix Gla protein-deficient mice. J. Histochem. Cytochem. 2007;55:375–386. doi: 10.1369/jhc.6A7087.2006. [DOI] [PubMed] [Google Scholar]
- Kameda T, Inoue T, Inatani M, Fujimoto T, Honjo M, Kasaoka N, Inoue-Mochita M, Yoshimura N, Tanihara H. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest. Ophthalmol. Vis. Sci. 2012;53:3092–3103. doi: 10.1167/iovs.11-8018. [DOI] [PubMed] [Google Scholar]
- Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 2011;109:e1–12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Kerr PG, Guerin AP. Arterial calcification and stiffness in chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2007;34:683–687. doi: 10.1111/j.1440-1681.2007.04660.x. [DOI] [PubMed] [Google Scholar]
- Kizu A, Shioi A, Jono S, Koyama H, Okuno Y, Nishizawa Y. Statins inhibit in vitro calcification of human vascular smooth muscle cells induced by inflammatory mediators. J. Cell Biochem. 2004;93:1011–1019. doi: 10.1002/jcb.20207. [DOI] [PubMed] [Google Scholar]
- Ko YH, Tsai MS, Lee PH, Liang JT, Chang KC. Methylprednisolone stiffens aortas in lipopolysaccharide-induced chronic inflammation in rats. PLoS One. 2013;8:e69636. doi: 10.1371/journal.pone.0069636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohn JB, Hutcheson JD, Martinez-Martinez E, Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J. Physiol. 2016;594:2895–2903. doi: 10.1113/JP271338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakari T, Sato T, Tokoro T. Regional scleral changes in form-deprivation myopia in chicks. Exp. Eye Res. 1997;64:465–476. doi: 10.1006/exer.1996.0242. [DOI] [PubMed] [Google Scholar]
- Kusakari T, Sato T, Tokoro T. Visual deprivation stimulates the exchange of the fibrous sclera into the cartilaginous sclera in chicks. Exp. Eye Res. 2001;73:533–546. doi: 10.1006/exer.2001.1064. [DOI] [PubMed] [Google Scholar]
- Lam WA, Rosenbluth MJ, Fletcher DA. Chemotherapy exposure increases leukemia cell stiffness. Blood. 2007;109:3505–3508. doi: 10.1182/blood-2006-08-043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last JA, Pan T, Ding Y, Reilly CM, Keller K, Acott TS, Fautsch MP, Murphy CJ, Russell P. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YA, Shih YF, Lin LL, Huang JY, Wang TH. Association between high myopia and progression of visual field loss in primary open-angle glaucoma. J. Formos. Med. Assoc. 2008;107:952–957. doi: 10.1016/S0929-6646(09)60019-X. [DOI] [PubMed] [Google Scholar]
- Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenes. Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transpl. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp. Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989–1994. doi: 10.1016/j.ophtha.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Marinou K, Christodoulides C, Antoniades C, Koutsilieris M. Wnt signaling in cardiovascular physiology. Trends Endocrinol. Metab. 2012;23:628–636. doi: 10.1016/j.tem.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Martin DD, Ahpin CY, Heit RJ, Perinpanayagam MA, Yap MC, Veldhoen RA, Goping IS, Berthiaume LG. Tandem reporter assay for myristoylated proteins post-translationally (TRAMPP) identifies novel substrates for posttranslational myristoylation: PKCepsilon, a case study. FASEB J. 2012;26:13–28. doi: 10.1096/fj.11-182360. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Mikhaylova L, Malmquist J, Nurminskaya M. Regulation of in vitro vascular calcification by BMP4, VEGF and Wnt3a. Calcif. Tissue Int. 2007;81:372–381. doi: 10.1007/s00223-007-9073-6. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. The intrinsic stiffness of human trabecular meshwork cells increases with senescence. Oncotarget. 2015a;6:15362–15374. doi: 10.18632/oncotarget.3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JT, Raghunathan VK, Chang YR, Murphy CJ, Russell P. Wnt inhibition induces persistent increases in intrinsic stiffness of human trabecular meshwork cells. Exp. Eye Res. 2015b;132:174–178. doi: 10.1016/j.exer.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Shioi A, Jono S, Nishizawa Y, Morii H. Dexamethasone enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:2112–2118. doi: 10.1161/01.atv.19.9.2112. [DOI] [PubMed] [Google Scholar]
- Mostafa NZ, Fitzsimmons R, Major PW, Adesida A, Jomha N, Jiang H, Uludag H. Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor, and bone morphogenetic protein-2. Connect. Tissue Res. 2012;53:117–131. doi: 10.3109/03008207.2011.611601. [DOI] [PubMed] [Google Scholar]
- Nguyen C, Cone FE, Nguyen TD, Coudrillier B, Pease ME, Steinhart MR, Oglesby EN, Jefferys JL, Quigley HA. Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma. Invest. Ophthalmol. Vis. Sci. 2013;54:1767–1780. doi: 10.1167/iovs.12-10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension. 1997;29:999–1006. doi: 10.1161/01.hyp.29.4.999. [DOI] [PubMed] [Google Scholar]
- O'Steen WK, Brodish A. Scleral calcification and photoreceptor cell death during aging and exposure to chronic stress. Am. J. Anat. 1990;189:62–68. doi: 10.1002/aja.1001890108. [DOI] [PubMed] [Google Scholar]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm's canal endothelial cells in glaucoma. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrinely JR, Green WR, Connor JM. Bilateral posterior scleral ossification. Am. J. Ophthalmol. 1982;94:351–356. doi: 10.1016/0002-9394(82)90361-0. [DOI] [PubMed] [Google Scholar]
- Pijanka JK, Kimball EC, Pease ME, Abass A, Sorensen T, Nguyen TD, Quigley HA, Boote C. Changes in scleral collagen organization in murine chronic experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55:6554–6564. doi: 10.1167/iovs.14-15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen JA, Vogt B, Martin PY, Burnier M, Bochud M. Inactive matrix gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension. 2015;66:85–92. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- Price PA, Urist MR, Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem. Biophys. Res. Commun. 1983;117:765–771. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- Raghunathan VK, Morgan JT, Park SA, Weber D, Phinney BS, Murphy CJ, Russell P. Dexamethasone stiffens trabecular meshwork, trabecular meshwork cells, and matrix. Invest. Ophthalmol. Vis. Sci. 2015;56:4447–4459. doi: 10.1167/iovs.15-16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- Robinson RF, Nahata MC, Sparks E, Daniels C, Batisky DL, Hayes JR, Mahan JD. Abnormal left ventricular mass and aortic distensibility in pediatric dialysis patients. Pediatr. Nephrol. 2005;20:64–68. doi: 10.1007/s00467-004-1667-x. [DOI] [PubMed] [Google Scholar]
- Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res. 1989;8:1233–1240. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Schuman JS, Chang W, Wang N, de Kater AW, Allingham RR. Excimer laser effects on outflow facility and outflow pathway morphology. Invest. Ophthalmol. Vis. Sci. 1999;40:1676–1680. [PubMed] [Google Scholar]
- Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol. Med. 2013;19:217–226. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- Shim SH, Kim CY, Kim JM, Kim DY, Kim YJ, Bae JH, Sung KC. The role of systemic arterial stiffness in open-angle glaucoma with diabetes mellitus. Biomed. Res. Int. 2015;2015:425835. doi: 10.1155/2015/425835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Geller AI, Escudero KW, Wilcox CL. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J. Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sung DC, Bowen CJ, Vaidya KA, Zhou J, Chapurin N, Recknagel A, Zhou B, Chen J, Kotlikoff M, Butcher JT. Cadherin-11 overexpression induces extracellular matrix remodeling and calcification in mature aortic valves. Arterioscler. Thromb. Vasc. Biol. 2016;36:1627–1637. doi: 10.1161/ATVBAHA.116.307812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanihara H, Inatani M, Honjo M, Tokushige H, Azuma J, Araie M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008;126:309–315. doi: 10.1001/archophthalmol.2007.76. [DOI] [PubMed] [Google Scholar]
- Thieme H, Nuskovski M, Nass JU, Pleyer U, Strauss O, Wiederholt M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest. Ophthalmol. Vis. Sci. 2000;41:4240–4246. [PubMed] [Google Scholar]
- Tiago DM, Conceicao N, Caiado H, Laize V, Cancela ML. Matrix Gla protein repression by miR-155 promotes oncogenic signals in breast cancer MCF-7 cells. FEBS Lett. 2016;590:1234–1241. doi: 10.1002/1873-3468.12155. [DOI] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp. Eye Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 2008;49:622–628. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Parapuram SK, Tripathi BJ, Zhong Y, Chalam KV. Corticosteroids and glaucoma risk. Drugs Aging. 1999;15:439–450. doi: 10.2165/00002512-199915060-00004. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz K, Oner V, Cicek Y, Kurt A, Durmus M. Systemic arterial stiffness in patients with pseudoexfoliation glaucoma. J. Glaucoma. 2014;23:e108–e111. doi: 10.1097/IJG.0b013e3182955d58. [DOI] [PubMed] [Google Scholar]
- Tüysüz B, Cinar B, Laciner S, Onay H, Mittaz-Crettol L. Clinical variability in two sisters with Keutel syndrome due to a homozygous mutation in MGP gene. Genet. Couns. 2015;26:187–194. [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Visontai Z, Mersich B, Hollo G. Carotid artery elasticity and baroreflex sensitivity in patients with glaucoma. J. Glaucoma. 2005;14:30–35. doi: 10.1097/01.ijg.0000145814.46848.76. [DOI] [PubMed] [Google Scholar]
- Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest. Ophthalmol. Vis. Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- Vittitow J, Borrás T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J. Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- Wallin R, Wajih N, Greenwood GT, Sane DC. Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med. Res. Rev. 2001;21:274–301. doi: 10.1002/med.1010. [DOI] [PubMed] [Google Scholar]
- Wang WH, McNatt LG, Pang IH, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Lütjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest. Ophthalmol. Vis. Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- Wirostko BM, Ehrlich R, Harris A. The vascular theory of glaucoma. Glaucoma Today April. 2009:25–27. [Google Scholar]
- Wirtz MK, Samples JR, Xu H, Severson T, Acott TS. Expression profile and genome location of cDNA clones from an infant human trabecular meshwork cell library. Invest. Ophthalmol. Vis. Sci. 2002;43:3698–3704. [PubMed] [Google Scholar]
- Wong S, Zakov ZN, Albert DM. Scleral and choroidal calcifications in a patient with pseudohypoparathyroidism. Br. J. Ophthalmol. 1979;63:177–180. doi: 10.1136/bjo.63.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Comes N, Borrás T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest. Ophthalmol. Vis. Sci. 2007;48:3184–3194. doi: 10.1167/iovs.06-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Wallin R, Olmsted-Davis EA, Borrás T. Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Invest. Ophthalmol. Vis. Sci. 2006;47:997–1007. doi: 10.1167/iovs.05-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh YT, Hur SS, Chang J, Wang KC, Chiu JJ, Li YS, Chien S. Matrix stiffness regulates endothelial cell proliferation through septin 9. PLoS One. 2012;7:e46889. doi: 10.1371/journal.pone.0046889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi M, Boorman G. Eye and associated glands. In: Boorman G, editor. Pathology of the Fisher Rat. Academic Press; San Diego, CA: 1990. pp. 239–259. [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]